ABSTRACT
Seasonal influenza can have serious morbid consequences and can even result in death, particularly in at-risk populations, including healthcare professionals (HCPs), elderly and those living with a medical risk condition. Although in Europe recommendations exist for annual influenza vaccination in these populations in most countries, the vaccination coverage rate (VCR) is often well below the World Health Organization target of 75% coverage. In our previous survey in 2009 we showed that some elements of national vaccination policies, e.g. reminder systems, strong official recommendation, and easy access, seemed to contribute to achieving higher influenza VCRs among elderly. We repeated the survey in 2016, using the same methodology to assess changes in influenza VCRs among the elderly and in the impact of policy elements on these VCRs. In addition, we collected information about VCRs among HCPs, and those living with a medical risk condition. The median VCR in the 21 countries that had recommendations for influenza vaccination in the elderly was 35.3%, ranging from 1.1% in Estonia to 74.5% in Scotland. The average VCRs for HCPs and those living with medical risk conditions, available in 17 and 10 countries, respectively, were 28.3% (range 7% in Czech Republic to 59.1% in Portugal) and 32.2% (range from 20.0% in the Czech Republic and Hungary to 59.6% in Portugal), respectively. Fewer countries were able to provide data from HCP and those living with medical risk conditions. Since the initial survey during the 2007–2008 influenza season, VCRs have decreased in the elderly in the majority of countries, thus, achieving high VCRs in the elderly and the other target groups is still a major public health challenge in Europe. This could be addressed by the identification, assessment and sharing of best practice for influenza vaccination policies.
KEYWORDS: Vaccination policies, Vaccination coverage, Seasonal influenza vaccination, healthcare professionals, Elderly, influenza, policy
Introduction
Influenza, a highly contagious respiratory infectious disease that can result in severe illness and death, is caused by influenza viruses which circulate throughout the world. The World Health Organization (WHO) have estimated that the annual influenza epidemics result in about 3 to 5 million cases of severe illness and about 250 000 to 500 000 deaths, thus influenza is a significant global public health burden.1 Influenza can trigger secondary infections and exacerbate pre-existing chronic medical conditions, leading to severe complications and increased morbidity and mortality. The annual number of deaths due to influenza in Europe, estimated at 38,500 in 2013, is higher than that due to car accidents, estimated at 25,900.2 In addition to this significant clinical impact, influenza has major economic consequences through a range of direct and indirect costs, e.g., medical treatment of influenza illness, loss of productivity and absenteeism.3
Safe and effective influenza vaccines are available and have been used for more than 60 years.4 These vaccines can provide protection even when the vaccine viruses do not exactly match the circulating viruses. WHO has stated that the most effective way to prevent influenza is vaccination.1 WHO has identified groups of people at higher risk of serious influenza complications and the people who live with, or care for high risk individuals as specific vaccination target groups. They recommend annual influenza vaccination for pregnant women (at any stage of pregnancy), children aged between 6 months to 5 years, elderly individuals (aged >65 years), individuals with chronic medical conditions and healthcare professionals (HCPs). In 2009 the Council of the European Union set a target of 75% coverage among all defined target groups by 2014/15.5 In Europe, in the 2012–2013 influenza season it was estimated that only about 80 million of the 180 million Europeans for whom seasonal influenza vaccination is recommended are vaccinated, giving <45% coverage.3 To achieve the 75% target rate, an additional 57.4 million people need to be vaccinated. If this target coverage were to be achieved, results from a statistical model suggest that the impact on the average annual influenza-related events averted would include 1.6 to 1.7 million fewer influenza cases, 678,500 to 767,800 fewer physician visits, 883,800 to 1,015,100 fewer lost days of work, 23,800 to 31,400 fewer hospitalizations and 9,800 to 14,000 deaths avoided resulting in 190€ to 226€ million savings of influenza-related costs.4
HCPs are at risk of being exposed to patients infected with influenza and if they become infected themselves they can play a role in nosocomial transmission.1,6 This makes them an important target group for influenza vaccination, not only for direct protection against influenza infection and prevent sick leave during epidemics but also to prevent transmission to patients and their families. Many studies have reported high rates of influenza vaccine hesitancy among HCPs, often due to misconceptions about their own risk and influenza vaccine effectiveness.6-8
We report the results from a follow-up study performed to assess changes in VCRs and changes in policy elements since 2008. We also expanded the survey to additional EU Member and Adriatic States and sought to identify national policy elements that may contribute to higher seasonal influenza VCRs for the elderly. This study was based on a similar survey process that we used previously to assess the impact of policy elements on VCR in the elderly (Blank et al, 2012). In the current study we expanded the populations of interest to include HCPs and those living with a medical risk condition (i.e. treatment-induced and/or disease-induced immunosuppression, metabolic disorders, and chronic pulmonary, cardiovascular and renal diseases).
Results
Vaccination coverage rates
The VCRs for the elderly were below the recommended 75% threshold set by the Council of the European Union for the 2013–14, 2014–15 and 2015–16 seasons. In The Netherlands, England and Scotland these were 64% or higher in the 2015–16 season, but the rates decreased over the three seasons (Figure 1A). In contrast with the other countries where the VCRs were either stationary or declined over the three seasons, the VCR in Portugal increased to 67.9% in the 2015–16 season from 57% and 55% in the two previous seasons.
Figure 1.
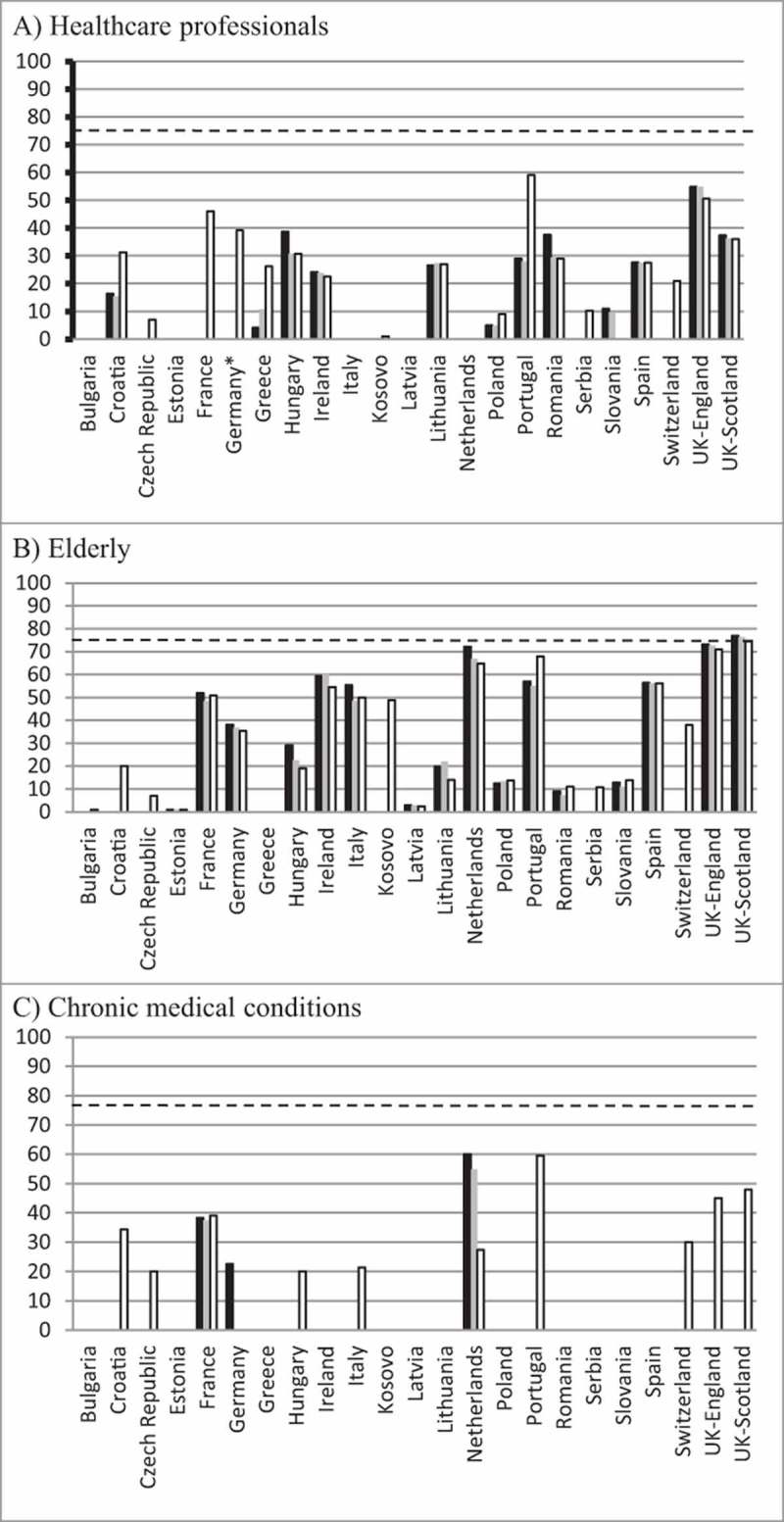
Vaccination coverage rates for; A) elderly, B) healthcare professionals, C) those with a medical risk condition for seasons 2013–14 (black bars), 2014–15 (grey bars) and 2015–16 (white bars). The dashed horizontal line indicates the 75% target vaccination coverage rate for all groups.
The VCRs for HCPs were also below the recommended 75% threshold in the 17 countries that could provide data (Figure 1B). The highest VCR reported was in Portugal (67.9%) in the 2015–16 season and the other VCRs were below 50% with the exception of England.
Only 11 countries were able to provide data for the VCRs in those living with a medical risk condition. England, France, The Netherlands and Scotland were the only countries to have data for all three seasons (Figure 1C). In the 2015–2016 season, the highest VCR was in Portugal (59.1%) with Scotland and England reporting VCRs of 48.0% and 45.1%, respectively. The VCRs for the other countries ranged from 20.0% in the Czech Republic and Hungary to 39.1% in France.
Implemented policy elements
The policy elements that are implemented in the countries that participated in the survey are summarized in Table 1.
Table 1.
Vaccination coverage rates and implemented policy elements aimed at increasing influenza vaccination coverage rates in 23 European countries in 2014/15.
| Bulgaria | Croatia | Czech Rep | Estonia | France | Germany | Greece | Hungary | Ireland (Eire) | Italy | Kosovo | Latvia | Lithuania | Netherlands | Poland | Portugal | Romania | Serbia | Slovakia | Spain | Switzerland | UK-England | UK-Scotland | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccine coveragerate | |||||||||||||||||||||||
| Elderly | ND | 20.1 | 24.9 | 1.1 | 50.8 | 35.3 | ND | 19.0 | 54.5 | 49.9 | 48.8 | 2.4 | 13.9 | 64.6 | 13.7 | 67.9 | 11.0 | 10.7 | 14.1 | 56.1 | 38.0 | 71.0 | 74.5 |
| Healthcare professionals | ND | 31.3 | 7.0 | ND | 46.0 | 39.2 | 26.3 | 30.7 | 22.5 | ND | 1.0 | ND | 27.0 | ND | 9.0 | 59.1 | 29.0 | 10.3 | ND | 27.5 | 21.0 | 50.6 | 36.0 |
| Living with a medical risk condition | ND | 34.4 | 20.0 | ND | 39.1 | ND | ND | 20.0 | ND | 21.4 | ND | ND | ND | 27.4 | ND | 59.6 | ND | ND | ND | ND | 30.0 | 45.1 | 48.0 |
| Influenza vaccination program policy element | |||||||||||||||||||||||
| Influenza vaccination recommendation for elderly | |||||||||||||||||||||||
| Influenza vaccination recommendation for HCPs | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes |
| Influenza vaccination recommendation for those living with a medical risk condition | |||||||||||||||||||||||
| Vaccination free for recommended patients (vaccine or administration) | no | yes | yes | no | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | no | yes | yes | yes | yes | yes | yes | yes | yes |
| National action plan to improve VCR | no | no | yes | no | NA | no | no | no | NA | NA | no | yes | no | no | yes | NA | no | no | no | NA | yes | yes | NA |
| Objective for 75% VCR in targeted groups | NA | NA | yes | NA | yes | yes | yes | no | yes | yes | no | yes | NA | no | no | yes | NA | NA | NA | NA | no | yes | NA |
| Yearly national VCR objective | no | no | no | no | NA | no | yes | no | NA | NA | no | no | no | no | no | NA | no | yes | no | NA | yes | yes | NA |
| Annual monitoring & communication of VCR, by target by HA | NA | NA | no | NA | NA | no | yes | yes | NA | NA | no | yes | NA | yes | no | NA | NA | NA | NA | NA | yes | yes | NA |
| Summit before or during annual campaign | no | no | no | no | NA | NA | yes | no | NA | NA | yes | yes | yes | no | yes | NA | yes | yes | yes | no | no | no | NA |
| HCP have clear objectives to achieve in high-risk groups (GP and/or other specialist) | no | no | yes | no | NA | no | no | yes | NA | NA | no | yes | no | no | no | NA | no | no | no | NA | no | yes | NA |
| HCP financial incentive | no | no | yes | no | yes | no | no | no | yes | no | no | NA | no | yes | no | NA | no | no | no | no | no | yes | yes |
| Awareness campaign by HA and/or NVIG | yes | no | no | no | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | no | yes | no | yes | yes | yes | yes | NA |
| Awareness campaign: TV / radio | no | yes | no | yes | no | no | yes | yes | yes | yes | yes | yes | no | yes | yes | yes | yes | yes | yes | yes | no | yes | yes |
| Awareness campaign: press advertisements for public | yes | yes | yes | yes | yes | yes | no | yes | yes | yes | yes | yes | yes | yes | yes | no | no | no | yes | yes | no | yes | yes |
| Awareness campaign: flyers / leaflets/ folders in medical waiting rooms | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | no | no | yes | no | yes | yes | yes | yes |
| Awareness campaign: website for public | yes | yes | no | yes | yes | yes | no | yes | no | yes | yes | yes | no | yes | yes | yes | yes | no | yes | yes | yes | yes | yes |
| Awareness campaign: advertisements or mailings to HCP | yes | no | no | yes | yes | yes | yes | no | yes | yes | yes | yes | no | yes | yes | no | yes | no | no | yes | yes | yes | yes |
| Additional data sources |
yes: fully or partially implemented; no: not implemented: NA: no answer.
65+ years and people with chronic respiratory or cardiovascular illness or diabetes on permanent medication
Slovak health insurance companies have paid vaccine and administration costs for everyone, not only for recommended target populations, for several years
Polish National Program for Influenza Prevention (NPIP) is a social initiative that does not receive financial support from the Ministry of Health or Sanitary Inspection
summits are organized by NPIP twice a year, with representatives from Polish Health Ministry, Sanitary Inspection, and Parliament. The most important activities are meetings: Flu Forum (at the beginning of the season, organized in September) and Flu Meeting (at the end of the season).
partially.
by the Hellenic Center for Disease Control and Prevention.
Data sources: ECDC22; Croatia -grip.hr – supported by NPH; France – Institut de veille sanitaire (InVS). Couverture vaccinale. http://invs.santepubliquefrance.fr//Dossiers-thematiques/Maladies-infectieuses/Maladies-a-prevention-vaccinale/Couverture-vaccinale/Donnees/Grippe, https://www.lequotidiendupharmacien.fr/actualite/article/2017/03/01/59-des-pharmaciens-vaccines-contre-la-grippe_264771?xtor = EPR-2-%5BNL_quotidienne%5D-20170301, Haut Conseil de la santé publique. Objectifs de santé publique: Évaluation des objectifs de la loi du 9 août 2004: Propositions (High Public Health Council. Public health objectives: Assessment of the objectives of the law of 9 August 2004: Proposals). 2010; Germany – Robert Koch Institut (RKI). RKI/ Epedimiologisches Bulletin 2017/1, http://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2016/47/Art_01.html; Hungary – OTH-ÁNTSZ (public health department of MoH), Ireland – http://www.hpsc.ie/a-z/respiratory/influenza/seasonalinfluenza/influenzaandhealthcareworkers/hcwinfluenzavaccineuptakereports/File,15542,en.pdf, Health Protection Surveillance Centre. Influenza vaccine uptake and adults 65 years and older http://www.hpsc.ie/a-z/respiratory/influenza/seasonalinfluenza/influenzaandadults65yearsandolder/Seasonal%20influenza%20uptake_Sep%202016_Feb%202017___12%2004%202017_10v_.pdf; Italy – Ministero della Salute. Piano Nazionale Prevenzione Vaccinale PNPV 2012‐2014 (Ministry of Health. National Vaccination Plan). 2012, Epicentro – Ministry of Health (http://www.epicentro.iss.it/problemi/influenza/CoperturaVaccinale2015-2016.asp, http://www.epicentro.iss.it/passi/dati/VaccinazioneAntinfluenzale.asp; Kosovo – National public health institute; Latvia – Center for disease prevention and control of Latvia, https://www.spkc.gov.lv/lv/informativi-izdevum; Netherlands – Heins M, Hooiveld M, ten Veen P, Korevaar J. Vaccine Coverage Dutch National Influenza Prevention Program 2015 – Brief monitor. Utrecht, NIVEL: 2016; Poland – Czarkowski MP, Kondej B, Staszewska-Jakubik E, Cieląbek E (2016) Vaccinations in Poland in 2015. National Institute of Public Health – National Institute of Hygiene – Department of Epidemiology, Warszawa; Portugal – Direção‐Geral da Saúde (DGS). Vacinação contra a gripe com a vacina trivalente para a época 2015/2016 (Directorate General for Health (DGS). Influenza vaccination with trivalent vaccine for the season 2015/2016). 2015; Serbia – National public health institute; Slovakia – Report of Influenza season 2015/16 in the Slovak Republik, web of Public Health Authority of SR: http://www.uvzsr.sk/docs/info/epida/Vyhodnotenie_chripkovej_sezony_2015_2016.pdf; Spain – Ministerio de Sanidad, Servicios Sociales e Igualdad. Coverturas de Vacunacion. Datos estadísticos. https://www.msssi.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/docs/CoberturasVacunacion/Tabla10.pdf; Switzerland – Office fédéral de la santé publique (OFSP). Recommandations pour la vaccination contre la grippe (Federal Office of Public Health. Recommendations for vaccination against influenza). 2011; Nationale Strategie zur Prävention der saisonalen Grippe (GRIPS), Bull OFSP 2016; Nr. 37:559-566; UK – Screening and Immunisations Team, Health and Social Care Information Centre. NHS Immunisation Statistics England, 2014‐15. 2015, https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/544552/Seasonal_flu_GP_patient_groups_annual_report_2015_2016.pdf, https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/508557/HCWs_Seasonal_Flu_Vaccine_February_Final_Report_170316.pdf, https://www.gov.uk/government/statistics/annual-flu-reports, https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/600880/annual_flu__letter_2017to2018.pdf, https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/600880/annual_flu__letter_2017to2018.pdf.
Recommendations and funding
All countries had recommendations for seasonal influenza vaccination for the elderly, healthcare professionals and persons with a medical risk condition, although the recommendations are heterogeneous. For example, the majority of countries recommend vaccination for those aged ≥65 years, while Germany, Greece, Hungary, The Netherlands and Portugal recommend vaccination for those aged and Slovakia for those aged≥59 years. In most countries vaccination is recommended for all HCPs, except in Slovakia where it is recommended for HCPs who have close contact with patients or foci of infection. The chronic medical conditions included in the recommendations vary between the countries. Season influenza vaccination is free (vaccine or administration) for those in the recommended target populations in all countries except Bulgaria, Estonia and Poland.
National target VCRs and annual monitoring and communication of VCRs
Nine countries reporting having an objective of 75% VCR in the target populations, and five reported having a national action plan to increase VCRs. Six countries reported annual monitoring and communication of influenza VCRs by target population, but 13 countries did not reply to this question. Among the six countries, three reported not having either a target VCR or a national plan to improve VCR (Hungary, Switzerland and the Netherlands), two reported an objective of 75% VCR and a national plan to increase VCR (Latvia and the UK), and one, Greece, reported having an objective of 75% VCR (Table 1).
Annual influenza summit
Eight countries reported have an annual influenza summit either before or during the influenza season: Greece, Kosovo, Latvia, Lithuania, Poland, Romania, Serbia and Slovakia.
HCP incentives
Six countries, Czech Republic, England, France, Ireland, The Netherlands, and Scotland reported having financial incentives (payment of a fee or additional revenue) for HCPs who administer the influenza vaccine.
Awareness campaigns
Seventeen countries said they had a seasonal influenza vaccination awareness campaign organized by their national health authority or vaccination committee (Table 1). The elements of this campaign varied, but most used flyers in doctors’ waiting rooms or advertisements in the press for the general public. The majority also organized advertising or mailings specifically for healthcare professionals. In the UK, GPs advise the vaccination on their practice websites and also contact patients mainly by text messages although some use letters or phone calls.
Policy elements for program implementation and VCRs
HCP financial incentive was significantly associated with higher VCRs for the elderly population (correlation coefficient 0.54, p = 0.018) while having a summit before or during annual influenza vaccination campaign was associated with lower VCRs in this population (correlation coefficient -0.53, p = 0.044). Free vaccination showed a borderline significant association with higher vaccination uptake rates in the elderly (correlation coefficient 0.43, p = 0.053). The combinations of HCP financial incentive with free vaccination and HCP financial incentive with advertisements or mailings to HCPs were both found to be significantly associated with higher VCRs in the elderly: correlation coefficient 0.49, p = 0.047 and correlation coefficient 0.79, p = 0.034, respectively, whereas the combination of free vaccination and annual summit was significantly associated with lower VCRs in the elderly: correlation coefficient -0.70, p = 0.008.
None of the individual policy elements were significantly associated with higher or lower VCRs for HCPs (Table 2). Two elements were found to be borderline significant: having an objective for 75% VCR in targeted groups (correlation coefficient 0.54, p = 0.088), and having a website for the general public (correlation coefficient 0.47, p = 0.054). The combination of free vaccination and annual monitoring & communication of VCR by the health authority and the combination of free vaccination and having a website for the general public were found to be significantly correlated with higher VCRs for HCP: correlation coefficient 0.83, p = 0.042 and correlation coefficient 0.57, p = 0.021, respectively.
Table 2.
Implementation of policy elements aimed at increasing influenza vaccination coverage rates and correlation with vaccination coverage rates in healthcare professionals and the elderly in countries with vaccination coverage rate data for the season 2015–16 available.
| HCPs |
Elderly |
||||
|---|---|---|---|---|---|
| Program implementation | Countries with 2015–16 VCR (n) | Average VCR % (95% CI) | Countries with 2015–16 VCR (n) | Average VCR % (95% CI) | |
| Recommendation for annual seasonal influenza vaccination | Yes | 17 | 27.9 (19.8, 35.9) | 21 | 35.3 (24.4, 46.3) |
| No | 0 | — | 0 | — | |
| Vaccination free for recommended patients (vaccine or administration)? | Yes | 16 | 29.0 (20.8, 37.2) | 19 | 38.3 (27.0, 49.5) |
| No | 1 | NN | 2 | 7.4 (NN) | |
| National action plan to improve VCR | Yes | 4 | 21.9 (0, 53.9) | 5 | 30.0 (0; 62.8) |
| No | 8 | 24 (13.9, 34.7) | 10 | 23.8 (9.7, 37.9) | |
| Objective for 75% VCR in targeted groups | Yes | 7 | 35.8 (19.0, 52.6) | 8 | 44.6 (25.5, 63.7) |
| No | 4 | 15.4 (0, 36.2) | 5 | 36.8 (10.7, 62.9) | |
| Yearly national VCR objective | Yes | 4 | 27.1 (0, 54.2) | 3 | 39.9 (NN) |
| No | 8 | 21.8 (10.1, 33.4) | 12 | 22.4 (10.4; 34.3) | |
| Annual monitoring & communication of VCR, by target by HA | Yes | 4 | 32.2 (11.6, 52.7) | 5 | 39.0 (2.7, 75.3) |
| No | 4 | 14.1 (0; 41.3) | 4 | 30.7 (6.9, 54.5) | |
| Summit before or during annual campaign | Yes | 6 | 17.1 (4.7, 29.5) | 7 | 16.3 (2.6, 30.1) |
| No | 6 | 28.0 (13.0, 42.9) | 8 | 36.8 (16.0, 57.6) | |
| HCP have clear objectives to achieve in high-risk groups (GP and/or other specialist) | Yes | 3 | 29.4 (0; 83.7) | 3 | 38.3 (0; 109) |
| No | 9 | 21.6 (12.1, 31.1) | 12 | 22.8 (10.3, 35.2) | |
| HCP financial incentive | Yes | 5 | 32.4 (10.3, 54.6) | 7 | 50.6 (29.2, 72.0) |
| No | 11 | 22.9 (15.2, 30.6) | 12 | 26.5 (14.8; 38.1) | |
| Awareness campaign by HA and/or NVIG | Yes | 12 | 27.5 (18.6, 36.3) | 15 | 36.2 (23.9, 48.5) |
| No | 4 | 26.9 (0; 65.1) | 5 | 24.9 (0; 56.8) | |
| Awareness campaign: TV / radio | Yes | 12 | 27.8 (17.3, 38.3) | 16 | 36.2 (21.9, 50.5) |
| No | 5 | 28.0 (9, 47.1) | 5 | 32.6 (15.3, 49.9) | |
| Awareness campaign: press advertisements for public | Yes | 12 | 27.3 (17.5, 37.1) | 16 | 37.5 (24.6, 50.4) |
| No | 5 | 29.1 (6.5, 51.8) | 5 | 28.3 (0; 59.2) | |
| Awareness campaign: flyers / leaflets/ folders in medical waiting rooms | Yes | 15 | 25,7 (17.7, 33.6) | 18 | 36.1 (24.3, 47.8) |
| No | 2 | 44.1 (NN) | 3 | 30.9 (NN) | |
| Awareness campaign: website for public | Yes | 12 | 31.7 (21.2, 42.2) | 17 | 37.5 (24.7, 50.4) |
| No | 5 | 18.6 (7, 30.2) | 4 | 26.0 (0, 57.7) | |
| Awareness campaign: advertisements or mailings to HCP | Yes | 11 | 28.1 (18.1, 38.0) | 14 | 40.8 (26.5, 55.1) |
| No | 6 | 27.6 (8.0, 47.1) | 7 | 24.3 (6.0, 42.6) | |
NN: not calculated due to small sample size.
The data for VCRs in those with medical risk condition were insufficient for correlation analyses to be performed.
Discussion
These results confirm that despite national and international recommendations for seasonal influenza vaccination for the elderly, HCPs and those with medical risk conditions, the VCRs that were available for these target groups have not reached WHO's target of 75% VCR. We also report that most of the policy elements did not seem to be associated with higher VCRs individually, although some combinations were statistically significantly associated with higher VCRs for the elderly and HCPs. Unexpectedly, we observed that having a summit before or during annual influenza vaccination campaign was associated with lower VCRs in the elderly. This could be because the countries where this occurred may not have had sufficiently support for an effective vaccination campaign. The VCRs for the elderly were generally lower than those reported for the 2007–2008 season in our previous survey in most countries.9 However, in Portugal the VCR increased from 51% in the previous survey to 68% in the 2015–2016 season and in the UK and Poland the VCRs were stable.
Best practices should be shared between countries to support policy changes aimed at improving influenza VCRs and thus achieving the European Union targets. To enable this, we need to understand better what facilitators and barriers have an impact on VCRs. Our previous survey in 16 European countries in the 2007–08 influenza season showed that monitoring and communicating VCRs and sending personal letters offering free vaccination were correlated with higher VCRs in the elderly.9 However, in this recent survey these elements were not associated with higher VCRs in the elderly. Monitoring VCRs is a key component in any vaccination program to help identify successful policy elements as well as gaps and weaknesses. This should be done annually and the results should be available before the next season to take advantage of the lessons learned and adapt the policy accordingly. Also this monitoring will provide material to communicate broadly on the outcomes of public health intervention, i.e., pubic money spent to reach an important public health goal. The VCRs we report are similar to those reported on the OECD website.10
Although the European countries in this survey all recommend seasonal influenza vaccination for people living with medical risk conditions, VCRs for this group were available only in 10 of the 23 countries. Only 17 of the reporting countries have vaccine coverage data for HCPs. In addition, VCR monitoring in these risk groups is not systemic in many countries and the methods used are heterogeneous. One of the problems is that it is often unclear how the number of individuals eligible for vaccination in these risk groups is determined. Since this number is used as the denominator in the VCR calculation, its accurate estimation is important. As monitoring VCRs is a key component of any vaccination campaign, monitoring should be done at least annual and the results should be available before the next campaign to help build on lessons learned and adapt the programs accordingly. These data would provide material for broad communicate on the outcomes of public health interventions and demonstrate that pubic money had been well spent to reach an important public health goal.
A Cochrane review of 57 randomized controlled trials assessing interventions to increase VCR in community-dwelling individuals aged ≥60 years concluded that, while some interventions effectively increased VCR the trials were heterogeneous, which limited their ability to perform meta-analyses.11 The trials assessed a variety of methods that can be grouped into increasing community demand, improving access to vaccination and HPC- or healthcare system/targeted interventions; but no trials assessing government policies were identified. Another systematic review assessed the effectiveness of interventions using new information and communication technologies to promote vaccination uptake and increase vaccination coverage generally.12 Based on 19 studies, mainly performed in the United States the conclusion was that although these interventions seem promising, there is a need for further, more robust data, including cost-effectiveness data. Our questionnaire did not collect data on the use of these new technologies but this will be included in future surveys.
A study in Israel sought nurses’ perspectives on facilitators and barriers for the elderly to accept influenza vaccination.13 The results showed that the healthcare team, particularly physicians, could encourage vaccination through recommendations and showing that they themselves were vaccinated. Other facilitators identified were easy access and free vaccine, influence from the media, family and social circles. The barriers identified included difficult access, not believing that the vaccine works, and fear of adverse effects or getting influenza from the vaccine. In addition, personal beliefs and influence from the media and social circles could also be barriers.
A study in Spain reported that influenza VCR in patients aged ≥ 65 years was higher in those whose primary care physician had been vaccinated than in those whose physician had not (57.3% vs. 55.2%, p = 0.008) and this remained significant after adjustment for age, region, and opinions on vaccine effectiveness.14 Although this observation needs to be confirmed, it may be useful to target HCPs with interventions to improve their opinions and attitudes about influenza vaccination as a means of increasing VCR among their at-risk patients.
In the USA, it has been reported that employer vaccination requirements for HCPs and offering free, on-site vaccination were associated with high VCRs.15 In Switzerland, a study to identify why nurses refused influenza vaccination reported three interrelated factors; their perception of being in an untrustworthy environment, which restricts their decisional autonomy and seems to work against their aim to maintain a strong and healthy body.16 In England, the national NHS leaders wrote to all staff to remind them of their ‘professional duty to protect their patients’ for the 2017–2018 season. They have urged all local NHS trusts to make influenza vaccination available to their staff for the season and they have said that all staff that refuse influenza vaccination will have to give their reasons, which will be recorded.17
We recognize that our analyses have some limitations which limit our ability to compare results between countries and to interpret the data. First, the policy elements were not examined in detail but there could be important differences between countries even for elements that are classified as the same. For example, we did not collect information about the timing and duration of awareness campaigns which would have an obvious impact on their efficacy. Second, we did not collect information about how long the individual elements had been in place and it is possible that if some elements were only recently introduced they would perhaps not have been reached their optimal efficacy. Lastly, despite all the countries surveyed being in Europe there will be differences in culture, religious beliefs and socioeconomic factors that can also have an impact on VCRs but our survey approach was unable to collect this level of information.
Vaccination in general, including influenza vaccination, is a much debated subject, which may have an impact on individuals’ confidence in vaccination and therefore influence their decision to be vaccinated or not. It has been suggested that evidence-based socio-psychological items could be included in national immunization surveys to evaluate the public's perception and identify emerging concerns which could then be addressed proactively in the awareness-raising campaigns.18
Although vaccination policies are decided at the national or regional level, the European Union is keen to support cooperation and exchange of information on national immunization programs between Member States and concrete joint actions are being planned.19,20 National immunization programs are organized differently between countries therefore awareness-raising campaigns are better organized at the national/regional level so that they can be tailored to the circumstances of each country/region. This collaborative approach should positively impact influenza vaccination policies and ultimately VCRs in the target groups.
Conclusion
European VCRs vary widely between the elderly, HCPs and those with medical risk conditions despite all countries identifying them as target groups in their recommendations for seasonal influenza vaccination. Over the three influenza seasons analyzed here, the VCRs did not reach the 75% target set by the WHO and many national health authorities; they were mainly stable, at best, but some decreases were observed. Since the initial survey during the 2007–2008 influenza season, VCRs have decreased in the elderly in the majority of countries, thus, achieving high VCRs in the elderly and the other target groups is still a major public health challenge in Europe which could be addressed by the identification, assessment and sharing of best practice for influenza vaccination policies.
Materials and methods
Data sources. At the time of the survey in February 2016 there were 22 members from 20 European countries in RAISE. RAISE is an informal group of national experts in the field of vaccination and influenza prevention who agreed to participate in RAISE (see list of current members in Appendix). They are mainly academic researchers or employed by public institutions. RAISE's goal is to achieve maximum influenza vaccination uptake in the at-risk groups, as defined by the WHO.21 The achieve this, the members meet regularly to exchange about what influenza prevention and influenza vaccination awareness activities are carried out in their country with the aim of developing synergy between the members and their activities. They discuss public health and individual health issues related to influenza control and prevention and propose and implement awareness activities to improve influenza vaccination coverage, targeting healthcare professionals and the general public. The meeting and logistical costs are paid by an unrestricted grant from Sanofi Pasteur; the members are not paid for their RAISE-related activities.
The methods used were similar to those used for the initial survey published in 2012.9 The survey questionnaire, written in English, contained closed questions (yes/no) but allowed the respondents to provide additional information as previously described. The questions were based on a previous literature review to identify policy elements that potentially influence VCRs. In this current study, we collected data about the elderly, as done previously, and also expanded to collect similar data for HCPs and those living with medical risk conditions. Although there was no formal validation of the survey, the data collected were compared those available from other sources, in particular in the latest ECDC report covering the 2007/08 to 2014/15 European influenza seasons.22
The questionnaire was sent out in February 2016 and returned between March 2016 and July 2016 to the 22 RAISE group members; 21 members responded (see Appendix for names of those who responded). This data collection was coordinated by one of the authors (PRB).In addition, in May 2017 we asked the RAISE members to verify the data and update them, if necessary.
Data analyses. The analyses were primarily descriptive, as previously described.9 Briefly, for the description of the presence or absence of policy elements covered by the questionnaire, a score of 1 or 0 was attributed for a given policy element, e.g., monitoring annual VCRs, if the country replied ‘yes’ or ‘no’, respectively. When the effect of two or three program elements was assessed, a score of 1 was attributed for a given policy element when countries responded ‘yes’ for all elements, and 0 if the responded ‘no’ for at least one element. Countries were excluded from the analysis for the element if there was no response (missing data), thereby avoiding inappropriately deflated averages by interpreting non-responses as negative responses. We assessed the association between implemented policy elements and vaccine coverage rates by Spearman's correlation coefficient (Spearman's rho). Correlation coefficients range from –1 for a perfect negative relationship to +1 for a perfect positive relationship, with 0 indicating no correlation. For all statistical tests, the threshold for statistical significance for the two-sided p values was set at <0.05. We performed multiple regression analyses but they provided no additional information and, given the limited data available, the results are not robust and interpretation is difficult (results not shown).
The data were analyzed using Microsoft Excel 2013. The statistical analyses were done using IBM SPSS version 22.
Appendix
List of members of the RAISE group (as of November 2017)
Bulgaria: Andrei Galev*; Croatia: Vladimir Drazenovic*; Cyprus: Evis Bagdades; Czech Republic: Jan Kyncl*; Estonia: Kadri Koivumagi*; France: Jean-Marie Cohen*; Germany: Jörg Schelling (Tom Schaberg*); Greece: Helena C. Maltezou*; Hungary: Daniel Eorsi*; Italy: Pierluigi Lopalco; Kosovo: Isme Humolli*; Latvia: Dace Zavadska*, Dana Isarova*; Lithuania: Aukse Mickiene*, Vytautas Griska; Netherlands: Gerrit Adrianus van Essen*; Poland: Ernest Kuchar*, Aneta Nitsch-Osuch*; Romania: Oana Falup Pecurariu*; Serbia: Snezana Medic*; Slovakia: Zuzana Kristufkova*; Spain: Raùl Ortiz de Lejarazu*; Switzerland: Patricia Blank*; UK: George Kassianos*
*indicates survey respondent
Disclosure of potential conflicts of interest
Patricia Blank is currently working for Roche Diagnostics Switzerland, however this work was done independently of this industry position. Gerrit Adrianus van Essen has given lectures for Sanofi Pasteur and Roche and participated in an advisory board for Seqirus. Raúl Ortiz de Lejarazu has acted as an advisor for GSK, Roche, Seqirus and Sanofi Pasteur-MSD and has received grants for meetings attendance and oral presentations from GSK, Roche, Seqirus, and Sanofi Pasteur-MSD. Aneta Nitsch-Osuch has given lectures for Sanofi Pasteur and GSK. George Kassianos participated in advisory boards and lectured in the last 12 months for Seqirus, Merck, Sanofi Pasteur and AstraZeneca. Jan Kyncl, Ernest Piotr Kuchar, Oana Falup-Pecurariu, Helena Maltezou, Dace Zavadska and Zuzana Kristufkova report no conflicts of interest.
Acknowledgments
This manuscript was written by a group of European experts (see Appendix for details), with financial support from Sanofi Pasteur for medical writing, meeting costs, and formatting. Dr Margaret Haugh (MediCom Consult, Villeurbanne) provided professional medical writing assistance in the preparation and development of the manuscript in accordance with the European Medical Writers Association guidelines and Good Publication Practice, funded by Sanofi Pasteur.
Funding
This manuscript was written by a group of European experts (see Appendix for details), with financial support from Sanofi Pasteur for medical writing, meeting costs, and formatting.
References
- 1.World Health Organisation Influenza (seasonal): fact sheet, November 2016 2016. Available at, http://www.who.int/mediacentre/factsheets/fs211/en/, Accessed: 28 November 2017.
- 2.European Centre for Disease Prevention and Control Revised estimates of deaths associated with seasonal influenza in the US. 2010. Available at, http://ecdc.europa.eu/en/activities/sciadvice/_layouts/forms/Review_DispForm.aspx?List=a3216f4c-f040-4f51-9f77-a96046dbfd72&ID=394, Accessed: 7 December 2016.
- 3.Preaud E, Durand L, Macabeo B, Farkas N, Sloesen B, Palache A, Shupo F, Samson SI. Annual public health and economic benefits of seasonal influenza vaccination: a European estimate. BMC Public Health. 2014;14:813. doi: 10.1186/1471-2458-14-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hannoun C. The evolving history of influenza viruses and influenza vaccines. Expert Rev Vaccines. 2013; 12:1085–94. doi: 10.1586/14760584.2013.824709. [DOI] [PubMed] [Google Scholar]
- 5.Council of the European Union Council Recommenation of 22 December 2009 on seasonal influenza vaccination (Text with EEA relevance) (2009/1019/EU). 2009. Available at, http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:348:0071:0072:EN:PDF, Accessed: 11 June 2011.
- 6.Ahmed F, Lindley MC, Allred N, Weinbaum CM, Grohskopf L. Effect of influenza vaccination of healthcare personnel on morbidity and mortality among patients: systematic review and grading of evidence. Clin Infect Dis. 2014;58:50–7. doi: 10.1093/cid/cit580. [DOI] [PubMed] [Google Scholar]
- 7.Corace K, Prematunge C, McCarthy A, Nair RC, Roth V, Hayes T, Suh KN, Balfour L, Garber G. Predicting influenza vaccination uptake among health care workers: what are the key motivators? Am J Infect Control. 2013;41:679–84. doi: 10.1016/j.ajic.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Hofmann F, Ferracin C, Marsh G, Dumas R. Influenza vaccination of healthcare workers: a literature review of attitudes and beliefs. Infection. 2006;34:142–7. doi: 10.1007/s15010-006-5109-5. [DOI] [PubMed] [Google Scholar]
- 9.Blank P, Schwenkglenks M, Szucs TD. The impact of European vaccination policies on seasonal influenza vaccination coverage rates in the elderly. Hum Vaccin Immunother. 2012;8:328–35. doi: 10.4161/hv.18629. [DOI] [PubMed] [Google Scholar]
- 10.OECD Influenza vaccination rates (indicator). 2018. Available at, data.oecd.org/healthcare/influenza-vaccination-rates.htm, Accessed: 24 April 2018. [Google Scholar]
- 11.Thomas RE, Lorenzetti DL. Interventions to increase influenza vaccination rates of those 60 years and older in the community. Cochrane Database Syst Rev. 2014:Cd005188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odone A, Ferrari A, Spagnoli F, Visciarelli S, Shefer A, Pasquarella C, Signorelli C. Effectiveness of interventions that apply new media to improve vaccine uptake and vaccine coverage. Hum Vaccin Immunother. 2015;11:72–82. doi: 10.4161/hv.34313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellen M. Factors that influence influenza vaccination rates among the elderly: nurses' perspectives. J Nurs Manag. 2017. [DOI] [PubMed] [Google Scholar]
- 14.Godoy P, Castilla J, Mayoral JM, Martin V, Astray J, Torner N, Toledo D, Soldevila N, Gonzalez-Candelas F, Garcia S, et al. . Influenza vaccination of primary healthcare physicians may be associated with vaccination in their patients: a vaccination coverage study. BMC Fam Pract. 2015;16:44. doi: 10.1186/s12875-015-0259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Black CL, Yue X, Ball SW, Fink R, de Perio MA, Laney AS, Williams WW, Lindley MC, Graitcer SB, Lu PJ, et al. . Influenza Vaccination Coverage Among Health Care Personnel – United States, 2016–17 Influenza Season. MMWR Morb Mortal Wkly Rep. 2017;66:1009–15. doi: 10.15585/mmwr.mm6638a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pless A, McLennan SR, Nicca D, Shaw DM, Elger BS. Reasons why nurses decline influenza vaccination: a qualitative study. BMC Nursing. 2017;16:20. doi: 10.1186/s12912-017-0215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iacobucci G. NHS staff who refuse flu vaccine this winter will have to give reasons. BMJ. 2017;359. [DOI] [PubMed] [Google Scholar]
- 18.Wheelock A, Miraldo M, Thomson A, Vincent C, Sevdalis N. Evaluating the importance of policy amenable factors in explaining influenza vaccination: a cross-sectional multinational study. BMJ Open. 2017;7:e014668. doi: 10.1136/bmjopen-2016-014668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haverkate M, D'Ancona F, Giambi C, Johansen K, Lopalco PL, Cozza V, Appelgren E. Mandatory and recommended vaccination in the EU, Iceland and Norway: results of the VENICE 2010 survey on the ways of implementing national vaccination programmes. Eurosurveillance. 2012;17:20183. doi: 10.2807/ese.17.22.20183-en. [DOI] [PubMed] [Google Scholar]
- 20.European Commission DG Public Health Workshop – Seeking new partnerships for EU action on vaccination. 2017. Available at, https://ec.europa.eu/health/vaccination/events/ev_20170531_en, Accessed: 28 November 2017. [Google Scholar]
- 21.World Health Organisation Influenza. Vaccine use. 2012. Available at, http://www.who.int/influenza/vaccines/use/en, Accessed: 16 May 2018. [Google Scholar]
- 22.European Centre for Disease Prevention and Control Seasonal influenza vaccination and antiviral use in Europe – Overview of vaccination recommendations and coverage rates in the EU Member States for the 2013–14 and 2014–15 influenza seasons. Stockholm: ECDC. 2016. [Google Scholar]


