Abstract
Chemopreventive effects and associated mechanisms of withaferin A (WA) against intestinal and colon carcinogenesis remain unknown. We investigated the chemopreventive effect of WA on transgenic adenomatous polyposis coli (APCMin/+) mouse and chemically induced azoxymethane/dextran sodium sulfate (AOM/DSS) models of intestinal and colon carcinogenesis. Oral WA administration (4 and 3 mg/kg) inhibited tumor initiation and progression of intestinal polyps formation in APCMin/+ mice and colon carcinogenesis in the AOM/DSS mouse model. WA-administered mice showed a significant reduction in both number [duodenum, 33% (P > 0.05); jejunum, 32% (P < 0.025); ileum, 43% ( P < 0.001); and colon 59% (P < 0.01] and size of polyps in APCMin/+ mice compared with the respective controls. Similarly, tumor multiplicity was significantly reduced (P < 0.05) in the colon of WA-administered AOM/DSS mice. Pathological analysis showed reduced adenomas and tissue inflammation in WA-administered mouse models. Molecular studies suggested that WA inhibited the expression of inflammatory (interluekin-6, tumor necrosis factor-alpha and cyclooxygenase-2), pro-survival (pAKT, Notch1 and NF-κB) markers in APCMin/+ and AOM/DSS models. The results suggest that WA is a potent agent for preventing colon carcinogenesis and further investigation is required to show clinical utility of the agent.
The present study demonstrates that Withaferin A (WA) a natural compound prevents colon carcinogenesis in both transgenic adenomatous polyposis coli (APCMin/+) mouse and chemically induced azoxymethane/dextran sodium sulfate (AOM/DSS) models.
Introduction
The risk of colorectal cancer (CRC) is linked to lifestyle, diet-related factors, chronic inflammatory states, increasing age and hereditary disorder (1). CRC remains the third most common cancer, causing significant morbidity and mortality (2). Approximately 135430 patients have been diagnosed with CRC and approximately 37% of CRC patients died in the United States in 2017 (3). Despite advances in surgery and chemotherapy to treat CRC, preventive measures are still required.
Spontaneous and chemical-induced mouse models are mostly used to study experimental CRC, because, these models exhibit a similar pathology, which correlates with the progression of human CRC (4). The genetic mouse model for CRC includes gastrointestinal tract studies using adenomatous polyposis coli (APCMin/+) mice in which CRC results from the mutation at codon 850 of the APC gene (5), which causes familial adenomatous polyposis, an autosomal dominantly inherited disease that eventually leads to colorectal malignancy (6). Similarly, the azoxymethane/dextran sodium sulfate (AOM/DSS) model is an excellent experimental model for studying CRC inflammation and pathogenesis (7). This model is strongly linked to chronic colitis-associated colon cancer (CAC) in humans (8). Moreover, the multistep process of carcinogenesis characterized by the canonical phases of initiation, promotion and progression has been entirely reproduced in this model (7).
Multiple signaling pathways are involved in CRC pathogenesis and more specifically, inflammatory activity is a significant contributing factor (9). Studies have shown that PI3K/AKT activates NF-κB signaling in the presence of tumor necrosis factor-alpha (TNF-α) and interleukin-1 (IL-1) by phosphorylating the p65 subunit (10). On the other hand, blocking TNF-α activation reduced the number and size of the tumors in the AOM/DSS model (11). Both, IL-6 and TNF-α are well-established tumor-promoting cytokines, and their upregulation has been frequently associated with sporadic and colitis-associated CRC (12). Among the different mediators of inflammation, the cyclooxygenases (COXs) are also linked as a causative factor for CRC (13). COX-2 is the inducible isoform, and it is controlled by growth factors and different cytokines such as IL-1β, IL-6, and TNF-α (14).
AKT, a serine/threonine-specific protein kinase is one of the most frequently deregulated signaling pathways in human cancers, including CRC (15). Activation of the AKT pathway plays a crucial role in cell survival, proliferation, migration and differentiation, contributing to tumorigenesis and tumor metastasis. CRC resistant to chemotherapy or radiotherapy is often associated with AKT overexpression and activation. Recent studies have demonstrated that AKT is an attractive target to recognize specific inhibitors with suitable pharmaceutical properties (16). Clinical studies have shown that AKT activation is increased in 46% of CRC, and it is associated with the Ki-67 proliferation index (17). Furthermore, the important function of AKT is to induce the pro-survival signaling by increasing the GSK-3β/mTOR/NF-κB expression in inflammation conditions, which ultimately leads to resistance to apoptosis in colon cancer (18). Notch signaling, which is constitutively activated in CRC, also cross talks with the p65 subunit of NF-κB; and inhibition of this signaling axis suppresses the cell growth and induced apoptosis (19). Notch signaling has been shown to regulate the colonic goblet cells and stem cells/progenitor cells (20). Thus, Notch signaling is critical for maintaining intestinal development and homeostasis.
The role of diet in modulating CRC risk is well-accepted, and natural compounds have been shown to be safe over time. Previous studies on nutraceutical foods have validated the significance of natural compounds which reduce the incidence of cancer including CRC (21). Withaferin A (WA) is a small natural molecule, an herbal dietary agent derived from the plant Withania somnifera that is used extensively in Asian and African traditional medicine to treat various ailments (22). Our earlier in vitro and in vivo studies demonstrated the therapeutic and preventive effect of WA against colon cancer (23,24). In this study, we demonstrate the chemoprevention efficacy of WA in the APCMin/+ and AOM/DSS mouse models by inhibiting cell proliferation (Ki67), survival (pAKT, Notch1 and NF-κB) and inflammation (IL-6, TNF-α and COX2) markers.
Materials and methods
WA was purchased from Nucleus Biopharma (King of Prussia, PA) for the in vivo mouse studies. Primary monoclonal antibodies specific for phospho-AKT (ser473), p65, Notch1 and Ki67 were purchased from Cell Signaling (Danvers, MA). All animals were housed and experiments were performed in accordance with Institutional Animal Care and Use Committee and approved by University of Louisville.
APC Min/+ model
C57BL/6 - APCMin/+ mice of both sexes with a starting age of 5–6 weeks were purchased from Jackson Laboratory (Bar Harbor, ME) and divided into two groups of 18 animals (9 males and 9 females) each. Control group received sesame oil as a vehicle and treatment groups received WA (4 mg/kg) by oral gavage for 5 days/week for 12 weeks. Mice were monitored every day for clinical signs of illness such as rectal bleeding, rectal prolapse and body weight loss. At the end of 12th week treatment, mice were sacrificed and the duodenum, jejunum, ileum and colon were removed. The organs were then flattened on filter paper and fixed overnight in 10% buffered formalin. The following day, segments were washed with phosphate-buffered saline and stained with 0.2% methylene blue in phosphate-buffered saline. The number, location and size of visible polyps were determined at ×10 magnification using a Nikon Smz800 microscope (Westmont, IL) (by two independent and blinded investigators). Based on polyps size, the intestinal tract was classified into four categories (0–1, 1–2, 2–3, >3 mm). After the count and size measurements of polyps, the segments were Swiss-rolled and then embedded in paraffin for histological analysis.
AOM/DSS model
For the AOM/DSS model, FVB/NJ mice of both sexes were purchased from Jackson Laboratory. Ten mice were assigned per group (control and treatment) AOM (10 mg/kg) was injected intraperitoneally. After 1 week, 2% DSS was administered in the drinking water for 7 days, followed by 2 weeks of regular water. This cycle was repeated three times. In the treatment group, mice received oral WA (3 mg/kg) for 5 days/week for 10 weeks.
At the end of the experimental period, mice were euthanized using CO2 and the entire colon was removed. The number of macroscopic tumors was counted and the tumor volume was measured. Subsequently, the colons were fixed in 10% neutral-buffered formalin for 24 h, and transferred to 70% ethanol and Swiss-rolled for paraffin embedding and histological analysis.
RNA extraction and RT–PCR
Total RNA was extracted from polyps from the duodenum, jejunum, ileum and colonic segments of the APCMin/+ mice and colonic tumors from the AOM/DSS mice using the TRIzol reagent and dissolved in diethyl pyrocarbonate water. Complementary DNA was then synthesized from total RNA with an Applied Biosystems complementary DNA synthesis kit using SYBR Green supermix (Qiagen, Valencia, CA). Quantitative RT–PCR was performed as described previously (25).
Histology and immunohistochemistry
For histopathological examination, the complete gastrointestinal tract (paraffin blocks) were sectioned into 4 μm lengths and processed for hematoxylin and eosin staining, as described previously (26). A board-certified pathologist examined the all stained slides. For immunohistochemical analysis, the complete gastrointestinal tract tissue was fixed in 10% buffered formalin and processed as described previously (26). The specific antibodies were obtained for pAKT (ser473), p65, Notch1 and Ki67 were obtained from Cell Signaling (Danvers, MA) and used to visualize the expression of specific proteins in tissue samples.
Statistical analysis
Multiplicity of the tumors (AOM/DSS mice), defined as mean number of tumors/mice, was analyzed by unpaired Student’s t-test with Welch’s Correction. The tumor incidence (AOM/DSS mice) (percentage of mice with colon tumors) was analyzed by Fisher’s exact two-tailed test. All the other data were expressed as the mean ± standard deviation or standard error of mean (SD or SEM). Significant differences between the groups were determined using the unpaired Student’s t-test or one-way analysis of variance. Significant differences were established at P < 0.05. All statistical calculations were performed using GraphPad Prism 7.03 software (La Jolla, CA). ***P < 0.001, **P < 0.01, *P < 0.05 versus vehicle control of APCMin/+ and AOM/DSS mice. Not significant (n.s).
Results
Oral administration of WA prevents the sporadic intestinal tumor in APCMin/+ mice
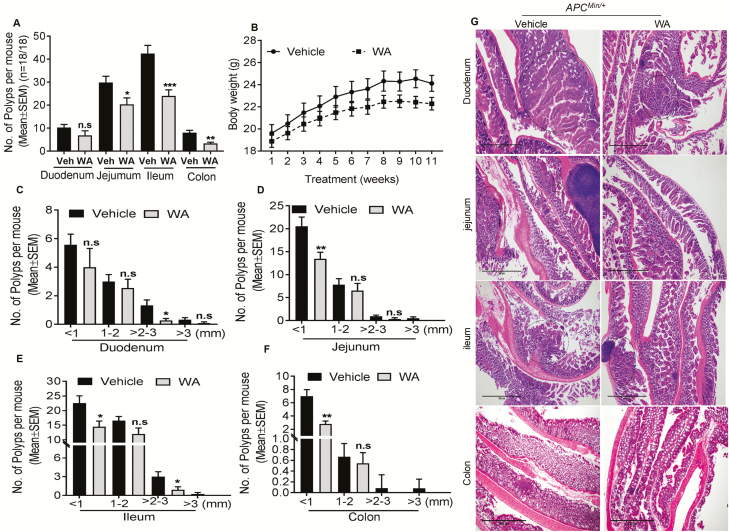
Control mice developed approximately 42 polyps in the ileum, 30 polyps in the jejunum, 10 polyps in the duodenum and 8 polyps in the colon after 12 weeks. However, WA-fed (4 mg/kg) mice showed a significant reduction in polyps in the ileum 43% (P < 0.001), jejunum 32% (P < 0.025), duodenum 33% (P > 0.05) and colon 59% (P < 0.01; Figure 1A). Size distribution analysis of adenomatous polyps in WA-treated mice showed a reduced number of <1-mm size polyps by 36% (P < 0.05) in the ileum, 35% (P < 0.01) in the jejunum and 28% in the duodenum (P > 0.05) compared with vehicle-treated groups. Polyps of 1–2 mm in size were reduced by 28% (P > 0.05) in the ileum, 16% (P > 0.05) in the jejunum and 15% (P > 0.05) in the duodenum. Polyps of 2–3 mm in size were reduced by 70% (P < 0.05) in the ileum, 60% (P > 0.05) in the jejunum and 80% (P < 0.05) in the duodenum (Figure 1C, D and E) compared with vehicle-treated mice, indicating differential effects of WA depending upon polyp size and the intestinal segment. The most prominent WA effect was observed on larger-sized polyps (>3 mm), which were undetectable in the jejunum and ileum and colon (Figure 1C, D and E). WA reduced the total number and size of colonic polyps <1 mm and 1–2 mm in size by 60% (P < 0.01) and 18% (P > 0.05), respectively (Figure 1F). None of the WA-treated mice showed 2–3 mm and >3 mm size polyps, showing a complete suppression, which suggests that WA effectively inhibits progression of colonic polyps. During the experimental period, WA-treated mice did not show any significant differences from vehicle-treated mice in body weight (Figure 1B). In APCMin/+ mice, all polyps were histologically identified as adenomas. However, the mice administrated WA showed less adenoma in both the duodenum and ileum, and there was no carcinogenesis or adenoma in the jejunum and colon (Figure 1G).
Figure 1.
WA reduced the number of intestinal polyps in APCMin/+ mice. Results are shown for the number of polyps and polyps size distribution in the small intestine and colon (A). The effect of WA on body weight of mice (B); WA reduced the number of polyps in duodenum, jejunum, ileum and colon (C–F); hematoxylin and eosin staining of the duodenum, jejunum, ileum and colon in control mice show adenomas with high-grade dysplasia compared with WA-treated APCMin/+ mice, scale bar: 300 µm (G). ***P < 0.001, **P < 0.01, *P < 0.05 versus vehicle control of APCMin/+. n.s, not significant. The bars represent mean ± SD or SEM of 18 mice/group
WA administration prevents chemically induced intestinal tumor in AOM/DSS mice
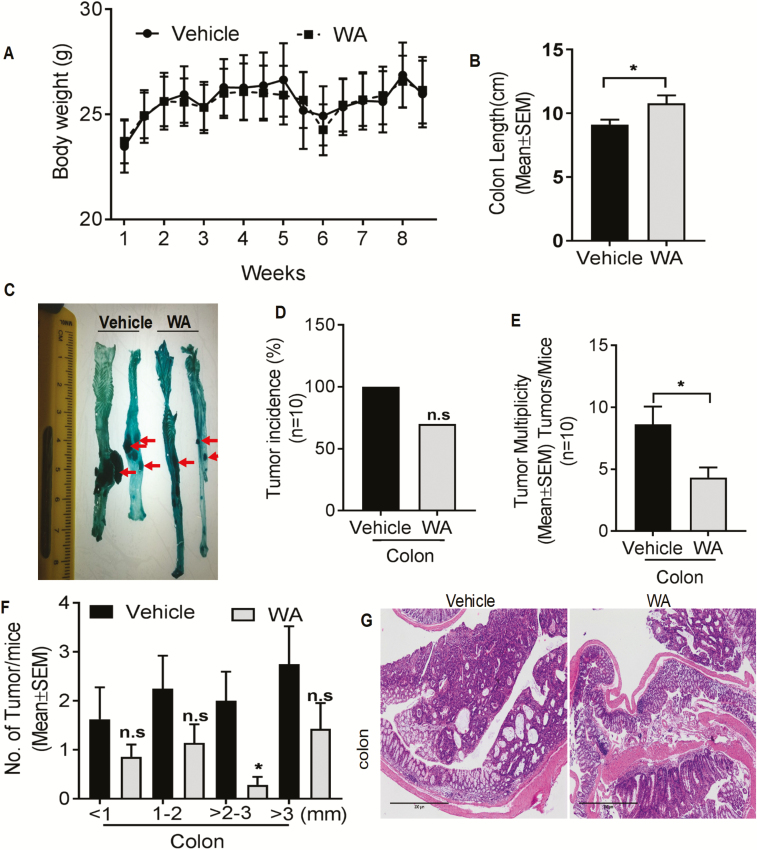
The antitumor effect of WA (3 mg/kg/body wt) was evaluated in AOM/DSS-induced tumorigenesis. Oral WA did not cause any apparent clinical toxicity or significant changes in body weight compared with AOM/DSS control (Figure 2A). AOM/DSS treatment shortened the colon length (Figure 2B) and increased the colon weight because of the tumor burden compared with the WA-treated group. Multiple colonic tumors were either flat or polypoid in control mice and the tumors were confined to the middle and distal colon (Figure 2C). No significant difference in the incidence of colon tumors were seen in WA-treated versus vehicle-treated AOM/DSS mice (Figure 2D). Further, WA treatment significantly reduced tumor multiplicity (50%, P < 0.05; Figure 2E) in AOM/DSS mice. Notably, WA-treated mice reduced the lesion frequency and size. A significant reduction (P < 0.05) was observed in tumor of size 2–3 mm in WA-treated group (Figure 2F). Correspondingly, the average tumor load was lower in WA-treated mice. In hematoxylin and eosin staining, AOM/DSS animals showed high-grade dysplasia, colonic adenoma and tissue inflammation. Administration of WA reduced all pathological abnormalities in the mice (Figure 2G).
Figure 2.
WA decreased the number of colonic tumors in AOM/DSS mice. Results are shown for the number of tumors and tumor size distribution in the colon. Effect of WA on body weight of mice (A); effect of WA on colon length of mice (B); representative macroscopic views of the mouse colons (C); effect of WA on tumor incidence of mice (% mice with colon tumors) (D); WA reduced the multiplicity of tumors in the colon (number of tumors/rats) (E); WA reduced the number of tumors of different sizes (F) hematoxylin and eosin staining on both control and WA-treated AOM/DSS mice, scale bar: 300 µm (G). *P < 0.05 versus vehicle control of AOM/DSS mice. n.s, not significant. The bars represent mean ± SD or SEM of 10 mice/group.
The expression profile of colonic inflammatory mediators was regulated by WA
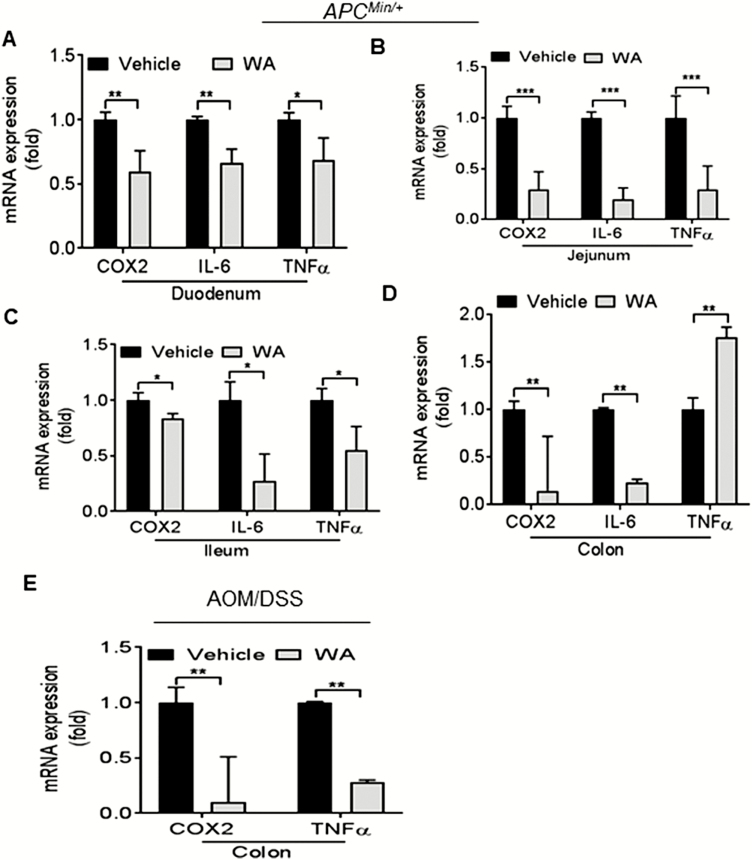
Chronic inflammation stimulates intestinal tumorigenesis by a variety of pro-inflammatory molecules and growth factors. In this experiment, we analyzed the cytokine and inflammatory profiles in the adenomatous polyps from the duodenum, jejunum, ileum and colon of APCMin/+ mice and colonic tumors from AOM/DSS mice. We observed that IL-6, TNF-α and COX-2 mRNA expression levels were significantly downregulated in adenomatous polyps or tumor from the duodenum, jejunum, ileum or colon of WA-fed APCMin/+ and AOM/DSS mice compared with controls (Figure 3A, B, C and E). A significant upregulation of TNF-α expression was seen in WA-treated colonic adenomatous polyps as compared with controls of APCMin/+ mice polyps (Figure 3D). These data suggested that the inhibition of pro-inflammatory cytokines and COX-2 expression by WA might play a role in the process of inflammation during intestinal tumorigenesis in APCMin/+ and AOM/DSS mice.
Figure 3.
Inhibition of inflammatory markers in WA-treated APCMin/+ and AOM/DSS mice. RNA was extracted from polyps or tumors from APCMin/+ and AOM/DSS mice, for real-time quantitative PCR analysis. The relative mRNA expression of inflammatory markers including COX-2, IL-6 and TNF-α is presented (A–E). ***P < 0.001, **P < 0.01, *P < 0.05 versus vehicle control of APCMin/+ and AOM/DSS.
WA treatment inhibits AKT activation in small and large intestinal polyps in APCMin/+ and AOM/DSS mice
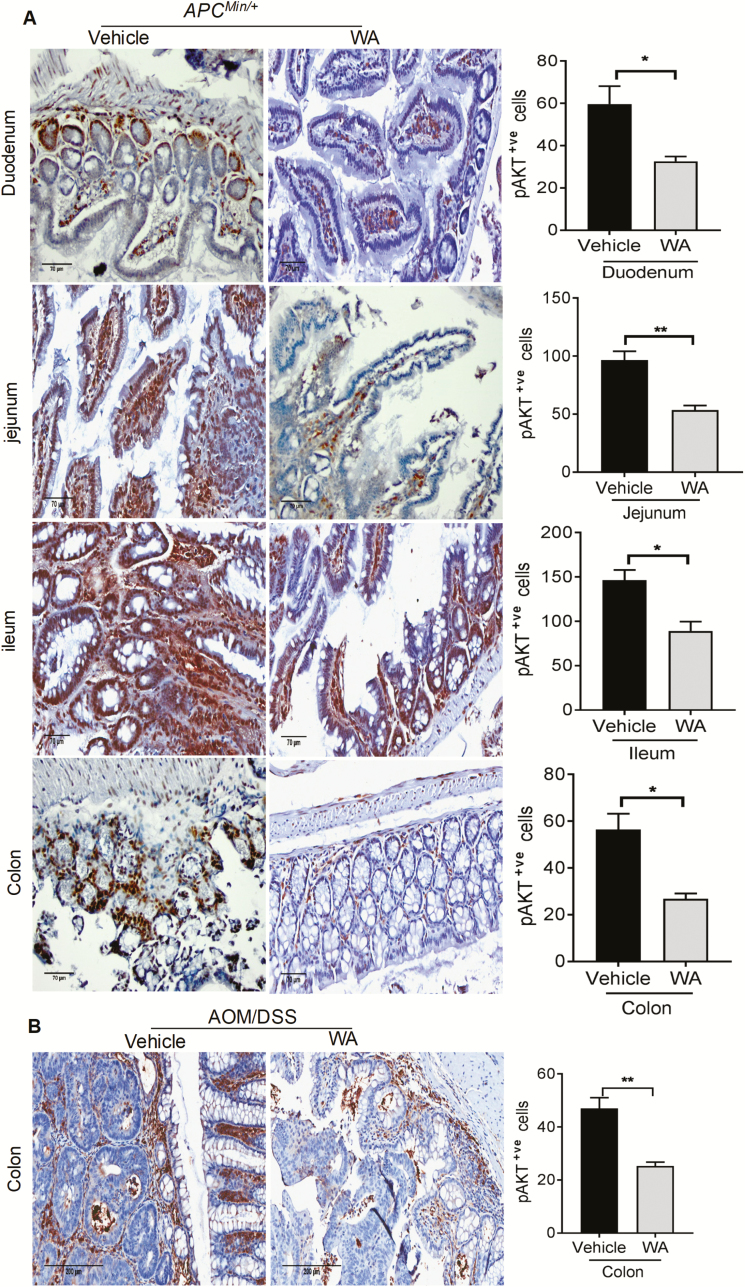
Tumor growth in CRC is often attributed to constitutive activation of AKT. Antiproliferative effects of WA on spontaneous and chemically induced intestinal tumorigenesis were evident. To dissect the underlying mechanism, we examined the effect of WA administration on AKT activation by immunohistochemistry. Microscopic quantification of all three small intestinal segments and colon indicated strong pAKT(ser473) expression in the nucleus and cytosol of adenomas polyps in the APCMin/+ and tumors in AOM/DSS controls, whereas significantly decreased pAKT expression was seen in the polyps or tumors of WA-administered group. The quantification of phospho-AKT expression showed a significant reduction by 39% in the polyps of ileum (P < 0.02), 45% (P < 0.01) in the polyps of jejunum, 45% (P < 0.05) in the polyps of duodenum and 53% (P < 0.05) in the polyps of colon in WA-treated mice compared with APCMin/+ control mice (Figure 4A). In AOM/DSS mice, pAKT-positive cells were significantly reduced by WA treatment by 46% (P < 0.01) in the tumors of colon compared with control tumors (Figure 4B).
Figure 4.
The pAKT expression in control and WA-treated APCMin/+ and AOM/DSS mice. Tissue sections from APCMin/+ and AOM/DSS control and WA groups show brown-colored pAKT-positive cells. Quantitative data were determined by the number of pAKT-positive cells in three randomly selected fields (A and B). Scale bar: 70 µm (APCMin/+), 200 µm (AOM/DSS). **P < 0.01, *P < 0.05 versus vehicle control of APCMin/+ and AOM/DSS mice.
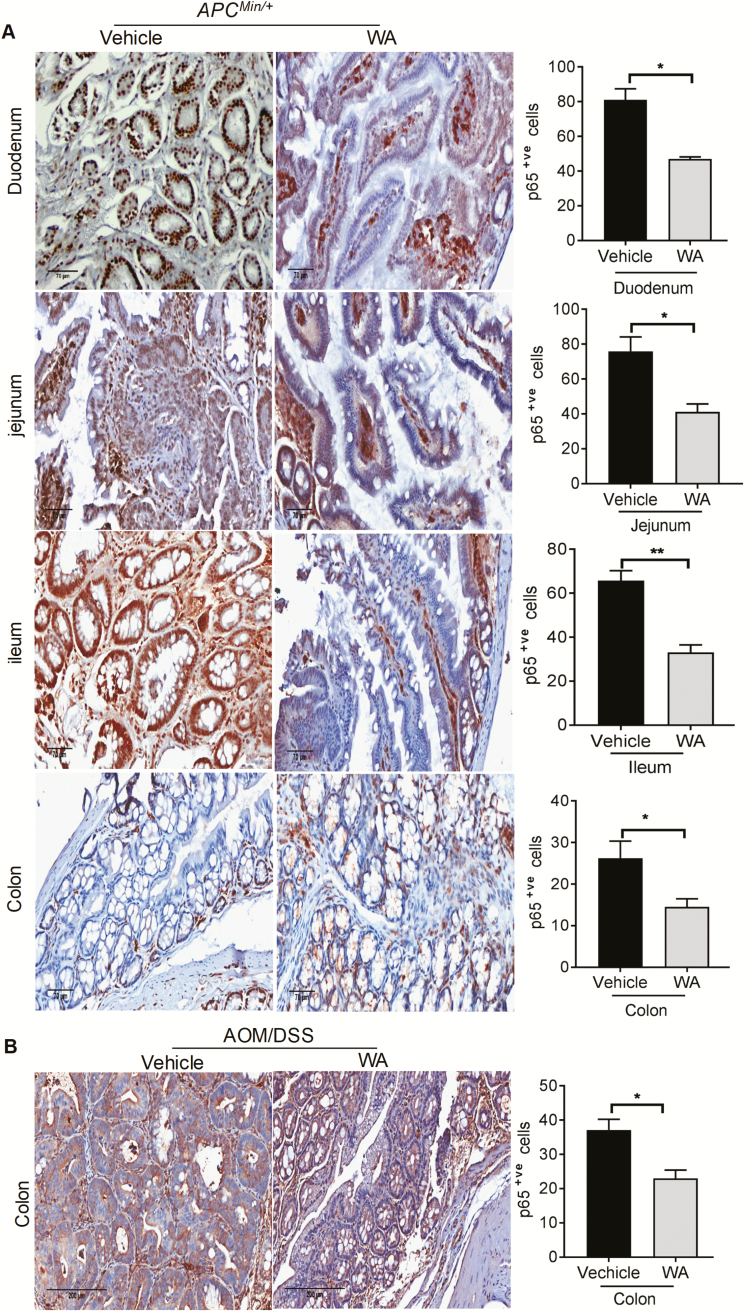
Inhibitory effects of WA on p65 expression in APCMin/+ and AOM/DSS models
We also used immunostaining to examine p65 expression in different portions of the small intestine and colon in the control and WA groups. Higher p65 expression is considered to be the most valuable malignant marker of adenomatous polyps in APCMin/+ and tumors of the AOM/DSS mice. Quantification of immunostaining showed that WA significantly decreased p65 expression by 50% (P < 0.002) in the polyps of ileum, 46% (P < 0.02) in the polyps of jejunum, 42% (P < 0.05) in the polyps of duodenum and 45% (P < 0.05) in the polyps of colon compared with the polyps of APCMin/+ control group (Figure 5A). WA administration in AOM/DSS mice significantly suppressed p65 expression in the colonic tumors by 37% (P < 0.05) compared with tumors of AOM/DSS control mice (Figure 5B).
Figure 5.
Inhibitory effects of WA on inflammatory markers in APCMin/+ and AOM-exposed mice. Immunohistochemistry staining of p65-positive cells in APCMin/+ and AOM/DSS alone and WA-treated groups, respectively. p65-positive cells as assessed by quantification of immunohistochemically stained mouse intestinal and colonic epithelium in three randomly selected fields from each tissue sample (A and B). Scale bar: 70 µm (APCMin/+), 200 µm (AOM/DSS). **P < 0.01, *P < 0.05 versus vehicle control of APCMin/+ and AOM/DSS mice.
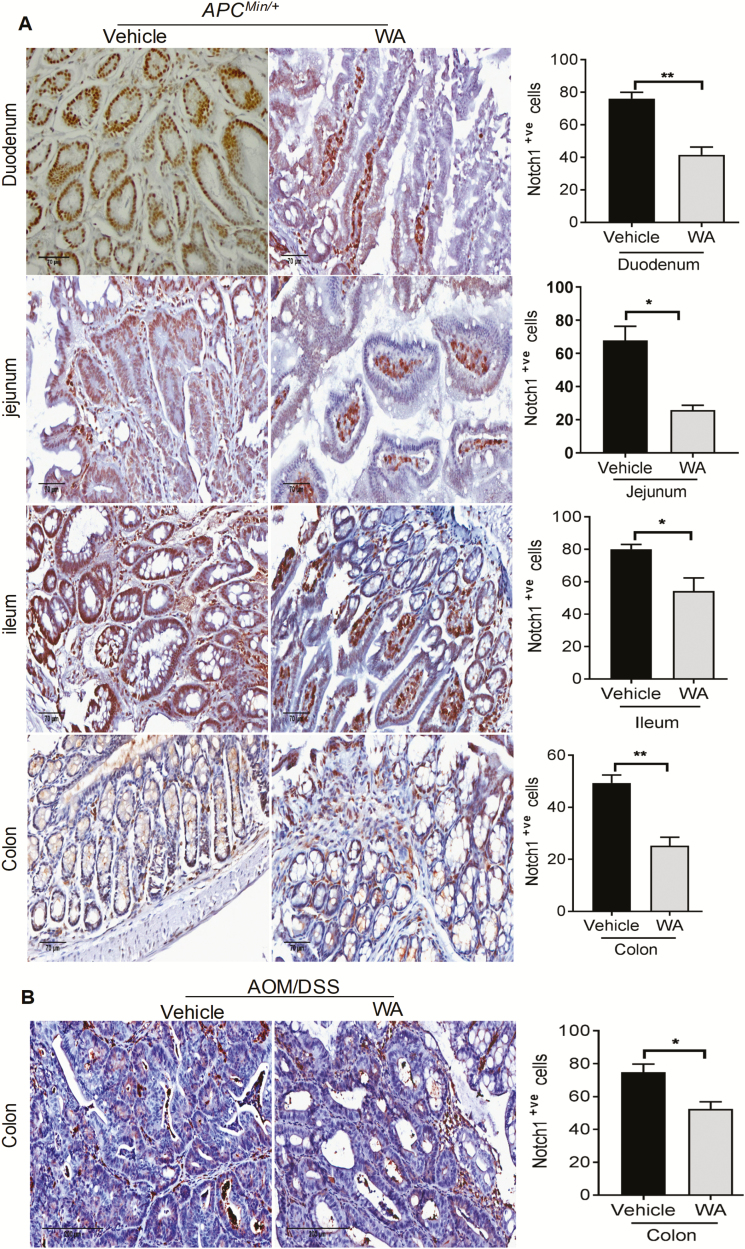
WA downregulates Notch1 expression in AOM/DSS and APCMin/+ mice
The cross talk between p65 and Notch1 is the mainstay of epithelial–mesenchymal transition (EMT) and thus tumorigenesis in CRC (27). Thus, it is important to examine the effect of any treatment on correlated signaling pathways such as p65 and Notch1. In APCMin/+ mice, Notch1 immunoreactivity was reduced by 45% (P < 0.002) in the polyps of duodenum, 62% (P < 0.05) in the polyps of jejunum, 32% (P < 0.05) in the polyps of ileum and 49% in the polyps of colon (P < 0.002) in WA-treated mice when compared with controls (Figure 6A). Notably, immunohistochemistry analysis suggested that WA significantly decreased Notch1 expression by 30% (P < 0.05) in the tumor of colon compared with the AOM/DSS control group (Figure 6B). Collectively, these results demonstrate the antiproliferative and antitumorigenic effect of WA.
Figure 6.
WA administration downregulated Notch1 expression in APCMin/+ and AOM/DSS mice. Notch1 expression in both animal models is shown by immunohistochemistry staining. Data were semi-quantified as the mean of positive cells in three randomly selected fields (A and B). Scale bar: 70 µm (APCMin/+), 200 µm (AOM/DSS). **P < 0.01, *P < 0.05 versus vehicle control of APCMin/+and AOM/DSS mice.
WA reduces proliferation in APCMin/+ and AOM/DSS mice
Excessive proliferation may be responsible for the progression of adenocarcinoma polyps. The reduction in size and frequency of polyps in WA-treated mice suggests the downregulation of genes responsible for proliferation compared with vehicle-treated cells. Thus, we evaluated vehicle- and WA-treated APCMin/+ and AOM/DSS mouse polyps or tumors for expression of proliferative markers. Duodenum, jejunum and ileum segments of the small intestine polyps and the colon polyps were analyzed immunohistochemically for Ki67 as a marker for tumor cell proliferation and growth. Microscopic analysis of tissue sections showed a decreased number of Ki67-positive cells from WA-treated animals polyps or tumors compared with APCMin/+ and AOM/DSS controls. Quantification of Ki67 staining showed that WA treatment decreases Ki67-positive cells by 42% (P < 0.05) in the polyps of duodenum, 55% (P < 0.01) in the polyps of jejunum, 30% (P < 0.05) in the polyps of ileum and 50% (P < 0.05) in the polyps of colon in WA-treated mice compared with vehicle-treated APCMin/+ mice (Supplementary Figure 1A). Additionally, 3 mg/kg WA-treated AOM/DSS mice tumors showed a reduction in the Ki67 proliferation index by 25% (P < 0.05) in the tumors of colon (Supplementary Figure 1B).
Discussion
Recently, cancer prevention involving dietary compounds has been shown to be a promising and cost-effective approach to reduce CRC incidence and morbidity by suppressing precancerous events before clinical disease is present. In this study, we demonstrated the chemopreventive effect of WA on spontaneous intestinal and chemically induced colon carcinogenesis in APCMin/+ and AOM/DSS mice.
Excessive growth and inadequate apoptosis are often associated with the development of intestinal tumorigenesis (28). The early stage of colon cancer is characterized by a benign adenoma that could progress to adenocarcinoma with high-grade dysplasia (29). The APCMin/+ mouse model is unique because tumors appear spontaneously in the gastrointestinal tract, rather than through induction using a carcinogen. APCMin/+ mice develop adenomas as the outcome of inactivation of the APC tumor suppressor gene, which is known to be associated with pathogenesis in most colon cancers in humans (30). In this study, we showed, for the first time, that WA significantly inhibits the formation of adenomatous polyps in APCMin/+ mice, which was demonstrated by WA administration that completely suppressed >3-mm polyps in the small intestine (jejunum and ileum) and colon.
The AOM/DSS-induced neoplasm mimics human CAC (31). Thus, experiments using these models are probably to be appropriate to use in the design of human chemoprevention clinical trials. AOM causes colonic carcinogenicity resulting in the formation of O6-methylguanine adducts upon metallic activation, whereas cycles of DSS treatment induce chronic inflammation in colons, which resembles inflammatory bowel disease (32). WA administration effectively reduced the inflammation associated with colitis in this study and decreased the multiplicity of colonic tumors in mice, as reflected by marked amelioration of clinical symptoms and a reduction in the incidence of colonic dysplasia. This protective effect was further confirmed in a decrease in the intensity of colonic damage and nuclear cell alterations, as evidenced by histological findings. However, no significant difference in tumor incidence between WA-treated and vehicle-treated mice was seen, which suggests that a higher concentration of WA may require to prevent the tumor incidence in AOM/DSS models. Similar results were shown in 7,12-dimethylbenz[a] anthracene (DMBA) and 12-O-tetradecanoylphorbol 13-acetate-induced skin tumor models and mammary tumor virus (MMTV)-neu mice that WA significantly inhibited the tumor multiplicity, but not the tumor incidence (33,34). Several naturally occurring dietary phytochemicals were shown to inhibit formation of adenomatous polyps and tumor multiplicity in APCMin/+ and AOM/DSS mice, which is consistent with our results (35,36).
Elevated AKT levels are present at the early stages of intestinal carcinogenesis (37). AKT is shown to be overexpressed in rat premalignant colonocytes and in 42% of the tumors that developed (38). We found that WA administration to APCMin/+ and AOM/DSS mice dramatically decreased AKT activation. These results are in agreement with our earlier findings, wherein WA administration downregulated pAKT expression in AKT overexpressing xenograft tumors and transgenic mice (TRAMP and PTEN) in prostate cancer mouse models (24,39,40). We previously reported that WA inhibited AKT-induced CRC cell growth in preclinical CRC models (24), which was further confirmed by another group in MMTV-neu mouse model (41). We and others have shown that WA appears to target only cancer cells and cause a minimal toxic effect on normal human gingival fibroblast (26), endothelial (42) and mammary epithelial cells (43). WA specifically targets cancer cells by inducing reactive oxygen species-mediated cell death without affecting normal mammary epithelial cells (44).
Many in vivo studies have further confirmed that WA exhibits selective cytotoxicity against tumor cells (24,39,45). A randomized, double-blind, clinical study suggested that three doses of W. somnifera extract (400 mg) for a month appears to be a well-tolerated dose, and also significantly reduced serum triglycerides in patients (46) and a similar study was reported earlier on the no adverse effect on human (47).
Another key player in inflammation and carcinogenesis is the NF-κB–COX-2 signaling pathway, which has also been shown to affect APC gene mutation in human intestine and colon cells. Thus, it is an important treatment target for CRC (48). An increase in inflammatory stress is reported to be correlated with the development of intestinal polyps in APCMin/+ mice. Notably, NF-κB is abnormally activated in 50% of CRC patients and those with colitis-associated tumors. Additionally, pro-inflammatory cytokines, such as TNF-α, IL-6 and IL-1β, or transcription factors such as NF-κB, which are induced by these cytokines, have been identified as potential targets for anticancer therapy (49). COX-2 is an immediate-early growth response gene product. NF-κB and Wnt signaling have both been shown to regulate COX-2 expression. COX-2 overexpression has been shown to increase AOM-induced tumor formation (50). COX-2 and cyclin D1 are induced by TNF-α in colonic cells that have the NF-κB binding site in their promoters (51). In this study, expression of the pro-inflammatory mediators, COX2 and NF-κB significantly suppressed by WA administration in both the mouse models. This decrease in NF-κB expression coupled with a dramatic downregulation of COX-2 and Notch1 expression could be the underlying mechanisms responsible for the anti-inflammatory and antitumorigenic effects of WA administration, ultimately leading to the suppression of polyps or tumors in the intestines and colon in APCMin/+ and AOM/DSS mice. Although the increased mRNA expression of TNF-α was seen in the colonic polyps of WA-treated mice, the number of polyps is significantly lesser than the controls that suggest TNF-α would not have played any significant role in tumor inhibition or WA could overcome TNF-α mediated carcinogenic effect.
This study demonstrates an in vivo antiproliferative efficacy of WA in intestinal and colonic polyps of APCMin/+ and tumors of AOM/DSS mice that is evidenced by Ki67 immunostaining. Proliferation is an integral part of cancer development and progression in the early stage of intestinal colon carcinogenesis. Thus, identification of chemopreventive agents that downregulate cell proliferation is recognized as a useful strategy to control colon tumor growth. The expression of Ki67 is widely used to study cell proliferative activity, that correlates with metastasis and clinical tumor stage. Additionally, Ki67 expression is significantly higher in malignant tissues with poorly differentiated tumor cells compared with normal tissue. The effect of WA observed in this study is supported by the reduction of cell proliferation in its suppression of early stage carcinogenesis, antiproliferative and anti-inflammatory mechanisms. Previous studies demonstrated that WA inhibits early event of colon carcinogenesis by inhibiting tumor promoter IDH1 gene activity and altering mitochondrial function (52). Interestingly, the half-life (t1/2) of WA (45 min) was higher than withanolide A, a group of secondary metabolites obtained from W. somnifera (the plant source of WA) in orally administered Swiss Albino female mice. Pharmacokinetics studies using high-performance liquid chromatography–mass spectrometry/mass spectrometry determined that WA has one and a half times more relative bioavailability as compared with withanolide A (53). Another study has shown that WA attains peak plasma concentration of up to 2 µm with a half-life of 1.36 h in 7- to 8-week-old BALB/c mice at a single dose of 4 mg/kg (54). These studies suggest that higher bioavailability of WA and thus impart better efficacy in diseased condition.
Overall, these results provide compelling evidence that WA prevents the development of intestinal and colonic neoplasia in APCMin/+ and AOM/DSS-induced CRC in mice by inhibiting cancer cell proliferation (Ki67), ameliorating colon inflammatory processes and limiting the activation of a transcription factor (NF-κB) that modulates the expression of Notch1 signaling. WA is thus a promising natural protective/preventive agent against sporadic and colitis-associated CRC with the potential to help maintain tissue homeostasis in the human colon.
Funding
This work was supported by 1R01CA185972–01.
Conflict of Interest Statement: None declared.
Supplementary Material
Abbreviations
- AOM
azoxymethane
- APC
adenomatous polyposis coli
- CAC
colitis-associated colon cancer
- COX
cyclooxygenase
- CRC
colorectal cancer
- DSS
dextran sodium sulfate
- IL
interleukin
- TNF-α
tumor necrosis factor-alpha
- WA
withaferin A
References
- 1. Kuipers E.J., et al. (2015)Colorectal cancer. Nat. Rev. Dis. Primers, 1, 15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bhandari A., et al. (2017)Colorectal cancer is a leading cause of cancer incidence and mortality among adults younger than 50 years in the USA: a SEER-based analysis with comparison to other young-onset cancers. J. Investig. Med., 65, 311–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siegel R.L., et al. (2017)Cancer Statistics, 2017. CA. Cancer J. Clin., 67, 7–30. [DOI] [PubMed] [Google Scholar]
- 4. Ward J.M., et al. (2014)Rodent intestinal epithelial carcinogenesis: pathology and preclinical models. Toxicol. Pathol., 42, 148–161. [DOI] [PubMed] [Google Scholar]
- 5. McIntyre R.E., et al. (2015)Mouse models of colorectal cancer as preclinical models. Bioessays, 37, 909–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leoz M.L., et al. (2015)The genetic basis of familial adenomatous polyposis and its implications for clinical practice and risk management. Appl. Clin. Genet., 8, 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tanaka T., et al. (2003)A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci., 94, 965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parang B., et al. (2016)Myeloid translocation genes differentially regulate colorectal cancer programs. Oncogene, 35, 6341–6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Figueroa-González G., et al. (2016)Anti-inflammatory and antitumor activity of a triple therapy for a colitis-related colorectal cancer. J. Cancer, 7, 1632–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sizemore N., et al. (2002)Distinct roles of the Ikappa B kinase alpha and beta subunits in liberating nuclear factor kappa B (NF-kappa B) from Ikappa B and in phosphorylating the p65 subunit of NF-kappa B. J. Biol. Chem., 277, 3863–3869. [DOI] [PubMed] [Google Scholar]
- 11. Popivanova B.K., et al. (2008)Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J. Clin. Invest., 118, 560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Terzić J., et al. (2010)Inflammation and colon cancer. Gastroenterology, 138, 2101–2114.e5. [DOI] [PubMed] [Google Scholar]
- 13. Staal F.J., et al. (2008)The canonical Wnt signaling pathway plays an important role in lymphopoiesis and hematopoiesis. Eur. J. Immunol., 38, 1788–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ramsay R.G., et al. (2003)Transcriptional regulation of cyclo-oxygenase expression: three pillars of control. Int. J. Immunopathol. Pharmacol., 16(2 suppl), 59–67. [PubMed] [Google Scholar]
- 15. Waniczek D., et al. (2018)Assessment of PI3K/AKT/PTEN signaling pathway activity in colorectal cancer using quantum dot-conjugated antibodies. Oncol. Lett., 15, 1236–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nitulescu G.M., et al. (2016)Akt inhibitors in cancer treatment: the long journey from drug discovery to clinical use (Review). Int. J. Oncol., 48, 869–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Henderson-Jackson E.B., et al. (2010)Correlation between Mcl-1 and pAKT protein expression in colorectal cancer. Int. J. Clin. Exp. Pathol., 3, 768–774. [PMC free article] [PubMed] [Google Scholar]
- 18. Mi W., et al. (2015)AKT inhibition overcomes rapamycin resistance by enhancing the repressive function of PRAS40 on mTORC1/4E-BP1 axis. Oncotarget, 6, 13962–13977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodilla V., et al. (2009)Jagged1 is the pathological link between Wnt and Notch pathways in colorectal cancer. Proc. Natl. Acad. Sci. U. S. A., 106, 6315–6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zheng H., et al. (2009)KLF4 gene expression is inhibited by the notch signaling pathway that controls goblet cell differentiation in mouse gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol., 296, G490–G498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Terry P., et al. (2001)Fruit, vegetables, dietary fiber, and risk of colorectal cancer. J. Natl. Cancer Inst., 93, 525–533. [DOI] [PubMed] [Google Scholar]
- 22. Rai M., et al. (2016)Anticancer activities of Withania somnifera: current research, formulations, and future perspectives. Pharm. Biol., 54, 189–197. [DOI] [PubMed] [Google Scholar]
- 23. Koduru S., et al. (2010)Notch-1 inhibition by Withaferin-A: a therapeutic target against colon carcinogenesis. Mol. Cancer Ther., 9, 202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Suman S., et al. (2016)Withaferin-A suppress AKT induced tumor growth in colorectal cancer cells. Oncotarget, 7, 13854–13864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Das T.P., et al. (2014)Induction of reactive oxygen species generation inhibits epithelial-mesenchymal transition and promotes growth arrest in prostate cancer cells. Mol. Carcinog., 53, 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chang H.W., et al. (2017)Withaferin A induces oxidative stress-mediated apoptosis and DNA damage in oral cancer cells. Front. Physiol., 8, 634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reedijk M., et al. (2008)Activation of Notch signaling in human colon adenocarcinoma. Int. J. Oncol., 33, 1223–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Giles R.H., et al. (2003)Caught up in a Wnt storm: wnt signaling in cancer. Biochim. Biophys. Acta, 1653, 1–24. [DOI] [PubMed] [Google Scholar]
- 29. Wang R., et al. (2014)The comparative study of acetyl-11-keto-beta-boswellic acid (AKBA) and aspirin in the prevention of intestinal adenomatous polyposis in APC(Min/+) mice. Drug Discov. Ther., 8, 25–32. [DOI] [PubMed] [Google Scholar]
- 30. Young M., et al. (2013)What are the best routes to effectively model human colorectal cancer?Mol. Oncol., 7, 178–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Neufert C., et al. (2007)An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat. Protoc., 2, 1998–2004. [DOI] [PubMed] [Google Scholar]
- 32. Greten F.R., et al. (2004)IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell, 118, 285–296. [DOI] [PubMed] [Google Scholar]
- 33. Li W., et al. (2016)Withaferin A suppresses the up-regulation of acetyl-coA carboxylase 1 and skin tumor formation in a skin carcinogenesis mouse model. Mol. Carcinog., 55, 1739–1746. [DOI] [PubMed] [Google Scholar]
- 34. Hahm E.R., et al. (2013)Metabolic alterations in mammary cancer prevention by withaferin A in a clinically relevant mouse model. J. Natl. Cancer Inst., 105, 1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tsai C.C., et al. (2015)Increase in apoptosis by combination of metformin with silibinin in human colorectal cancer cells. World J. Gastroenterol., 21, 4169–4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huo X., et al. (2016)Flavonoids extracted from licorice prevents colitis-associated carcinogenesis in AOM/DSS mouse model. Int. J. Mol. Sci., 17, pii: E1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ju J., et al. (2005)Inhibition of intestinal tumorigenesis in Apcmin/+ mice by (-)-epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res., 65, 10623–10631. [DOI] [PubMed] [Google Scholar]
- 38. Roy H.K., et al. (2002)AKT proto-oncogene overexpression is an early event during sporadic colon carcinogenesis. Carcinogenesis, 23, 201–205. [DOI] [PubMed] [Google Scholar]
- 39. Moselhy J., et al. (2017)Withaferin A inhibits prostate carcinogenesis in a PTEN-deficient mouse model of prostate cancer. Neoplasia, 19, 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Suman S., et al. (2016)Oral administration of withaferin A inhibits carcinogenesis of prostate in TRAMP model. Oncotarget, 7, 53751–53761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nagalingam A., et al. (2014)Mechanistic elucidation of the antitumor properties of withaferin a in breast cancer. Cancer Res., 74, 2617–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Heyninck K., et al. (2016)Withaferin A induces heme oxygenase (HO-1) expression in endothelial cells via activation of the Keap1/Nrf2 pathway. Biochem. Pharmacol., 109, 48–61. [DOI] [PubMed] [Google Scholar]
- 43. Stan S.D., et al. (2008)Withaferin A causes FOXO3a- and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo. Cancer Res., 68, 7661–7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hahm E.R., et al. (2011)Withaferin A-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLoS One, 6, e23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Choi B.Y., et al. (2015)Withaferin-A inhibits colon cancer cell growth by blocking STAT3 transcriptional activity. J. Cancer Prev., 20, 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Agnihotri A.P., et al. (2013)Effects of Withania somnifera in patients of schizophrenia: a randomized, double blind, placebo controlled pilot trial study. Indian J. Pharmacol., 45, 417–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Andallu B., et al. (2000)Hypoglycemic, diuretic and hypocholesterolemic effect of winter cherry (Withania somnifera, Dunal) root. Indian J. Exp. Biol., 38, 607–609. [PubMed] [Google Scholar]
- 48. Nigam J., et al. (2014)Expression of survivin mRNA in gallbladder cancer: a diagnostic and prognostic marker?Tumour Biol., 35, 9241–9246. [DOI] [PubMed] [Google Scholar]
- 49. Klampfer L. (2011)Cytokines, inflammation and colon cancer. Curr. Cancer Drug Targets, 11, 451–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Al-Salihi M.A., et al. (2009)Transgenic expression of cyclooxygenase-2 in mouse intestine epithelium is insufficient to initiate tumorigenesis but promotes tumor progression. Cancer Lett., 273, 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sze S.C., et al. (2011)Regulation of p21, MMP-1, and MDR-1 expression in human colon carcinoma HT29 cells by Tian Xian liquid, a Chinese medicinal formula, in vitro and in vivo. Integr. Cancer Ther., 10, 58–69. [DOI] [PubMed] [Google Scholar]
- 52. Li W., et al. (2013)Withaferin A suppresses tumor promoter 12-O-tetradecanoylphorbol 13-acetate-induced decreases in isocitrate dehydrogenase 1 activity and mitochondrial function in skin epidermal JB6 cells. Cancer Sci., 104, 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Patil D., et al. (2013)Determination of withaferin A and withanolide A in mice plasma using high-performance liquid chromatography-tandem mass spectrometry: application to pharmacokinetics after oral administration of Withania somnifera aqueous extract. J. Pharm. Biomed. Anal., 80, 203–212. [DOI] [PubMed] [Google Scholar]
- 54. Thaiparambil J.T., et al. (2011)Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int. J. Cancer, 129, 2744–2755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.