Cardiac troponin activators could be beneficial in systolic heart failure. Tikunova et al. demonstrate that, unlike previously known calcium sensitizers, the small molecule 3-chlorodiphenylamine does not activate isolated cardiac troponin C but instead activates the intact troponin complex.
Abstract
Despite extensive efforts spanning multiple decades, the development of highly effective Ca2+ sensitizers for the heart remains an elusive goal. Existing Ca2+ sensitizers have other targets in addition to cardiac troponin (cTn), which can lead to adverse side effects, such as hypotension or arrhythmias. Thus, there is a need to design Ca2+-sensitizing drugs with higher affinity and selectivity for cTn. Previously, we determined that many compounds based on diphenylamine (DPA) were able to bind to a cTnC–cTnI chimera with moderate affinity (Kd ∼10–120 µM). Of these compounds, 3-chlorodiphenylamine (3-Cl-DPA) bound most tightly (Kd of 10 µM). Here, we investigate 3-Cl-DPA further and find that it increases the Ca2+ sensitivity of force development in skinned cardiac muscle. Using NMR, we show that, like the known Ca2+ sensitizers, trifluoperazine (TFP) and bepridil, 3-Cl-DPA is able to bind to the isolated N-terminal domain (N-domain) of cTnC (Kd of 6 µM). However, while the bulky molecules of TFP and bepridil stabilize the open state of the N-domain of cTnC, the small and flexible 3-Cl-DPA molecule is able to bind without stabilizing this open state. Thus, unlike TFP, which drastically slows the rate of Ca2+ dissociation from the N-domain of isolated cTnC in a dose-dependent manner, 3-Cl-DPA has no effect on the rate of Ca2+ dissociation. On the other hand, the affinity of 3-Cl-DPA for a cTnC–TnI chimera is at least an order of magnitude higher than that of TFP or bepridil, likely because 3-Cl-DPA is less disruptive of cTnI binding to cTnC. Therefore, 3-Cl-DPA has a bigger effect on the rate of Ca2+ dissociation from the entire cTn complex than TFP and bepridil. Our data suggest that 3-Cl-DPA activates the cTn complex via a unique mechanism and could be a suitable scaffold for the development of novel treatments for systolic heart failure.
Introduction
Heart failure occurs when the heart is unable to pump enough blood to meet the demands of the body (Erdmann, 1998; Kemp and Conte, 2012; Johnson, 2014). In the United States, over six million adults are living with heart failure, with the number of affected individuals projected to rise to over eight million by the year 2030 (Benjamin et al., 2018). Heart failure patients face poor long-term outcomes, with ∼50% and 10% survival rates at 5 and 10 yr after diagnosis, respectively (Roger, 2013). In systolic heart failure, contraction of cardiac muscle is impaired, leading to a reduced ejection fraction (Chatterjee, 2012; Komamura, 2013). One way to enhance the strength of cardiac muscle contraction is to increase the responsiveness of the contractile apparatus to Ca2+ (Kass and Solaro, 2006; Davis et al., 2016). Thus, compounds that sensitize the contractile apparatus to Ca2+ are expected to be beneficial in the treatment of systolic heart failure.
Because of its key role in the regulation of muscle contraction, cardiac troponin C (cTnC) represents a logical target for the development of positive inotropic drugs (Kass and Solaro, 2006; Endoh, 2008; Li and Hwang, 2015). In fact, increasing Ca2+ sensitivity of the regulatory N-domain of cTnC by mutagenesis resulted in improved heart function, without adverse side effects, in a model of heart failure caused by myocardial infarction (Shettigar et al., 2016). However, despite extensive efforts, development of highly effective Ca2+-sensitizing compounds has remained elusive. Currently, two Ca2+-sensitizing compounds, levosimendan and pimobendan, are being used in clinical practice to treat heart failure in humans, albeit not in the United States (Lehmann et al., 2003; Kass and Solaro, 2006; Pollesello et al., 2016). One of these compounds, pimobendan, has been approved in the United States and worldwide to treat heart failure in dogs (Boyle and Leech, 2012). Both levosimendan and pimobendan are believed to elicit their positive inotropic effect through sensitization of the regulatory N-domain of cTnC to Ca2+ (Fujino et al., 1988; Pollesello et al., 1994). However, both of these compounds have molecular targets other than cTnC, potentially leading to undesirable side effects, such as hypotension (Hwang and Sykes, 2015; Pollesello et al., 2016).
Many compounds with Ca2+-sensitizing properties have not entered clinical practice for use in heart failure patients for various reasons (Li et al., 2008). For instance, use of positive inotropes such as trifluoperazine (TFP) and bepridil in the treatment of heart failure is prevented by their off-target actions (Li et al., 2008). Although these compounds are not used clinically to treat heart failure, studies of interactions between these molecules and cTnC provide insight into features important for Ca2+ sensitization. A crystal structure suggests that the Ca2+-sensitizing effect of bepridil arises from the ability of the molecule to bind into the hydrophobic cavity in the regulatory N-domain of cTnC, stabilizing the fully open state (Li et al., 2000). However, binding of bepridil into the hydrophobic pocket of cTnC competes with binding of cTnI (Wang et al., 2002), diminishing the overall cTn-activating effect. The ability of cTnC to bind the switch peptide of cTnI is a critical determinant of the overall Ca2+ sensitivity of the contractile apparatus (Siddiqui et al., 2016).
The ultimate goal of our research is to develop effective treatments for cardiovascular diseases. Recently, we determined that despite their small size, many compounds based on diphenylamine were able to bind moderately well (Kd ∼10–120 µM) to a chimeric protein consisting of the regulatory N-domain of cTnC (cNTnC) and the switch region of cTnI (cNTnC–cSp chimera), with 3-chlordiphenylamine (3-Cl-DPA) binding most tightly (Kd of 10 µM; Cai et al., 2016). The goal of this work was to determine whether 3-Cl-DPA is able to sensitize cardiac muscle to Ca2+ and, if so, to explore the molecular mechanism of its action compared with that of bepridil and TFP, compounds known to sensitize the N-domain of isolated cTnC to Ca2+ (Li et al., 2008).
Materials and methods
Preparation of proteins for fluorescence studies
The recombinant human cTnCT53C protein (with C35S and C84S substitutions) was generated, expressed, purified, and labeled with 2-(4′-(iodoacetamido)anilino)naphthalene-6-sulfonic acid (IAANS) as previously described (Davis et al., 2007). The recombinant human cTnI and cTnT proteins were expressed and purified as previously described (Davis et al., 2007). Reconstitution of the cTn complex was performed as previously described (Davis et al., 2007).
Measurements of force versus pCa (−log [Ca2+]) in skinned ventricular trabeculae
The care and use of animals in this study were in accordance with a protocol approved by The Ohio State University Institutional Animal Care and Use Committee. The hearts of four female Wistar rats (3–5 months old) were rapidly isolated after induction of a deep level of anesthesia with isoflurane and placed in cold relaxing solution. A single cut was made through the free wall of the right and left ventricles, and the hearts were soaked in 1% Triton X-100 for 30 min, with occasional manual swirling, to permeabilize the trabeculae on the endocardial surface. The trabeculae were studied as previously described (McConnell et al., 2015). Briefly, a trabecula was mounted in a temperature-controlled experimental chamber that was maintained at 15°C. The chamber was placed on the stage of an upright microscope (Labophot; Nikon). The trabecula was connected at one end to a servo-controlled torque motor (model 322C; Aurora Scientific) and at the other end to an isometric force transducer (model 403; Aurora Scientific). The motor and transducer were attached to three-way positioners, and the length of the trabecula was set by moving the motor or transducer to set the resting striation spacing (equated with sarcomere length). Striation spacing was determined using a digital camera that was mounted on the microscope and image analysis software. The distance spanned by ∼20 striations was measured to calculate resting sarcomere length. Fiber width and depth were measured and fiber cross-sectional area was calculated, assuming an ellipsoidal cross section. The average resting sarcomere length of all 13 trabeculae was set to 2.12 ± 0.03 µm. The force versus pCa relationship was studied in ten trabeculae, first without, then with 100 µM 3-Cl-DPA (100 mM stock dissolved in DMSO). The force versus pCa relationship was also studied in three trabeculae, first without and then with 1 µl/ml DMSO. The trabecula was exposed to a series of pCa solutions with complete relaxation (in pCa 9.0 solution) between each activation. Resting force was measured (in pCa 9.0 solution) and was subtracted from the total peak force during each activation to calculate peak active force. Every third activation was performed with pCa 4.0 solution, and the force in that solution was used to normalize the force during each temporally neighboring submaximal activation. All of the solutions (preactivating, activating, and relaxing) that were used for a series of measurements (with or without 3-Cl-DPA and DMSO) were identical with respect to the concentration of 3-Cl-DPA and DMSO, so that the only variation was the pCa of the activating solution. All of the measurements were made at pH 7.0 and 15°C. The composition and preparation of the solutions was as previously described (Reiser et al., 2013). The force versus pCa data were fit with the logistic sigmoid function, mathematically equivalent to the Hill equation, as previously described (Black et al., 2000; Tikunova et al., 2002).
Determination of Ca2+-binding sensitivities
All steady-state fluorescence measurements were performed using a Perkin-Elmer LS55 fluorescence spectrometer at 15°C. IAANS fluorescence was excited at 330 nm and monitored at 445 nm as microliter amounts of CaCl2 were added to 2 ml of IAANS-labeled cTnCT53C reconstituted into 0.15 µM cTn complex, in the absence or presence of increasing concentrations of 3-Cl-DPA or bepridil, in titration buffer (200 mM MOPS [to prevent pH changes upon addition of Ca2+], 150 mM KCl, 2 mM EGTA, 3 mM MgCl2, and 0.025% Tween-80, pH 7.0). Tween-80 was excluded from the titration buffer for experiments performed in the presence of 3-Cl-DPA. Free [Ca2+] was calculated using a program (EGCA02) developed by Robertson and Potter (1984). Each reported pCa50 represents an average of at least three titrations ± SEM. The data were fit with a logistic sigmoid function, mathematically equivalent to the Hill equation, as previously described (Black et al., 2000; Tikunova et al., 2002).
Determination of Ca2+ dissociation rates
All kinetic measurements were performed using an Applied Photophysics model SX.18MV stopped-flow apparatus with a dead time of ∼1.4 ms. IAANS fluorescence was excited at 330 nm with emission monitored through a 420–470 nm band-pass interference filter (Oriel). 10 mM EGTA in a stopped-flow buffer (10 mM MOPS and 150 mM KCl, pH 7.0) was used to remove 500 µM Ca2+ from IAANS-labeled cTnCT53C in isolation (1 µM) or reconstituted into the cTn complex (0.3 µM), in the absence or presence of compounds, in the stopped-flow buffer at 15°C. Increasing concentration of compounds were individually added to both stopped-flow reactants. The data were fit using a program by P. J. King (Applied Photophysics) that utilizes the nonlinear Levenberg–Marquardt algorithm. Each koff represents an average of at least three separate experiments ± SEM, each averaging at least five shots, which were fit with a single exponential equation.
NMR titration of cTnC and cNTnC–cSp chimera with 3-Cl-DPA, TFP, and bepridil
Sample preparation
Recombinant human cTnC, with the mutations C35S and C84S, was used for these experiments. The expression and purification of [15N]-cTnC and [15N]-cNTnC–cSp chimera (also containing C35S and C84S mutations) in Escherichia coli were as described previously (Cai et al., 2016). In this construct, the N-domain of cTnC with C35S, C84S substitutions (residues 1–90) is fused to cTnI136–163. The cNTnC–cSp chimera also contains a C-terminal Gly-His6 tag to aid purification. The full amino acid sequence of the cNTnC–cSp chimera was reported previously (Cai et al., 2016).
For each NMR sample, solid [15N]-cTnC or cNTnC–cSp chimera was dissolved into 500 µl NMR buffer containing 100 mM KCl, 10 mM imidazole in 90% H2O/10% D2O, and 0.5 mM 4,4-dimethyl-4-silapentane-1-sulfonic acid as chemical shift standard. Protein concentration was determined by integrating 1D 1H and 2D [1H,15N] heteronuclear single quantum coherence (HSQC) NMR spectral signals. For titrations into [15N]-cTnC, the starting protein concentration was 0.48 mM. For titrations into [15N]-cNTnC–cSp chimera, the starting protein concentration was 0.11 mM. To each sample, 5 µl of 1 M CaCl2 was added to ensure that the protein was Ca2+ saturated and the pH was adjusted with 1 M NaOH or 1 M HCl to 6.7. 1H,15N-NMR experiments are usually performed in slightly acidic conditions, because this slows the solvent hydrogen exchange rate of unprotected backbone amides so that they can be observed by NMR (Bai et al., 1993).
3-Cl-DPA was purchased from Toronto Research Chemicals. TFP and bepridil were purchased from Sigma. The compounds are insoluble in aqueous solutions and were thus dissolved in d6-DMSO to make stock solutions (97.2 mM 3-Cl-DPA, 67 mM TFP, and 67 mM bepridil), with concentrations determined by integrating 1D 1H NMR spectrum using 4,4-dimethyl-4-silapentane-1-sulfonic acid as an internal standard. The purity and structure of the compounds were verified by 1D 1H NMR spectroscopy. The vials containing the compound solutions were wrapped in aluminum foil to protect the molecules from light-catalyzed degradation. To a 500-µl NMR sample, aliquots of 0.2–10 µl compound stock solution were added for each titration point. The sample was mixed thoroughly with each addition. Total volume increases were taken into account for data analyses. Both 1D 1H and 2D [1H,15N]-HSQC spectra were acquired at every titration point.
NMR spectroscopy
All NMR experiments were run on a Varian Inova 500-MHz spectrometer. All data were collected at 30°C. Both 1D 1H and 2D [1H,15N]-HSQC spectra were acquired at every titration point. The spectrometer is equipped with a triple resonance 1H,13C,15N probe and z-pulsed field gradients.
Kd values were calculated from NMR-based compound titrations. Amide NMR signals that showed large and linear perturbations during a titration were chosen for Kd calculation. For each individual amide signal, chemical shift changes were plotted as a function of ligand-to-protein ratio and fit to a 1:1 binding stoichiometry (protein + compound ↔ protein • compound) using xcrvfit, which fully accounts for protein concentration, ligand concentration, and dilution effects (www.bionmr.ualberta.ca/bds/software/xcrvfit). This yielded a Kd value for each amino acid residue analyzed that was then averaged to a single value ± SEM.
Statistical analysis
Statistical significance was determined by a paired or unpaired two-sample t test (wherever applicable), using the statistical analysis software Minitab. The two means were considered to be significantly different when the P value was <0.05. All data are reported as mean ± SEM.
Results
Effect of 3-Cl-DPA on the Ca2+ sensitivity of force development in skinned ventricular trabeculae
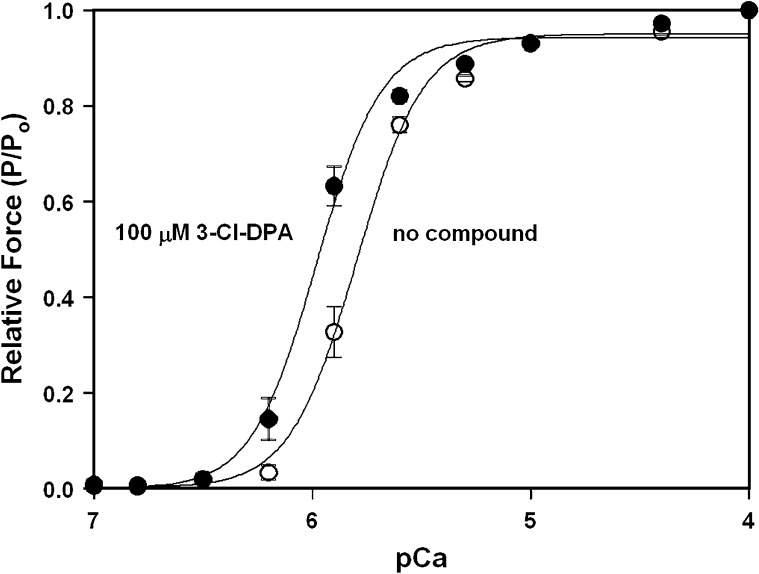
Previously, we determined that 3-Cl-DPA was able to bind to the cNTnC–cSp chimera with an affinity of 10 µM (Cai et al., 2016). For this study, we wanted to determine whether 3-Cl-DPA was a Ca2+ sensitizer of cardiac muscle. Fig. 1 shows that in the absence of 3-Cl-DPA, rat ventricular trabeculae develop force with a pCa50 (−log[Ca2+] at which force is at 50% of maximal force) of 5.81 ± 0.03. When force versus pCa experiments are repeated in the presence of 100 µM 3-Cl-DPA, trabeculae develop force with a significantly higher pCa50 of 5.99 ± 0.03. The presence of 3-Cl-DPA does not significantly affect the steepness of the force versus pCa curve (Hill coefficient [nH] = 3.3 ± 0.2 in the absence of a compound versus nH = 3.5 ± 0.3 in the presence of 100 µM 3-Cl-DPA). DMSO alone did not cause a significant shift in the force versus pCa relationship (data not shown). The resting tension, in pCa 9.0, was measured after all of the force versus pCa data were collected in seven of the trabeculae that were used to study the effects of 3-Cl-DPA. This value was compared with the initial resting tension value immediately after the trabecula was mounted in the chamber and before exposure to 3-Cl-DPA. The initial and final resting tensions, normalized with trabecula cross-sectional area (1.2 ± 0.4 kN/m2 and 1.1 ± 0.4 kN/m2, respectively), were not significantly different.
Figure 1.
Effect of 3-Cl-DPA on the Ca2+ sensitivity of force development in skinned ventricular trabeculae. The curves show the Ca2+ dependence of force development in skinned ventricular trabeculae in the absence and presence of 100 µM 3-Cl-DPA. Data represent the mean ± SEM for 10 trabeculae. Data sets were individually normalized and fit with a logistic sigmoid.
There was a small but significant decrease in maximal force (Po) during the course of the control (no 3-Cl-DPA) force versus pCa measurements. The mean Po in the first and the last activations with pCa 4.0 was 12.8 ± 1.1 and 11.2 ± 0.8 kN/m2, respectively. There was also a small but significant decrease in Po during the course of measurements with 3-Cl-DPA (10.8 ± 0.7 [first activation] and 10.0 ± 0.7 [last activation] kN/m2). We compared Po in pCa 4.0 during the first activation with 3-Cl-DPA to the last activation in pCa 4.0 without 3-Cl-DPA to test whether the compound had any effect on Po, and there was not a significant difference. Therefore, we conclude that 3-Cl-DPA had no significant effect on maximal force. Thus, our results indicate that 3-Cl-DPA results in ∼1.5-fold increase in the Ca2+ sensitivity of force development without altering the maximal or resting forces.
Effect of 3-Cl-DPA on the rate of Ca2+ dissociation from the regulatory N-domain of intact cTnC
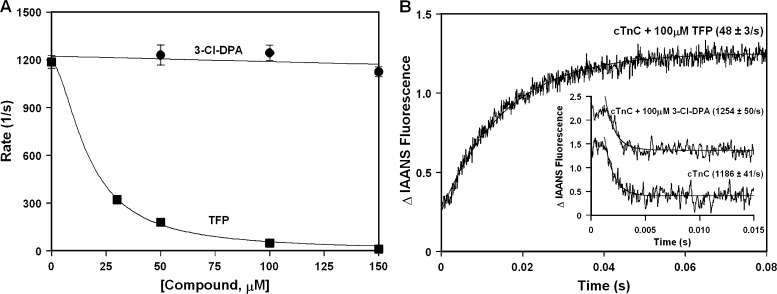
Ca2+-sensitizing compounds typically increase the Ca2+-binding affinity of cTnC by slowing the rate of Ca2+ dissociation from the N-terminal regulatory domain of cTnC (for review, see Davis and Tikunova, 2008). To further elucidate the molecular mechanism by which 3-Cl-DPA sensitizes cardiac muscle to Ca2+, we compared the effect of 3-Cl-DPA on the rate of Ca2+ dissociation from the regulatory N-domain of intact IAANS-labeled cTnCT53C to that of a known Ca2+-sensitizing compound, TFP. Fig. 2 A (with representative stopped-flow traces shown in Fig. 2 B) shows that while TFP dramatically slows the rate of Ca2+ dissociation from the N-domain of intact cTnC in a concentration-dependent manner, 3-Cl-DPA has no effect on the rate of Ca2+ dissociation at a concentration of up to 150 µM. Note that in the presence of TFP, removal of Ca2+ from the N-domain of cTnC led to an increase rather than decrease in IAANS fluorescence observed in the absence of compound or presence of 3-Cl-DPA.
Figure 2.
Effect of 3-Cl-DPA on the rate of Ca2+ dissociation from the regulatory N-domain of intact cTnC. (A) Plot of the apparent rates of Ca2+ dissociation from the N-domain of intact cTnC in the presence of increased concentrations of 3-Cl-DPA or TFP. Each data point represents an average of at least three measurements ± SEM. (B) Representative stopped-flow traces as Ca2+ is removed from the N-domain of intact cTnC in the absence or presence of 100 µM 3-Cl-DPA or TFP. The traces have been normalized and staggered for clarity. Rates of Ca2+ dissociation were measured following fluorescence of IAANS attached to Cys53 of cTnC with C35S, T53C, and C84S substitutions.
We are not able to unambiguously measure the Ca2+-binding affinity of the regulatory N-domain of IAANS-labeled cTnCT53C, because, in addition to undergoing an increase in fluorescence upon Ca2+ binding to the N-terminal regulatory site of cTnC, the environmentally sensitive IAANS probe attached to Cys53 undergoes a large increase in fluorescence upon Ca2+ binding to the C-terminal structural sites of isolated cTnC. Since the rate of Ca2+ dissociation from the N-domain of isolated cTnC is more than 1,000-fold faster than that from the C-terminal domain (C-domain) , we are able to distinguish the rate of Ca2+ dissociation from the N-domain of IAANS-labeled cTnCT53C versus the rate from the C-domain using a stopped-flow apparatus, but we were not able to accurately distinguish the Ca2+-binding sensitivity of the N-domain versus C-domainusing steady-state fluorescence. However, we were able to determine that 100 µM 3-Cl-DPA did not significantly change the Ca2+ sensitivity of the regulatory N-domain of isolated cTnC using IAANS attached to Cys84 of cTnCC35S, whereas 100 µM bepridil led to ∼6.5-fold increase in the Ca2+ sensitivity of the N-domain of IAANS-labeled cTnCC35S (data not shown). Thus, our results suggest that the molecular mechanism by which 3-Cl-DPA interacts with cTnC is different from that of TFP, bepridil, and several other Ca2+-sensitizing compounds.
Titrations of 3-Cl-DPA, TFP, and bepridil into Ca2+-saturated intact cTnC
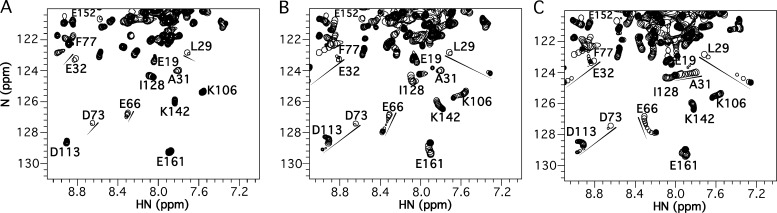
Ca2+-bound cNTnC exists in a predominantly closed form that makes transient excursions to more open states (Sia et al., 1997; Eichmüller and Skrynnikov, 2005; Cordina et al., 2013). A previously published x-ray crystal structure demonstrated bepridil binds to the central hydrophobic cavity of the cNTnC domain in its open conformation (Li et al., 2000). As bepridil is titrated into cTnC, NMR signals corresponding to the cNTnC domain move as the conformational equilibrium shifts from the closed state to the open bepridil-bound form (Fig. 3 C). A previous study showed that bepridil binds cNTnC with a Kd of 20 µM (Wang et al., 2002). Most peaks shift linearly, suggesting a 1:1 binding process. Some residue peaks move in a curved path during the titration, indicating that these residues are influenced by additional lower-affinity binding sites on cNTnC (e.g., E66). Some peaks with large chemical shift changes (e.g., L29, E32, and D73) disappear during the titration and then reappear at saturation, consistent with an intermediate timescale exchange process, suggesting that bepridil binding to cNTnC occurs on a timescale similar to the frequency difference between free and bound states for these residues (∼200 Hz on a 500-MHz spectrometer). Some peaks in the C-terminal domain also shift, indicating fast timescale weak binding to this domain as well. The NMR spectra of the TFP titration into cTnC (Fig. 3 B) looks very similar to that of bepridil, suggesting a similar binding mode and accompanying closed-to-open conformational transition, except that TFP appears to bind with slightly slower kinetics (L29, E32, and D73 are in a slow-to-intermediate exchange timescale) and correspondingly tighter binding (Kd <20 µM, though the exact Kd cannot be calculated because of multiple binding sites on the two cTnC domains).
Figure 3.
Titrations of 3-Cl-DPA, TFP, and bepridil into Ca2+-saturated intact cTnC. (A–C) Titrations of 3-Cl-DPA (A), TFP (B), or bepridil (C) into 15N-labeled cTnC, observed by 2D [1H,15N]-HSQC NMR spectra (amide HN 1H chemical shift plotted along x axis, amide 15N chemical shift plotted along y axis). For each titration, all spectra have been overlaid, with peaks plotted as a single contour level so that their progression can be tracked (shown by arrows). The final spectrum at the end of the titration is plotted conventionally with multiple contour levels. For 3-Cl-DPA, note many peaks disappear and do not reappear at the end of the titration.
For 3-Cl-DPA, the start of the titration begins similarly to bepridil and TFP, with peaks shifting in the same direction (see E32, D73, E66, A31, and L29), signifying a similar closed-to-open conformational change to accommodate small-molecule binding (Fig. 3 A). However, as the 3-Cl-DPA titration progresses, most cNTnC peaks weaken in intensity and then disappear, except for those that are less influenced by the closed-to-open transition, mainly the first 20 amino acid residues in the sequence. Unlike the effects of bepridil and TFP, the disappearing signals do not reemerge at the end of the titration, indicating conformational flexibility within the fully saturated cNTnC–3-Cl-DPA complex. Thus, it appears that 3-Cl-DPA binding does not stabilize a single dominant open conformation in cNTnC like bepridil or TFP, instead giving rise to an ensemble of structural states.
Also seen in Fig. 3 A, NMR signals from the C-terminal domain of cTnC shift only slightly during the 3-Cl-DPA titration (e.g., K106, I128, K142, and E161), indicating very weak binding. Thus, 3-Cl-DPA binding appears to be much more specific for the N-terminal domain, which is also unlike bepridil or TFP. Although we cannot definitively determine the Kd of 3-Cl-DPA for intact cTnC, a Kd of 6 ± 1 µM was determined from the titration of 3-Cl-DPA into the isolated cNTnC domain (data not shown).
Effect of 3-Cl-DPA on the Ca2+-binding properties of the regulatory N-domain of cTnC reconstituted into the cTn complex
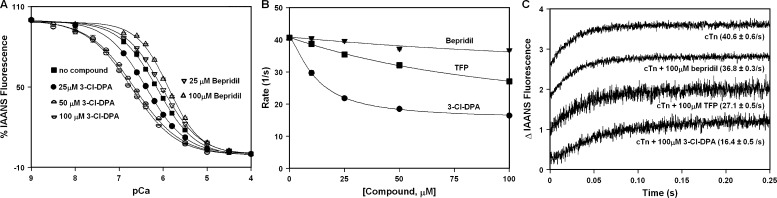
Surprisingly, our results indicate that despite binding to cTnC and sensitizing cardiac muscle to Ca2+, 3-Cl-DPA has no effect on the rate of Ca2+ dissociation from the N-domain of intact cTnC. One possibility is that 3-Cl-DPA requires the presence of the other cTn subunits to exert its effect on Ca2+ binding. To test this hypothesis, we compared the effect of 3-Cl-DPA on the Ca2+ sensitivity of the N-domain of intact cTnC after reconstitution into the cTn complex (cTnC complexed with cTnI and cTnT) to that of bepridil. After reconstitution into the cTn complex, changes in fluorescence of IAANS-labeled cTnCT53C primarily report N-domain Ca2+ binding and exchange (Davis et al., 2007). The Ca2+-induced decreases in IAANS fluorescence, which occur when Ca2+ binds to the N-domain of intact labeled cTnCT53C reconstituted into the cTn complex in the absence or presence of small molecules, are shown in Fig. 4 A. In the absence of small molecules, the cTn complex exhibited a half-maximal Ca2+-dependent decrease in IAANS fluorescence with a pCa50 of 6.13 ± 0.02. In the presence of increasing concentrations of 3-Cl-DPA, the Ca2+ sensitivity of the cTn complex significantly increases in a concentration-dependent manner (pCa50 = 6.39 ± 0.01, pCa50 = 6.65 ± 0.01, and pCa50= 6.73 ± 0.02 in the presence of 25, 50, and 100 µM 3-Cl-DPA, respectively). In contrast, bepridil actually significantly desensitizes the cTn complex to Ca2+, also in a concentration-dependent manner (pCa50 = 6.02 ± 0.2 and pCa50 = 5.88 ± 0.01 in the presence of 25 and 100 µM bepridil, respectively).
Figure 4.
Effect of 3-Cl-DPA on the Ca2+ binding properties of the regulatory N-domain of cTnC reconstituted into the cTn complex. (A) Decreases in IAANS fluorescence occur when Ca2+ binds to labeled cTnC with C35S, T53C, and C84S substitutions, reconstituted into the cTn complex in the absence or presence of increasing concentrations of 3-Cl-DPA or bepridil. Data sets were individually normalized and fit with a logistic sigmoid. (B) Plot of the apparent rates of Ca2+ dissociation from the cTn complex in the presence of increased concentrations of 3-Cl-DPA, TFP, or bepridil. (C) Representative stopped-flow traces as Ca2+ is removed from the cTn complex in the absence or presence of 100 µM 3-Cl-DPA, TFP, or bepridil. The traces have been normalized and staggered for clarity. Rates of Ca2+ dissociation were measured following the fluorescence of IAANS attached to Cys53 of cTnC with C35S, T53C, and C84S substitutions after reconstitution into the cTn complex.
We also compared the effect of 3-Cl-DPA on the rate of Ca2+ dissociation from the N-domain of intact cTnC reconstituted into the cTn complex to that of bepridil and TFP. We have previously demonstrated that under identical experimental conditions, the rate of Ca2+ dissociation measured from IAANS-labeled cTnCT53C reconstituted into the cTn complex was similar to that measured from the unlabeled wild-type cTnC reconstituted into the cTn complex (Davis et al., 2007). Thus, we are confident that IAANS-labeled cTnCT53C accurately reports Ca2+ dissociation from the cTn complex. Stopped-flow experiments were conducted to determine the effect of increasing concentrations (ranging from 12.5 to 100 µM) of 3-Cl-DPA, bepridil, and TFP on the rate of Ca2+ dissociation from the cTn complex. Our results, shown in Fig. 4 B (with representative stopped-flow traces shown in Fig. 4 C), indicate that the ability of 3-Cl-DPA to slow the rate of Ca2+ dissociation from the cTn complex is much greater than that of either bepridil or TFP. Thus, 3-Cl-DPA requires the addition of cTnI and cTnT to sensitize cTnC to Ca2+, with the most likely essential binding partner being cTnI, since cTnT is not known to interact with cNTnC. Indeed, 3-Cl-DPA is able to slow Ca2+ dissociation from a cTnC-cTnI chimeric protein (data not shown).
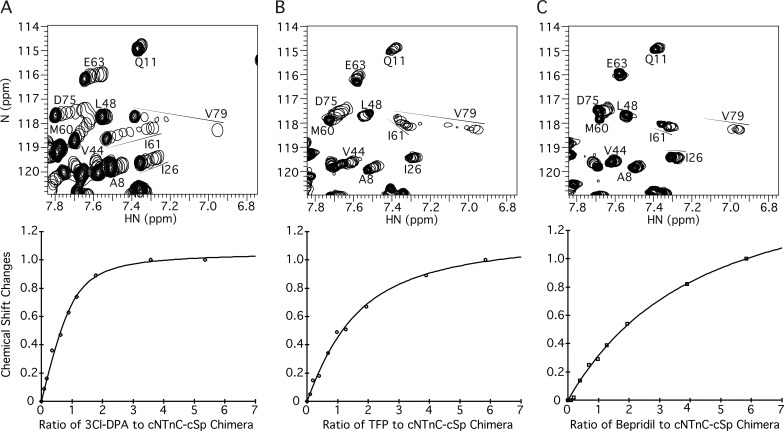
Titrations of 3-Cl-DPA, TFP, and bepridil into Ca2+-saturated cNTnC–cSp chimera
The constant presence of cTnI residues 146–158 in the cNTnC–cSp chimera keeps the Ca2+-saturated cNTnC domain in an open conformation for >90% of the time, consistent with a high effective concentration of cTnI (Pineda-Sanabria et al., 2014; Siddiqui et al., 2016). As shown in Fig. 5 A, titration of 3-Cl-DPA into cNTnC–cSp chimera yields large NMR chemical shift changes that are linear, indicative of a 1:1 complex, and all peaks corresponding to cNTnC are present at 3-Cl-DPA saturation; that is, they do not disappear as they did in the cTnC–3-Cl-DPA complex (compare with Fig. 3 A). Thus, binding of cTnI prevents the conformational exchange processes that lead to NMR signal broadening in the cTnC–3-Cl-DPA complex. 3-Cl-DPA binds to cNTnC–cSp chimera with a Kd of 10 ± 2 µM, demonstrating that 3-Cl-DPA binding is compatible with cTnI switch region binding, though it is slightly weakened in its presence (Kd of 6 µM for 3-Cl-DPA binding to the isolated cNTnC domain).
Figure 5.
Titrations of 3-Cl-DPA, TFP, and bepridil into Ca2+-saturated cNTnC–cSp chimera. (A–C) Titrations of 3-Cl-DPA (A), TFP (B), or bepridil (C) into 15N-labeled cNTnC–cSp chimera, as observed by 2D [1H,15N]-HSQC NMR spectra (amide HN 1H chemical shift plotted along x axis, amide 15N chemical shift plotted along y axis). For each titration, all spectra have been overlaid, with peaks plotted as a single contour level so that their progression can be tracked (shown by arrows). The final spectrum at the end of the titration is plotted conventionally with multiple contour levels. Below each spectrum is a representative plot of chemical shift changes as a function of compound added, from which Kd is calculated. Note that the ratio of compound to cNTnC–cSp chimera, rather than the absolute concentration of compound, is on the x axis. This is because plots of saturation versus compound concentration are typically performed when the protein concentration is much smaller than the Kd. However, because of the sensitivity limitation of multidimensional solution NMR, the protein concentration (0.11 mM) is greater than the Kd (10 µM in the case of 3-Cl-DPA). Thus, to avoid confusion, the protein/compound ratio is used instead.
In contrast to 3-Cl-DPA, when bepridil is titrated into cNTnC–cSp chimera, NMR signals corresponding to cNTnC weaken or disappear, indicating conformational exchange (Fig. 5 C). The binding affinity of bepridil to the cNTnC–cSp chimera is very weak (Kd = 380 ± 60 µM; an order of magnitude weaker than it is to cNTnC domain alone), indicating that the cTnI switch region strongly competes against bepridil for binding to cNTnC. The binding of TFP (Fig. 5 B) is also similarly weakened (Kd = 110 ± 20 µM), indicating competition with cTnI for cTnC binding, too. Thus, bepridil and TFP both compete with the cTnI switch region for binding to cNTnC, whereas 3-Cl-DPA binding is more compatible.
Discussion
Recently, we screened over 40 diphenylamine (DPA)-based compounds for binding to cNTnC–cSp chimera in order to explore whether any of these compounds have potential for drug development (Cai et al., 2016). Of the DPA-based compounds, 3-Cl-DPA was able to bind to cNTnC–cSp chimera most tightly (Kd of 10 µM), and thus, it was selected for further studies. Since molecules that bind to cTnC are not necessarily Ca2+ sensitizers, we evaluated the ability of 3-Cl-DPA to increase the Ca2+ sensitivity of force development in cardiac muscle. Our results show that 3-Cl-DPA is a Ca2+ sensitizer of cardiac muscle.
The Ca2+ sensitivity of cardiac muscle is determined by the Ca2+-binding affinity of the regulatory N-domain of cTnC, which in turn is modulated by many factors (Li and Hwang, 2015). Physiologically, the most important of these factors is the effective concentration of cTnI switch region (Siddiqui et al., 2016). Binding of the cTnI switch region to the N-domain of cTnC locks it into an open conformation, in which the Ca2+ ion is fully ligated (either directly or indirectly) by Ca2+-binding residues of the second EF hand (Ca2+-binding helix–loop–helix motif) (Takeda et al., 2003). In contrast, when the Ca2+-bound N-domain of cTnC is not bound to the cTnI switch region, it remains predominantly in the closed conformation (Sia et al., 1997), in which the EF hand loop residues Asp67 and Ser69 do not directly chelate the Ca2+ (Zhang et al., 2013) as they do in the open state (Takeda et al., 2003). Consequently, the N-domain of cTnC (dominated by the closed conformation) has a Ca2+-binding affinity approximately one order of magnitude lower than that of the intact cTn complex (Davis and Tikunova, 2008). Like the cTnI switch region, small molecules can act as Ca2+ sensitizers by stabilizing the open form of the N-domain of cTnC (for review, see Li et al., 2008; Li and Hwang, 2015).
Our results show that unlike bepridil or TFP, 3-Cl-DPA is unable to sensitize the N-domain of isolated cTnC to Ca2+. Our NMR data suggest that saturation with bepridil or TFP stabilizes the open conformation of the N-domain of cTnC, consistent with a crystal structure of the complex between bepridil and Ca2+-bound cTnC (Li et al., 2000). Our stopped-flow experiments demonstrate that stabilization of the open state by TFP results in dramatic slowing of the rate of Ca2+ dissociation from the N-domain of cTnC. On the other hand, our NMR studies suggest that 3-Cl-DPA binding does not stabilize the open state in the same manner as TFP or bepridil. In agreement with NMR studies, 3-Cl-DPA is not able to slow the rate of Ca2+ dissociation from the N-domain of cTnC. Thus, unlike bulky molecules such as TFP and bepridil, the small and flexible molecule 3-Cl-DPA is able to bind into the hydrophobic cavity of cNTnC without formation of the fully open state. The inability of 3-Cl-DPA to stabilize the open state of cNTnC likely results in its ineffectiveness in Ca2+-sensitizing the N-domain of isolated cTnC and diminishes its Ca2+-sensitizing potency in cardiac muscle.
We originally designed diphenylamine-based compounds based on structures of bepridil bound to the N-domain of cTnC (Li et al., 2000; Wang et al., 2002). We suspected that most of the free energy of bepridil binding derived from its benzylphenylamine group, whereas its bulky isopropyl group displaces the switch region of cTnI from its optimal binding site. We found that benzylphenylamine bound to the cNTnC–cSp chimera with a Kd of 150 µM, and the smaller diphenylamine molecule bound even better, with a Kd of 120 µM (Cai et al., 2016). Out of all the diphenylamine derivatives we tested, 3-Cl-DPA bound most tightly, but we chose to determine the structure of the similar compound, 3-methyl-DPA bound to the cNTnC–cSp chimera, because the methyl group provides additional useful information for structural NMR studies (Cai et al., 2016). Our structures showed that 3-methyl-DPA was less disruptive of cTnI switch region binding than bepridil, as it was designed to be. Ile148 and Met153 of cTnI are displaced slightly, but all other major contacts between cNTnC and cTnI are preserved. 3-Cl-DPA likely binds in a manner similar to 3-methyl-DPA, because the chemical shift changes upon binding are very similar. In contrast, bepridil binding causes a marked displacement of the entire switch region as well as an additional opening of the cNTnC structure (Wang et al., 2002).
Our results suggest that 3-Cl-DPA can stabilize the open state of the N-domain of cTnC only in the presence of cTnI. In fact, 3-Cl-DPA acts as a Ca2+ sensitizer of the cTn complex, while bepridil actually desensitizes the cTn complex to Ca2+. In that regard, bepridil acts similarly to the V44Q mutation in cTnC, which sensitizes isolated cTnC and cardiac muscle to Ca2+ (Tikunova and Davis, 2004; Norman et al., 2007) but has a Ca2+-desensitizing effect on the cTn complex (Tikunova et al., 2010). Our results are consistent with that of an earlier study showing that bepridil had almost no effect on the rate of Ca2+ dissociation from the cTn complex (Varughese et al., 2011). The interference of bepridil with the binding of the switch region of cTnI to the hydrophobic cavity likely weakens the extent of Ca2+ sensitization that bepridil is able to achieve in cardiac muscle, compared with its large Ca2+ sensitizing effect on isolated cTnC (Solaro et al., 1986; Kischel et al., 1999; Piroddi et al., 2007).
The ability of cTnC to bind cTnI plays a major role in influencing its Ca2+-binding properties (Siddiqui et al., 2016). Because cTnC transitions through multiple states during each heartbeat (Davis and Tikunova, 2008; Davis et al., 2016), the effect of compounds on each state of cTnC needs to be considered. We theorize that the most potent Ca2+ sensitization could be achieved by compounds that are able to stabilize the open state of cNTnC while enhancing or at least not interfering with the binding of cTnI, thus increasing Ca2+ sensitivity of cNTnC before and after binding of cTnI. In that regard, an “ideal” Ca2+-sensitizing compound would act as the L48Q mutation in cTnC, which sensitizes isolated cTnC and the cTn complex to Ca2+ (Tikunova and Davis, 2004; Tikunova et al., 2010) while slightly enhancing the affinity of cTnC for cTnI (Wang et al., 2012). There are numerous ways in which the Ca2+ sensitivity of the heart can be altered. Although there is evidence that some cardiomyopathy-linked modifications that alter the Ca2+ sensitivity may be a part of the disease process (Gomes and Potter, 2004; Kimura, 2010), we have recently demonstrated that this is not always the case. For instance, the L48Q mutation in cTnC increases in vivo cardiac contractility without causing adverse side effects in a mouse model of heart failure (Shettigar et al., 2016). Thus, we believe that it is possible to enhance cardiac contractility through sensitizing cTnC to Ca2+ without causing disease.
Moreover, it should be noted that Ca2+-sensitizing drugs are not intended for use in normal well-functioning hearts, in which the delicate balance between contraction and relaxation is properly regulated. Rather, the drugs are intended for diseased hearts with primarily systolic failure, as typically seen in familial dilated cardiomyopathy or ischemic heart disease. In such cases, it is anticipated that Ca2+ sensitizers will shift the contractile balance, improving the efficiency of cardiac contraction and attenuating (and possibly even reversing) the pathological remodeling process that leads to progressively dilated and thinned ventricles.
In conclusion, we determined that 3-Cl-DPA is a Ca2+ sensitizer of cardiac muscle, with a mechanism of action very different from that of known Ca2+ sensitizers, such as TFP and bepridil. The small size of the 3-Cl-DPA molecule makes it an excellent starting scaffold for the development of more potent Ca2+-sensitizing compounds. Understanding the molecular mechanisms by which small molecules modulate Ca2+ and cTnI binding and exchange with cTnC is the first step in the development of drugs with high potency and specificity that could one day be used to treat systolic heart failure.
Acknowledgments
This research was supported, in part, by National Institute of Health grants R01 HL132213 and R21 AG051913 to J.P. Davis. P. Hwang was supported by a Canadian Institutes of Health Research Phase 2 Clinician Scientist Salary Award and by a Heart and Stroke Foundation of Canada – Mazankowski Alberta Heart Institute Emerging Research Leaders Initiative award. M. Li was supported by funding from the Hwang Professional Corporation.
The authors declare no competing financial interests.
Author contributions: S.B. Tikunova, P.M. Hwang, and J.P. Davis conceived and designed the study; S. B. Tikunova, A. Cuesta, M. Price, M.X. Li, N. Belevych, and P.J. Reiser collected and analyzed data; B.J. Biesiadecki contributed resources; and S.B. Tikunova, M.X. Li, P.J. Reiser, P.M. Hwang, and J.P. Davis drafted, reviewed, and edited the manuscript.
Henk L. Granzier served as editor.
Footnotes
This work is part of a special collection on myofilament function.
References
- Bai Y., Milne J.S., Mayne L., and Englander S.W.. 1993. Primary structure effects on peptide group hydrogen exchange. Proteins. 17:75–86. 10.1002/prot.340170110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin E.J., Virani S.S., Callaway C.W., Chamberlain A.M., Chang A.R., Cheng S., Chiuve S.E., Cushman M., Delling F.N., Deo R., et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . 2018. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 137:e67–e492. 10.1161/CIR.0000000000000558 [DOI] [PubMed] [Google Scholar]
- Black D.J., Tikunova S.B., Johnson J.D., and Davis J.P.. 2000. Acid pairs increase the N-terminal Ca2+ affinity of CaM by increasing the rate of Ca2+ association. Biochemistry. 39:13831–13837. 10.1021/bi001106+ [DOI] [PubMed] [Google Scholar]
- Boyle K.L., and Leech E.. 2012. A review of the pharmacology and clinical uses of pimobendan. J. Vet. Emerg. Crit. Care (San Antonio). 22:398–408. 10.1111/j.1476-4431.2012.00768.x [DOI] [PubMed] [Google Scholar]
- Cai F., Li M.X., Pineda-Sanabria S.E., Gelozia S., Lindert S., West F., Sykes B.D., and Hwang P.M.. 2016. Structures reveal details of small molecule binding to cardiac troponin. J. Mol. Cell. Cardiol. 101:134–144. 10.1016/j.yjmcc.2016.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee K. 2012. Pathophysiology of systolic and diastolic heart failure. Med. Clin. North Am. 96:891–899. 10.1016/j.mcna.2012.07.001 [DOI] [PubMed] [Google Scholar]
- Cordina N.M., Liew C.K., Gell D.A., Fajer P.G., Mackay J.P., and Brown L.J.. 2013. Effects of calcium binding and the hypertrophic cardiomyopathy A8V mutation on the dynamic equilibrium between closed and open conformations of the regulatory N-domain of isolated cardiac troponin C. Biochemistry. 52:1950–1962. 10.1021/bi4000172 [DOI] [PubMed] [Google Scholar]
- Davis J.P., and Tikunova S.B.. 2008. Ca(2+) exchange with troponin C and cardiac muscle dynamics. Cardiovasc. Res. 77:619–626. 10.1093/cvr/cvm098 [DOI] [PubMed] [Google Scholar]
- Davis J.P., Norman C., Kobayashi T., Solaro R.J., Swartz D.R., and Tikunova S.B.. 2007. Effects of thin and thick filament proteins on calcium binding and exchange with cardiac troponin C. Biophys. J. 92:3195–3206. 10.1529/biophysj.106.095406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J.P., Shettigar V., Tikunova S.B., Little S.C., Liu B., Siddiqui J.K., Janssen P.M., Ziolo M.T., and Walton S.D.. 2016. Designing proteins to combat disease: Cardiac troponin C as an example. Arch. Biochem. Biophys. 601:4–10. 10.1016/j.abb.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichmüller C., and Skrynnikov N.R.. 2005. A new amide proton R1rho experiment permits accurate characterization of microsecond time-scale conformational exchange. J. Biomol. NMR. 32:281–293. 10.1007/s10858-005-0658-y [DOI] [PubMed] [Google Scholar]
- Endoh M. 2008. Cardiac Ca2+ signaling and Ca2+ sensitizers. Circ. J. 72:1915–1925. 10.1253/circj.CJ-08-0838 [DOI] [PubMed] [Google Scholar]
- Erdmann E. 1998. Pathophysiology of heart failure. Heart. 79(Suppl 2):S3–S5. 10.1136/hrt.79.2008.3S [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino K., Sperelakis N., and Solaro R.J.. 1988. Sensitization of dog and guinea pig heart myofilaments to Ca2+ activation and the inotropic effect of pimobendan: comparison with milrinone. Circ. Res. 63:911–922. 10.1161/01.RES.63.5.911 [DOI] [PubMed] [Google Scholar]
- Gomes A.V., and Potter J.D.. 2004. Molecular and cellular aspects of troponin cardiomyopathies. Ann. N. Y. Acad. Sci. 1015:214–224. 10.1196/annals.1302.018 [DOI] [PubMed] [Google Scholar]
- Hwang P.M., and Sykes B.D.. 2015. Targeting the sarcomere to correct muscle function. Nat. Rev. Drug Discov. 14:313–328. 10.1038/nrd4554 [DOI] [PubMed] [Google Scholar]
- Johnson F.L. 2014. Pathophysiology and etiology of heart failure. Cardiol. Clin. 32:9–19: vii. 10.1016/j.ccl.2013.09.015 [DOI] [PubMed] [Google Scholar]
- Kass D.A., and Solaro R.J.. 2006. Mechanisms and use of calcium-sensitizing agents in the failing heart. Circulation. 113:305–315. 10.1161/CIRCULATIONAHA.105.542407 [DOI] [PubMed] [Google Scholar]
- Kemp C.D., and Conte J.V.. 2012. The pathophysiology of heart failure. Cardiovasc. Pathol. 21:365–371. 10.1016/j.carpath.2011.11.007 [DOI] [PubMed] [Google Scholar]
- Kimura A. 2010. Molecular basis of hereditary cardiomyopathy: abnormalities in calcium sensitivity, stretch response, stress response and beyond. J. Hum. Genet. 55:81–90. 10.1038/jhg.2009.138 [DOI] [PubMed] [Google Scholar]
- Kischel P., Stevens L., and Mounier Y.. 1999. Differential effects of bepridil on functional properties of troponin C in slow and fast skeletal muscles. Br. J. Pharmacol. 128:767–773. 10.1038/sj.bjp.0702820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komamura K. 2013. Similarities and Differences between the Pathogenesis and Pathophysiology of Diastolic and Systolic Heart Failure. Cardiol. Res. Pract. 2013:824135 10.1155/2013/824135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann A., Boldt J., and Kirchner J.. 2003. The role of Ca++-sensitizers for the treatment of heart failure. Curr. Opin. Crit. Care. 9:337–344. 10.1097/00075198-200310000-00002 [DOI] [PubMed] [Google Scholar]
- Li M.X., and Hwang P.M.. 2015. Structure and function of cardiac troponin C (TNNC1): Implications for heart failure, cardiomyopathies, and troponin modulating drugs. Gene. 571:153–166. 10.1016/j.gene.2015.07.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.X., Robertson I.M., and Sykes B.D.. 2008. Interaction of cardiac troponin with cardiotonic drugs: a structural perspective. Biochem. Biophys. Res. Commun. 369:88–99. 10.1016/j.bbrc.2007.12.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Love M.L., Putkey J.A., and Cohen C.. 2000. Bepridil opens the regulatory N-terminal lobe of cardiac troponin C. Proc. Natl. Acad. Sci. USA. 97:5140–5145. 10.1073/pnas.090098997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell B.K., Singh S., Fan Q., Hernandez A., Portillo J.P., Reiser P.J., and Tikunova S.B.. 2015. Knock-in mice harboring a Ca(2+) desensitizing mutation in cardiac troponin C develop early onset dilated cardiomyopathy. Front. Physiol. 6:242 10.3389/fphys.2015.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman C., Rall J.A., Tikunova S.B., and Davis J.P.. 2007. Modulation of the rate of cardiac muscle contraction by troponin C constructs with various calcium binding affinities. Am. J. Physiol. Heart Circ. Physiol. 293:H2580–H2587. 10.1152/ajpheart.00039.2007 [DOI] [PubMed] [Google Scholar]
- Pineda-Sanabria S.E., Julien O., and Sykes B.D.. 2014. Versatile cardiac troponin chimera for muscle protein structural biology and drug discovery. ACS Chem. Biol. 9:2121–2130. 10.1021/cb500249j [DOI] [PubMed] [Google Scholar]
- Piroddi N., Belus A., Scellini B., Tesi C., Giunti G., Cerbai E., Mugelli A., and Poggesi C.. 2007. Tension generation and relaxation in single myofibrils from human atrial and ventricular myocardium. Pflugers Arch. 454:63–73. 10.1007/s00424-006-0181-3 [DOI] [PubMed] [Google Scholar]
- Pollesello P., Ovaska M., Kaivola J., Tilgmann C., Lundström K., Kalkkinen N., Ulmanen I., Nissinen E., and Taskinen J.. 1994. Binding of a new Ca2+ sensitizer, levosimendan, to recombinant human cardiac troponin C. A molecular modelling, fluorescence probe, and proton nuclear magnetic resonance study. J. Biol. Chem. 269:28584–28590. [PubMed] [Google Scholar]
- Pollesello P., Papp Z., and Papp J.G.. 2016. Calcium sensitizers: What have we learned over the last 25 years? Int. J. Cardiol. 203:543–548. 10.1016/j.ijcard.2015.10.240 [DOI] [PubMed] [Google Scholar]
- Reiser P.J., Welch K.C. Jr., Suarez R.K., and Altshuler D.L.. 2013. Very low force-generating ability and unusually high temperature dependency in hummingbird flight muscle fibers. J. Exp. Biol. 216:2247–2256. 10.1242/jeb.068825 [DOI] [PubMed] [Google Scholar]
- Robertson S., and Potter J.. 1984. The regulation of free Ca2+ ion concentration by metal chelators. In Myocardial Biology. A. Schwartz, editor. Springer, Boston. 63–75. [Google Scholar]
- Roger V.L. 2013. Epidemiology of heart failure. Circ. Res. 113:646–659. 10.1161/CIRCRESAHA.113.300268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shettigar V., Zhang B., Little S.C., Salhi H.E., Hansen B.J., Li N., Zhang J., Roof S.R., Ho H.T., Brunello L., et al. . 2016. Rationally engineered Troponin C modulates in vivo cardiac function and performance in health and disease. Nat. Commun. 7:10794 10.1038/ncomms10794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia S.K., Li M.X., Spyracopoulos L., Gagné S.M., Liu W., Putkey J.A., and Sykes B.D.. 1997. Structure of cardiac muscle troponin C unexpectedly reveals a closed regulatory domain. J. Biol. Chem. 272:18216–18221. 10.1074/jbc.272.29.18216 [DOI] [PubMed] [Google Scholar]
- Siddiqui J.K., Tikunova S.B., Walton S.D., Liu B., Meyer M., de Tombe P.P., Neilson N., Kekenes-Huskey P.M., Salhi H.E., Janssen P.M., et al. . 2016. Myofilament Calcium Sensitivity: Consequences of the Effective Concentration of Troponin I. Front. Physiol. 7:632 10.3389/fphys.2016.00632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaro R.J., Bousquet P., and Johnson J.D.. 1986. Stimulation of cardiac myofilament force, ATPase activity and troponin C Ca++ binding by bepridil. J. Pharmacol. Exp. Ther. 238:502–507. [PubMed] [Google Scholar]
- Takeda S., Yamashita A., Maeda K., and Maéda Y.. 2003. Structure of the core domain of human cardiac troponin in the Ca(2+)-saturated form. Nature. 424:35–41. 10.1038/nature01780 [DOI] [PubMed] [Google Scholar]
- Tikunova S.B., and Davis J.P.. 2004. Designing calcium-sensitizing mutations in the regulatory domain of cardiac troponin C. J. Biol. Chem. 279:35341–35352. 10.1074/jbc.M405413200 [DOI] [PubMed] [Google Scholar]
- Tikunova S.B., Rall J.A., and Davis J.P.. 2002. Effect of hydrophobic residue substitutions with glutamine on Ca(2+) binding and exchange with the N-domain of troponin C. Biochemistry. 41:6697–6705. 10.1021/bi011763h [DOI] [PubMed] [Google Scholar]
- Tikunova S.B., Liu B., Swindle N., Little S.C., Gomes A.V., Swartz D.R., and Davis J.P.. 2010. Effect of calcium-sensitizing mutations on calcium binding and exchange with troponin C in increasingly complex biochemical systems. Biochemistry. 49:1975–1984. 10.1021/bi901867s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varughese J.F., Baxley T., Chalovich J.M., and Li Y.. 2011. A computational and experimental approach to investigate bepridil binding with cardiac troponin. J. Phys. Chem. B. 115:2392–2400. 10.1021/jp1094504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Robertson I.M., Li M.X., McCully M.E., Crane M.L., Luo Z., Tu A.Y., Daggett V., Sykes B.D., and Regnier M.. 2012. Structural and functional consequences of the cardiac troponin C L48Q Ca(2+)-sensitizing mutation. Biochemistry. 51:4473–4487. 10.1021/bi3003007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Li M.X., and Sykes B.D.. 2002. Structure of the regulatory N-domain of human cardiac troponin C in complex with human cardiac troponin I147-163 and bepridil. J. Biol. Chem. 277:31124–31133. 10.1074/jbc.M203896200 [DOI] [PubMed] [Google Scholar]
- Zhang X.L., Tibbits G.F., and Paetzel M.. 2013. The structure of cardiac troponin C regulatory domain with bound Cd2+ reveals a closed conformation and unique ion coordination. Acta Crystallogr. D Biol. Crystallogr. 69:722–734. 10.1107/S0907444913001182 [DOI] [PubMed] [Google Scholar]