Abstract
Introduction:
Leptomeningeal carcinomatosis (LM) is a severe complication of NSCLC historically associated with poor prognosis. New chemotherapeutic and targeted treatments could potentially impact the natural history of LM.
Methods:
Patients with a pathologic diagnosis of NSCLC with LM treated at Stanford between 2003 and 2011 were identified via institutional databases and medical records. LM was defined by positive CSF for malignant cells or LM enhancement by MRI with gadolinium contrast. Retrospective, landmark analyses were performed to estimate survival. Statistical analyses performed using SAS Enterprise Guide v4.3.
Results:
LM was identified in 30 patients. All cases were adenocarcinoma, 60% of patients had a known or suspected driver mutations, mean age was 58, 67% were women, 70% were non-smokers, 27% initially presented with LM, 84% received systemic treatment at or after development of LM and 53% of these patients received modern systemic therapy for their LM defined as a regimen containing pemetrexed, bevacizumab or a tyrosine kinase inhibitor.
Mean OS after LM diagnosis was 6 months (95% CI 3–12 months). Patients who received modern systemic treatment for LM had decreased hazard of death (HR 0.24, p=0.007).
Conclusion:
In this retrospective, single institution analysis median survival with LM was higher compared with historical experience. Patients who received modern systemic treatment for their LM had particularly good outcomes. Our data provides evidence for improving survival outcomes in the modern treatment era for this difficult to treat complication.
Keywords: Leptomeningeal Carcinomatosis, Chemotherapy, Non-Small Cell Lung Cancer
MicroAbstract
LM is a severe complication of NSCLC historically associated with poor prognosis. New chemotherapeutic agents and targeted treatments could potentially impact the natural history of LM. Our data in 30 NSCLC patients with LM provides evidence for improving survival outcomes in the modern treatment era for this difficult to treat complication.
Introduction:
Of the over 220,000 cases of new lung cancer diagnosed in the United States annually, over 85% of cases are non-small cell lung cancer (NSCLC), and 30–40% of those patients will develop central nervous system (CNS) metastases1. Parenchymal brain metastases represent the vast majority of CNS disease in NSCLC: only about 510% of these patients will develop leptomeningeal carcinomatosis (LM)2,3.
LM is a devastating complication of non-small cell lung cancer historically associated with poor prognosis. A recent retrospective analysis of LM outcomes in NSCLC indicated a poor median survival for patients with LM of only 3 months and no difference in survival in patients who received whole brain radiotherapy. There was, however, a survival benefit in the small number of patients who received intrathecal chemotherapy, but this may be due to selection bias4. A Korean retrospective analysis showed a longer median survival for NSCLC patients with LM of 4.3 months and an overall survival benefit in patients with a good performance status who received intrathecal chemotherapy, epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) therapy or systemic chemotherapy with modern treatment5.
Recently approved chemotherapeutics and targeted agents have improved survival and clinical outcomes in patients with NSCLC. Newly FDA approved agents over the past decade include: the EGFR-inhibitors erlotinib and afatinib, pemetrexed and bevacizumab for non-squamous NSCLC, nab-paclitaxel and the ALK targeted agent crizotinib for patients with ALK-rearranged NSCLC. Pemetrexed improves survival in non-squamous NSCLC patients both in the frontline and relapsed/refractory setting and has activity in patients with CNS metastases6–9. EGFR-TKIs substantially improve progression free survival in patients whose tumors harbor EGFR-activating mutations10,11. Erlotinib, in particular, has demonstrated CSF penetration and CNS activity; pulsatile dosing schemes have been utilized to treat CNS disease with some effect12,13. Bevacizumab improves survival in patients with metastatic non-squamous NSCLC when given in combination with first-line carboplatin and paclitaxel14. Though bevacizumab is contraindicated in untreated parenchymal brain metastases, it is safe in treated stable brain metastases15. Bevacizumab also demonstrated activity in CNS choroidal metastases and is used to treat radiation necrosis of the brain and glioblastoma multiforme16–18. Recent data also support the safety of this agent, and even efficacy, in patients with untreated asymptomatic brain metastases19.
These targeted agents and chemotherapeutics may alter the natural history of LM in NSCLC. We undertook this retrospective analysis to explore how adoption of these new therapies potentially improve outcomes of NSCLC patients with LM in a US population.
Methods:
Patients with a pathologic diagnosis of NSCLC with LM who were treated at Stanford University Medical Center and Clinics between 2003 and 2012 were identified via institutional databases and medical records under an approved Stanford University School of Medicine Institutional Review Board Protocol. Identified cases had pathology proven NSCLC with either positive CSF for malignant or atypical cells or LM enhancement by MRI with gadolinium contrast in the appropriate clinical context (NSCLC diagnosis with no other apparent cause of LM). Medical records were reviewed for patient demographics, pathologic characteristics, treatment regimens and clinical outcomes. Patients where molecular testing was not available, but whose tumor demonstrated radiographic response to an EGFR-TKI were defined as having “suspected” EGFR activating mutations. Known or suspected EGFR activating mutations was used since many patients in this analysis were treated with an EGFR-TKI before EGFR mutation testing to select for EGFR-TKI therapy became standard of care. Patients were considered to receive modern systemic treatment for LM if they received pemetrexed, bevacizumab and/or a tyrosine kinase inhibitor (either erlotinib, gefitinib or crizotinib) for treatment of LM.
Retrospective, landmark analyses were performed to estimate survival from the time of LM diagnosis using Kaplan-Meier method. A two sided p-value of < 0.05 was considered significant. Overall survival from development of LM and from development of initial metastatic disease was examined. Treatment for LM was defined as therapy given for LM after diagnosis as noted in the medical record. Time to LM was calculated as the time of metastatic diagnosis on imaging to time to development of LM. A limited cox-regression was performed to estimate hazard ratios (HR) of factors hypothesized to impact survival with LM. The proportionalhazards assumption was checked using Schoenfeld residuals and was found to be valid for all factors in the forms presented. Statistical analyses were performed using SAS Enterprise Guide v4.3 (Cary, NC).
Results:
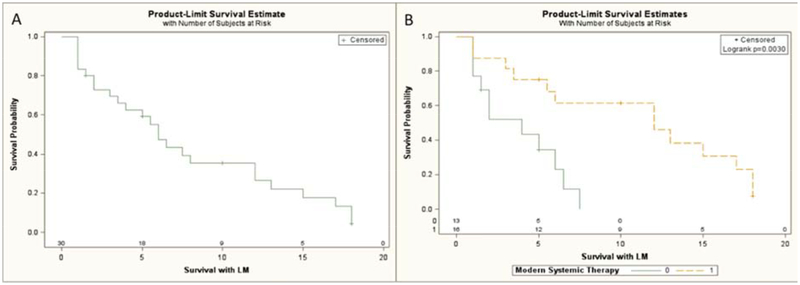
The demographics and molecular alterations of the thirty identified NSCLC patients diagnosed with LM are presented in tables 1 and 2. Table 3 summarizes the treatments these patients received. Patients had a median survival of 6 months from time of LM diagnosis (95% CI 3–12 months, Figure 1a). Patients who received modern systemic treatment for their LM (defined as either a erlotinib, gefitinib, pemetrexed, bevacizumab or crizotinib) had a prolonged survival with LM compared to patients who did not receive these treatments (Fig 1b, p=0.003 logrank). All patients in this cohort had adenocarcinoma histology. Mean time to development of LM from initial diagnosis of metastatic lung adenocarcinoma was lengthy at 16.4 months (95% CI: 11.3 – 21.5 months). A univariate cox-regression of pertinent factors thought to be associated with prolonged survival with LM highlights that patients diagnosed with LM at metastatic diagnosis (HR 0.063, p=0.008) or who received modern systemic treatment for LM (HR 0.24, p=0.007) had the best overall survival with LM (Table 4). Patient characteristics comparing groups who received or did not receive modern systemic treatment for LM are highlighted in Table 5. Forty-three percent of patients who did not receive modern systemic treatment received other older chemotherapy regimens and 71% received whole brain radiotherapy for LM.
Table 1:
Clinical and Tumor Characteristics of NSCLC Patients Diagnosed with Leptomeningeal Carcinomatosis.
| Patient Characteristic | Percent (Number/Total) |
|---|---|
| Number of Patients | 30 |
| Adenocarcinoma Histology | 100% (30/30) |
| Women | 67% (20/30) |
| Age (Mean) | 58 |
| Stage IV at NSCLC Diagnosis | 73% (22/30) |
| Current or Former Smoker | 30% (9/30) |
| LM Disease at Time of Metastatic Presentation | 27% (8/30) |
| Diagnosis by MRI | 97% (29/30) |
| Diagnosis by Lumbar Puncture | 33% (10/30) |
| ECOG PS at LM Diagnosis | |
| - ECOG PS 0–1 | 46% (14/30) |
| - ECOG PS ≥ 2 | 54% (16/30) |
| Neurologic Symptoms | 70% (21/30) |
| Brain metastases | |
| - Prior to LM Diagnosis | 50% (11/22) |
| - At Diagnosis of LM | 73% (22/30) |
| Race | |
| - Asian | 53% (16/30) |
| - White | 37% (11/30) |
| - Hispanic | 7% (2/30) |
| - Not Known | 3% (1/30) |
Table 2:
Molecular Alterations Present in Tumor of Patients with LM. a. Suspected EGFR activating mutations defined as a patient who had a documented response to gefitinib or erlotinib on imaging in the absence of EGFR mutation testing.
| Molecular Alteration | Percent (number/total) |
|---|---|
| - K-ras mutation | 7% (2/30) |
| - Known or Suspected EGFR activating mutationa | 43% (13/30) |
| - ALK translocation | 7% (2/30) |
| - EGFR Exon 20 insertion/PI3K mutation | 3% (1/30) |
| - None/Unknown | 40% (12/30) |
Table 3:
Treatment Received in NSCLC Patients Diagnoses with LM.
| Treatment | Percent (Number/Total) |
|---|---|
| Whole Brain Radiotherapy | |
| - Before LM | 10% (3/22) |
| - At/During LM | 63% (19/30) |
| Intrathecal Chemotherapy | 7% (2/30) |
| Chemotherapy or TKI for LM | 70% (21/30) |
| Pemetrexed or TKI or Bevacizumab at/during LM diagnosis | 53% (16/30) |
| Pemetrexed or TKI or Bevacizumab prior to LM diagnosis | 82% (18/22) |
| Pemetrexed or TKI or Bevacizumab at any Point in Treatment | 87% (26/30) |
Figure 1:
Overall Survival from: A) Time of LM Diagnosis of Patients Diagnosed with Leptomeningeal Carcinomatosis. B) Time of LM Diagnosis of Patients Who Received Modern Systemic Therapy for LM (Orange) Compared to Patients Who Did Not Receive Modern Systemic Therapy for LM (Green).
Table 4:
Cox-Regression of Pertinent Factors Associated With Survival With LM. a) Modern systemic treatment includes patients who received pemetrexed, bevacizumab and/or a tyrosine kinase inhibitor (either erlotinib, gefitinib or crizotinib for treatment of LM). b) Suspected EGFR activating mutations were patients who had a response to an EGFR-TKI, by imaging but were not formally tested for an EGFR activating mutation.
| Variable | HR | 95% CI | p-value | Number/Total |
|---|---|---|---|---|
| LM at Diagnosis | 0.062 | (0.008–0.492) | 0.008 | 8/30 |
| Any Systemic Treatment for LM | 0.415 | (0.162–1.066) | 0.068 | 21/30 |
| Modern Systemic Treatment for LMa | 0.24 | (0.087–0.69) | 0.007 | 16/30 |
| Whole Brain Radiation Treatment for LM | 0.93 | (0.391–2.251) | 0.87 | 19/30 |
| Known Or Suspected EGFR Activating Mutationsb | 0.99 | (0.445–2.186) | 0.97 | 13/30 |
Table 5:
Clinical and Tumor Characteristics of NSCLC Patients Whether Patients Received Modern Systemic Treatment for LM. Modern systemic treatment includes patients who received pemetrexed, bevacizumab and/or a tyrosine kinase inhibitor (either erlotinib, gefitinib or crizotinib) for treatment of LM.
| Patient Characteristic | Modern Systemic Treatment (N=16) | No Modern Systemic Treatment (N=14) |
|---|---|---|
| Women | 62% (10/16) | 71% (10/14) |
| Age (Mean) | 54 | 60 |
| Current or Former Smoker | 25% (4/16) | 50% (5/14) |
| ECOG PS at LM Diagnosis | ||
| - ECOG PS 0–1 | 44% (7/16) | 50% (7/14) |
| - ECOG PS ≥ 2 | 56% (9/16) | 50% (7/14) |
| Whole Brain Radiotherapy | 56% (9/16) | 71% (10/14) |
| Systemic Therapy for LM | 100% (16/16) | 43% (6/14) |
| Known of Suspected EGFR activating mutation | 56% (9/16) | 29% (4/14) |
Discussion:
In this single institution, retrospective analysis we observed a lengthy median survival with LM of 6 months, which compares favorably to previously published median overall survivals with LM4. Other recent retrospective analysis of patients with LM in the modern treatment era show similar results in an Asian patient population5. Our results in a US patient population provide further evidence (albeit retrospective) that clinical outcomes of NSCLC patients with LM may be improving.
The population with LM described in this analysis (Tables 1 and 2) is not the typical metastatic NSCLC population (even for lung adenocarcinoma) with high percentages of women, Asian ethnicity, non-smokers and patients with known or suspected oncogenic driver mutations. This may be partly reflective of the lung cancer population seen at our institution or may represent a population more prone to develop LM. A recently published trial of NSCLC patients with CNS metastases treated with erlotinib and concurrent whole brain radiotherapy, where a high percentage (50%) of enrolled patients had tumors with EGFR-activating mutations, also supports the hypothesis that certain lung adenocarcinoma subtypes and populations may be more predisposed to developing CNS metastases.20. Patients who developed LM after initial diagnosis of metastatic lung cancer had a prolonged time to development of LM (mean 16.4 months (95% CI: 11.3 – 21.5 months)), suggesting that LM may be a late complication of advanced NSCLC in many patients and thus may occur more frequently in patient populations that often do well with treatment for long periods of time (women, non-smokers, patients with EGFR and ALK driver mutations). A similar analysis from Korea showed a prolonged time to development of LM in metastatic patients with lung cancers harboring EGFR activating mutations and treated with EGFR-TKI (mean time to LM > 21 months)21.
In patients who had LM at the time of diagnosis of metastatic disease, median survival with LM was particularly lengthy at 18 months. A recent analysis of another institutions experience with LM correlated intrathecal treatment (IT) of LM with increased overall survival. Only two patients in our cohort were treated with IT chemotherapy, limiting our analysis of outcomes for this treatment modality4. Another study from Korea showed WBRT or systemic chemotherapy was associated with improved overall survival in NSCLC patients with LM5. Analysis of our cohort also suggests that patients who received systemic treatment for LM, especially with modern or targeted therapy did well with a decreased hazard of death that was statistically significant. These differences in survival comparing patients who received modern systemic therapy with those who did not could be biased due to the retrospective nature of our study, but the magnitude of the effect is noteworthy. Confounding by performance status between does not account for these differences as 56% of patients who received modern systemic therapy had Zubrod PS > 1 compared to 50% for patients who did not receive these modern therapies (Table 5). There were some differences in frequency between these treatment groups in regarding whole brain radiotherapy or suspected EGFR activating mutations, but Cox Regression did not show that they affected overall survival, though the sample size of this retrospective analysis was too small to reach a definitive conclusion (Tables 4, 5). Many of the patients who did not receive modern systemic therapy for LM received whole brain radiotherapy (71%) or older chemotherapies (43%). Multivariate analysis was not performed due to the small sample size of patients analyzed.
Conclusion:
Prospective clinical trials are difficult in this patient population. Thus, we must rely on retrospective analyses like this one and others that are limited by small sample size and biases that include: selection, lead and length time bias. Nevertheless, we see a striking lengthening of survival in patients with LM compared to historical controls, particularly in patients with LM at diagnosis who received systemic treatment and patients with LM who received modern treatment regimens—including systemic therapies with CNS activity. Thus the magnitude of the effect we observed in this retrospective analysis suggests that systemic therapy, particularly with modern agents in not heavily pretreated patients can have good survival outcomes compared with the historically poor outcomes of this uncommon complication of non-small cell lung cancer.
Clinical Practice Points:
Leptomeningeal carcinomatosis in NSCLC has historically indicated a poor prognosis. Compared to historical controls this retrospective study suggests that prognosis for these patients may be improving. Patients with LM who are naïve to treatment at the time of LM diagnosis may preferentially benefit from systemic treatment. Modern systemic treatments may be improving patient outcomes in this difficult to treat patient population.
Acknowledgements:
We are grateful for the statistical assistance of Ray Balise, Ph.D in the Department of Health Research and Policy at Stanford University School of Medicine.
The project was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant 5 KL2 RR025743 (JWR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
None of the authors have any conflicts of interest related to the content of this manuscript.
References:
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. January 2013;63(1):11–30. [DOI] [PubMed] [Google Scholar]
- 2.Chen AM, Jahan TM, Jablons DM, Garcia J, Larson DA. Risk of cerebral metastases and neurological death after pathological complete response to neoadjuvant therapy for locally advanced nonsmall-cell lung cancer: clinical implications for the subsequent management of the brain. Cancer. April 15 2007;109(8):1668–1675. [DOI] [PubMed] [Google Scholar]
- 3.Eichler AF, Kahle KT, Wang DL, et al. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro-oncology. November 2010;12(11):1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris PG, Reiner AS, Szenberg OR, et al. Leptomeningeal Metastasis from Non-small Cell Lung Cancer: Survival and the Impact of Whole Brain Radiotherapy. J Thorac Oncol. November 15 2011. [DOI] [PubMed] [Google Scholar]
- 5.Park JH, Kim YJ, Lee JO, et al. Clinical outcomes of leptomeningeal metastasis in patients with non-small cell lung cancer in the modern chemotherapy era. Lung Cancer. June 2012;76(3):387–392. [DOI] [PubMed] [Google Scholar]
- 6.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. July 20 2008;26(21):3543–3551. [DOI] [PubMed] [Google Scholar]
- 7.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. October 24 2009;374(9699):1432–1440. [DOI] [PubMed] [Google Scholar]
- 8.Bearz A, Garassino I, Tiseo M, et al. Activity of Pemetrexed on brain metastases from Non-Small Cell Lung Cancer. Lung Cancer. May 2010;68(2):264–268. [DOI] [PubMed] [Google Scholar]
- 9.Barlesi F, Gervais R, Lena H, et al. Pemetrexed and cisplatin as first-line chemotherapy for advanced non-small-cell lung cancer (NSCLC) with asymptomatic inoperable brain metastases: a multicenter phase II trial (GFPC 07–01). Ann Oncol. November 2011;22(11):2466–2470. [DOI] [PubMed] [Google Scholar]
- 10.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. September 3 2009;361(10):947–957. [DOI] [PubMed] [Google Scholar]
- 11.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. March 2012;13(3):239–246. [DOI] [PubMed] [Google Scholar]
- 12.Grommes C, Oxnard GR, Kris MG, et al. “Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neurooncology. December 2011;13(12):1364–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke JL, Pao W, Wu N, Miller VA, Lassman AB. High dose weekly erlotinib achieves therapeutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer. J Neurooncol. September 2010;99(2):283–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. December 14 2006;355(24):2542–2550. [DOI] [PubMed] [Google Scholar]
- 15.Socinski MA, Langer CJ, Huang JE, et al. Safety of bevacizumab in patients with non-small-cell lung cancer and brain metastases. J Clin Oncol. November 1 2009;27(31):5255–5261. [DOI] [PubMed] [Google Scholar]
- 16.Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. April 1 2011;79(5):1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George B, Wirostko WJ, Connor TB, Choong NW. Complete and durable response of choroid metastasis from non-small cell lung cancer with systemic bevacizumab and chemotherapy. J Thorac Oncol. May 2009;4(5):661–662. [DOI] [PubMed] [Google Scholar]
- 18.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. February 10 2009;27(5):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benjamin Besse SLM, Hélène Senellart, Julien Mazieres, Fabrice Barlesi, Eric Dansin, Gilles Robinet, Maurice Perol, Denis Moro-Sibilot, Jean-Charles Soria. Phase II Study of Bevacizumab in Combination with First-line Chemotherapy or Second-line Erlotinib in Non-squamous NSCLC Patients with Asymptomatic Untreated Brain Metastases. ESMO. 2012:873. [Google Scholar]
- 20.Welsh JW, Komaki R, Amini A, et al. Phase II Trial of Erlotinib Plus Concurrent Whole-Brain Radiation Therapy for Patients With Brain Metastases From Non-Small-Cell Lung Cancer. J Clin Oncol. January 22 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee Y, Han JY, Kim HT, et al. Impact of EGFR tyrosine kinase inhibitors versus chemotherapy on the development of leptomeningeal metastasis in never smokers with advanced adenocarcinoma of the lung. J Neurooncol. July 6 2013. [DOI] [PubMed] [Google Scholar]