Abstract
This study examined the coupling between visual information and body sway in patients with Parkinson’s disease (PD) compared with healthy controls. Postural control performance was compared between 14 patients with PD (age: 69.6 ± 8.8 years - stages 1 to 3 of the Hoehn and Yahr scale) and 14 healthy control participants (age: 68.6 ± 3.0 years). Participants stood upright in a moving room that remained motionless or continuously oscillated in the anterior-posterior direction. Ten trials were performed in the following conditions: no movement of the room (1 trial) and with the room moving at frequencies of 0.1, 0.17, and 0.5 Hz (3 trials each frequency). Body sway and moving room displacement were recorded. The results indicated that patients with PD displayed larger body sway magnitude in the stationary room condition. Body sway of patients with PD was induced by visual manipulation in all three visual stimulus frequencies, but body sway of patients with PD was less coherent compared to that of the control participants. However, no difference was observed in the visual-body sway coupling structure. These results indicate that patients with PD can unconsciously couple body sway to visual information in order to control postural sway in a similar manner to healthy participants with intact visual-motor coupling for posture control. However, this coupling is marked by greater variability, indicating that people with PD have a motor system with greater inherent noise leading to a more varied behavior.
Keywords: Sensorimotor coupling, posture, vision, Parkinson’s disease
Introduction
Parkinson’s disease (PD) is characterized by disruption of many types of sensorimotor control, including postural control. Although postural instability might not be an initial symptom of the disease [1], it is associated with an increased risk of falling [2] and a decline in the ability to independently perform daily living activities. Even in the initial stages, patients with PD display larger body sway magnitude [3], reduced limits of stability [4,5], and higher incidence of falls [6], which worsen with disease progression. Poor postural control performance in patients with PD is not surprising, considering the many changes in motor [1,7,8] and sensory [9,10] systems.
The use of sensory cues for postural control may be examined by manipulating cues from a specific source, leaving the remaining cues unaltered, and observing the body sway induced by this manipulation [11]. After the pioneering studies of Lee and Lishman [12], the moving room paradigm has been extensively employed to examine and elucidate the underlying aspects of visual-motor coupling in different populations: young [13,14] and older adults [15], typical infants [16,17], infants [18] and adults with Down Syndrome [19], typical children [20], children with cerebral palsy [21], and children with dyslexia [22]. The moving room strategy has also been used to examine the impact of visual flow manipulation on the postural control of patients with PD [23], leading to the finding that patients with PD were susceptible to visual manipulation, as they displayed body sway corresponding to the discrete movement of the room. Such results are surprising at first glance, considering that patients with PD experience several visual changes such as visual acuity, color, and contrast sensitivity [24] that may lead to changes in control of stance and gait [25].
Bronstein et al. [23] also demonstrated that patients with PD exhibited a larger body sway magnitude response when exposed to displacements of the visual environment than other patients, suggesting that patients with PD have an abnormal reliance on visual information for postural control. Following these studies, several reports have suggested that patients with PD are more dependent on visual information, which leads to difficulties in performing certain motor tasks [26–29], most likely to compensate for poor and less informative somatosensory cues [30]. Conversely, visual cues have been used to improve motor performance, particularly gait, as a typical therapeutic approach to minimize the lack of automatic control [31].
Despite these conflicting suggestions regarding the use of visual cues for postural and motor control, overreliance on visual optical flow has been also observed in older adults with no PD. Wade et al. [15] observed that older adults were more influenced by the discrete movement of a moving room than young adults. Similar results were observed when older adults were exposed to discrete [32] and continuous periodic oscillation [33,34] of the moving room. Based on these results, the overreliance due to optical flow manipulation observed in patients with PD [23] might not be due to the disease but due to the natural aging process, which impacts the quality of sensory cues and leads to less accurate information regarding body position [33,34]. In this case, under any visual manipulation, postural control mechanisms would induce an exaggerated response and produce larger body sway in both older adults and in patients with PD of a similar age. Therefore, the aim of this study was to compare postural control performance and the use of visual information in controlling body sway in patients with PD and healthy older adults.
Materials and methods
Participants
Fourteen patients with idiopathic PD (age: 69.6 ± 8.8 years, 4 females and 10 males), who obtained a severity score of 1–3 on the Hoehn and Yahr scale [35] and received dopamine replacement medication, and 14 healthy older people (control group, age: 68.6 ± 3.0 years, 5 females and 9 males) participated in this study. Participants with PD were recruited from the Brazilian Parkinson Association and were tested in their “on levodopa” state. Inclusion criteria involved: (1) idiopathic PD diagnosed by an experienced specialist, following the UK Brain Bank criteria; (2) absence of neurological diseases, except for PD, and detectable sensory and/or motor disturbances in the hands and arms; (3) a minimum score of 24 on the Mini-Mental State Examination; (4) normal or corrected visual acuity; and (5) lack of auditory losses. All these criteria were based upon previous evaluations performed in the Brazilian Parkinson Association. Participants of the control group were recruited using personal contacts. All participants provided informed written consent, according to procedures approved by Institutional Review Ethics Committee.
Procedures
In a single visit to the laboratory, participants were asked to stand inside a moving room. The room consisted of three walls (2 m length, 2 m width, 2 m height) and a ceiling mounted on wheels, allowing for movement in the anterior-posterior (AP) direction while the floor remained motionless. The walls were covered with a pattern of white (33 cm wide) and black (22 cm wide) stripes. The movement of the room was produced by a servomotor mechanism consisting of a linear guide (Ottime, model PL6–90C-LD-MT-RC), stepper motor (Ottime, model SM3452808), and motor drive (Ottime, model MBD-8080DC) controlled by Motion Planner software. Two fluorescent lights (20 W) were placed on the room ceiling to maintain constant illumination.
Participants were asked to stand upright as stable as possible, with their feet placed comfortably at hip width apart, and to look at a target attached to the front wall of the room. An experimenter remained aside and close to the participant to assure that the task requirements were accomplished and in case participants would need any assistance. Participants of both groups performed a total of ten trials of 60 s each. In the first trial, the room remained motionless. The other nine trials were grouped in three blocks of three trials, in which the room oscillated at frequencies of 0.1, 0.17, and 0.5 Hz (one trial at each frequency, in randomized order). The peak-to-peak velocity of 0.6 cm/s was maintained for all three frequencies as amplitude was varied. These frequencies were selected based on the postural sway characteristics during upright stance, aiming to drive the postural control system close to the natural frequency (0.17 Hz) and to frequencies below (0.1 Hz) and above (0.5 Hz) the natural frequency.
All participants were unaware of the movement of the room. In addition, a random sound (white noise) was provided to mask possible auditory cues that emanated from the room. At the end of experimental procedures, participants were asked if they had noted any unusual condition and none of them reported anything related to the movement of the room and, therefore, it was assumed that body sway induced by the visual manipulation occurred unconsciously by the participants.
One infrared emitting diode (IRED) was placed centrally on the participant’s back at the scapula level (~8th thoracic vertebra), and another IRED was placed on the front wall of the room to record body and room position, respectively. One OPTOTRAK™ camera block (Northern Digital Inc., Waterloo, Canada) was positioned behind the participants to track the IREDs at a sampling rate of 100 Hz.
Data analysis
In the stationary room condition, the mean sway amplitude for both AP and medial-lateral (ML) directions was obtained. The mean sway amplitude was calculated by subtracting a first-order polynomial and the average of the time series from each data point and obtaining the standard deviation of the time series, indicating sway variability.
Because the room oscillated in the AP direction, mean sway amplitude in the room oscillation conditions was obtained only for the AP direction. Similarly, the relationship between room movement and postural sway was also obtained only for the AP direction, using coherence, gain, and phase. Coherence indicated the strength of the relationship between room movement and body sway, at the respective frequency of the driving signal in each condition (0.1, 0.17, and 0.5 Hz). Coherence values close to one/zero indicated strong/weak dependency between these two signals, respectively. Gain and phase indicated the magnitude and the temporal influence of room movement on body oscillation. Altogether, these two variables indicated the coupling structure between body sway and visual information. These variables were calculated by obtaining a transfer function (frequency response function), which was computed by dividing the Fourier transforms of body sway by the Fourier transforms of the respective driving signal (moving room). Gain corresponded to the absolute value of the frequency response function, indicating 1 when body sway matched the moving room amplitude, and lower/higher values indicated that the response amplitude was lower/higher than the stimulus driving amplitude. Phase corresponded to the argument of the frequency response function, thereby indicating the temporal relationship between body sway and the moving room position. Phase values of zero indicated that body sway was occurring in-phase with the room movement, and positive/negative phase values indicated that body sway led/lagged behind the room movement, respectively.
All of the above-described procedures were performed using specific custom software written in Matlab (Math Works, Inc.).
Statistical analysis
A multivariate analysis of variance (MANOVA) was performed using group as a factor and the mean sway amplitude, for both AP and ML directions in the no visual manipulation condition, as dependent variables. Two analyses of variance (ANOVA) and one MANOVA were performed using group and frequency (repeated measure) as factors. The dependent variables for each ANOVA were mean sway amplitude, for the AP direction in the visual manipulation conditions, and coherence. The dependent variables for the MANOVA were gain and phase. When applicable, appropriate follow-up univariate analyses and Tukey’s Honestly Significant Difference post hoc tests were performed. The significant level was maintained at 0.05 and analyses were performed using SPSS software.
Results
Body sway: no visual manipulation
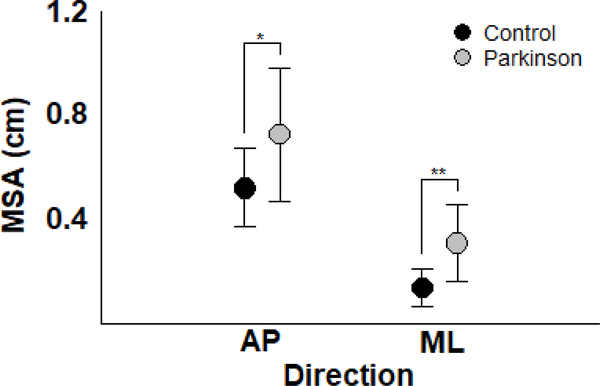
Patients with PD exhibited larger body sway than control participants when the moving room remained stationary. Figure 1 depicts mean sway amplitude in AP and ML directions for both PD and control groups. MANOVA revealed group effect (Wilks’ Lambda=0.625, F(2,25)=7.50, p<0.005). Univariate analysis indicated that patients with PD exhibited a larger magnitude of body sway than the control group in both AP (F(1,26)=6.73, p<0.05) and ML (F(1,26)=15.22, p<0.005) directions.
Figure 1.
Mean and standard deviation of the mean sway amplitude in the anterior-posterior and medial-lateral directions for the Parkinson’s disease and control participants. *p<0.05, **p<0.005.
Coupling between visual manipulation and body sway
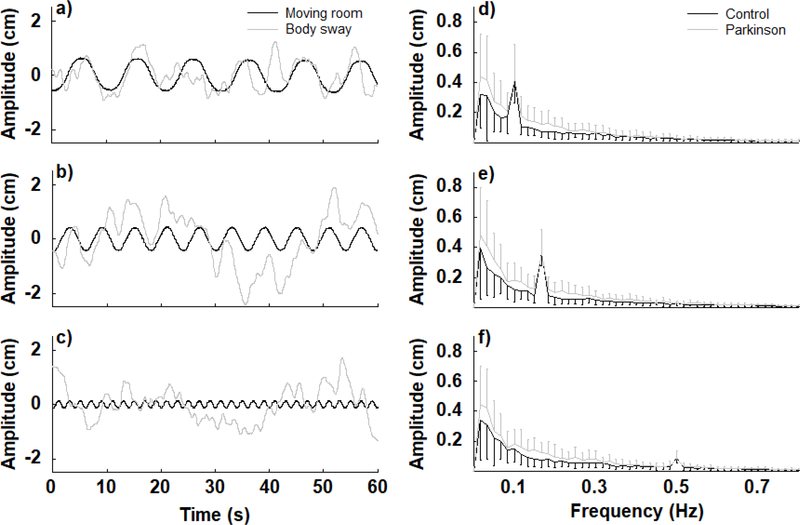
Visual motion induced body sway in both PD and control participants. Figure 2 depicts timeseries of body sway and moving room displacement from a representative participant with PD and the amplitude spectra for both PD and control groups at the three frequencies of the moving room. For both PD and control participants, body sway characteristics changed according to the visual manipulation condition, adopting the same oscillation frequency as the room and depicting a well-defined peak in the spectra according to the frequency that the room oscillated at.
Figure 2.
Time-series of body oscillations and moving room displacements of a representative Parkinson’s disease participant at three room oscillation frequencies (0.1, 0.17, and 0.5 Hz for a,b,c, respectively) and the amplitude spectra for the Parkinson’s disease and control groups at all three room oscillation frequencies (d,e,f).
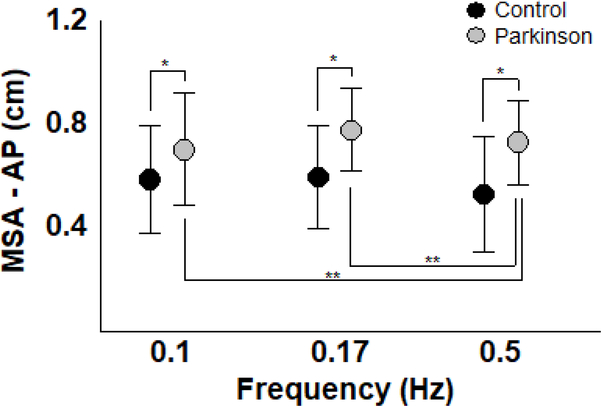
Body sway was largest for the lowest (0.1 Hz) and smallest for the highest (0.5 Hz) visual oscillation frequency for both groups. Figure 3 depicts mean sway amplitude in the AP direction for both PD and control participants. ANOVA revealed a larger body sway magnitude for PD than control participants (F(1,26)=4.47, p<0.05). ANOVA also indicated a frequency effect (F(2,52)=7.89, p<0.005). Post hoc tests indicated that mean sway amplitude for both groups was larger at 0.1 and 0.17 than at 0.5 Hz.
Figure 3.
Mean and standard deviation of the mean sway amplitude in the anterior-posterior direction, across all three moving room frequencies, for the Parkinson’s disease and control groups. *p<0.05, **p<0.005.
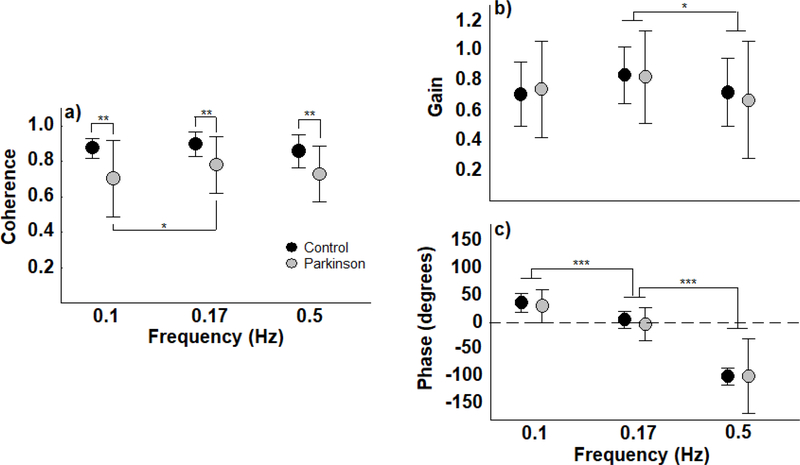
The control group obtained higher coherence values in all three moving room frequencies than the PD group, suggesting stronger coupling between visual information and body sway in the control participants than in the participants with PD. Figure 4 depicts coherence, gain, and phase values between the moving room and body position for both PD and control participants. For coherence, ANOVA revealed group (F(1,26)=8.52, p<0.01) and frequency effect (F(2,52)=4.40, p<0.05). Post hoc tests also indicated that coherence was higher at 0.17 than at 0.1 and 0.5 Hz.
Figure 4.
Mean and standard deviation of coherence, gain, and phase between moving room and body sway position in the anterior-posterior direction, across all three moving room frequencies, for the Parkinson’s disease and control groups. *p<0.05, **p<0.01, ***p<0.001.
The coupling structure between the moving room and body sway was similar for the PD and control participants. MANOVA for gain and phase revealed no group effect (Wilks’ Lambda=0.987, F(2,25)=0.16, p>0.05), but revealed a frequency effect (Wilks’ Lambda=0.061, F(4,23)=89.04, p<0.001). Univariate tests indicated that there was a significant difference among frequencies for both gain (F(1,31)=4.53, p<0.05) and phase (F(1,31)=191.50, p<0.001). Post hoc tests indicated that both groups exhibited higher gain at 0.17 Hz than at 0.5 Hz. Moreover, phase post hoc tests indicated that body sway led the room position at a visual room frequency of 0.1 Hz, was in-phase at a frequency of 0.17 Hz, and lagged behind the room at a frequency of 0.5 Hz.
Discussion
PD reduces postural control performance, such that participants with PD exhibited larger sway magnitude than their peers in both stationary and visual oscillatory environments. Although participants with PD coupled their postural sway to the visual cues with a similar coupling structure as the control participants, with similar gain and phase values observed in both groups, patients with PD exhibited less coherent body sway when exposed to visual manipulation at all frequencies. Based on these results, we suggest that patients with PD do not exhibit any difference in the use of visual cues to control body orientation compared to control participants, albeit with more variable control.
Control of postural sway in stance is reduced in patients with PD [3–5] and our results corroborate such previous observations, as patients with PD were found to sway with larger magnitude in both AP and ML directions than control participants. Our results also indicated that patients with PD swayed with larger magnitude than their peers even when they were exposed to visual manipulation, thereby corroborating the results of previous studies wherein patients with PD were exposed to discrete visual manipulation in the moving room [23]. Our results add to these previous findings, clearly indicating that patients with PD were capable of swaying at the same frequency as the visual stimulus manipulation provided by the moving room in addition to frequencies other than the driving signal, leading to larger sway magnitude (Figure 3).
To our knowledge, this is the first study demonstrating that postural sway in participants with PD is influenced by visual manipulation in a similar manner to their elderly peers. Moreover, our results resemble those of previous studies demonstrating that older people are more visually dependent than younger people, indicating larger postural responses consistent with visual compensation for loss of somatosensory control [15,32]. However, the frequency spectrum indicated a clear peak at the stimulus driving frequency condition, for both older adults and patients with PD, indicating that the visual stimuli induced corresponding body sway for both groups. Based on this observation, we suggest not only that patients with PD can use visual stimuli to drive body sway, but also that such use of visual information occurs across a spectrum of different frequencies in such manner that the postural control system can incorporate visual characteristics into motor action to control body dynamics.
Although patients with PD swayed with larger magnitude, sway induced at specific frequencies by visual manipulation was similar to their peers, with gain and phase values indicating a similar visual stimulus and body sway coupling structure in patients with PD. Similar gain values in patients with PD and older adults indicate that postural control of both groups used the visual stimuli to produce similar sway magnitude at the stimulus frequency [36]. Similarly, similar phase values indicated that, although frequency-dependent, both groups incorporated the visual stimuli to produce sway with the same temporal structure [36] – in this case, producing sway that led, was in-phase, and was behind the visual stimulus, respective to the frequencies employed in this study.
The observation of similar visual and sway coupling structures in patients with PD and control participants is a surprising finding for several reasons. First, it has been suggested that patients with PD are overly reliant on vision for motor and postural control [37] and even for gait navigation [31], due to reduced proprioception [30]. However, if this were the case, we would expect that patients with PD would also produce an exaggerated response to the visual manipulations implemented in this study, and exhibit higher gain values than their peers. Similar gain values between the PD and the control group across all three visual stimulus frequencies contradict such a suggestion, and indicate that the postural control system of patients with PD uses visual cues to control postural sway in a similar manner to that of the healthy older adults. Over-reliance on vision has been observed in conditions in which patients with PD need to discriminate stance surface changes [38] or navigate cluttered environments [39]; such situations also involve discriminative perception and judgment. In contrast, our moving room strategy does not involve discriminative perception, with participants being influenced by visual manipulation with no conscious awareness of body sway induction [40,41], as was the case for all participants in the present study. Therefore, the issue that patients with PD are overly reliant on visual cues needs to be better examined in order to further understand this notion and to uncover the basis for such use of visual cues in different specific environmental conditions.
The observation that phase values were comparable in both groups across all three visual frequencies also provides important information regarding the mechanisms underlying the use of visual cues in patients with PD. Phase frequency-dependent values, with participants’ oscillations leading the moving room at the lowest frequency (0.1 Hz), in-phase with the moving room at 0.17 Hz, and lagging behind at the highest frequency (0.5 Hz), are comparable to several other studies using visual [42] and somatosensory [43] stimuli, indicating that participants couple to both position and velocity of these stimuli [36]. Based on our present results, we can suggest that the mechanisms underlying the postural control system coupling with visual stimuli in patients with PD functions in a similar manner to that of their peers. This indicates that these mechanisms are intact and have not being affected by the loss of dopamine in this disease.
Although our results clearly indicate that patients with PD couple visual information to body sway in a similar manner to their peers, a previous study demonstrated that optic flow generates a larger than normal response in subjects with PD, albeit with reduced visual cortex activation [44]. A possible explanation for such divergent results is that the visual-body sway coupling in our study may have occurred automatically, reflecting intrinsic central nervous system dynamics with little (if any) cognitive or attentional effort [45]. Automatic visual-postural coupling is rather clever because it avoids overloading the higher centers of the central nervous system with control of low-level tasks, such as using environmental sensory cues to control postural orientation. If this is the case, these sensory-motor properties appear to be intact or at least still functioning efficiently in patients with PD. There is a need, although, to examine these sensory-motor properties in conditions of more challenging stimulus (e.g. complex and non-periodic visual trials) and such tests are underway.
Considering the high impact of postural instability on the ability to independently perform daily living activities and on the quality of life in patients with PD, clarification of the role of visual cues in compensating for the deficiency in automaticity or sensory integration in motor control tasks, such as maintaining upright stance, may contribute to the improvement of rehabilitation approach efficacy.
Highlights.
Patients with PD show larger body sway magnitude than control participants.
Visual manipulation induces correspondent sway in patients with PD.
Coupling structure between visual information and body sway is unaltered in participants with PD.
Patients with PD show unaltered automatic visual-motor coupling in postural control under visual manipulation.
Results question any significant change in sensory integration in patients with PD due to overreliance on visual stimuli.
Acknowledgments
The authors thank Milena Razuk, Ivan E.P. Vargas, and Giovanna G. Genoves for assistance in data collection. This work was supported in part by Higher Education Personnel Improvement Coordination (Capes) of the Brazilian Federal Government and the NIH National Institutes on Aging R01 AG006457 29.
Footnotes
Declarations of interest
The authors have no conflict of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Mazzoni P, Shabbott B, Cortes JC, Motor control abnormalities in Parkinson’s disease, Cold Spring Harb. Perspect. Med 2 (2012) 1–17. doi: 10.1101/cshperspect.a009282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM, Silburn PA, Predictors of future falls in Parkinson disease, Neurology. 75 (2010) 116–124. doi: 10.1212/WNL.0b013e3181e7b688. [DOI] [PubMed] [Google Scholar]
- [3].Horak FB, Dimitrova D, Nutt JG, Direction-specific postural instability in subjects with Parkinson’s disease, Exp. Neurol 193 (2005) 504–521. doi: 10.1016/j.expneurol.2004.12.008. [DOI] [PubMed] [Google Scholar]
- [4].Doná F, Aquino CC, Gazzola JM, Borges V, Silva SMCA, Ganança FF, Caovilla HH, Ferraz HB, Changes in postural control in patients with Parkinson’s disease: a posturographic study, Physiotherapy. 102 (2016) 272–279. doi: 10.1016/j.physio.2015.08.009. [DOI] [PubMed] [Google Scholar]
- [5].Horak FB, Nutt JG, Nashner LM, Postural inflexibility in parkinsonian subjects, J. Neurol. Sci 111 (1992) 46–58. doi: 10.1016/0022-510X(92)90111-W. [DOI] [PubMed] [Google Scholar]
- [6].Allen NE, Schwarzel AK, Canning CG, Recurrent falls in Parkinson’s disease: a systematic review, Parkinsons. Dis 2013 (2013) 1–16. doi: 10.1155/2013/906274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Carpenter MG, Allum JHJ, Honegger F, Adkin AL, Bloem BR, Postural abnormalities to multidirectional stance perturbations in Parkinson’s disease, J. Neurol. Neurosurg. Psychiatry 75 (2004) 1245–1254. doi: 10.1136/jnnp.2003.021147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dimitrova D, Horak FB, Nutt JG, Postural muscle responses to multidirectional translations in patients with Parkinson’s disease, J. Neurophysiol 91 (2004) 489–501. doi: 10.1152/jn.00094.2003. [DOI] [PubMed] [Google Scholar]
- [9].Bertolini G, Wicki A, Baumann CR, Straumann D, Palla A, Impaired tilt perception in Parkinson’s disease: a central vestibular integration failure, PLoS One. 10 (2015) e0124253. doi: 10.1371/journal.pone.0124253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Konczak J, Krawczewski K, Tuite P, Maschke M, The perception of passive motion in Parkinson’s disease, J. Neurol 254 (2007) 655–663. doi: 10.1007/s00415-006-0426-2. [DOI] [PubMed] [Google Scholar]
- [11].Schöner G, Dijkstra TMH, Jeka JJ, Action-perception pattterns emerge from coupling and adaptation, Ecol. Psychol 10 (1998) 323–346. [Google Scholar]
- [12].Lee DN, Lishman JR, Visual proprioceptive control of stance, J. Hum. Mov. Stud 1 (1975) 87–95. [Google Scholar]
- [13].Barela AMF, Barela JA, Rinaldi NM, Toledo DR, Influence of imposed optic flow characteristics and intention on postural responses, Motor Control. 13 (2009) 119–129. [DOI] [PubMed] [Google Scholar]
- [14].Jeka JJ, Oie KS, Kiemel T, Asymmetric adaptation with functional advantage in human sensorimotor control, Exp. Brain Res 191 (2008) 453–463. doi: 10.1007/s00221-008-1539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wade MG, Lindquist R, Taylor JR, Treat-Jacobson D, Optical flow, spatial orientation, and the control of posture in the elderly, Journals Gerontol. Ser. B Psychol. Sci. Soc. Sci 50B (1995) P51–P54. doi: 10.1093/geronb/50B.1.P51. [DOI] [PubMed] [Google Scholar]
- [16].Barela JA, Godoi D, Freitas Júnior PB, Polastri PF, Visual information and body sway coupling in infants during sitting acquisition, Infant Behav. Dev 23 (2000) 285–297. [Google Scholar]
- [17].Bertenthal BI, Boker SM, Xu M, Analysis of the perception-action cycle for visually induced postural sway in 9-month-old sitting infants, Infant Behav. Dev 23 (2000) 299–315. doi: 10.1016/S0163-6383(01)00046-7. [DOI] [Google Scholar]
- [18].Polastri PF, Barela JA, Perception-action coupling in infants with Down syndrome: effects of experience and practice, Adapt. Phys. Act. Q 22 (2005) 39–58. [Google Scholar]
- [19].Gomes MM, Moraes R, Barela JA, Coupling between visual information and body sway in adults with Down syndrome, Res. Dev. Disabil 58 (2016) 9–19. doi: 10.1016/j.ridd.2016.08.011. [DOI] [PubMed] [Google Scholar]
- [20].Rinaldi NM, Polastri PF, Barela JA, Age-related changes in postural control sensory reweighting, Neurosci. Lett 467 (2009) 225–229. doi: 10.1016/j.neulet.2009.10.042. [DOI] [PubMed] [Google Scholar]
- [21].Barela JA, Focks GMJ, Hilgeholt T, Barela AMF, de P. Carvalho R, Savelsbergh GJP, Perception–action and adaptation in postural control of children and adolescents with cerebral palsy, Res. Dev. Disabil 32 (2011) 2075–2083. doi: 10.1016/j.ridd.2011.08.018. [DOI] [PubMed] [Google Scholar]
- [22].Barela JA, Dias JL, Godoi D, Viana AR, de Freitas PB, Postural control and automaticity in dyslexic children: the relationship between visual information and body sway, Res. Dev. Disabil 32 (2011) 1814–1821. doi: 10.1016/j.ridd.2011.03.011. [DOI] [PubMed] [Google Scholar]
- [23].Bronstein AM, Hood JD, Gresty MA, Panagi C, Visual control of balance in cerebellar and parkinsonian syndromes, Brain. 113 (1990) 767–779. doi: 10.1093/brain/113.3.767. [DOI] [PubMed] [Google Scholar]
- [24].Weil RS, Schrag AE, Warren JD, Crutch SJ, Lees AJ, Morris HR, Visual dysfunction in Parkinson’s disease, Brain. 139 (2016) 2827–2843. doi: 10.1093/brain/aww175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Piro A, Tagarelli A, Nicoletti G, Fletcher R, Quattrone A, Color vision impairment in Parkinson’s disease, J. Parkinsons. Dis 4 (2014) 317–9. [DOI] [PubMed] [Google Scholar]
- [26].Jacobs JV, Horak FB, Abnormal proprioceptive-motor integration contributes to hypometric postural responses of subjects with Parkinson’s disease, Neuroscience. 141 (2006) 999–1009. [DOI] [PubMed] [Google Scholar]
- [27].Caudron S, Guerraz M, Eusebio A, Gros JP, Azulay JP, Vaugoyeau M, Evaluation of a visual biofeedback on the postural control in Parkinson’s disease, Neurophysiol. Clin. Neurophysiol 44 (2014) 77–86. doi: 10.1016/j.neucli.2013.10.134. [DOI] [PubMed] [Google Scholar]
- [28].Pieruccini-Faria F, Ehgoetz Martens KA, Silveira CR, Jones JA, Almeida QJ, Interactions between cognitive and sensory load while planning and controlling complex gait adaptations in Parkinson’s disease, BMC Neurol. 14 (2014) 250. doi: 10.1186/s12883-014-0250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Panyakaew P, Anan C, Bhidayasiri R, Visual deprivation elicits subclinical postural inflexibilities in early Parkinson’s disease, J. Neurol. Sci 349 (2015) 214–219. doi: 10.1016/j.jns.2015.01.022. [DOI] [PubMed] [Google Scholar]
- [30].Vaugoyeau M, Viel S, Assaiante C, Amblard B, Azulay JP, Impaired vertical postural control and proprioceptive integration deficits in Parkinson’s disease, Neuroscience. 146 (2007) 852–863. doi: 10.1016/j.neuroscience.2007.01.052. [DOI] [PubMed] [Google Scholar]
- [31].Almeida QJ, Bhatt H, A manipulation of visual feedback during gait training in Parkinson’s disease, Parkinsons. Dis 2012 (2012) 1–7. doi: 10.1155/2012/508720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Prioli AC, Freitas Júnior PB, Barela JA, Physical activity and postural control in the elderly: coupling between visual information and body sway, Gerontology. 51 (2005) 145–148. doi: 10.1159/000083984. [DOI] [PubMed] [Google Scholar]
- [33].Toledo DR, Barela JA, Sensory and motor differences between young and older adults: somatosensory contribution to postural control, Rev. Bras. Fisioter 14 (2010) 267–75. http://www.ncbi.nlm.nih.gov/pubmed/20730372. [PubMed] [Google Scholar]
- [34].Toledo DR, Barela JA, Age-related differences in postural control: effects of the complexity of visual manipulation and sensorimotor contribution to postural performance, Exp. Brain Res 232 (2014) 493–502. doi: 10.1007/s00221-013-3756-1. [DOI] [PubMed] [Google Scholar]
- [35].Hoehn MM, Yahr MD, Parkinsonism: onset, progression, and mortality, Neurology. 17 (1967) 427–442. http://www.neurology.org/content/17/5/427.citation. [DOI] [PubMed] [Google Scholar]
- [36].Jeka JJ, Oie KS, Schöner G, Dijkstra T, Henson E, Position and velocity coupling of postural sway to somatosensory drive, J. Neurophysiol 79 (1998) 1661–1674. [DOI] [PubMed] [Google Scholar]
- [37].Park J-H, Kang Y-J, Horak FB, What is wrong with balance in Parkinson’s disease?, J. Mov. Disord 8 (2015) 109–114. doi: 10.14802/jmd.15018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wright WG, Gurfinkel V, King L, Horak F, Parkinson’s disease shows perceptuomotor asymmetry unrelated to motor symptoms, Neurosci. Lett 417 (2007) 10–15. doi: 10.1016/j.neulet.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Beck EN, Ehgoetz Martens KA, Almeida QJ, Freezing of gait in Parkinson’s disease: an overload problem?, PLoS One. 10 (2015) e0144986. doi: 10.1371/journal.pone.0144986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Barela JA, Weigelt M, Polastri PF, Godoi D, Aguiar SA, Jeka JJ, Explicit and implicit knowledge of environment states induce adaptation in postural control, Neurosci. Lett 566 (2014) 6–10. doi: 10.1016/j.neulet.2014.02.029. [DOI] [PubMed] [Google Scholar]
- [41].Freitas PB Júnior, Barela JA, Postural control as a function of self- and object-motion perception, Neurosci. Lett 369 (2004) 64–68. doi: 10.1016/j.neulet.2004.07.075. [DOI] [PubMed] [Google Scholar]
- [42].Dijkstra TMH, Schöner G, Giese MA, Gielen CCAM, Frequency dependence of the action-perception cycle for postural control in a moving visual environment: relative phase dynamics, Biol. Cybern 71 (1994) 489–501. doi: 10.1007/BF00198467. [DOI] [PubMed] [Google Scholar]
- [43].Jeka JJ, Ribeiro P, Oie KS, Lackner JR, The structure of somatosensory information for human postural control, Motor Control. 2 (1998) 13–33. doi: 10.1123/mcj.2.1.13. [DOI] [PubMed] [Google Scholar]
- [44].Van Der Hoorn A, Renken RJ, Leenders KL, De Jong BM, Parkinson-related changes of activation in visuomotor brain regions during perceived forward self-motion, PLoS One. 9 (2014). doi: 10.1371/journal.pone.0095861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Genoves GG, Barela AMF, Sanches C, Barela JA, Attentional artifacts in sensorimotor coupling in the postural control of young adults, Exp. Brain Res 234 (2016) 3641–3647. doi: 10.1007/s00221-016-4762-x. [DOI] [PubMed] [Google Scholar]