Abstract
Background
The recommended timing of surgical intervention for vertebral osteomyelitis (VO) is controversial; however, most studies are not sufficiently powered. Our goal was to investigate the associated effects of delaying surgery in VO patients on in-hospital complications, neurologic deficits, and mortality.
Methods
Retrospective review of the National Inpatient Sample. Patients who underwent surgery for VO from 1998 to 2013 were identified using codes from the International Classification of Disease, Ninth Revision, Clinical Modification. Patients were stratified into groups based on incremental delay of surgery: 0-day delay (same-day surgery), 1-day delay, 2-day delay, 3- to 6-day delay, 7- to 14-day delay, and 14- to 30-day delay. Univariate analysis compared demographics and outcomes between groups. Multivariate logistic regression models calculated independent predictors of any complication, mortality, and neurologic deficits. A 0-day delay was the reference group.
Results
A total of 34 465 patients were identified. Delayed groups were older (same day: 53.5 vs. 7–14-day delay: 61.1) and had a higher Deyo-Charlson score (same day: 0.4901 vs. 14–30-day delay: 1.66), length of stay (same day: 4.2 vs. 14–30-day delay: 34.04 days), and total charges (same day: $63,390.78 vs. 14-30-day delay: $245,752.4), all P < .001. Delayed groups had higher surgical combined-approach rates (same day: 9.1% vs. 14–30-day delay: 31.5%) and lower anterior-approach rates (same day: 42.4% vs. 14–30-day delay: 24.2%). Delayed groups had increased mortality and complication rates. Regressions showed delayed groups as the strongest independent indicators of any complication (14–30-day delay: odds ratio [OR] 3.384), mortality (14–30-day delay: OR 10.658), and neurologic deficits (14-30-day delay: OR 3.464), all P < .001.
Conclusion
VO patients who operate within 24 hours of admission are more likely to have desirable outcomes. Patients with delayed surgery had a significantly increased risk of developing any complication, mortality, and discharging with neurologic deficits.
Level of Evidence
III.
Clinical Relevance
Medically fit patients may benefit from earlier surgical management in order to reduce risk of postoperative complications, improve outcomes, and reduce overall hospital costs.
Keywords: vertebral osteomyelitis, surgical delay, spinal fusion, spondylodiscitis
INTRODUCTION
Vertebral osteomyelitis (VO) is a rare spine infection often developing from open spinal trauma, infections in adjacent anatomic structures, or hematogenous spread of bacteria or as a postoperative complication to spinal surgery.1–4 Although VO remains uncommon, with yearly rates of 5 to 20 cases per million in the United States, its incidence is increasing.3,5–7 This is believed to be attributed to the increase of patients with advanced age, diabetes mellitus, chronic renal or liver disease, intravenous drug use, HIV infection, chronic corticosteroid use, chemotherapy, and severe trauma—all predisposing factors to VO.8,9 Since 1974, the incidence has nearly doubled from 0.2 to 2 cases per 10 000 hospital admissions to 2 to 3 cases per 10 000 hospital admissions,10–12 with a 20-fold-higher incidence in older patients.13 If left untreated, VO can lead to irreversible spinal cord injury, deformity, neurologic deficits, septicemia, and mortality (mortality rates ranges 4%–29%).4,14 VO is traditionally treated conservatively with antimicrobial therapies, but up to 40% to 50% of VO patients suffering will eventually require surgical intervention.4,15
VO surgical intervention is indicated when patients express progressive loss of motor and/or neurological functions, cauda equine syndrome, progressive deformities, spinal instability, abscess formation, and delayed diagnosis. Failure of conservative treatment categorized as persistent pain, residual neurologic deficits, and systemic inflammation/infection also warrants surgical intervention. While clinicians have agreed on surgical indications for VO, a persistent area of controversy is the optimal timing of surgery that most benefits the patient.14 Prior studies report conflicting findings: Ghobrial et al16 in 2014 and Connor et al18 in 2013 reported a relative advantage to earlier surgical intervention with regard to discharge neurological status, while Adogwa et al17 in 2014 and Karikari et al10 in 2009 reported no statistical benefit from early surgical intervention on postoperative outcomes. While these studies present relative insight into the timing of surgical intervention, they are limited by small sample sizes (82–104 patients), minimal subgroup analysis, and institutional bias.
This study evaluates the outcomes of early (< 24 hours) versus delayed surgical treatment of VO using a large nationwide inpatient database. Our study also aimed to investigate the demographics, comorbidities, socioeconomics, and prevalence of multiple complications associated with variant surgical timing of the VO patient population.
MATERIALS AND METHODS
Data Source
This is a retrospective review of the National Inpatient Sample (NIS) from 1998 to 2013. The NIS is the largest available all-payer database for US inpatient care and includes data approximating 8 million discharges from 1000 hospitals annually. Forty-five states are represented in the NIS, with approximately a 20% stratified random sample of all US community hospitals. The Health Care Cost and Utilization Project provides support for the NIS, with further support coming from federal, state, and industrial partnerships. The NIS presents clinical, demographic, diagnosis, and procedural data elements in the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) format. More information can be found at http://hcup-us.ahrq.gov/db/nation/nis/NIS_Introduction_2012.jsp. This study was deemed exempt by our institutional review board due to the deidentified nature of the data.
Inclusion Criteria
Patients of all ages who underwent surgical treatment for VO from 1998 to 2013 were identified. ICD-9 codes identified patients who underwent surgery for VO. Patients were then stratified based on procedural delay into 6 groups: 0 days (same day as admission surgery), 1 day, 2 days, 3 to 6 days, 7 to 14 days, and 14 to 30 days. Patients with delays > 30 days, representing less than 0.1% of the total subset, were excluded due to wide variability in surgical delays often related to the presence of significant comorbidities.
Data Collection
Demographics, including age, gender, race, length of hospital stay, total charges, comorbidity status, and insurance type, were analyzed. The Deyo-Charlson Index, a modification by Deyo et al19 of the Charlson comorbidity severity index for the utilization of ICD-9-CM diagnostic and procedural codes, was used to describe comorbidity severity within our cohorts. The comorbidities assessed in this study and included within the index were myocardial infarction, congestive heart failure, pulmonary vascular disease, stroke, dementia, chronic pulmonary disease, rheumatological conditions, peptic ulcer disease, liver disease, diabetes, diabetes with complications, renal disease, cancer, severe liver disease, metastasis, and AIDS. Subsets of VO surgical patients were investigated by the method of surgical approach (anterior, posterior, and combined anterior/posterior) and the spinal level (cervical, thoracic, lumbosacral) of the operation. Perioperative surgical and medical complications were identified and further classified into the following subsets: the top 3 most common complications, rate of any complications, and mortality. All elements were identified using ICD-9 codes (Appendix A).
Neurologic Index
A neurologic index was created using the design of the American Spinal Injury Association (ASIA) impairment scale as a reference. Numerical scores between 0 and 2 were assigned to patients with corresponding neurological deficits. A score of 0 was assigned to patients without any neurologic deficits (ASIA grade E), a score of 1 to patients with incomplete neurologic deficit (ASIA grades B-D), and a score of 2 to patients with complete neurologic deficit (ASIA grade A). Incomplete neurologic deficits include paraplegia (upper or lower), radiculopathy, myelopathy, cauda equina syndrome, and spondylosis with compression. Complete neurologic deficits include quadriplegia and complete paralysis.
Study Design and Statistical Analysis
Descriptive analysis was used to analyze the demographics and comorbidities. Trends of same-day surgeries and delayed surgeries between 1998 and 2013 were plotted. Varying surgical groups were compared via univariate analysis. Independent-sample t tests and 1-way analysis of variance elucidated significant variation in age, Deyo-Charlson Index, total hospital charges, and length of stay. Chi-square tests evaluated variations in patient distribution between the surgical delay groups with regard to demographic data (insurance, race, gender) as well as surgical information (complication type, complication rates, spinal level, surgical approach, neurologic index). Three binary multiple logistic forward stepwise regression models were generated to identify independent predictors of any complication, mortality, and neurologic index > 0. Models controlled for age, gender, Deyo-Charlson score, and surgical approach. The level of significance was set to P < .05. Statistical analyses were performed using IBM SPSS Statistics 23.
RESULTS
Trends of Surgical Timing
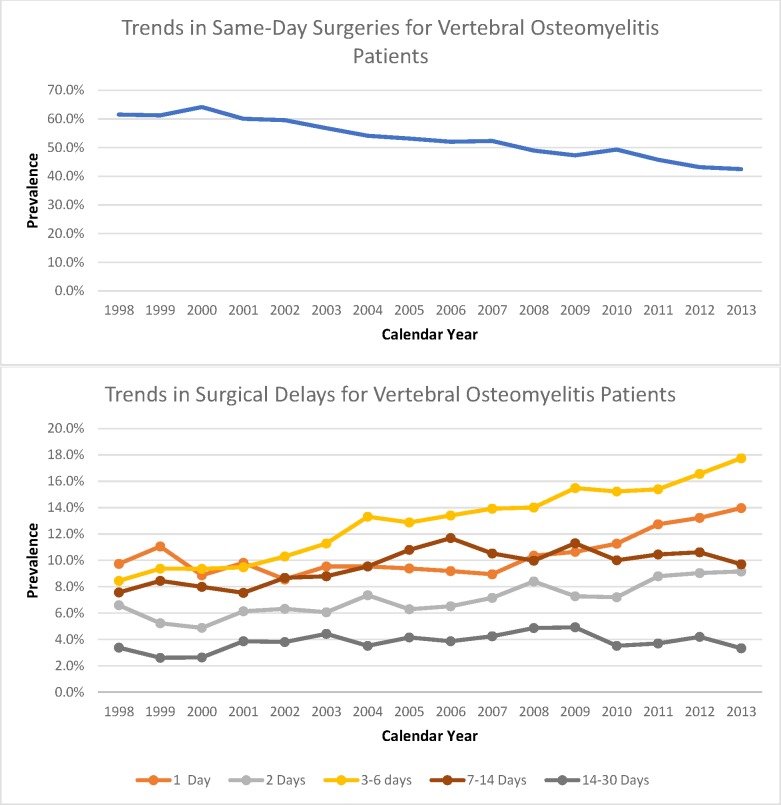
Since 1998, the incidence of patients undergoing surgical treatment has drastically increased. In 1998, 1243 patients underwent surgical intervention. Since then, there has been an incremental increase to 3155 patients undergoing surgical treatment in 2013. The prevalence of VO patients undergoing same-day-admission surgeries decreased from 61.5% in 1998 to 42.5% in 2013, while the prevalence of VO patients undergoing surgical delays has increased. In 1998, patients with 3 to 6 days of surgical delay had a rate of 8.4%, which increased to 17.7% in 2013 (Figure 1). Of all years examined, 2013 showed the highest prevalence of patients undergoing surgery with a 1-day delay (14%), 2-day delay (9.2%), and 3- to 6-day delay (17.7%) (Figure 1).
Figure 1.
Trends in same-day surgeries and surgical delays for vertebral osteomyelitis (VO) patients from 1998 to 2013. The y-axis percentages represent the prevalence of patients in a specified surgical group in relation to the total amount of surgical VO patients for each corresponding x-axis year.
General Analysis Between Surgical Delay Groups
A total of 34 465 patients fulfilled the inclusion criteria for this study. Patients undergoing same-day surgery had a mean age of 53.48 years. Mean age increased incrementally in successive surgical delay groups (7–14 days: 61.05 years), apart from patients with 14- to 30-day delays (60.22 years). Same-day-surgery patients had a significantly lower Deyo-Charlson Index (0.49) than those with surgical delays as short as 1 day (1.08). Further, the Deyo-Charlson Index increased in every successive surgical delay group. The proportion of black and Hispanic patients increased in successive surgical delay groups. The prevalence of those with Medicare (same day: 29.3% vs. 14–30-day delay: 51.2%) and Medicaid (same day: 6.1% vs. 14–30-day delay: 16.2%) increased in successive surgical delay groups. The variations between surgical groups for all demographic variables tested were statistically significant (P < .001) (Table 1).
Table 1.
Demographics of vertebral osteomyelitis surgical patients with varied surgical delays. Bolded cells with an asterisk represent a significance of P < .05.
|
Same Day |
1 d |
2 d |
3–6 d |
7–14 d |
14–30 d |
P
Value |
|
| Sample size | 17 693 | 3725 | 2536 | 5780 | 3391 | 1340 | |
| Mean age | 53.48 | 57.95 | 58.98 | 60.23 | 61.05 | 60.22 | < .001* |
| Length of stay | 4.20 | 10.10 | 11.19 | 13.95 | 20.64 | 34.04 | < .001* |
| Total charges | 63 390.78 | 84 995.54 | 95 425.43 | 111 013.7 | 155 282.86 | 245 752.4 | < .001* |
| Deyo-Charlson Index | 0.49 | 1.08 | 1.19 | 1.39 | 1.57 | 1.67 | < .001* |
| Gender | |||||||
| Male | 51.20% | 59.80% | 58.30% | 57.90% | 56.70% | 56.70% | < .001* |
| Female | 48.80% | 40.20% | 41.70% | 42.10% | 43.30% | 43.30% | |
| Race | |||||||
| White | 79.10% | 75.30% | 71.80% | 69.0% | 66.00% | 61.40% | < .001* |
| Black | 8.10% | 11.20% | 12.90% | 15.3% | 17.00% | 19.50% | |
| Hispanic | 8.70% | 9.00% | 9.80% | 10.3% | 10.70% | 12.80% | |
| Other | 4.20% | 4.50% | 5.50% | 5.4% | 6.20% | 6.20% | |
| Insurance | |||||||
| Medicare | 29.3% | 42.5% | 46.2% | 51.8% | 55.5% | 51.2% | < .001* |
| Medicaid | 6.1% | 11.1% | 12.2% | 12.6% | 12.4% | 16.2% | |
| Private insurance | 46.6% | 33.3% | 30.1% | 25.2% | 22.6% | 22.1% | |
| Self-Pay | 1.6% | 5.4% | 5.1% | 4.9% | 4.4% | 5.2% | |
| No charge | 0.2% | 0.6% | 0.8% | 0.6% | 0.7% | 0.8% | |
| Other | 16.2% | 7.1% | 5.6% | 4.9% | 4.6% | 4.5% | |
The most prevalent comorbidities identified in same-day-surgery patients were diabetes (12.9%), chronic pulmonary disease (11.9%), and myocardial infarction (2.2%). All investigated comorbidities showed significant increases in prevalence with successive surgical delay (P < .001). Comorbidities that were most prevalent in the 14- to 30-day-delay group included congestive heart failure (14.8%), renal disease (14.8%), diabetes with complications (7.9%), cancer (6.4%), and stroke (4.0%) (Table 2).
Table 2.
Comorbidities of patients with varying surgical delays. Bolded cells with an asterisk represent a significance of P < .05.
|
Same Day, % |
1 d, % |
2 d, % |
3–6 d, % |
7–14 d, % |
14–30 d, % |
P
Value |
|
| Diabetes | 12.90 | 17.60 | 18.10 | 19.30 | 19.20 | 18.90 | < .001* |
| Chronic pulmonary disease | 11.90 | 12.10 | 12.30 | 14.50 | 14.90 | 12.40 | < .001* |
| Myocardial infarction | 2.20 | 2.90 | 2.20 | 3.10 | 3.50 | 2.60 | < .001* |
| Rheumatological conditions | 2.00 | 3.00 | 3.20 | 3.30 | 2.90 | 3.10 | < .001* |
| Renal disease | 1.90 | 7.00 | 9.40 | 11.90 | 14.20 | 14.80 | < .001* |
| Congestive heart failure | 1.80 | 5.30 | 8.20 | 9.40 | 12.90 | 14.80 | < .001* |
| Diabetes with complications | 1.30 | 3.90 | 5.60 | 6.10 | 7.60 | 7.90 | < .001* |
| Pulmonary vascular disease | 1.20 | 2.10 | 3.40 | 4.30 | 3.70 | 3.20 | < .001* |
| Cancer | 1.10 | 4.20 | 5.00 | 4.90 | 6.20 | 6.40 | < .001* |
| Any stroke | 0.80 | 1.90 | 1.40 | 2.50 | 3.40 | 4.00 | < .001* |
| Peptic ulcer disease | 0.60 | 0.90 | 1.30 | 1.00 | 1.40 | 2.30 | < .001* |
| Liver disease | 0.40 | 2.50 | 2.10 | 2.50 | 2.50 | 2.80 | < .001* |
| Metastasis | 0.30 | 1.60 | 1.70 | 2.00 | 1.70 | 2.80 | < .001* |
| AIDS | 0.20 | 0.90 | 0.80 | 1.10 | 1.40 | 1.00 | < .001* |
| Severe liver disease | 0.10 | 0.50 | 0.80 | 1.00 | 1.20 | 2.00 | < .001* |
| Dementia | 0.00 | 0.20 | 0.00 | 0.30 | 0.60 | 0.60 | < .001* |
In-Hospital Perioperative Outcomes of Patients With Varying Surgical Delays
Patients in all groups were more likely to undergo posterior-only approaches (range: 41.7%–48.4%). In comparison to surgically delayed patients, same-day-surgery patients had higher anterior-only approach rates (same day: 42% vs. 7–14-day delay: 24.6%, P < .05). Surgically delayed patients were more likely to undergo combined approaches (same day: 9.1% vs. 14–30-day delay: 31.5%, P < .05). The lumbosacral region had the highest operative rates among all groups (range: 41.6%–62.8%). Cervical procedures were most prevalent less delayed groups (same day: 32.6%, P < .001), while thoracic procedures were most prevalent in surgically delayed patients (14-30-day delay: 34.6%, P < .001) (Table 3).
Table 3.
Overview of surgical approach in patients with varied surgical delays. Bolded cells with an asterisk represent a significance of P < .05.
|
Same Day, % |
1 d, % |
2 d, % |
3–6 d, % |
7–14 d, % |
14–30 d, % |
P
Value |
|
| Surgical approach | |||||||
| Anterior | 42.4 | 36.5 | 33.8 | 33.3 | 24.6 | 24.2 | < .001* |
| Posterior | 48.4 | 47.6 | 41.7 | 44.1 | 46.5 | 44.3 | |
| Combined | 9.1 | 15.9 | 24.4 | 22.6 | 28.9 | 31.5 | |
| Surgical location | |||||||
| Cervical | 32.6 | 33.6 | 29.1 | 27.8 | 22.4 | 20.1 | < .001* |
| Thoracic | 4.6 | 24.8 | 28.3 | 27.9 | 33.9 | 34.6 | |
| Lumbosacral | 62.8 | 41.6 | 42.6 | 44.3 | 43.7 | 45.3 | |
Anemia was the most common complication in same-day-surgery patients (6.1%). Infection was the most common complication in patients with 1-day delay (7.9%). Subsequently, each successive surgical delay group had sepsis as the most common complication (range: 8.2%–19.3%). Mortality in same-day-surgery patients was 0.3%, with successive surgical delay groups experiencing increased mortality rates, peaking at 5.5% (14–30-day delay, P < .001). The occurrence of any complication progressively increased from 16.7% (same day) to 42.5% (14–30-day delay, P < .001). At discharge, same-day-surgery patients had a 7.2% rate of incomplete neurological deficits. Surgically delayed patients showed significantly higher rates of incomplete neurologic deficits (range: 11.5%–14.7%, P < .001) at discharge. Rates of complete neurologic deficit also showed significant variation among groups, with a higher prevalence in delayed groups (14–30-day delay: 0.4%, P < .05) (Table 4).
Table 4.
Prevalence of most common complications and neurologic deficits in groups with varied surgical delays. Bolded cells with an asterisk represent a significance of P < .05.
|
Complications |
Same Day, % |
1 d, % |
2 d, % |
3–6 d, % |
7–14 d, % |
14–30 d, % |
P
Value |
| Most common | Anemia (6.1) | Infection (7.9) | Sepsis (8.2) | Sepsis (10.6) | Sepsis (14.5) | Sepsis (19.3) | — |
| Second most common | Device (3.3) | Sepsis (7.1) | Anemia (6.1) | Anemia (6.8) | Anemia (8.4) | Anemia (10.5) | — |
| Third most common | Infection (2.0) | Anemia (6.9) | Infection (6.0) | Infection (5.4) | Infection (5.4) | Infection (8.5) | — |
| Mortality | 0.3 | 1.7 | 2.15 | 2.3 | 3.1 | 5.5 | < .001* |
| Rate of any complication | 16.7 | 25.6 | 23.8 | 27.1 | 33.2 | 42.5 | < .001* |
| Neurologic index | |||||||
| Incomplete neurologic deficit | 7.2 | 11.9 | 11.5 | 11.7 | 12.9 | 14.7 | < .001* |
| Complete neurologic deficit | 0.0 | 0.0 | 0.1 | 0.0 | 0.1 | 0.4 | < .001* |
Regression models revealed that all surgical delay groups ≥ 1 day were the strongest predictors of complications, mortality, and a neurologic index > 0. The most significant independent predictor of the occurrence of any complication was a surgical delay of 14 to 30 days (B = 1.219, odds ratio [OR] = 3.384 [2.830–4.046], P < .001), while a surgical delay of 7 to 14 days was the second most significant independent predictor of any complication (B = 0.984, OR = 2.675 [2.346–3.051], P < .001). A combined surgical approach further predicted complication rate (B = 0.973, OR = 2.647 [2.360–2.969], P < .001). After surgical delay groups, the Deyo-Charlson Index was the next most significant predictor of mortality (B = 0.232, OR = 1.261 [1.179–1.348], P < .001) and neurologic index > 0 (B = 0.493, OR = 1.637 [1.566–1.711], P < .001) (Table 5).
Table 5.
Demographic information, surgical delay, and characteristics of surgery as independent predictors of any complication, mortality, and neurologic index. “—” indicates that the variable failed to enter the regression model. Bolded cells with an asterisk represent a significance of P < .05.
|
Predictor |
B-Coefficient |
Odds Ratio (95% Confidence Interval) |
P
Value |
| Any complication | |||
| Age | 0.017 | 1.017 (1.014–1.020) | < .001* |
| Gender | — | — | — |
| Deyo-Charlson Index | 0.030 | 1.031 (1.002–1.061) | .038* |
| 1-d delay | 0.802 | 2.230 (1.935–2.570) | < .001* |
| 2-d delay | 0.637 | 1.891 (1.591–2.247) | < .001* |
| 3- to 6-d delay | 0.889 | 2.433 (2.165–2.735) | < .001* |
| 1-wk to 14-d delay | 0.984 | 2.675 (2.346–3.051) | < .001* |
| 14-d to 1-mo delay | 1.219 | 3.384 (2.830–4.046) | < .001* |
| Year of surgery | — | — | — |
| Posterior surgical approach | 0.537 | 1.710 (1.561–1.874) | < .001* |
| Anterior surgical approach | 0.973 | 2.647 (2.360–2.969) | < .001* |
| Surgical invasiveness | 0.051 | 1.052 (1.041–1.065) | < .001* |
| Mortality | |||
| Age | 0.051 | 1.053 (1.040–1.066) | < .001* |
| Gender | −0.327 | 0.721 (0.525–0.990) | .043* |
| Deyo Index | 0.232 | 1.261 (1.179–1.348) | < .001* |
| 1-d delay | 1.730 | 5.643 (3.138–10.146) | < .001* |
| 2-d delay | 1.580 | 4.854 (2.447–9.626) | < .001* |
| 3- to 6-d delay | 1.945 | 6.994 (4.256–11.493) | < .001* |
| 1-wk to 14-d delay | 2.031 | 7.623 (4.538–12.807) | < .001* |
| 14-d to 1-mo delay | 2.366 | 10.658 (6.020–18.868) | < .001* |
| Year of surgery | −0.76 | 0.927 (0.896–0.959) | < .001* |
| Posterior surgical approach | −0.625 | 0.535 (0.377–0.760) | < .001* |
| Anterior surgical approach | −0.274 | 0.760 (0.503–1.151) | .195 |
| Surgical invasiveness | — | — | — |
| Neurologic index > 0 | |||
| Age | −0.12 | 0.989 (0.981–0.996) | .002* |
| Gender | −0.290 | 0.748 (0.611–0.916) | .005* |
| Deyo Index | 0.493 | 1.637 (1.566–1.711) | < .001* |
| 1-d delay | 1.573 | 4.823 (3.536–6.578) | < .001* |
| 2-d delay | 0.960 | 2.611 (1.718–3.966) | < .001* |
| 3- to 6-d delay | 1.118 | 3.060 (2.271–4.121) | < .001* |
| 1-wk to 14-d delay | 1.295 | 3.652 (2.685–4.969) | < .001* |
| 14-d to 1-mo delay | 1.242 | 3.464 (2.357–5.086) | < .001* |
| Year of surgery | — | — | — |
| Posterior surgical approach | 0.368 | 1.445 (1.102–1.895) | .008* |
| Anterior surgical approach | 0.344 | 1.410 (1.034–1.923) | .030* |
| Surgical invasiveness | 0.123 | 1.131 (1.105–1.158) | < .001* |
DISCUSSION
Trends over the past 2 decades have shown a progressive increase of incidence in VO.3,5–7 Elderly patients are particularly at risk,13 and with the US annual increase in life expectancy,20 VO has become an increasingly relevant concern for health care providers. VO's high operative and mortality rates4,14,15 pose a need for more information concerning surgical treatment and perioperative outcomes. Currently, the recommended timing of surgical intervention for VO patients is very controversial.
This study found that VO patients who underwent surgery after 24 hours of admission had higher chances of mortality, being discharged with an impaired neurologic status, and having any type of perioperative complication. Specifically, there were higher rates of sepsis, anemia, and recurrent infection in surgically delayed patients. These patients were also more likely to have a combined anterior-posterior surgery and less likely to undergo an anterior approach. Surgeries were more likely to be performed at the thoracic than cervical level in surgically delayed patients. Demographically, our results indicate that surgically delayed VO patients were more likely to be older, male, black or Hispanic, and on Medicare or Medicaid and to have increased total hospital charges. Patients experiencing surgical delays predictably had higher Deyo-Charlson scores and increased comorbidity rates. While the Deyo-Charlson score was shown to be a significant predictor of adverse outcomes, controlled regression models showed that surgical delay of any degree most greatly predicted mortality, impaired neurologic status, and the chance of developing any complication. This reduces the confounding nature of the association between the Deyo-Charlson score and surgical delay, further justifying our findings.
This study is the first to review a large VO cohort, derived from a nationwide database, to delineate associated effects of surgical delay. Comparatively, the majority of papers investigating surgical indications and treatment outcomes for VO patients have cohorts ranging between 10 and 300 patients. Previous studies by Miller et al,21 Adogwa et al,17 Kothari et al,22 Arnold et al,23 and Mavrogenis et al24 all had cohort sizes of 50, 82, 16, 94, and 13 patients, respectively. Of the few studies that did use large database cohorts, such as Akiyama et al25 (n = 7118), none investigated the effects of delayed surgical treatment. Previous studies focusing on the timing of surgical intervention, such as Ghobrial et al,16 reported that surgical evacuation of abscess within 24 hours of admission showed a relative advantage to discharge neurologic grade in comparison to delayed surgical patients. The findings of Connor et al18 strongly support immediate surgical decompression, combined with appropriately tailored antibiotic therapy for optimal neurologic outcomes. Alternatively, studies such as those by Karikari et al10 and Adogwa et al17 reported no benefit to early surgical intervention for abscess removal.
Despite the controversial nature of our findings, the mortality rates presented in our cohort (which range between 0.3% and 5.5%) seem to be relatively more consistent than the majority of reports in the literature. Akiyama et al,25 McHenry et al,26 Grammatico et al,7 Hutchinson et al,27 and Mylona et al1 reported in-hospital mortality rates of 6%, 11%, 3%, 27%, and 6%, respectively. The slightly lower rates observed in our cohort may be an implication of our VO patient population being limited to surgical candidates only. Miller et al21 (n = 50) reported a mortality rate of 2% within the 30-day postoperative period, while Matsui et al28 (n = 38) reported a postoperative mortality rate of 0%.
For the majority of patients, anterior vertebral elements are most commonly involved with infection, while posterior elements are affected mainly in patients with advanced infection. In general, most authors advocate anterior procedures for extensive disc and vertebral body debridement in that early surgical patients, who may be in better health and of higher bone quality, may not need the additional stabilization provided by the posterior approach.5 Arnold et al23 observed that in patients with successful surgical treatments, 32% had an anterior approach, 24% had a posterior approach, and 44% had a combined approach. Pourtaheri et al29 observed that in patients with instrumented surgical treatment, 58% had an anterior approach, 13% had a posterior approach, and 29% had a combined approach. While our rates for the anterior approach seem to align with previous literary reports, our high posterior-approach rates seem to be discrepant. This may be explained by the belief that the posterior approach is associated with fewer overall postoperative complications30 regardless of higher wound infection rates.31 Cohort size differences and/or surgeon preference of smaller studies may also be to blame. Our data also showed that combined surgical approach rates incrementally increased with further delay of surgery. This may be attributed to the complexity of the procedure that typically requires a 2-stage operation with extensive planning.14
We also found a higher prevalence of surgically delayed VO patients with Medicare and Medicaid insurance. Previous studies have indicated that patients on Medicaid have a hindered access to health care,32 longer and more costly hospitalizations,33 and higher rates of delayed cancer diagnosis, resulting in higher mortality rates,34,35 and are less likely to receive cancer-directed surgery36 in comparison to privately insured patients. If these trends hold true for patients with VO, delay of diagnosis and health care providers' reluctance to provide surgical care is extremely detrimental to a patient suffering from VO. Timely diagnoses and earlier surgical care may reduce inpatient length of stay, decrease total hospital costs, lower mortality rates, and improve a patient's overall quality of care. Effectively, Furlan et al37 provided an economic cost-utility analysis for patients undergoing early versus delayed surgical spinal cord decompression. They found that early surgical decompression was more cost effective and resulted in improved patient's quality of life in comparison to delayed surgical decompression.
Information is needed with regard to the causative factors that are at play in patients receiving surgical treatment for VO. While the NIS is one of the largest health care databases available, there are confounding variables that are neither identifiable nor controlled for. For example, patient frailty may play a significant role in surgical decision making and influence the decision to delay a surgical procedure. While frailty indexes have been developed for other large databases, such as the National Surgical Quality Improvement Program,38 to the best of our knowledge, no previous frailty measures have been validated for use on the NIS, most likely due to a lack of specificity among clinical metrics. The use of ICD-9 codes also limits our ability to determine causation of outcomes. Further, the NIS includes only inpatient data, without outpatient claims, follow-up, and patient-reported outcomes. This is an important limitation to consider, specifically with the usage of the Deyo-Charlson Index. The Deyo-Charlson Index, which includes only data from the inpatient setting, may not include important comorbidities that are recorded on outpatient claims, creating a misrepresentation of a patient's true comorbidity severity.39 The NIS database also lacks specific information regarding the patient's clinical course and operation. Finally, this paper does not detail the varying organisms responsible for infection, which may have a profound effect on outcomes. Further information on patient history, clinical course, and follow-up outcomes is needed to create a complete understanding with regard to the management of VO.
CONCLUSION
This study explores the associated effects of surgical delays in patients with VO. Using a nationwide database, the prevalence of various outcomes in VO patients who underwent early versus delayed surgical procedures was compared. Patients with surgical delays were more likely to have higher mortality rates, develop any complication, or discharge with neurologic deficits. Surgically delayed patients were also more likely to have a greater length of stay and higher total hospital charges. Medically fit patients may benefit from earlier surgical management in order to reduce risk of postoperative complications, improve outcomes, and reduce overall hospital costs.
APPENDIX
Surgical Procedure ICD-9 Codes: 03.09, 03.53, 03.99, 77.49, 77.70, 80.51, 81.00, 81.01, 81.02, 82.03, 81.04, 81.05, 81.06, 81.07, 81.08, 81.09, 81.62, 81.63, 81.64, 84.51, 84.52, 81.30, 81.32, 81.33, 81.34, 81.35, 81.36, 81.37, 81.38
Vertebral Osteomyelitis ICD-9 Codes: 730.08, 730.28, 722.90, 722.91, 722.93 and 722.93
Incomplete Neurologic Deficit ICD-9 Codes: 344.0, 344.00, 344.02, 344.04, 344.1, 344.2, 344.30, 344.31, 344.32, 344.40, 344.41 344.42, 344.5, 722.71 722.73, 722.72, 724.4, 723.4, 721.1, 721.42, 721.41, 336.8
Complete Neurologic Deficit ICD-9 Codes: 344.01, 344.03
Diabetes: 2900 29010 29011 29012 29013 29020 29021 2903 29040 29041 29042 29043 2908 2909
Chronic Pulmonary Disease: 490 4910 4911 49120 49121 49122 4918 4919 4920 4928 49300 49301 49302 49310 49311 49312 49320 49321 49322 49381 49382 49390 49391 49392 4940 4941 4950 4951 4952 4953 4954 4955 4956 4957 4958 4959 496 500 501 502 503 504 505 5064
Myocardial Infarction: 41000 4100 41010 41011 41012 41020 41021 41022 41030 41031 41032 41040 41040 41041 41042 41050 41051 41052 41060 41061 41062 41070 41072 41080 41081 41082 41090 41091 41092 412
Rheumatological Conditions:7100 7101 7104 7140 7141 7142 71481 725
Renal: 5820 5821 5822 5824 58281 58289 5829 5830 5831 5832 5834 5836 5837 5851 5852 5853 5853 5854 5855 5856 5859 586 5880 5881 58881 58889 5889
Congestive Heart Failure: 4280 4281 42820 42821 42822 42823 42830 42831 42833 42840 42841 42842 42843 4289
Diabetes with Complications: 25040 25041 25042 25043 25050 25051 25052 25053 25060 25061 250062 25063
PVD: 4439 44100 44101 44102 44103 4411 4412 4413 4414 4415 4416 4417 4419 7854 38487 38482
Cancer: 1400 1401 1403 1404 1405 1406 1408 1409 1410 1411 1412 1413 1414 1415 1416 1418 1419 1420 1421 1422 1428 1429 1430 1431 1438 1439 1440 1441 1448 1449 1450 1451 1452 1453 1454 1455 1456 1458 1459 1460 1461 4162 1463 1464 1465 1466 1467 1468 1469 1470 1471 1472 1473 1478 1479 1480 1481 1482 1483 1488 1489 1490 1491 1498 1499 1500 1501 1502 1503 1504 1505 1508 1509 1510 1511 1512 1513 1514 1515 1516 1518 1519 1520 1521 1522 1523 1528 1529 1530 1531 1532 1533 1534 1535 1536 1537 1538 1539 1540 1541 1542 1543 1548 1550 1551 1552 1560 1561 1562 1568 1569 1570 1571 1572 1573 1574 1589 1579 1580 1588 1589 1590 1891 1598 1599 1600 1601 1602 1603 1604 1605 1608 1609 1610 1611 1612 1613 1618 1619 1620 1622 1623 1624 1625 1628 1629 1630 1631 1638 1639 1640 1641 1642 1643 1648 1649 1650 1658 1659 1701 1702 1703 1704 1705 1706 1707 1708 1709 1710 1712 1713 1714 1715 1716 1717 1718 1719 1720 1721 1722 1723 1724 1725 1726 1727 1728 1729 1740 1741 1742 1743 1744 1745 1746 1748 1749 1750 1759 1760 1761 1762 1763 1764 1765 1768 1769 179 1800 1801 1808 1809 181 1820 1821 1828 1830 1832 1833 1834 1835 1838 1839 1840 1841 1842 1843 1844 1848 1849 185 1860 1869 1871 1872 1873 1874 1875 1876 1877 1878 1879 1880 1881 1882 1883 1884 1885 1886 1887 1888 1889 1890 1891 1892 1893 1894 1898 1899 1900 1901 1902 1903 1904 1905 1906 1907 1908 1909 1910 1911 1912 1913 1914 1915 1916 1917 1918 1919 1920 1921 1922 1923 1928 1929 193 1940 1941 1943 1944 1945 1946 1948 1949 1950 1951 1952 1953 1954 1955 1958 20000 20001 20002 20003 20004 2005 20006 20007 20008 20010 20011 20012 20013 20014 20015 20016 20017 20018 20020 20021 20022 20023 20024 20025 20026 20027 20028 20030 20031 20032 20033 20034 20035 20036 20037 20038 20040 20041 20042 20043 20044 20045 20046 20047 20048 20050 20051 20052 20023 20024 20055 20056 20057 20058 20060 20061 20062 20063 20064 20065 20066 20067 20070 20071 20072 20073 20074 20075 20076 20077 20078 20080 20081 20082 20083 20084 20085 20086 20087 20088 20100 20101 20102 20103 20104 20105 20106 20107 20108 20110 20111 20112 20113 20114 20115 20116 20117 20118 20120 20121 20122 20123 20124 20125 20126 20127 20128 20130 20131 20132 20133 20134 20135 20136 20137 20138 20140 20141 20142 20143 20144 20145 20146 20147 20148 20150 20151 20152 20153 20154 20155 20156 20157 20158 20160 20161 20162 20163 20164 20165 20166 20167 20168 20170 20171 20172 20173 20174 20175 20176 20177 20178 20190 20191 20192 20193 20194 20195 20196 20197 20198 20200 20201 20202 20203 20204 20205 20206 20207 20208 20210 20211 20212 20213 20214 20215 20216 20217 20218 20220 20221 20222 20223 20224 20225 20226 20227 20228 20230 20231 20232 20233 20234 20235 20236 20237 20238 20240 20241 20242 20243 20244 20245 20246 20247 20248 20250 20251 20252 20253 20254 20255 20256 20257 20258 20260 20261 20262 20263 20264 20265 20266 20267 20268 20270 20271 20272 20273 20274 20275 20276 20277 20278 20280 20281 20282 20283 20284 20285 20286 20287 20288 20290 20291 20292 20293 20294 20295 20296 20297 20298 20300 20301 20302 20310 20311 20312 20380 20381 20382 20400 20401 20402 20410 20411 20412 20420 20421 20422 20480 20481 20490 20491 20942 20500 50201 20502 20510 20511 20512 20520 20521 20522 20530 20531 20532 20580 20581 20582 20590 20591 20592 20600 20601 20602 20610 20611 20612 20620 20621 20622 20680 20681 20682 20690 20691 20692 20700 20701 20702 20710 20711 20712 20720 20721 20722 20780 20781 20782 20800 20801 20802 20810 20811 20812 20821 20822 20880 20881 20882 20890 20891 20892
Any Stroke: 430 431 4320 4321 4329 43300 43301 43311 43320 43321 43330 43331 43380 43381 43390 43391 43400 43401 43410 43411 43490 43491 4350 4351 4352 4353 4358 4359 436 4370 4371 4372 4373 4374 4375 4376 4377 4378 4379 4380 43810 43811 43812 43813 43814 43819 43820 43821 43822 43830 43831 43832 43840 43841 43842 43850 43851 43852 43853 4386 4387 43881 43882 43883 43884 43885 43889 4389
Peptic Ulcer Disease: 53100 53101 53110 53111 53120 53121 53130 53131 53140 53141 53150 53151 53160 53161 53170 53171 53190 53191 53200 53201 53210 53211 53220 53221 53230 53231 53240 53241 53250 53251 53260 53261 53270 53271 53290 53291 53300 53301 53310 53320 53321 53330 53331 53340 53341 53350 53351 53360 53361 53370 53371 53390 53391 53400 53401 53410 53411 53420 53421 53430 53431 53440 53441 53450 53451 53460 53461 53470 53471 53490 53491
Liver Disease: 4560 4561 45620 46521 5722 5723 5724 5728
Metastasis: 1960 1961 1962 1963 1965 1966 1968 1969 1970 1971 1972 1973 1974 1975 1976 1977 1978 1980 1981 1982 1983 1984 1985 1986 1987 19881 19882 19889 1990 1991
AIDS: 42 420 421 422 429 430 431 432 433 439 440 449
Severe Liver Disease: 4560 4561 45620 46521 5722 5723 5724 5728
Dementia: 2900 29010 29011 29012 29013 29020 29021 2903 29040 29041 29042 29043 2908 2909
Anemia: 2851
Device: 99600 9964 99640 99641 99642 99643 99644 99645 99646 99647 99649
Infection: 9985 99851 99859
Sepsis: 99591 99592
REFERENCES
- 1.Mylona E, Samarkos M, Kakalou E, Fanourgiakis P, Skoutelis A. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum. 2009;39(1):10–17. doi: 10.1016/j.semarthrit.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Schimmer RC, Jeanneret C, Nunley PD, Jeanneret B. Osteomyelitis of the cervical spine: a potentially dramatic disease. J Spinal Disord Tech. 2002;15(2):110–117. doi: 10.1097/00024720-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Jaramillo-de la Torre JJ, Bohinski RJ, Kuntz Iv C. Vertebral osteomyelitis. Neurosurg Clin N Am. 2006;17(3):339–351. doi: 10.1016/j.nec.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Berbari EF, Kanj SS, Kowalski TJ, et al. Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clin Infect Dis. 2015;61(6):e26–e46. doi: 10.1093/cid/civ482. [DOI] [PubMed] [Google Scholar]
- 5.Skaf GS, Domloj NT, Fehlings MG, et al. Pyogenic spondylodiscitis: an overview. J Infect Public Health. 2010;3(1):5–16. doi: 10.1016/j.jiph.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Torda AJ, Gottlieb T, Bradbury R. Pyogenic vertebral osteomyelitis: analysis of 20 cases and review. Clin Infect Dis. 1995;20(2):320–328. doi: 10.1093/clinids/20.2.320. [DOI] [PubMed] [Google Scholar]
- 7.Grammatico L, Baron S, Rusch E, et al. Epidemiology of vertebral osteomyelitis (VO) in France: analysis of hospital-discharge data 2002-2003. Epidemiol Infect. 2008;136(5):653–660. doi: 10.1017/S0950268807008850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carragee EJ. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am. 1997;79(6):874–880. doi: 10.2106/00004623-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Cottle L, Riordan T. Infectious spondylodiscitis. J Infect. 2008;56(6):401–412. doi: 10.1016/j.jinf.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Karikari IO, Powers CJ, Reynolds RM, Mehta AI, Isaacs RE. Management of a spontaneous spinal epidural abscess: a single-center 10-year experience. Neurosurgery. 2009;65(5):919–923. doi: 10.1227/01.NEU.0000356972.97356.C5. [DOI] [PubMed] [Google Scholar]
- 11.Darouiche R. Spinal epidural abscess. N Engl J Med. 2006;355(19):2012–2020. doi: 10.1056/NEJMra055111. [DOI] [PubMed] [Google Scholar]
- 12.Zimmerer SME, Conen A, Müller AA, et al. Spinal epidural abscess: aetiology, predisponent factors and clinical outcomes in a 4-year prospective study. Eur Spine J. 2011;20(12):2228–2234. doi: 10.1007/s00586-011-1838-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chenoweth CE, Bassin BJ, Hartley SE, et al. Vertebral Osteomyelitis Guideline Team. Vertebral Osteomyelitis, Discitis, and Spinal Epidural Abscess in Adults. Ann Arbor, MI: University of Michigan; 2013. http://www.med.umich.edu/1info/FHP/practiceguides/vertebral/VO.pdf. [PubMed] [Google Scholar]
- 14.Nickerson EK, Sinha R. Vertebral osteomyelitis in adults: an update. Br Med Bull. 2016;117(1):121–138. doi: 10.1093/bmb/ldw003. [DOI] [PubMed] [Google Scholar]
- 15.Pola E, Logroscino CA, Gentiempo M, et al. Medical and surgical treatment of pyogenic spondylodiscitis. Eur Rev Med Pharmacol Sci. 2012;16(suppl 2):35–49. http://www.ncbi.nlm.nih.gov/pubmed/22655482. [PubMed] [Google Scholar]
- 16.Ghobrial GM, Beygi S, Viereck MJ, et al. Timing in the surgical evacuation of spinal epidural abscesses. Neurosurg Focus. 2014;37(August):E1. doi: 10.3171/2014.6.FOCUS14120. [DOI] [PubMed] [Google Scholar]
- 17.Adogwa O, Karikari IO, Carr KR, et al. Spontaneous spinal epidural abscess in patients 50 years of age and older: a 15-year institutional perspective and review of the literature. J Neurosurg Spine. 2014;20(3):344–349. doi: 10.3171/2013.11.SPINE13527. [DOI] [PubMed] [Google Scholar]
- 18.Connor DE, Chittiboina P, Caldito G, Nanda A. Comparison of operative and nonoperative management of spinal epidural abscess: a retrospective review of clinical and laboratory predictors of neurological outcome. J Neurosurg Spine. 2013;19(1):119–127. doi: 10.3171/2013.3.SPINE12762. [DOI] [PubMed] [Google Scholar]
- 19.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9 administrative databases. J Clin Epidemiol. 1992;45(10):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 20.Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States. Econ Stat Adm US Dep Commer. 2014;1964:1–28. [Google Scholar]
- 21.Miller JA, Achey RL, Derakhshan A, Lubelski D, Benzel EC, Mroz TE. Neurologic complications, reoperation, and clinical outcomes following surgery for vertebral osteomyelitis. Spine (Phila Pa 1976) 2015;41(4):1. doi: 10.1097/BRS.0000000000001157. [DOI] [PubMed] [Google Scholar]
- 22.Kothari M, Shah K, Tikoo A, Nene A. Short to mid-term term surgical outcome study with posterior only approach on tuberculous spondylodiscitis in an elderly population. Asian Spine J. 2016;10(2):258–266. doi: 10.4184/asj.2016.10.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnold R, Rock C, Croft L, Gilliam BL, Morgan DJ. Factors associated with treatment failure in vertebral osteomyelitis requiring spinal instrumentation. Antimicrob Agents Chemother. 2014;58(2):880–884. doi: 10.1128/AAC.01452-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mavrogenis AF, Igoumenou V, Tsiavos K, et al. When and how to operate on spondylodiscitis: a report of 13 patients. Eur J Orthop Surg Traumatol. 2016;26(1):31–40. doi: 10.1007/s00590-015-1674-6. [DOI] [PubMed] [Google Scholar]
- 25.Akiyama T, Chikuda H, Yasunaga H, Horiguchi H, Fushimi K, Saita K. Incidence and risk factors for mortality of vertebral osteomyelitis: a retrospective analysis using the Japanese diagnosis procedure combination database. BMJ Open. 2013;3(3):1–6. doi: 10.1136/bmjopen-2012-002412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McHenry MC, Easley KA, Locker GA. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. 2002;34(10):1342–1350. doi: 10.1086/340102. [DOI] [PubMed] [Google Scholar]
- 27.Hutchinson C, Hanger C, Wilkinson T, Sainsbury R, Pithie A. Spontaneous spinal infections in older people. Intern Med J. 2009;39(12):845–848. doi: 10.1111/j.1445-5994.2009.02052.x. [DOI] [PubMed] [Google Scholar]
- 28.Matsui H, Hirano N, Sakaguchi Y. Vertebral osteomyelitis: an analysis of 38 surgically treated cases. Eur Spine J. 1998;7(1):50–54. doi: 10.1007/s005860050027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pourtaheri S, Issa K, Stewart T, et al. Comparison of instrumented and noninstrumented surgical treatment of severe vertebral osteomyelitis. Orthopedics. 2016;39(3):e504–e508. doi: 10.3928/01477447-20160427-07. [DOI] [PubMed] [Google Scholar]
- 30.Hee HT, Majd ME, Holt RT, Pienkowski D. Better treatment of vertebral osteomyelitis using posterior stabilization and titanium mesh cages. J Spinal Disord Tech. 2002;15(2):149–156. doi: 10.1097/00024720-200204000-00010. discussion 156. [DOI] [PubMed] [Google Scholar]
- 31.Rayes M, Colen C, Bahgat D, et al. Safety of instrumentation in patients with spinal infection. J Neurosurg Spine. 2010;12(6):647–659. doi: 10.3171/2009.12.SPINE09428. [DOI] [PubMed] [Google Scholar]
- 32.Cook NL, Hicks LS, O'Malley AJ, Keegan T, Guadagnoli E, Landon BE. Access to specialty care and medical services in community health centers. Health Aff. 2007;26(5):1459–1468. doi: 10.1377/hlthaff.26.5.1459. [DOI] [PubMed] [Google Scholar]
- 33.Hasan O, Orav EJ, Hicks LS. Insurance status and hospital care for myocardial infarction, stroke, and pneumonia. J Hosp Med. 2010;5(8):452–459. doi: 10.1002/jhm.687. [DOI] [PubMed] [Google Scholar]
- 34.McDavid K, Tucker TC, Sloggett A. Cancer survival in Kentucky and health insurance coverage. Arch Intern Med. 2003;163(18):2135–2144. doi: 10.1001/archinte.163.18.2135. [DOI] [PubMed] [Google Scholar]
- 35.Bradley CJ. Disparities in cancer diagnosis and survival. Cancer. 2001;91(1):178–188. doi: 10.1002/1097-0142(20010101)91:1<178::aid-cncr23>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 36.Roetzheim RG, Pal N, Gonzalez EC, Ferrante JM, Van Durme DJ, Krischer JP. Effects of health insurance and race on colorectal cancer treatments and outcomes. Am J Public Health. 2000;90(11):1746–1754. doi: 10.2105/ajph.90.11.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furlan JC, Craven BC, Massicotte EM, Fehlings MG. Early versus delayed surgical decompression of spinal cord after traumatic cervical spinal cord injury: a cost-utility analysis. World Neurosurg. 2016;88:166–174. doi: 10.1016/j.wneu.2015.12.072. [DOI] [PubMed] [Google Scholar]
- 38.Mogal H, Vermilion SA, Dodson R, et al. Modified frailty index predicts morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol. 2017;24(6):1714–1721. doi: 10.1245/s10434-016-5715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]