ABSTRACT
Inflammatory bowel disease (IBD) is a chronic disabling inflammatory process that affects young individuals, with growing incidence. The etiopathogenesis of IBD remains poorly understood. A combination of genetic and environmental factors triggers an inadequate immune response against the commensal intestinal flora in IBD patients. Thus, a better understanding of the immunological mechanisms involved in IBD pathogenesis is central to the development of new therapeutic options.
Current pharmacological treatments used in clinical practice like thiopurines or anti-TNF are effective but can produce significant side effects and their efficacy may diminish over time. In fact, up to one third of the patients do not have a satisfactory response to these therapies. Consequently, the search for new therapeutic strategies targeting alternative immunological pathways has intensified. Several new oral and parenteral substances are in the pipeline for IBD.
In this review we discuss novel therapies targeting alternative pro-inflammatory pathways like IL-12/23 axis, IL-6 pathway or Janus Kinase inhibitors; as well as others modulating anti-inflammatory signalling pathways like transforming growth factor-β1 (TGF-β1). We also highlight new emerging therapies targeting the adhesion and migration of leukocytes into the inflamed intestinal mucosa by blocking selectively different subunits of α4β7 integrins or binding alternative adhesion molecules like MAdCAM-1. Drugs reducing the circulating lymphocytes by sequestering them in secondary lymphoid organs (sphingosine-1-phosphate (S1P) receptor modulators) are also discussed. Finally, the latest advances in cell therapies using mesenchymal stem cells or engineered T regs are reviewed. In addition, we provide an update on the current status in clinical trials of these new immune-regulating therapies that open a new era in the treatment of IBD.
KEYWORDS: inflammatory bowel disease, therapy, Crohn's disease, ulcerative colitis, Janus kinases, adhesion molecules, cell therapy
Inflammatory bowel disease (IBD) is a chronic disabling inflammatory process that affects mainly the gastrointestinal tract and may present associated extraintestinal manifestations.1 IBD includes both ulcerative colitis (UC) and Crohn's disease (CD). In UC, the inflammation takes place in the colon and rectum, is limited to the mucosa and always extends in oral direction from the rectum.2 CD can affect any part of the gastrointestinal tract (typically the ileocecal area) and is characterised by transmural inflammation and local complications like stenosis, fistulae and abscesses.3
It predominantly affects young individuals with symptoms like abdominal pain, chronic diarrhea, fever, rectal bleeding and weight loss; alternating flares and periods of remission.3 The prevalence of IBD in western countries is estimated to be up to 0.5% of the general population, with growing incidence.4 The treatment of IBD requires the continuous use of powerful anti-inflammatory drugs (corticosteroids, thiopurines, anti-tumour necrosis factor [anti-TNF] agents etc.) and hospitalisation or surgery to manage its complications.1-3 This results in significantly compromised quality of life as well as a huge economic burden for society with high associated healthcare costs.5 In fact, only the direct costs in Europe are estimated to exceed €5.6 billion annually.6
The etiopathogenesis of the disease remains largely unknown. It is currently considered a polygenic immune disorder involving: 1) individual genetic factors; 2) environmental factors; 3) intestinal flora (microbiome); and 4) immune response. A combination of these factors triggers an inadequate immune response against the commensal flora in genetically predisposed subjects.7,8
Several alterations in some key innate immunity mechanisms have been reported in recent years involving both the recognition and clearance of intracellular organisms and bacteria, such as alterations in the nucleotide-binding oligomerisation domain-containing protein 2 (NOD2)9 or in the autophagy related protein 16L (ATG16L).10 In addition, other factors can contribute to the appearance of recurrent infections and chronic intestinal inflammation, such as: alterations in the intestinal permeability11,12; alterations in the mucosal layer13,14; dysfunction in the production of defensins by Paneth cells15 or alterations in the stress mechanisms of the endoplasmic reticulum.16
CD is considered to be a predominant type 1 T helper cell (Th1)- and Th17-mediated disease with an increased production of IL-17, IFN-γ and TNF-α; while UC has been associated with a dysregulated Th2 response.2
However, the IL-23 pathway – that is crucial to the function of Th17 cells – is altered in both conditions.17 The importance of regulatory T cells (Tregs) and other unconventional T cells like natural killer T cells (NKT), innate lymphoid cells (ILC) or gammadelta (γδ) T cells have been increasingly recognised in IBD pathogenesis, underlining its complexity.7,18-20
This impaired immune response leads to an increased infiltration of leukocytes into the inflamed intestinal mucosa, which contributes to persistent inflammation.21 Understanding the interplay between these cytokines and the different immune cells is central to the development of new therapeutic options in IBD.
Heterogeneity is an important issue in the clinical management of IBD patients since both clinical manifestations, disease location and behavior (phenotypes) and response to different therapies varies widely from patient to patient.1
The current treatment of IBD includes mesalazine (oral and rectal formulations), glucocorticoids (conventional and other forms like budesonide or beclomethasone), antibiotics (typically ciprofloxacine and metronidazole), immunosuppressants (mostly azathioprine/6-mercaptopurine or methotrexate) and anti-TNF agents (infliximab, adalimumab, certolizumab pegol and golimumab). Recently, the anti-integrin antibody vedolizumab and the antibody against IL-12/23 ustekinumab have been approved for IBD.22,23
The introduction of anti-TNF agents into clinical practice (infliximab was first approved in 1998 and 2005 for CD and UC, respectively) both for the induction of remission and as maintanence therapy, has improved the outcomes of IBD patients significantly.24 However these drugs have several limitations: 1) they are injectable (intravenous or subcutaneous) complicating the compliance; 2) they work only in a subset of patients (one third of the patients show no benefit); 3) they are immunogenic, causing allergic reactions and secondary loss of response (in approximately another third of the cases) due to antibody formation; 4) they are expensive; and 5) can lead to reactivation of infections – like tuberculosis or hepatitis B – and may increase the risk of some cancers.25-27 Moreover, the need for surgery remains high despite of the wide use of biologics in clinical practice (the risk of surgery 10 years after diagnosis is still 16% in UC and as high as 47% in CD patients).28,29
Thus, there is an urge for the development of new therapeutic targets for IBD. An increased insight into IBD pathogenesis (and especially the immunological aspects) can provide an excellent opportunity for therapeutic advances. Consequently, the search for new therapeutic strategies targeting alternative inflammatory cytokines and immunological pathways has intensified in the recent years. New oral and parenteral substances regulating alternative immune pathways like IL-12/23 axis, IL-6, Janus Kinase inhibition, TGF-β pathway, as well as the regulation of adhesion/migration of leucocytes or novel cell therapies using mesenchymal stem cells (MSC) or engineered T cells are in the pipeline. Here, we review the most promising of those new coming approaches in immunotherapy for IBD (summarized in Figure 1 and Table 1).
Figure 1.
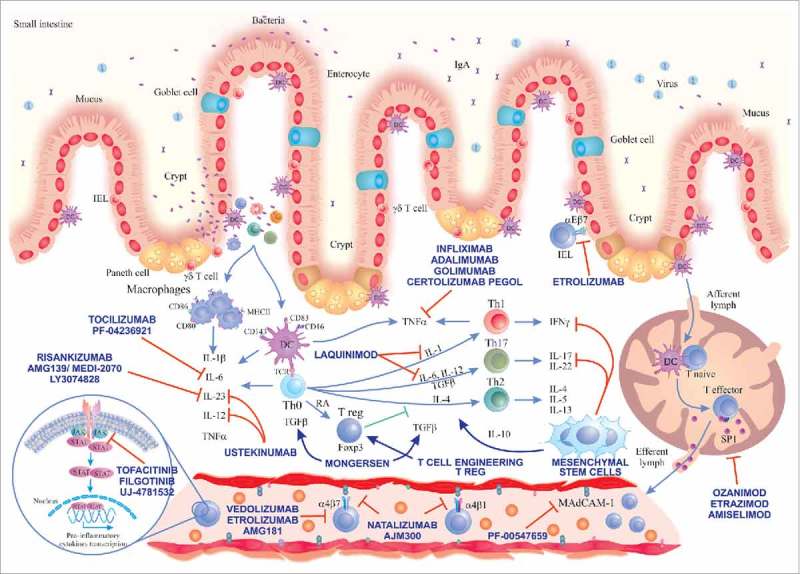
Immunological pathways targeted by the main novel therapies for IBD. A loss of intestinal barrier integrity takes place in IBD, leading to translocation of bacteria that triggers an exaggerated immune response with secondary activation of Th cell responses (Th1, Th2, Th17). We show the main drugs inhibiting IL-6 pathway, IL-12/23 axis, Jak inhibitors, laquinimod and stimulators of TGF-β1 pathway (mongersen). Drugs inhibiting TNF-α already approved for use in IBD and cell therapies using MSCs and Tregs and their main immune-modulation functions are depicted in the centre. Substances targeting different adhesion molecules are described in the blood vessel and in the gut epithelium (etrolizumab). On the bottom right, we show the mechanism of action of drugs reducing the circulating lymphocytes by sequestering them in secondary lymphoid organs (sphingosine-1-phosphate receptor modulators). Main abbreviations: IEL, intraepithelial lymphocyte; DC, dendritic cell; JAK, Janus kinase; STAT, signal transducer and activator of transcription; IL, interleukin; Th, T helper lymphocyte; TGF-β, transforming growth factor-beta; RA, retinoic acid; IFNγ, interferon gamma; S1P, sphingosine-1-phosphate.
Table 1.
Novel drugs, therapeutic targets and current status in clinical studies.
| Drug | Target | Pathway/mechanism of action | Administration | Tested in UC/CD | Main issues and side effects |
|---|---|---|---|---|---|
| Ustekinumab46 | p40 | IL-12/IL-23 | IV, SC | CD (approved) UC phase III | Cardiovascular safety. |
| ABT-874 (briakinumab)49 | p40 | IL-12/IL-23 | IV | CD phase II | No clear benefit. Cardiovascular safety. Discontinued. |
| AMG 139/MEDI2070 (brazikumab)53 | p19 | IL-12/IL-23 | IV, SC | CD phase II | — |
| BI 655066 (risankizumab)54 | p19 | IL-12/IL-23 | IV, SC | CD phase II | — |
| Tocilizumab68 | IL-6R | IL-6 | IV | CD | No further studies since 2004. |
| PF-0423692169 | IL-6 | IL-6 | SC | CD phase II | Serious adverse events including 1 death, perforations and abscesses. |
| Tofacitinib74,79 | JAK-1, JAK-2, JAK-3 | Jak inhibitor | Oral | UC phase III | Increased risk for infections. Alterations in serum lipids observed. Unclear efficacy in CD. |
| CD phase III | |||||
| Filgotinib80 | JAK-1 | Jak inhibitor | Oral | UC phase III | Increased risk for infections. |
| CD phase III | Endoscopic improvement did not reach significance in UC. | ||||
| Laquinimod86 | — | Th1/Th17 | Oral | CD phase II | Good safety profile. Ongoing long-term trials |
| Morgensen94 | SMAD7 | TGF-β1 pathway | Oral | UC phase II | Lack of endoscopic outcomes in the main studies. Concerns about stricture formation (fibrosis). Premature discontinuation of long-term studies for CD by October 2017. |
| CD phase II | |||||
| Alicaforsen105 | ICAM-1 | Blocking ICAM-1 production by complementary hybridization to mRNA target gene | IV | CD, UC, pouchitis | Intravenous formulation ineffective in CD. |
| Enema | |||||
| Natalizumab110 | α4-subunit of α4β1 and α4β7 integrins | Inhibition of lymphocyte adhesion to VCAM-1 and MAdCAM-1 | IV | CD | Not gut specific. PML. |
| Vedolizumab114,115,119 | α4β7 | Inhibition of lymphocyte adhesion to MAdCAM-1 | IV | CD, UC | Currently approved for UC and CD. |
| AMG 181/MEDI7183 (abrilumab)120,121 | α4β7 | Inhibition of lymphocyte adhesion to MAdCAM-1 | SC | CD, UC | Primary end point not met in CD. |
| phase II | |||||
| PF-00547659122,123 | MAdCAM-1 | Inhibition of lymphocyte adhesion to α4β7 | SC | CD, UC | Not better than placebo in CD. |
| phase II | |||||
| Etrolizumab124 | β7 of αEβ7 and α4β7 integrins | Inhibition of lymphocyte adhesion to E-cadherin and to MAdCAM-1 | SC | CD, UC | — |
| phase III | |||||
| AJM300113 | α4 of α4β1 and α4β7 integrins | Adhesion to VCAM-1 and MAdCAM-1 | Oral | UC phase II | Concern for PML-risk as it targets α4β1 (as natalizumab) |
| Ozanimod126 | S1PR1/5 | Sphingosine-1-Phosphate (S1P) receptor agonist | Oral | UC phase III | Lymphopenia and risk for infections in the long term need to be addressed. |
| CD phase II | |||||
| Etrasimod (APD334) | S1PR1 | Sphingosine-1-Phosphate (S1P) agonist | Oral | UC phase II | — |
| Amiselimod (MT-1303) | S1PR1/5 | Sphingosine-1-Phosphate (S1P) agonist | Oral | CD phase II | _ |
| Mesechymal Stem cells150-152 | Several immune-regulating targets | Several immune-regulating targets | IV | UC, CD | Small studies |
| Unclear dosing | |||||
| Unknown dose or long-term safety | |||||
| Tregs168,169 | Production of IL-10 and TGF-β | Several anti-inflammatory effects | IV | CD | Type of Treg to use not fully clear. Lack of clinical studies with placebo arm |
Targeting pro-inflammatory pathways
The interleukin 12-family
The interleukin-12 (IL-12) family of five cytokines are heterodimeric cytokines composed of two covalently linked chains.30 IL-12 and IL-23 are pro-inflammatory and have been found to be pathogenic in animal models of intestinal inflammation and in IBD.31-33
IL-12 consist of the heterodimeric proteins p40 and p35, while IL-23 consists of the heterodimeric proteins p40 and p19. Therefore, the shared p40 protein is a common target of both these cytokines. IL-12 and IL-23 secreted from antigen presenting cells (dendritic cells, macrophages) spark off intestinal inflammation and maintain the inflammatory response by secretion of inflammatory cytokines like IL-6, IL-17 and TNF-α from macrophages, neutrophils and natural killer cells.17,31,33 IL-12 and IL-23 favour Th1 and Th17 differentiation of naïve T-cells, in CD, as opposed to the postulated Th2 domination in UC.17,34 IL-12 and IL-23 signal through heterodimeric receptors: ILRβ1 and IL12Rβ2 for p40 and p35 subunits of IL-12; and IL-23R and ILRβ1 for p19 and p40 subunits of IL-23.32 Even though IL-23 might favour Th17 differentiation in CD, single nucleotide polymorphisms (SNPs) have been found in candidate gene encoding the IL-23 receptor (IL23R), which is associated with both increased and reduced risk for CD and UC.35-37 IL-12 and IL-23 signalling is mediated via Janus kinase (JAK)-signal transducer and activator of transcription (STAT)-proteins33 and induces expression of interferon gamma (IFN-γ) and IL-17 from Th1 and Th17 cells, respectively.
Given the importance of the IL-23–IL-17 axis and IL-17 overexpression in CD, blocking IL-17 could be beneficial. However, the anti-IL-17A monoclonal antibody secukinumab and anti-IL-17 receptor antibody brodalumab were both ineffective in CD.38,39 This is consistent with a study in mice showing the importance of IL-17 production by γδ T cells (independent of IL-23) for maintenance and protection of the epithelial barrier in the intestinal mucosa.40 Even though IL-23 contributes to tissue damage in IBD, it has an important role controlling infections. In fact, IL-23 and IL-17RA knockout mice have increased susceptibility and mortality to pulmonary infection with Klebsiella pneumoniae and IL-23 knockout mice showed increased mortality after enteric infection with Citrobacter rodentium.41 Moreover, mice with IL-23R deficiency in intestinal epithelial cells have reduced IL-22 induction, leading to increase in pro-inflammatory flagellated bacteria and increased mortality to dextran sodium sulfate colitis.42 In addition, intestinal epithelium derived IL-23 mediates mucosal healing via IL-22.43 Of note, IL-23R have shown opposing roles in different colitis models.44,45
Ustekinumab is a human anti-p40 IgG1 antibody blocking both IL-12 and IL-23. It is administered intravenously, and it has proven to be effective in CD both for induction and maintenance therapy after anti-TNF failure.46 Another antibody against the p40 subunit – briakinumab – has been tested in CD with some clinical benefit,47,48 but the overall quality of the evidence for the outcome clinical remission was rated as low in a recent meta-analysis.49 In fact, briakinumab studies have been discontinued due to limited clinical efficacy.49,50
Concerns have been raised about cardiovascular safety for both ustekinumab and briakinumab,51,52 but a Cochrane review concludes that both drugs are safe.49 The new drug brazikumab – AMG 139/MEDI2070 – blocking the p19 subunit (specific for IL-23) was associated with clinical improvement53 in patients with CD and TNF-antagonist failure. One study with risankizumab (BI 655066) – also targeting the p19 subunit – has shown some efficacy in CD.54 Trials with other drugs targeting the p19 subunit, like mirikizumab (LY3074828) are underway.50,55
IL-6 pathway
IL-6 is a key pleiotropic cytokine with an immunoregulatory role in innate and adaptive immune responses synthesized by a wide variety of immune cells.56 In fact, IL-6 can display pro- and anti-inflammatory properties depending on the context.
IL-6 has a protective role in many infections, where a transient production of IL-6 contributes to host defense and tissue repair.57 In addition IL-6 induces the hepatic synthesis of C-reactive protein and other acute-phase proteins.58 However, an excessive production of IL-6 can contribute to the maintenance of chronic inflammatory diseases like rheumatoid arthritis or IBD.59,60 In fact, IL-6 promotes specific differentiation of naïve CD4-positive cells into Th17 cells and inhibits TGF-β-induced Treg development.61,62 This dysregulation of Th17/Treg balance by IL-6 is considered to be key in the development of autoimmune and chronic inflammatory diseases, like IBD.63
IL-6 can display its effects through a transmembrane receptor (IL-6R) and a soluble form (sIL-6R).64 Blocking the IL-6 pathway ameliorates colitis in mice.65 Both IL-6 and sIL-6R are highly expressed in the colonic mucosa of patients with IBD66 and several studies show that high serum concentration of IL-6 is predictive of relapse in IBD.67 Thus, IL-6 antagonism has been explored as a novel therapeutic target in IBD.
Intravenous tocilizumab, a humanized anti-IL6R antibody, was well tolerated but showed a modest effect in a pilot study including 36 CD patients. Clinical remission was obtained in 20% of treated patients versus none in the placebo group, but no differences in the endoscopic or histological examination were found.68
PF-04236921, a fully human monoclonal IgG2 antibody that binds to IL-6, was given subcutaneously in 3 different doses (10, 50 and 200 mg) on days 1 and 28 in a phase II randomized, double-blind, placebo-controlled trial in 247 subjects with refractory CD.69 Only the 50 mg s.c. arm achieved the primary endpoint (a clinical response defined as a CDAI-70 at week 8 or 12). This occurred in 49% and 47% of treated patients versus 31% and 29%, respectively, in the placebo arm (P <0.05 for both). However, the 200 mg arm was terminated early because of safety concerns. There was a troubling number of adverse events including 1 death because of post-operative respiratory failure and 6 patients experiencing complications like abscess or perforation during the induction study.69 These serious side effects may be due to the pleiotropic functions of IL-6 including epithelial regeneration.70 The inhibition of these beneficial functions – like the stimulation of mucosal healing – can lead to complications, and may limit the use of IL-6 inhibition in the future.
Janus Kinase inhibitors
The inhibition of JAK by small oral molecules has been tested in several autoimmune diseases like rheumatoid arthritis or myelofibrosis; and lately in IBD.71-74 JAK inhibitors can target signalling pathways used by multiple cytokines contributing to intestinal inflammation in IBD like IL-2, IL-6, IL-12, IL-21, IL-23 or IFN-γ (reviewed in refs. 75-77).
JAKs are non-receptor tyrosine kinases expressed in multiple immune cells comprising 4 members: JAK1, JAK2, JAK3, and Tyrosine kinase 2 (TYK2). These proteins are bound to the intracellular domain of several cytokine and hormone receptors where they facilitate signal transduction. The binding of the cytokine to its receptor results in JAK activation and auto-phosphorylation as well as phosphorylation of the receptor chains, forming binding sites and activating STATs. As a result, STATs form homo- or heterodimers and translocate to the nucleus where they modulate the transcription of target genes. There are seven STAT family members (STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6). Thus, each cytokine will activate different patterns in the JAK-STAT pathway leading to different immunomodulatory effects.76,77
The possibility of inhibiting several pro-inflammatory cytokines interacting with this pathway led to the test of tofacitinib (an oral JAK inhibitor that mainly inhibits JAK1, JAK2 and JAK3) in UC. A phase II randomized, placebo-controlled trial including 194 active UC patients showed that tofacitinib 15 mg oral twice daily produced a statistically significant clinical response at week 8 (primary endpoint), as well as clinical remission and endoscopic response (secondary outcomes) compared with placebo. A dose-dependent increase in both LDL and HDL cholesterol concentrations which reversed after discontinuation of the drug was observed. In addition, 3 patients treated with tofacitinib had an absolute neutrophil count of less than 1500/mm3 during the study period and 2 presented serious adverse events in form of infection.73
The results of two phase III trials testing tofacitinib 10 mg twice daily for the induction of remission in active UC (OCTAVE Induction 1 and 2) have been recently presented.74 More patients receiving tofacitinib achieved remission, mucosal healing and clinical response in both studies compared with placebo at week 8. No differences regarding previous anti-TNF treatment were reported. Tofacitinib showed a quick onset of action (as for week 2) and the rate of serious adverse events was similar across groups. However, increases in serum lipids (cholesterol, LDL and HDL) and creatine kinase levels were reported with tofacitinib.74
A phase III study assessing the effectiveness and safety of tofacitinib in the maintenance of remission in UC has recently been completed and a multicenter open label extension phase III study to address the safety at 12 months is still recruiting patients (NCT01458574 and NCT01470612, respectively).
In contrast to the results of tofacitinib in UC, recent trials in patients with CD have produced disappointing results.78,79 A phase II trial failed to show any significant differences in the percentage of CD patients who achieved clinical responses or clinical remission after 4 weeks administration of tofacitinib (1, 5, or 15 mg) or placebo twice daily. However, the high placebo response and short duration of treatment, together with the biological effects observed with high doses of tofacitinib (significant reductions in CRP and faecal calprotectin), encouraged the continuation of the trials in CD. Consequently, two phase IIb studies evaluated the efficacy and safety of tofacitinib for induction and maintenance treatment in patients with moderate-to-severe CD.79 Again, both primary efficacy endpoints (proportion of patients in clinical remission at week 8 and clinical response or remission at week 26) were not significantly different from placebo.79 Thus, further development of tofacitinib in CD has been discontinued.
Filgotinib (GLPG0634, GS-6034), a novel once daily oral JAK1-selective inhibitor, was also tested in a large multicentre phase II study. Forty seven percent of patients treated with filgotinib 200 mg/day achieved clinical remission at week 10 (primary endpoint) versus 23% in the placebo group.80 However, the proportion of patients achieving endoscopic remission or mucosal healing was similar in both groups. Serious treatment-emergent adverse effects were reported more often in patients treated with filgotinib than in the placebo group (9% versus 4%) and serious infections occured in 3% of patients. In addition, exposure to filgotinib for 20 weeks resulted in 12% increase in mean LDL levels.80
The results of two phase III studies for the induction and long-term maintenance for CD (NCT02914561 and NCT02914600) will help test the efficacy and safety of filgotinib. Moreover, filgotinib has also entered two phase III studies for UC (NCT02914522 and NCT02914535). Another selective oral JAK1/3 inhibitor (JNJ-54781532) has completed a phase IIb trial to investigate its safety and effectiveness in active UC (NCT01959282). The results have not been communicated yet.
Laquinimod
Laquinimod is a new oral medication with several anti-inflammatory properties and a good safety profile that has been already clinically tested in multiple sclerosis81,82 and lupus nephritis.83
Its mechanism of action is not fully understood. Laquinimod suppresses Th1 and Th17-responses (inhibiting the production of TNF-α, IL-17 and IL-12) and induces a Th2 shift (increasing the production TGF-β, IL-10 and IL-4). It can also stimulate the action of Tregs and is able to inhibit leucocyte migration (reviewed in refs. 84, 85).
A dose escalation multicentre double-blind phase II study evaluated the safety and efficacy of laquinimod as induction therapy in patients with active CD, showing a significant clinical improvement and a favourable safety profile.86 Patients received laquinimod orally (0.5, 1, 1.5 or 2 mg/day) or placebo for 8 weeks with 4-week follow-up. The proportion of patients in clinical remission at week 8 was higher for the laquinimod 0.5 mg (48.3%) and 1 mg (26.7%) groups compared to higher doses of laquinimod or placebo (15.9%).
The incidence of side effects was similar. An elevation of liver enzymes reported in previous MS trials was not observed in the 0.5 mg group. Laquinimod was well tolerated and decreased significantly faecal calprotectin levels.86 Thus, laquinimod is a promising oral drug with a good safety profile and broad-spectrum anti-inflammatory and immune-regulatory properties. A phase III clinical development programme exploring the effect of the most effective dose (0.5 mg/day) along with a lower dose (0.25 mg/day) for the induction and maintenance of remission in moderate to severe CD is planned.86
Targeting anti-inflammatory pathways
The transforming growth factor β (TGF–β) pathway. SMAD7 antisense oligonucleotide
TGF–β1 is a multifunctional cytokine produced by many immune cells. It has been shown to down-regulate immune responses in the intestine and participates in several anti-inflammatory mechanisms. TGF–β1 suppresses the activation macrophages and effector T cells, stimulates the differentiation of Tregs and induces mucosal healing by promoting margination of epithelial cells and production of collagen (reviewed in refs. 87-89).
TGF–β1 signals through two transmembrane protein kinase receptors (TGFβR1 and TGFβR2) that, upon activation, phosphorylates SMAD2 and SMAD3 that subsequently associate with SMAD4. This complex translocates to the nucleus where it regulates the expression of target genes. SMAD7 is an intracellular negative regulator of TGF–β1 signaling that prevents the phosphorylation of SMAD2 and SMAD3.90,91
IBD patients present a decreased activity of the anti-inflammatory cytokine TGF–β1 caused by increased levels of SMAD7 secondary to decreased degradation.92 In addition, previous studies showed that specific antisense oligonucleotides for SMAD7 restores TGF–β1 signaling decreasing pro-inflammatory cytokine production.93 Consequently, the inhibition of SMAD7 could be a novel potential therapeutic target in IBD.
Mongersen is a new oral antisense oligonucleotide that targets SMAD7 mRNA facilitating the degradation of SMAD7 and thus, restoring the anti-inflammatory effects of TGF–β1.94 Mongersen is enveloped in a pH-dependent release tablet that makes it optimal to treat ileocolonic CD. Pharmacokinetic studies suggested that it acts locally and is not systemically available in plasma.95
After assessing the safety and tolerability of mongersen in a phase I trial,95 166 patients with moderate-to-severe CD were enrolled in a phase II study. Three oral doses of mongersen (10, 40, or 160 mg/day) or placebo were administered for two weeks. The primary endpoint was defined as CDAI <150 points at week two and maintained for two weeks. Fifty-five and 65% of patients achieved clinical remission in the 40 mg and 160 mg mongersen groups respectively, as compared with 10% in the placebo group (P < 0.001).94
Interestingly, no statistically significant differences were found in the number of participants achieving normalization of CRP levels after treatment, raising the question of its effect on mucosal inflammation. The authors argument that the short duration of the study could have been insufficient to reach CRP normalization.94 In addition, the lack of endoscopic evaluation is a major drawback of the study. To address this issue, a phase Ib randomized study investigated the endoscopic outcomes in 63 CD patients after therapy with a high dose of mongersen (160 mg daily for 4, 8 or 12 weeks), showing endoscopic response in 37% of patients (defined as a reduction in Simple Endoscopic Score for CD of at least 25% in comparison to baseline).96
The rate of adverse events did not differ among groups and most adverse events were considered to be related to complications of the disease in the pivotal phase II study.94 However, the short duration of therapy (2 weeks) may also limit the evaluation of safety in a chronic disease like CD.94
TGF–β1 is a profibrotic agent that can activate fibroblasts and smooth muscle cells and increase the production of collagen, which raises concerns regarding the possible effect of its therapeutic stimulation in the production of strictures and eventually in the incidence of colon cancer.97 Of note, patients with a history of strictures or fistulae were excluded from the main study by Monteleone et al.94
A previous study tried to address this issue. A small open label study including 15 patients treated with mongersen daily for a week showed no association with the development of small bowel strictures at 6 months.98 However, the question regarding the development of fibrosis on the long term needs to be further addressed. A phase II study exploring the efficacy and safety of mongersen in UC has recently been completed (NCT02601300). Two ongoing randomized multicentre phase III trials for induction and maintenance of remission in CD were prematurely discontinued in October 2017 by the sponsor pharma company after assessing overall benefit/risk in an interim futility analysis.
Targeting adhesion, trafficking and migration of immune cells
Adhesion molecules
Lymphocyte migration and retention in the intestinal mucosa and epithelium is mediated by adhesion molecules expressed on lymphocytes, the endothelium, the epithelium and the extracellular matrix (cell adhesion molecules [CAMs], integrins, selectins and cadherins).99 Blocking the adhesion of lymphocytes to the endothelium could alleviate the inappropriate immune reaction in IBD, stopping T-cell recruitment and retention to the inflamed mucosa. Endothelial adhesion molecules are induced during inflammation through cytokine (IL-1 and TNF) activation.100-102
The intercellular adhesion molecule 1 (ICAM-1) antisense oligonucleotide alicaforsen showed no effect in CD given intravenously. However, it is effective in distal UC and pouchitis when used in form of enemas.103-105
Gut activated T-lymphocytes express the α4β7 integrin for specific homing to the intestinal mucosa,106 while the corresponding ligand mucosal vascular addressin cell adhesion molecule (MAdCAM-1) is primarily expressed in the gastrointestinal tract (endothelium and lymphoid tissue).107 Thus, targeting the gut-specific binding between α4β7 expression on effector/memory T-cells and MAdCAM-1 of the inflamed intestinal endothelium is a therapeutic option.
Natalizumab, a monoclonal antibody against human α4 integrin blocks the α4-subunit of α4β1 and α4β7 integrins, inhibiting the adhesion to vascular cell adhesion molecule-1 (VCAM-1) and MAdCAM-1, respectively.108 Therefore, inhibition of lymphocyte – endothelial adhesion is not gut specific for natalizumab. Natalizumab is effective in the treatment of multiple sclerosis109 and in inducing and maintaining remission in CD.110,111 Enthusiasm for natalizumab in the treatment of CD has been curbed due to the increased risk of developing John Cunningham (JC) virus-related progressive multifocal leukoencephalopathy (PML).112 Due to this and also because of the development of vedolizumab, a monoclonal antibody against the α4β7 integrin, natalizumab is no longer a first option for anti-adhesion treatment in IBD.
AJM300 is another drug that targets the α4-subunit of α4β1 and α4β7 integrins, thus blocking binding to VCAM-1 and MAdCAM-1. In contrast to natalizumab, which is administered intravenously, AJM300 is given orally. It has been studied in patients with active UC and was well tolerated (no serious adverse events, including PML); and was better than placebo for induction of clinical response, remission and mucosal healing.113
Targeting α4β7 makes vedolizumab gut-specific. It inhibits the gut homing of lymphocytes by blocking α4β7 binding to MAdCAM-1. Vedolizumab was evaluated in UC and CD in the GEMINI 1 and GEMINI 2 studies, respectively.114,115 Vedolizumab was significantly better for induction and maintenance of remission in UC compared to placebo.114 CD patients receiving vedolizumab were more likely to have a remission, but not a CDAI-100 response, and significantly more patients who had a response to induction therapy and continued to receive vedolizumab were in clinical remission after one year.115 Another study evaluating the effect of vedolizumab found that 21–25% of patients starting vedolizumab for active IBD (21% for CD and 25% for UC) were in clinical remission after 54 weeks.116 Long term efficacy (152 weeks) has been observed for anti-TNF failure and naïve patient with both UC and CD treated with vedolizumab, and patients losing effect with conventional 8-weekly dosing might benefit from increased dosing frequency.117,118 Vedolizumab has an excellent safety profile in both UC and CD: 2830 patients with 4811 person-years of vedolizumab exposure had no cases of PML and no increased risk of infection, serious infection or malignancy.119 Vedolizumab is approved and in clinical use for both UC and CD.
Another antibody against α4β7 integrin – abrilumab (AMG 181/MEDI7183) – has been evaluated in both UC and CD in phase II studies with good safety and promising efficacy profiles.120,121 Anti-MAdCAM-1 therapy targeting the receptor of the α4β7 ligand is theoretically as promising as vedolizumab. The OPERA and TURANDOT studies evaluated the efficacy and safety of PF-00547659, a human monoclonal antibody that binds to MAdCAM-1, in patients with moderate to severe CD and moderate to severe UC, respectively.122,123 In CD, PF-00547659 was not better than placebo, but was safe.122 In UC, the drug was better than placebo for induction of remission after 12 weeks and was safe and well tolerated.123
Etrolizumab, a new agent targeting the β7 subunit of both the α4β7 and αEβ7 integrin, has also been studied. It acts inhibiting both T-cell mucosal recruitment and epithelial retention of intraepithelial lymphocytes through inhibition of α4β7-MAdCAM-1 binding (similar to vedolizumab) and epithelial αEβ7-E-cadherin bindings, respectively. Etrolizumab was more likely to achieve clinical remission at week 10 than placebo in moderately to severely active UC (21% for etrolizumab 100 mg, 10% for etrolizumab 300 mg and loading dose and 0% for placebo). Adverse events were similar in the three groups and no serious opportunistic infections including PML were recorded.124 Several ongoing studies (NCT02136069, NCT02165215, NCT02118584, NCT02403323 and NCT02394028) are evaluating etrolizumab in both UC and CD.125
Sphingosine-1-Phosphate (S1P) pathways
Reducing the circulating lymphocytes by sequestering them in secondary lymphoid organs is an attractive approach to reduce inflammation in the intestinal mucosa. A new class of oral small molecules modulating sphingosine-1-phosphate (S1P) receptor, has recently shown efficacy in IBD.126
Sphingosine derives from the catabolism of endogenous cellular sphingolipids, that are essential constituents of cellular membranes. S1P is the 1-phosphorylated form of sphingosine. S1P can activate a family of five receptors (S1P1–5 receptors) exerting a wide range of immunological functions (reviewed in refs. 127-129).
Of interest, the sphingosine 1-phosphate receptor 1 (S1P1) promotes lymphocyte egress from lymphoid organs to blood. A new generation of oral S1P receptor agonists induce internalization and degradation of the S1P1 receptor. That makes lymphocytes incapable of migrating from secondary lymphoid organs reducing the circulating lymphocytes in the blood and as a consequence, in the intestinal mucosa.130-132
Ozanimod is a new oral selective small-molecule agonist for S1P1 and to a lesser extent for S1P5 that reduces circulating lymphocytes by sequestering them in secondary lymphoid organs that showed efficacy in multiple sclerosis.133 A recent double-blind, placebo-controlled phase II trial examined the safety and efficacy of oral 0.5 and 1 mg of ozanimod daily compared with placebo in active UC (TOUCHSTONE). The primary outcome (clinical remission at 8 weeks) was achieved in 16% and 14% respectively, versus 6% in the placebo arm (P = 0.048 and P = 0.14, respectively).126 Significant differences in clinical response at week 8 were achieved only for the 1 mg group. As expected because of the mechanism of action of the drug, absolute lymphocyte counts in blood decreased after treatment (32% and 49% from baseline in patients who received 0.5 mg and 1 mg, respectively). Mucosal healing – but not histological remission – was achieved in both ozanimod groups.126
Ozanimod showed a good safety profile. No important differences were observed in the most commonly reported adverse events between groups. Of note, 4 patients treated with ozanimod had an increase in the alanine aminotransferase level of more than three times the upper limit.126 Since most patients that received 1 mg had lymphocyte counts below the lower limit of the normal range at week 8, future long-term studies are needed to assess the risk of infections.126 Ozanimod has recently entered two phase III trials for induction and/or maintenance in UC (NCT02435992 and NCT02531126) that are still recruiting (as for January 2018); as well as a phase II multicentre study in moderately to severely active CD (NCT02531113).
Moreover, similar agents such as another S1P1 agonist etrasimod (APD334) have entered phase II trials for UC (NCT02447302 and NCT02536404). Amiselimod (MT-1303), an S1P1/S1P5 agonist, has recently completed two phase II trials for CD (NCT02389790 and NCT02378688). The results are awaited.
Cellular therapy
Mesenchymal stem cells (MSCs)
MSCs are nonhematopoietic multipotent cells that can be isolated from the connective tissues of most organs including the bone marrow (BM), adipose tissue and the umbilical cord. MSCs have self-renewal ability, can differentiate into various cell types and exert several interesting immunomodulatory properties (excellent reviews in refs. 134 and 135).
Since they constitute a heterogeneous group of cells, the International Society for Cellular Therapy proposed 3 criteria to define human MSCs: 1. must be plastic-adherent when maintained in standard culture conditions; 2. must express CD105, CD73 and CD90, and lack expression of CD45, CD34, CD14 or CD11b, CD79α or CD19 and HLA-DR surface molecules; and 3. must differentiate to osteoblasts, adipocytes and chondroblasts in vitro.136
MSCs can inhibit Th1 and Th17 cell proliferation and promote Treg differentiation accompanied by a systemic reduction in pro-inflammatory cytokines (Il-6, IL-17 and IFN-γ) and an elevation of anti-inflammatory cytokines like TGF-β and IL-10.137-142
MSC also have the unique ability to migrate selectively into various sites of tissue injury and inflammation (like the intestinal mucosa), where they actually can be detected several days after intravenous injection in mice.141,143,144 Locally, MSCs promote tissue repair and wound healing through stimulation of angiogenesis and inhibition of apoptosis,145,146 restoring the epithelial barrier integrity147 and secreting potent growth factors like vascular endothelial growth factor (VEGF) and TGF-β1.144
Those interesting features prompted the study of MSCs in mice models of colitis with promising results.137-141 In human studies, MSCs have been administered mainly by two methods: intravenously (i.v.) for the treatment of luminal IBD, or by local injection for perianal fistulizing CD.
A recent meta-analysis suggests that systemic infusion of MSCs is a relatively well tolerated therapy for luminal IBD. A total of 40.5% of patients (95% CI 7.5% – 78.5%) achieved remission after MSCs infusion. However, the studies included had high heterogeneity and risk for bias.148
The first report using allogeneic BM-derived MSCs by systemic infusion in CD was published in abstract form by Onken J et al. in 2006.149 Four out of 10 patients with active luminal CD refractory to steroids showed clinical response (one even achieved clinical remission).149 The same approach using allogeneic BM-derived MSCs obtained from the sternum or the iliac crest and cultured for 5–6 weeks also showed some clinical efficacy. Moreover, an important number of the IBD patients treated were able to taper off steroids after treatment (34 out of 50 IBD patients).150
A subsequent study revealed that 5 out of 7 IBD patients (3 UC and 4 CD) achieved clinical remission at 3 months after the infusion of MSC derived from BM and umbilical cord.151 The use of 4 weekly infusions of BM-derived MSCs was effective in active luminal CD refractory to immunomodulators (clinical response in 12/15 patients, clinical remission in 8/15 and endoscopic improvement in 7/15 at day 42).152 Most of those studies used doses that ranged from 1–8 × 106 MSC/kg.
An alternative approach for the use of MSCs in IBD has been its combination with standard therapy. Knyazev et al. recently reported that the addition of BM-derived MSCs to conventional therapy in UC patients decreased fecal calprotectin and histological indexes at 2, 6 and 12 months.153 The same group reported that the addition of MSCs to infliximab decreased the relapse rate in luminal CD at 3 years.154 However, both studies have been published only in abstract form, complicating further investigations regarding study design, methods and safety issues.
Serious adverse events related to allogenic MSCs are relatively uncommon and injections appear to be safe, as recently confirmed in a meta-analysis.155 Commonly reported non-serious adverse events after infusion are headache, diarrhea, mild transfusion-reactions or dysgeusia, all of them self-limited.152 Of note, the study by Forbes et al. reported an adenocarcinoma arising in a dysplasia associated lesion in one patient. After retrospective chart reviews, the authors suggested the possibility that the cancer was present prior to MSC infusion.152 However, further large controlled trials are needed to address the long-term safety of allogeneic MSCs treatment in IBD.
Only two small studies used injections of autologous MSCs in refractory CD, showing a more modest effect and worse safety profile.156,157 Although clinical response was achieved in both studies, a worsening of the disease was reported in almost half of the patients,156,157 and two serious events possibly related to the treatment were noted (appendicitis and Clostridium difficile colitis).157
Several trials are ongoing in both UC and CD, mostly using allogenic MSCs derived from the BM or the umbilical cord (NCT 02000362, NCT 02150551), both recruiting by January 2018. A phase II study exploring the use of BM-derived MSC in active CD has recently been completed (NCT00294112). Results for this novel therapeutic approach are awaited.
In addition, the use of local injection of MSCs has shown efficacy in the treatment of refractory perianal CD fistulas. The review of these studies is out of the scope of the present work (see recent extensive reviews in refs. 135, 148, 158).
T cell engineering
Tregs are a subset of T lymphocytes that are able to suppress the activation and effector function of multiple immune cells involved in intestinal inflammation and help maintain immune tolerance. Tregs are characterized by the expression of the transcription factor Foxp3 and the production of potent anti-inflammatory cytokines like IL-10 and TGF-β. They are considered to play a major role in the pathogenesis of IBD (reviewed in refs. 18 and 159).
Several studies using mice models resembling IBD support an anti-inflammatory role for Tregs.160,161 In most human studies a decreased number of Tregs in the peripheral blood of IBD patients is observed, while greater numbers accumulate in active inflammatory lesions suggesting an increased migration in active phases.162-164 However, Tregs' suppressive function is not compromised in IBD patients compared to healthy controls.162 Furthermore, some studies showed that effector T cells that accumulated in the intestine of patients are partially resistant to Tregs,165 which might suggest an effect of the intestinal inflammatory milieu in the function of Tregs in IBD.18
Treg cell therapy has already shown efficacy in other inflammatory diseases like graft versus host disease166 and type 1 diabetes.167 The first study testing the efficacy of Tregs in IBD was published by Desreumaux et al. in 2012 in a phase I/IIa study including 20 CD refractory patients (Crohn's And Treg Cells Study [CATS1]).168 Ovalbumin-specific type 1 Tregs (ova-Tregs) were isolated from patients' peripheral blood mononuclear cells, exposed to ovalbumin, and administrated intravenously in a single injection in escalating doses. In order to promote gut migration of ova-Tregs, patients ingested an ovoalbumin enriched diet (a meringue cake). The injections of ova-Tregs were well tolerated and 40% of the patients had a clinical response at weeks 5 and 8 (CDAI reduction of 100 points), but only 10% of patients achieved clinical remission (CDAI ≤150) and the clinical effect after a single dose was transient.168 In addition, no information about the numbers of ova-Tregs that reached the intestinal mucosa or their stability and plasticity features was provided in this study. The results from a phase IIb multicenter placebo-controlled clinical trial with ova-Tregs in refractory CD (CATS29) are expected during 2018 (NCT02327221).
A recent study aimed to define the optimal population for Treg cell therapy comparing CD4+CD25+CD127loCD45RA+ and CD4+CD25+CD127loCD45RA−Treg subsets. Tregs were isolated from CD patients' blood, expanded in vitro and tested in a xenotransplant model of human intestine. The study showed that CD45RA+ Tregs do not convert to a Th17 phenotype in vitro, express gut homing molecules (like α4β7integrin) and suppress activation of lymphocytes isolated from inflamed mucosa of CD patients. Thus, the authors propose CD4+CD25+CD127loCD45RA+ as the most appropriate from which to expand Tregs for T cell therapy in future studies.169
Some authors have pointed the possibility of manipulating γδ T cells to treat IBD. γδ T cells are unconventional T cells with interesting immunoregulatory and tissue healing properties recently implicated in CD pathogenesis.20 γδ T cells have shown a protecting function against colitis in several murine models170,171 and appeared safe and effective in clinical trials in cancer immunotherapy.172 However, no clinical studies to treat IBD have been published to this date.
Thus, although cellular therapies are emerging as safe and effective therapies, many unresolved questions like type of cells to use, adequate doses and long-term effects need to be addressed in larger clinical trials.
Other immune-regulating therapies
Finally, other therapeutic approaches have shown immune-modulating and anti-inflammatory properties in IBD. Promising candidates that showed efficacy in clinical trials are: fecal microbiota transplantation173,174; antibiotics with immune-regulating properties like metronidazole or ciprofloxacin175-178; modulation of mucosal immunity by helminths179,180; dietary induction of Tregs by short fatty acids or prebiotics181-183; substitution of phosphatidylcholine to increase the mucus layer184; or certain herbs and plants with immune-regulating characteristics like: curcumin, artemisia absinthium, myrrh, chamomile or wheatgrass.185
Concluding remarks and future perspectives
IBD is a chronic disabling inflammatory process that affects young individuals, with growing incidence. The etiopathogenesis of IBD remains poorly understood, but recent studies show that improved understanding of the immunological mechanisms involved in IBD pathogenesis is key to the development of new therapeutic options.
Current pharmacological treatments used in clinical practice like thiopurines or anti-TNF are effective. However, some of these drugs have significant side effects like infections or an increased risk for certain cancers and their efficacy may diminish over time. In fact, up to one third of the patients do not have a satisfactory response to these therapies.
Consequently, the search for new therapeutic strategies targeting alternative immunological pathways has intensified. New therapies targeting alternative pro-inflammatory pathways like IL-12/23 axis, IL-6 pathway or Janus Kinase inhibitors are on its way. Alternatively, some emerging oral substances that aim to stimulate canonical immune-modulating pathways, like the TGF-β pathway, have shown clinical efficacy. The inhibition of adhesion and migration of leukocytes into the inflamed intestinal mucosa has also received much attention. Molecules like vedolizumab are currently approved for IBD and other approaches targeting alternative adhesion or migration mechanisms are in advanced phases in clinical trials. Finally, the possibility of engineering immune-modulating cells like MSCs or Tregs is a promising alternative approach, but cell therapies still need to prove safety and efficacy in larger clinical trials.
In conclusion, several novel treatment strategies for IBD are on their way and will certainly expand our therapeutic armamentarium in the next future. However, IBD is a very heterogeneous disorder where patients have different genetic and environmental backgrounds and can display a wide variety of clinical phenotypes. In addition, IBD treatment is still based basically on clinical and endoscopical findings and patients may have unpredictable responses to different therapies. This makes it difficult for the clinician to choose the appropiate drug according to risk factors and clinical course.
A better understanding of the immunopathogenesis of IBD is crucial to help the clinician to select the most appropiate therapeutic approach to maximize cost-efficacy and minimize risks and undesirable side-effects derived from the immune-regulation (like infections or the inhibition of other protective properties like epithelial healing). Furthermore, a combination of some of these novel drugs with the ones currently in use could be a plausible startegy to improve therapeutic outcomes by targeting different pathways.
Thus, it will be crucial to include an examination of immune responses before and after therapy and integrate these data with other genetic, serologic and mucosal variables to tailor our therapeutic decisions towards a real personalized medicine in IBD. We are in the opening of a new era in the treatment of IBD and immunotherapy is definitely going to play a major role in the next future.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgements
We would like to thank Dr. Miriam Giambelluca for her contribution and technical suggestions in the elaboration of the figure in this article; and Dr. Arne Kristian Sandvik for reviewing this manuscript.
References
- 1.Abraham C, Cho JH. Inflammatory Bowel Disease. N Engl J Med. 2009;361:2066–78. doi: 10.1056/NEJMra0804647. PMID:19923578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ordas I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606–19. doi: 10.1016/S0140-6736(12)60150-0. PMID:22914296. [DOI] [PubMed] [Google Scholar]
- 3.Baumgart DC, Sandborn WJ. Crohn's disease. Lancet. 2012;380:1590–605. doi: 10.1016/S0140-6736(12)60026-9. PMID:22914295. [DOI] [PubMed] [Google Scholar]
- 4.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, et al.. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42. doi: 10.1053/j.gastro.2011.10.001. PMID:22001864. [DOI] [PubMed] [Google Scholar]
- 5.Rocchi A, Benchimol EI, Bernstein CN, Bitton A, Feagan B, Panaccione R. Inflammatory bowel disease: a Canadian burden of illness review. Can J Gastroenterol. 2012;26(11):811–817. doi: 10.1155/2012/984575. PMID:23166905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burisch J, Jess T, Martinato M, Lakatos PL. The burden of inflammatory bowel disease in Europe. Journal of Crohn's and colitis. 2013;7:322. doi: 10.1016/j.crohns.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 7.de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13–27. doi: 10.1038/nrgastro.2015.186. PMID:26627550. [DOI] [PubMed] [Google Scholar]
- 8.de Souza HSP, Fiocchi C, Iliopoulos D. The IBD interactome: an integrated view of aetiology, pathogenesis and therapy. Nat Rev Gastroenterol Hepatol. 2017;14:739–49. doi: 10.1038/nrgastro.2017.110. PMID:28831186. [DOI] [PubMed] [Google Scholar]
- 9.Hugot J-P, Chamaillard M, Zouali H, Lesage S, Cezard J-P, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, et al.. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. PMID:11385576. [DOI] [PubMed] [Google Scholar]
- 10.Hampe J, Franke A, Rosenstiel P, Till A, Teuber M, Huse K, Albrecht M, Mayr G, De La Vega FM, Briggs J, et al.. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007;39:207–11. doi: 10.1038/ng1954. PMID:17200669. [DOI] [PubMed] [Google Scholar]
- 11.Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. PMID:16822808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrett JC, Lee JC, Lees CW, Prescott NJ, Anderson CA, Phillips A, Wesley E, Parnell K, Zhang H, Drummond H, et al.. Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat Genet. 2009;41:1330–4. doi: 10.1038/ng.483. PMID:19915572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buisine MP, Desreumaux P, Debailleul V, Gambiez L, Geboes K, Ectors N, Delescaut MP, Degand P, Aubert JP, Colombel JF, et al.. Abnormalities in mucin gene expression in Crohn's disease. Inflamm Bowel Dis 1999;5:24–32. doi: 10.1097/00054725-199902000-00004. PMID:10028446. [DOI] [PubMed] [Google Scholar]
- 14.Braun A, Treede I, Gotthardt D, Tietje A, Zahn A, Ruhwald R, Schoenfeld U, Welsch T, Kienle P, Erben G, et al.. Alterations of phospholipid concentration and species composition of the intestinal mucus barrier in ulcerative colitis: a clue to pathogenesis. Inflamm Bowel Dis. 2009;15:1705–20. doi: 10.1002/ibd.20993. PMID:19504612. [DOI] [PubMed] [Google Scholar]
- 15.Wehkamp J, Salzman NH, Porter E, Nuding S, Weichenthal M, Petras RE, Shen B, Schaeffeler E, Schwab M, Linzmeier R, et al.. Reduced Paneth cell α-defensins in ileal Crohn's disease. Proc Natl Acad Sci U S A. 2005;102:18129–34. doi: 10.1073/pnas.0505256102. PMID:16330776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH, et al.. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–56. doi: 10.1016/j.cell.2008.07.021. PMID:18775308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–42. doi: 10.1038/nri3661. PMID:24751956. [DOI] [PubMed] [Google Scholar]
- 18.Mayne CG, Williams CB. Induced and natural regulatory T cells in the development of inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1772–88. doi: 10.1097/MIB.0b013e318281f5a3. PMID:23656897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters CP, Mjosberg JM, Bernink JH, Spits H. Innate lymphoid cells in inflammatory bowel diseases. Immunology letters. 2016;172:124–31. doi: 10.1016/j.imlet.2015.10.004. PMID:26470815. [DOI] [PubMed] [Google Scholar]
- 20.Catalan-Serra I, Sandvik AK, Bruland T, Andreu-Ballester JC. Gammadelta T Cells in Crohn's Disease: A New Player in the Disease Pathogenesis? Journal of Crohn's & colitis. 2017;11:1135–45. [DOI] [PubMed] [Google Scholar]
- 21.Danese S, Panes J. Development of drugs to target interactions between leukocytes and endothelial cells and treatment algorithms for inflammatory bowel diseases. Gastroenterology. 2014;147:981–9. doi: 10.1053/j.gastro.2014.08.044. PMID:25220794. [DOI] [PubMed] [Google Scholar]
- 22.Gomollon F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, Peyrin-Biroulet L, Cullen GJ, Daperno M, Kucharzik T, et al.. 3rd european evidence-based consensus on the diagnosis and management of crohn's disease 2016: part 1: diagnosis and medical management. Journal of Crohn's & colitis. 2017;11:3–25. doi: 10.1093/ecco-jcc/jjw168. [DOI] [PubMed] [Google Scholar]
- 23.Harbord M, Eliakim R, Bettenworth D, Karmiris K, Katsanos K, Kopylov U, Kucharzik T, Molnar T, Raine T, Sebastian S, et al.. third european evidence-based consensus on diagnosis and management of ulcerative colitis. part 2: Current management. Journal of Crohn's & colitis. 2017;11:769–84. doi: 10.1093/ecco-jcc/jjx009.. [DOI] [PubMed] [Google Scholar]
- 24.Cohen BL, Sachar DB. Update on anti-tumor necrosis factor agents and other new drugs for inflammatory bowel disease. BMJ (Clinical research ed). 2017;357:j2505. doi: 10.1136/bmj.j2505. PMID:28630047. [DOI] [PubMed] [Google Scholar]
- 25.Ding NS, Hart A, De Cruz P. Systematic review: predicting and optimising response to anti-TNF therapy in Crohn's disease – algorithm for practical management. Aliment Pharmacol Ther. 2016;43:30–51. doi: 10.1111/apt.13445. PMID:26515897. [DOI] [PubMed] [Google Scholar]
- 26.Dulai PS, Thompson KD, Blunt HB, Dubinsky MC, Siegel CA. Risks of serious infection or lymphoma with anti-tumor necrosis factor therapy for pediatric inflammatory bowel disease: a systematic review. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12:1443–51; quiz e88-9. doi: 10.1016/j.cgh.2014.01.021. PMID:24462626. [DOI] [PubMed] [Google Scholar]
- 27.Dulai PS, Siegel CA. The risk of malignancy associated with the use of biological agents in patients with inflammatory bowel disease. Gastroenterol Clin North Am. 2014;43:525–41. doi: 10.1016/j.gtc.2014.05.010. PMID:25110257. [DOI] [PubMed] [Google Scholar]
- 28.Frolkis AD, Dykeman J, Negrón ME, deBruyn J, Jette N, Fiest KM, Frolkis T, Barkema HW, Rioux KP, Panaccione R, et al.. Risk of Surgery for Inflammatory Bowel Diseases Has Decreased Over Time: A Systematic Review and Meta-analysis of Population-Based Studies. Gastroenterology. 2013;145:996–1006. doi: 10.1053/j.gastro.2013.07.041. PMID:23896172. [DOI] [PubMed] [Google Scholar]
- 29.Gionchetti P, Dignass A, Danese S, Magro Dias FJ, Rogler G, Lakatos PL, Adamina M, Ardizzone S, Buskens CJ, Sebastian S, et al.. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 2: Surgical Management and Special Situations. Journal of Crohn's & colitis. 2017;11:135–49. doi: 10.1093/ecco-jcc/jjw169.. [DOI] [PubMed] [Google Scholar]
- 30.Trinchieri G, Pflanz S, Kastelein RA. The IL-12 Family of Heterodimeric Cytokines: New Players in the Regulation of T Cell Responses. Immunity. 2003;19:641–4. doi: 10.1016/S1074-7613(03)00296-6. PMID:14614851. [DOI] [PubMed] [Google Scholar]
- 31.McGovern D, Powrie F. The IL23 axis plays a key role in the pathogenesis of IBD. Gut. 2007;56:1333–6. doi: 10.1136/gut.2006.115402. PMID:17872562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abraham C, Dulai PS, Vermeire S, Sandborn WJ. Lessons Learned From Trials Targeting Cytokine Pathways in Patients With Inflammatory Bowel Diseases. Gastroenterology. 2017;152:374–88.e4. doi: 10.1053/j.gastro.2016.10.018. PMID:27780712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vignali DAA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. 2012;13:722. doi: 10.1038/ni.2366. PMID:22814351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yadav V, Varum F, Bravo R, Furrer E, Bojic D, Basit AW. Inflammatory bowel disease: exploring gut pathophysiology for novel therapeutic targets. Translational research : the journal of laboratory and clinical medicine. 2016;176:38–68. doi: 10.1016/j.trsl.2016.04.009. PMID:27220087. [DOI] [PubMed] [Google Scholar]
- 35.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, et al.. A Genome-Wide Association Study Identifies IL23R as an Inflammatory Bowel Disease Gene. Science. 2006;314:1461–3. doi: 10.1126/science.1135245. PMID:17068223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silverberg MS, Cho JH, Rioux JD, McGovern DP, Wu J, Annese V, Achkar JP, Goyette P, Scott R, Xu W, et al.. Ulcerative colitis-risk loci on chromosomes 1p36 and 12q15 found by genome-wide association study. Nat Genet. 2009;41:216–20. doi: 10.1038/ng.275. PMID:19122664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pidasheva S, Trifari S, Phillips A, Hackney JA, Ma Y, Smith A, Sohn SJ, Spits H, Little RD, Behrens TW, et al.. Functional Studies on the IBD Susceptibility Gene IL23R Implicate Reduced Receptor Function in the Protective Genetic Variant R381Q. PLOS ONE. 2011;6:e25038. doi: 10.1371/journal.pone.0025038. PMID:22022372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, Wehkamp J, Feagan BG, Yao MD, Karczewski M, et al.. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693–700. doi: 10.1136/gutjnl-2011-301668. PMID:22595313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Targan SR, Feagan B, Vermeire S, Panaccione R, Melmed GY, Landers C, Li D, Russell C, Newmark R, Zhang N, et al.. A Randomized, Double-Blind, Placebo-Controlled Phase 2 Study of Brodalumab in Patients With Moderate-to-Severe Crohn's Disease. Am J Gastroenterol. 2016;111:1599–607. doi: 10.1038/ajg.2016.298. PMID:27481309. [DOI] [PubMed] [Google Scholar]
- 40.Lee Jacob S, Tato Cristina M, Joyce-Shaikh B, Gulen Muhammet F, Cayatte C, Chen Y, Blumenschein Wendy M, Judo M, Ayanoglu G, McClanahan Terrill K, et al.. Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability. Immunity. 2015;43:727–38. doi: 10.1016/j.immuni.2015.09.003. PMID:26431948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maloy KJ, Kullberg MC. IL-23 and Th17 cytokines in intestinal homeostasis. Mucosal Immunol. 2008;1:339. doi: 10.1038/mi.2008.28. PMID:19079198. [DOI] [PubMed] [Google Scholar]
- 42.Aden K, Rehman A, Falk-Paulsen M, Secher T, Kuiper J, Tran F, Pfeuffer S, Sheibani-Tezerji R, Breuer A, Luzius A, et al.. Epithelial IL-23R Signaling Licenses Protective IL-22 Responses in Intestinal Inflammation. Cell Rep. 2016;16:2208–18. doi: 10.1016/j.celrep.2016.07.054. PMID:27524624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macho-Fernandez E, Koroleva EP, Spencer CM, Tighe M, Torrado E, Cooper AM, Fu YX, Tumanov AV. Lymphotoxin beta receptor signaling limits mucosal damage through driving IL-23 production by epithelial cells. Mucosal Immunol. 2015;8:403–13. doi: 10.1038/mi.2014.78. PMID:25183367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cox JH, Kljavin NM, Ota N, Leonard J, Roose-Girma M, Diehl L, Ouyang W, Ghilardi N. Opposing consequences of IL-23 signaling mediated by innate and adaptive cells in chemically induced colitis in mice. Mucosal Immunol. 2012;5:99–109. doi: 10.1038/mi.2011.54. PMID:22089030. [DOI] [PubMed] [Google Scholar]
- 45.Eken A, Singh AK, Treuting PM, Oukka M. IL-23R+ innate lymphoid cells induce colitis via interleukin-22-dependent mechanism. Mucosal Immunol. 2014;7:143–54. doi: 10.1038/mi.2013.33. PMID:23715173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandborn WJ, Gasink C, Gao L-L, Blank MA, Johanns J, Guzzo C, Sands BE, Hanauer SB, Targan S, Rutgeerts P, et al.. Ustekinumab Induction and Maintenance Therapy in Refractory Crohn's Disease. N Engl J Med. 2012;367:1519–28. doi: 10.1056/NEJMoa1203572. PMID:23075178. [DOI] [PubMed] [Google Scholar]
- 47.Mannon PJ, Fuss IJ, Mayer L, Elson CO, Sandborn WJ, Present D, Dolin B, Goodman N, Groden C, Hornung RL, et al.. Anti–Interleukin-12 Antibody for Active Crohn's Disease. N Engl J Med. 2004;351:2069–79. doi: 10.1056/NEJMoa033402. PMID:15537905. [DOI] [PubMed] [Google Scholar]
- 48.Panaccione R, Sandborn WJ, Gordon GL, Lee SD, Safdi A, Sedghi S, Feagan BG, Hanauer S, Reinisch W, Valentine JF, et al.. Briakinumab for treatment of Crohn's disease: results of a randomized trial. Inflamm Bowel Dis. 2015;21:1329–40. PMID:25989338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacDonald JK, Nguyen TM, Khanna R, Timmer A. Anti-IL-12/23p40 antibodies for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2016;11:Cd007572. PMID:27885650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker KF, Isaacs JD. Novel therapies for immune-mediated inflammatory diseases: What can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn's disease and ulcerative colitis? Ann Rheum Dis. 2018;77:175–87. doi: 10.1136/annrheumdis-2017-211555. PMID:28765121. [DOI] [PubMed] [Google Scholar]
- 51.Ryan C, Leonardi CL, Krueger JG, Kimball AB, Strober BE, Gordon KB, Langley RG, de Lemos JA, Daoud Y, Blankenship D, et al.. Association between biologic therapies for chronic plaque psoriasis and cardiovascular events: a meta-analysis of randomized controlled trials. Jama. 2011;306:864–71. PMID:21862748. [DOI] [PubMed] [Google Scholar]
- 52.Tzellos T, Kyrgidis A, Trigoni A, Zouboulis CC. Association of ustekinumab and briakinumab with major adverse cardiovascular events: An appraisal of meta-analyses and industry sponsored pooled analyses to date. Dermato-endocrinology. 2012;4:320–3. doi: 10.4161/derm.23100. PMID:23467502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sands BE, Chen J, Feagan BG, Penney M, Rees WA, Danese S, Higgins PDR, Newbold P, Faggioni R, Patra K, et al.. Efficacy and Safety of MEDI2070, an Antibody Against Interleukin 23, in Patients With Moderate to Severe Crohn's Disease: A Phase 2a Study. Gastroenterology. 2017;153:77–86.e6. doi: 10.1053/j.gastro.2017.03.049. PMID:28390867. [DOI] [PubMed] [Google Scholar]
- 54.Feagan BG, Sandborn W, Panés J, Ferrante M, Louis E, D'Haens GR, Franchimont D, Kaser A, Dewit O, Seidler U, et al.. 812a Efficacy and Safety of Induction Therapy With the Selective IL-23 Inhibitor BI 655066, in Patients With Moderate-to-Severe Crohn's Disease: Results of a Randomized, Double-Blind, Placebo-Controlled Phase II Study. Gastroenterology. 2016;150:S1266. doi: 10.1016/S0016-5085(16)34278-0.. [DOI] [Google Scholar]
- 55.Coskun M, Vermeire S, Nielsen OH. Novel Targeted Therapies for Inflammatory Bowel Disease. Trends in pharmacological sciences. 2017;38:127–42. doi: 10.1016/j.tips.2016.10.014. PMID:27916280. [DOI] [PubMed] [Google Scholar]
- 56.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–57. doi: 10.1038/ni.3153. PMID:25898198. [DOI] [PubMed] [Google Scholar]
- 57.Tanaka T, Narazaki M, Ogata A, Kishimoto T. A new era for the treatment of inflammatory autoimmune diseases by interleukin-6 blockade strategy. Semin Immunol. 2014;26:88–96. doi: 10.1016/j.smim.2014.01.009. PMID:24594001. [DOI] [PubMed] [Google Scholar]
- 58.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–36. doi: 10.1042/bj2650621. PMID:1689567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Calabrese LH, Rose-John S. IL-6 biology: implications for clinical targeting in rheumatic disease. Nat Rev Rheumatol. 2014;10:720. doi: 10.1038/nrrheum.2014.127. PMID:25136784. [DOI] [PubMed] [Google Scholar]
- 60.Gross V, Andus T, Caesar I, Roth M, Scholmerich J. Evidence for continuous stimulation of interleukin-6 production in Crohn's disease. Gastroenterology 1992;102:514–9. doi: 10.1016/0016-5085(92)90098-J. PMID:1370661. [DOI] [PubMed] [Google Scholar]
- 61.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. PMID:19132915. [DOI] [PubMed] [Google Scholar]
- 62.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. PMID:16648838. [DOI] [PubMed] [Google Scholar]
- 63.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–5. doi: 10.1002/eji.201040391. PMID:20583029. [DOI] [PubMed] [Google Scholar]
- 64.Wolf J, Rose-John S, Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine. 2014;70:11–20. doi: 10.1016/j.cyto.2014.05.024. PMID:24986424. [DOI] [PubMed] [Google Scholar]
- 65.Yamamoto M, Yoshizaki K, Kishimoto T, Ito H. IL-6 is required for the development of Th1 cell-mediated murine colitis. Journal of immunology (Baltimore, Md : 1950). 2000;164:4878–82. doi: 10.4049/jimmunol.164.9.4878. PMID:10779797. [DOI] [PubMed] [Google Scholar]
- 66.Hosokawa T, Kusugami K, Ina K, Ando T, Shinoda M, Imada A, Ohsuga M, Sakai T, Matsuura T, Ito K, et al.. Interleukin-6 and soluble interleukin-6 receptor in the colonic mucosa of inflammatory bowel disease. J Gastroenterol Hepatol. 1999;14:987–96. doi: 10.1046/j.1440-1746.1999.01989.x. PMID:10530495. [DOI] [PubMed] [Google Scholar]
- 67.Louis E, Belaiche J, van Kemseke C, Franchimont D, de Groote D, Gueenen V, Mary JY. A high serum concentration of interleukin-6 is predictive of relapse in quiescent Crohn's disease. Eur J Gastroenterol Hepatol. 1997;9:939–44. doi: 10.1097/00042737-199710000-00004.. [DOI] [PubMed] [Google Scholar]
- 68.Ito H, Takazoe M, Fukuda Y, Hibi T, Kusugami K, Andoh A, Matsumoto T, Yamamura T, Azuma J, Nishimoto N, et al.. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn's disease. Gastroenterology. 2004;126:989–96;discussion 47. doi: 10.1053/j.gastro.2004.01.012. PMID:15057738. [DOI] [PubMed] [Google Scholar]
- 69.Danese S, Vermeire S, Hellstern P, Panaccione R, Rogler G, Fraser G, Kohn A, Desreumaux P, Leong RW, Comer GM, et al.. Randomised trial and open-label extension study of an anti-interleukin-6 antibody in Crohn's disease (ANDANTE I and II). Gut. 2017;1–9. doi: 10.1136/gutjnl-2017-314562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuhn KA, Manieri NA, Liu T-C, Stappenbeck TS. IL-6 Stimulates Intestinal Epithelial Proliferation and Repair after Injury. PLOS ONE. 2014;9:e114195. doi: 10.1371/journal.pone.0114195. PMID:25478789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, Gruben D, Wallenstein GV, Zwillich SH, Kanik KS. Placebo-Controlled Trial of Tofacitinib Monotherapy in Rheumatoid Arthritis. N Engl J Med. 2012;367:495–507. doi: 10.1056/NEJMoa1109071. PMID:22873530. [DOI] [PubMed] [Google Scholar]
- 72.Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, Estrov Z, Fridman JS, Bradley EC, Erickson-Viitanen S, et al.. Safety and Efficacy of INCB018424, a JAK1 and JAK2 Inhibitor, in Myelofibrosis. N Engl J Med. 2010;363:1117–27. doi: 10.1056/NEJMoa1002028. PMID:20843246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, Niezychowski W. Tofacitinib, an Oral Janus Kinase Inhibitor, in Active Ulcerative Colitis. N Engl J Med. 2012;367:616–24. doi: 10.1056/NEJMoa1112168. PMID:22894574. [DOI] [PubMed] [Google Scholar]
- 74.Sandborn WJ, Su C, Sands BE, D'Haens GR, Vermeire S, Schreiber S, Danese S, Feagan BG, Reinisch W, Niezychowski W, et al.. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2017;376:1723–36. doi: 10.1056/NEJMoa1606910. PMID:28467869. [DOI] [PubMed] [Google Scholar]
- 75.Coskun M, Salem M, Pedersen J, Nielsen OH. Involvement of JAK/STAT signaling in the pathogenesis of inflammatory bowel disease. Pharmacol Res. 2013;76:1–8. doi: 10.1016/j.phrs.2013.06.007. PMID:23827161. [DOI] [PubMed] [Google Scholar]
- 76.Ghoreschi K, Laurence A, O'Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273–87. doi: 10.1111/j.1600-065X.2008.00754.x. PMID:19290934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O'Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36:542–50. doi: 10.1016/j.immuni.2012.03.014. PMID:22520847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sandborn WJ, Ghosh S, Panes J, Vranic I, Wang W, Niezychowski W. A phase 2 study of tofacitinib, an oral Janus kinase inhibitor, in patients with Crohn's disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12:1485–93.e2. doi: 10.1016/j.cgh.2014.01.029. PMID:24480677. [DOI] [PubMed] [Google Scholar]
- 79.Panés J, Sandborn WJ, Schreiber S, Sands BE, Vermeire S, D'Haens G, Panaccione R, Higgins PDR, Colombel J-F, Feagan BG, et al.. Tofacitinib for induction and maintenance therapy of Crohn's disease: results of two phase IIb randomised placebo-controlled trials. Gut. 2017;66(6):1049–1059. doi: 10.1136/gutjnl-2016-312735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vermeire S, Schreiber S, Petryka R, Kuehbacher T, Hebuterne X, Roblin X, Klopocka M, Goldis A, Wisniewska-Jarosinska M, Baranovsky A, et al.. Clinical remission in patients with moderate-to-severe Crohn's disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet. 2017;389:266–75. doi: 10.1016/S0140-6736(16)32537-5. PMID:27988142. [DOI] [PubMed] [Google Scholar]
- 81.Comi G, Jeffery D, Kappos L, Montalban X, Boyko A, Rocca MA, Filippi M. Placebo-Controlled Trial of Oral Laquinimod for Multiple Sclerosis. N Engl J Med. 2012;366:1000–9. doi: 10.1056/NEJMoa1104318. PMID:22417253. [DOI] [PubMed] [Google Scholar]
- 82.Vollmer TL, Sorensen PS, Selmaj K, Zipp F, Havrdova E, Cohen JA, Sasson N, Gilgun-Sherki Y, Arnold DL. A randomized placebo-controlled phase III trial of oral laquinimod for multiple sclerosis. J Neurol. 2014;261:773–83. doi: 10.1007/s00415-014-7264-4. PMID:24535134. [DOI] [PubMed] [Google Scholar]
- 83.Jayne D, Appel G, Chan TM, Barkay H, Weiss R, Wofsy D. LB0003 A Randomized Controlled Study of Laquinimod in Active Lupus Nephritis Patients in Combination with Standard of Care. Ann Rheum Dis. 2013;72:A164–A. doi: 10.1136/annrheumdis-2013-eular.528.. [DOI] [Google Scholar]
- 84.Bruck W, Wegner C. Insight into the mechanism of laquinimod action. J Neurol Sci. 2011;306:173–9. doi: 10.1016/j.jns.2011.02.019. PMID:21429524. [DOI] [PubMed] [Google Scholar]
- 85.Thöne J, Linker RA. Laquinimod in the treatment of multiple sclerosis: a review of the data so far. Drug Design, Development and Therapy. 2016;10:1111–8. doi: 10.2147/DDDT.S55308. PMID:27042003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.D'Haens G, Sandborn WJ, Colombel JF, Rutgeerts P, Brown K, Barkay H, Sakov A, Haviv A, Feagan BG. Laquinimod for Crohn's Disease I. A phase II study of laquinimod in Crohn's disease. Gut. 2015;64:1227–35. doi: 10.1136/gutjnl-2014-307118. PMID:25281416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nat Rev Immunol. 2002;2:46–53. doi: 10.1038/nri704. PMID:11905837. [DOI] [PubMed] [Google Scholar]
- 88.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–61. doi: 10.1146/annurev.immunol.16.1.137. PMID:9597127. [DOI] [PubMed] [Google Scholar]
- 89.Sedda S, Marafini I, Dinallo V, Di Fusco D, Monteleone G. The TGF-beta/Smad System in IBD Pathogenesis. Inflamm Bowel Dis. 2015;21:2921–5. doi: 10.1097/MIB.0000000000000542. PMID:26230862. [DOI] [PubMed] [Google Scholar]
- 90.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–71. doi: 10.1038/37284. PMID:9393997. [DOI] [PubMed] [Google Scholar]
- 91.Derynck R, Zhang Y, Feng XH. Smads: transcriptional activators of TGF-beta responses. Cell 1998;95:737–40. doi: 10.1016/S0092-8674(00)81696-7. PMID:9865691. [DOI] [PubMed] [Google Scholar]
- 92.Monteleone G, Del Vecchio Blanco G, Monteleone I, Fina D, Caruso R, Gioia V, Ballerini S, Federici G, Bernardini S, Pallone F, et al.. Post-transcriptional regulation of Smad7 in the gut of patients with inflammatory bowel disease. Gastroenterology. 2005;129:1420–9. doi: 10.1053/j.gastro.2005.09.005. PMID:16285943. [DOI] [PubMed] [Google Scholar]
- 93.Monteleone G, Kumberova A, Croft NM, McKenzie C, Steer HW, MacDonald TT. Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease. J Clin Invest. 2001;108:601–9. doi: 10.1172/JCI12821. PMID:11518734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Monteleone G, Neurath MF, Ardizzone S, Di Sabatino A, Fantini MC, Castiglione F, Scribano ML, Armuzzi A, Caprioli F, Sturniolo GC, et al.. Mongersen, an Oral SMAD7 Antisense Oligonucleotide, and Crohn's Disease. N Engl J Med. 2015;372:1104–13. doi: 10.1056/NEJMoa1407250. PMID:25785968. [DOI] [PubMed] [Google Scholar]
- 95.Monteleone G, Fantini MC, Onali S, Zorzi F, Sancesario G, Bernardini S, Calabrese E, Viti F, Monteleone I, Biancone L, et al.. Phase I clinical trial of Smad7 knockdown using antisense oligonucleotide in patients with active Crohn's disease. Molecular therapy : the journal of the American Society of Gene Therapy. 2012;20:870–6. doi: 10.1038/mt.2011.290. PMID:22252452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Feagan B, Sands B, Rossiter G, Li X, Usiskin K, Zhan X, Colombel JF. PD-008 A Study of Oral Mongersen (GED-0301) on Endoscopy and Clinical Activity (Stool Frequency and Abdominal Pain) in Crohn's Disease. Inflamm Bowel Dis. 2017;23:S8–S. [Google Scholar]
- 97.Li C, Kuemmerle JF. Mechanisms that mediate the development of fibrosis in patients with Crohn's disease. Inflamm Bowel Dis. 2014;20:1250–8. doi: 10.1097/MIB.0000000000000043. PMID:24831560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zorzi F, Calabrese E, Monteleone I, Fantini M, Onali S, Biancone L, Pallone F, Monteleone G. A phase 1 open-label trial shows that smad7 antisense oligonucleotide (GED0301) does not increase the risk of small bowel strictures in Crohn's disease. Aliment Pharmacol Ther. 2012;36:850–7. doi: 10.1111/apt.12051. PMID:22971085. [DOI] [PubMed] [Google Scholar]
- 99.Lobaton T, Vermeire S, Van Assche G, Rutgeerts P. Review article: anti-adhesion therapies for inflammatory bowel disease. Aliment Pharmacol Ther. 2014;39:579–94. doi: 10.1111/apt.12639. PMID:24479980. [DOI] [PubMed] [Google Scholar]
- 100.Jones SC, Banks RE, Haidar A, Gearing AJ, Hemingway IK, Ibbotson SH, Dixon MF, Axon AT. Adhesion molecules in inflammatory bowel disease. Gut 1995;36:724–30. doi: 10.1136/gut.36.5.724. PMID:7541009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Souza HS, Elia CCS, Spencer J, MacDonald TT. Expression of lymphocyte-endothelial receptor-ligand pairs, α4β7/MAdCAM-1 and OX40/OX40 ligand in the colon and jejunum of patients with inflammatory bowel disease. Gut. 1999;45:856–63. doi: 10.1136/gut.45.6.856. PMID:10562584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oshima T, Jordan P, Grisham MB, Alexander JS, Jennings M, Sasaki M, Manas K. TNF-alpha induced endothelial MAdCAM-1 expression is regulated by exogenous, not endogenous nitric oxide. BMC Gastroenterol. 2001;1:5. doi: 10.1186/1471-230X-1-5. PMID:11481030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yacyshyn BR, Chey WY, Goff J, Salzberg B, Baerg R, Buchman AL, Tami J, Yu R, Gibiansky E, Shanahan WR. Double blind, placebo controlled trial of the remission inducing and steroid sparing properties of an ICAM-1 antisense oligodeoxynucleotide, alicaforsen (ISIS 2302), in active steroid dependent Crohn's disease. Gut. 2002;51:30–6. doi: 10.1136/gut.51.1.30. PMID:12077088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vegter S, Tolley K, Wilson Waterworth T, Jones H, Jones S, Jewell D. Meta-analysis using individual patient data: efficacy and durability of topical alicaforsen for the treatment of active ulcerative colitis. Aliment Pharmacol Ther. 2013;38:284–93. doi: 10.1111/apt.12369.. [DOI] [PubMed] [Google Scholar]
- 105.Jairath V, Khanna R, Feagan BG. Alicaforsen for the treatment of inflammatory bowel disease. Expert opinion on investigational drugs. 2017;26:991–7. doi: 10.1080/13543784.2017.1349753. PMID:28670932. [DOI] [PubMed] [Google Scholar]
- 106.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, von Andrian UH. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. PMID:12840763. [DOI] [PubMed] [Google Scholar]
- 107.Briskin M, Winsor-Hines D, Shyjan A, Cochran N, Bloom S, Wilson J, McEvoy LM, Butcher EC, Kassam N, Mackay CR, et al.. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol 1997;151:97–110. PMID:9212736. [PMC free article] [PubMed] [Google Scholar]
- 108.Ghosh S, Panaccione R. Anti-adhesion molecule therapy for inflammatory bowel disease. Ther Adv Gastroenterol. 2010;3:239–58. doi: 10.1177/1756283X10373176. PMID:21180606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Miller DH, Khan OA, Sheremata WA, Blumhardt LD, Rice GPA, Libonati MA, Willmer-Hulme AJ, Dalton CM, Miszkiel KA, O'Connor PW. A Controlled Trial of Natalizumab for Relapsing Multiple Sclerosis. N Engl J Med. 2003;348:15–23. doi: 10.1056/NEJMoa020696. PMID:12510038. [DOI] [PubMed] [Google Scholar]
- 110.Sandborn WJ, Colombel JF, Enns R, Feagan BG, Hanauer SB, Lawrance IC, Panaccione R, Sanders M, Schreiber S, Targan S, et al.. Natalizumab Induction and Maintenance Therapy for Crohn's Disease. N Engl J Med. 2005;353:1912–25. doi: 10.1056/NEJMoa043335. PMID:16267322. [DOI] [PubMed] [Google Scholar]
- 111.Targan SR, Feagan BG, Fedorak RN, Lashner BA, Panaccione R, Present DH, Spehlmann ME, Rutgeerts PJ, Tulassay Z, Volfova M, et al.. Natalizumab for the treatment of active Crohn's disease: results of the ENCORE Trial. Gastroenterology. 2007;132:1672–83. doi: 10.1053/j.gastro.2007.03.024. PMID:17484865. [DOI] [PubMed] [Google Scholar]
- 112.Pagnini C, Arseneau KO, Cominelli F. Natalizumab in the treatment of Crohn's disease patients. Expert opinion on biological therapy. 2017;17:1433–8. PMID:28832222. [DOI] [PubMed] [Google Scholar]
- 113.Yoshimura N, Watanabe M, Motoya S, Tominaga K, Matsuoka K, Iwakiri R, Watanabe K, Hibi T. Safety and Efficacy of AJM300, an Oral Antagonist of alpha4 Integrin, in Induction Therapy for Patients With Active Ulcerative Colitis. Gastroenterology. 2015;149:1775–83.e2. doi: 10.1053/j.gastro.2015.08.044. PMID:26327130. [DOI] [PubMed] [Google Scholar]
- 114.Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel J-F, Sandborn WJ, Van Assche G, Axler J, Kim H-J, Danese S, et al.. Vedolizumab as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2013;369:699–710. doi: 10.1056/NEJMoa1215734. PMID:23964932. [DOI] [PubMed] [Google Scholar]
- 115.Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel J-F, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B, et al.. Vedolizumab as Induction and Maintenance Therapy for Crohn's Disease. N Engl J Med. 2013;369:711–21. doi: 10.1056/NEJMoa1215739. PMID:23964933. [DOI] [PubMed] [Google Scholar]
- 116.Stallmach A, Langbein C, Atreya R, Bruns T, Dignass A, Ende K, Hampe J, Hartmann F, Neurath MF, Maul J, et al.. Vedolizumab provides clinical benefit over 1 year in patients with active inflammatory bowel disease – a prospective multicenter observational study. Aliment Pharmacol Ther. 2016;44(11–12):1199–1212. doi: 10.1111/apt.13813. [DOI] [PubMed] [Google Scholar]
- 117.Loftus EV Jr., Colombel JF, Feagan BG, Vermeire S, Sandborn WJ, Sands BE, Danese S, D'Haens GR, Kaser A, Panaccione R, et al.. Long-term Efficacy of Vedolizumab for Ulcerative Colitis. Journal of Crohn's & colitis. 2017;11:400–11. [DOI] [PubMed] [Google Scholar]
- 118.Vermeire S, Loftus EV Jr., Colombel JF, Feagan BG, Sandborn WJ, Sands BE, Danese S, D'Haens GR, Kaser A, Panaccione R, et al.. Long-term Efficacy of Vedolizumab for Crohn's Disease. Journal of Crohn's & colitis. 2017;11:412–24. [DOI] [PubMed] [Google Scholar]
- 119.Colombel JF, Sands BE, Rutgeerts P, Sandborn W, Danese S, D'Haens G, Panaccione R, Loftus EV Jr., Sankoh S, Fox I, et al.. The safety of vedolizumab for ulcerative colitis and Crohn's disease. Gut. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sandborn WJ, Cyrille M, Hansen MB, Feagan BG, Loftus JEV, Rogler G, Vermeire S, Cruz ML, Yang J, Sullivan BA, et al.. OP034 Efficacy and safety of abrilumab in subjects with moderate to severe ulcerative colitis: results of a phase 2b, randomised, double-blind, multiple-dose, placebo-controlled study. Journal of Crohn's and Colitis. 2017;11:S21–S2. doi: 10.1093/ecco-jcc/jjx002.033.. [DOI] [Google Scholar]
- 121.Sandborn WJ, Cyrille M, Berner Hansen M, Feagan BG, Loftus JEV, Vermeire S, Cruz ML, Mo M, Sullivan BA, Reinisch W. OP035 Efficacy and safety of abrilumab (AMG 181/MEDI 7183) therapy for moderate to severe Crohn's disease. Journal of Crohn's and Colitis. 2017;11:S22–S3. doi: 10.1093/ecco-jcc/jjx002.034.. [DOI] [Google Scholar]
- 122.Sandborn WJ, Lee SD, Tarabar D, Louis E, Klopocka M, Klaus J, Reinisch W, Hebuterne X, Park DI, Schreiber S, et al.. Phase II evaluation of anti-MAdCAM antibody PF-00547659 in the treatment of Crohn's disease: report of the OPERA study. Gut. 2017;1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vermeire S, Sandborn WJ, Danese S, Hebuterne X, Salzberg BA, Klopocka M, Tarabar D, Vanasek T, Gregus M, Hellstern PA, et al.. Anti-MAdCAM antibody (PF-00547659) for ulcerative colitis (TURANDOT): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:135–44. doi: 10.1016/S0140-6736(17)30930-3. PMID:28527704. [DOI] [PubMed] [Google Scholar]
- 124.Vermeire S, O'Byrne S, Keir M, Williams M, Lu TT, Mansfield JC, Lamb CA, Feagan BG, Panes J, Salas A, et al.. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet. 2014;384:309–18. doi: 10.1016/S0140-6736(14)60661-9.. [DOI] [PubMed] [Google Scholar]
- 125.Argollo M, Fiorino G, Hindryckx P, Peyrin-Biroulet L, Danese S. Novel therapeutic targets for inflammatory bowel disease. J Autoimmun. 2017;85:103–16. doi: 10.1016/j.jaut.2017.07.004. PMID:28711286. [DOI] [PubMed] [Google Scholar]
- 126.Sandborn WJ, Feagan BG, Wolf DC, D'Haens G, Vermeire S, Hanauer SB, Ghosh S, Smith H, Cravets M, Frohna PA, et al.. Ozanimod Induction and Maintenance Treatment for Ulcerative Colitis. N Engl J Med. 2016;374:1754–62. doi: 10.1056/NEJMoa1513248. PMID:27144850. [DOI] [PubMed] [Google Scholar]
- 127.Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11:403–15. doi: 10.1038/nri2974. PMID:21546914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fyrst H, Saba JD. An update on sphingosine-1-phosphate and other sphingolipid mediators. Nat Chem Biol. 2010;6:489–97. doi: 10.1038/nchembio.392. PMID:20559316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nielsen OH, Li Y, Johansson-Lindbom B, Coskun M. Sphingosine-1-Phosphate Signaling in Inflammatory Bowel Disease. Trends Mol Med. 2017;23:362–74. doi: 10.1016/j.molmed.2017.02.002. PMID:28283249. [DOI] [PubMed] [Google Scholar]
- 130.Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, et al.. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–9. doi: 10.1126/science.1070238. PMID:11923495. [DOI] [PubMed] [Google Scholar]
- 131.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, et al.. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–8. doi: 10.1126/science.1139221. PMID:17363629. [DOI] [PubMed] [Google Scholar]
- 132.Thangada S, Khanna KM, Blaho VA, Oo ML, Im DS, Guo C, Lefrancois L, Hla T. Cell-surface residence of sphingosine 1-phosphate receptor 1 on lymphocytes determines lymphocyte egress kinetics. J Exp Med. 2010;207:1475–83. doi: 10.1084/jem.20091343. PMID:20584883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cohen JA, Arnold DL, Comi G, Bar-Or A, Gujrathi S, Hartung JP, Cravets M, Olson A, Frohna PA, Selmaj KW. Safety and efficacy of the selective sphingosine 1-phosphate receptor modulator ozanimod in relapsing multiple sclerosis (RADIANCE): a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15:373–81. doi: 10.1016/S1474-4422(16)00018-1. PMID:26879276. [DOI] [PubMed] [Google Scholar]
- 134.Mao F, Tu Q, Wang L, Chu F, Li X, Li HS, Xu W. Mesenchymal stem cells and their therapeutic applications in inflammatory bowel disease. Oncotarget. 2017;8:38008–21. doi: 10.18632/oncotarget.16682. PMID:28402942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gregoire C, Lechanteur C, Briquet A, Baudoux E, Baron F, Louis E, Beguin Y. Review article: mesenchymal stromal cell therapy for inflammatory bowel diseases. Aliment Pharmacol Ther. 2017;45:205–21. doi: 10.1111/apt.13864. PMID:27878827. [DOI] [PubMed] [Google Scholar]
- 136.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. PMID:16923606. [DOI] [PubMed] [Google Scholar]
- 137.Li L, Liu S, Xu Y, Zhang A, Jiang J, Tan W, Xing J, Feng G, Liu H, Huo F, et al.. Human umbilical cord-derived mesenchymal stem cells downregulate inflammatory responses by shifting the Treg/Th17 profile in experimental colitis. Pharmacology. 2013;92:257–64. doi: 10.1159/000354883. PMID:24280970. [DOI] [PubMed] [Google Scholar]
- 138.Choi YS, Jeong JA, Lim DS. Mesenchymal stem cell-mediated immature dendritic cells induce regulatory T cell-based immunosuppressive effect. Immunol Invest. 2012;41:214–29. doi: 10.3109/08820139.2011.619022. PMID:22017637. [DOI] [PubMed] [Google Scholar]
- 139.Gonzalez MA, Gonzalez-Rey E, Rico L, Buscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978–89. doi: 10.1053/j.gastro.2008.11.041. PMID:19135996. [DOI] [PubMed] [Google Scholar]
- 140.He XW, He XS, Lian L, Wu XJ, Lan P. Systemic infusion of bone marrow-derived mesenchymal stem cells for treatment of experimental colitis in mice. Dig Dis Sci. 2012;57:3136–44. doi: 10.1007/s10620-012-2290-5. PMID:22752635. [DOI] [PubMed] [Google Scholar]
- 141.Liang L, Dong C, Chen X, Fang Z, Xu J, Liu M, Zhang X, Gu DS, Wang D, Du W, et al.. Human umbilical cord mesenchymal stem cells ameliorate mice trinitrobenzene sulfonic acid (TNBS)-induced colitis. Cell Transplant. 2011;20:1395–408. doi: 10.3727/096368910X557245. PMID:21396175. [DOI] [PubMed] [Google Scholar]
- 142.Gonzalez-Rey E, Anderson P, Gonzalez MA, Rico L, Buscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–39. doi: 10.1136/gut.2008.168534. PMID:19136511. [DOI] [PubMed] [Google Scholar]
- 143.Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. PMID:12480709. [DOI] [PubMed] [Google Scholar]
- 144.Hayashi Y, Tsuji S, Tsujii M, Nishida T, Ishii S, Iijima H, Nakamura T, Eguchi H, Miyoshi E, Hayashi N, et al.. Topical implantation of mesenchymal stem cells has beneficial effects on healing of experimental colitis in rats. J Pharmacol Exp Ther. 2008;326:523–31. doi: 10.1124/jpet.108.137083. PMID:18448866. [DOI] [PubMed] [Google Scholar]
- 145.Kachgal S, Putnam AJ. Mesenchymal stem cells from adipose and bone marrow promote angiogenesis via distinct cytokine and protease expression mechanisms. Angiogenesis. 2011;14:47–59. doi: 10.1007/s10456-010-9194-9. PMID:21104120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, Cai T, Chen W, Sun L, Shi S. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10:544–55. doi: 10.1016/j.stem.2012.03.007. PMID:22542159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yabana T, Arimura Y, Tanaka H, Goto A, Hosokawa M, Nagaishi K, Yamashita K, Yamamoto H, Adachi Y, Sasaki Y, et al.. Enhancing epithelial engraftment of rat mesenchymal stem cells restores epithelial barrier integrity. J Pathol. 2009;218:350–9. doi: 10.1002/path.2535. PMID:19291714. [DOI] [PubMed] [Google Scholar]
- 148.Dave M, Mehta K, Luther J, Baruah A, Dietz AB, Faubion WA Jr.. Mesenchymal Stem Cell Therapy for Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Inflamm Bowel Dis. 2015;21:2696–707. doi: 10.1097/MIB.0000000000000543. PMID:26230863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Onken J, Gallup D, Hanson J, Pandak M, Custer L. Successful outpatient treatment of refractory Crohn's disease using adult mesenchymal stem cells. Abstract 121. American College of Gastroenterology Conference Las Vegas, NV, 2006. [Google Scholar]
- 150.Lazebnik LB, Konopliannikov AG, Kniazev OV, Parfenov AI, Tsaregorodtseva TM, Ruchkina IN, Khomeriki SG, Rogozina VA, Konopliannikova OA. [Use of allogeneic mesenchymal stem cells in the treatment of intestinal inflammatory diseases]. Terapevticheskii arkhiv. 2010;82:38–43. PMID:20387674. [PubMed] [Google Scholar]
- 151.Liang J, Zhang H, Wang D, Feng X, Wang H, Hua B, Liu B, Sun L. Allogeneic mesenchymal stem cell transplantation in seven patients with refractory inflammatory bowel disease. Gut. 2012;61:468–9. doi: 10.1136/gutjnl-2011-300083. PMID:21617158. [DOI] [PubMed] [Google Scholar]
- 152.Forbes GM, Sturm MJ, Leong RW, Sparrow MP, Segarajasingam D, Cummins AG, Phillips M, Herrmann RP. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn's disease refractory to biologic therapy. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12:64–71. doi: 10.1016/j.cgh.2013.06.021. PMID:23872668. [DOI] [PubMed] [Google Scholar]
- 153.Knyazev O, Kagramanova A, Churikova A, Konoplyannikov A, Khomeriki S, Parfenov A, Ruchkina I. P575. The use of mesenchymal stromal cells in order to achieve deep (biological) remission of Ulcerative Colitis. Journal of Crohn's and Colitis. 2015;9:S367–S8. doi: 10.1093/ecco-jcc/jju027.693.. [DOI] [Google Scholar]
- 154.Knyazev O, Fadeeva N, Kagramanova A, Shcherbakov P, Ruchkina I, Parfenov A, Konoplyannikov A. P485. The combined of mesenchymal stem cells and infliximab reduces the recurrence rate of Crohn's disease. Journal of Crohn's and Colitis. 2016;10:S346–S. [Google Scholar]
- 155.Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ. Safety of Cell Therapy with Mesenchymal Stromal Cells (SafeCell): A Systematic Review and Meta-Analysis of Clinical Trials. PLOS ONE. 2012;7:e47559. doi: 10.1371/journal.pone.0047559. PMID:23133515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Duijvestein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW, Kooy-Winkelaar EM, Koning F, Zwaginga JJ, Fidder HH, et al.. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn's disease: results of a phase I study. Gut. 2010;59:1662–9. doi: 10.1136/gut.2010.215152. PMID:20921206. [DOI] [PubMed] [Google Scholar]
- 157.Dhere T, Copland I, Garcia M, Chiang KY, Chinnadurai R, Prasad M, Galipeau J, Kugathasan S. The safety of autologous and metabolically fit bone marrow mesenchymal stromal cells in medically refractory Crohn's disease – a phase 1 trial with three doses. Aliment Pharmacol Ther. 2016;44:471–81. doi: 10.1111/apt.13717. PMID:27385373. [DOI] [PubMed] [Google Scholar]
- 158.Dave M, Jaiswal P, Cominelli F. Mesenchymal stem/stromal cell therapy for inflammatory bowel disease: an updated review with maintenance of remission. Curr Opin Gastroenterol. 2017;33:59–68. doi: 10.1097/MOG.0000000000000327. PMID:28134690. [DOI] [PubMed] [Google Scholar]
- 159.Fantini MC, Monteleone G. Update on the Therapeutic Efficacy of Tregs in IBD: Thumbs up or Thumbs down? Inflamm Bowel Dis. 2017;23:1682–8. doi: 10.1097/MIB.0000000000001272. PMID:28906289. [DOI] [PubMed] [Google Scholar]
- 160.Elinav E, Waks T, Eshhar Z. Redirection of regulatory T cells with predetermined specificity for the treatment of experimental colitis in mice. Gastroenterology. 2008;134:2014–24. doi: 10.1053/j.gastro.2008.02.060. PMID:18424268. [DOI] [PubMed] [Google Scholar]
- 161.Huber S, Schramm C, Lehr HA, Mann A, Schmitt S, Becker C, Protschka M, Galle PR, Neurath MF, Blessing M. Cutting edge: TGF-beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. Journal of immunology (Baltimore, Md : 1950). 2004;173:6526–31. doi: 10.4049/jimmunol.173.11.6526. PMID:15557141. [DOI] [PubMed] [Google Scholar]
- 162.Maul J, Loddenkemper C, Mundt P, Berg E, Giese T, Stallmach A, Zeitz M, Duchmann R. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–78. doi: 10.1053/j.gastro.2005.03.043. PMID:15940622. [DOI] [PubMed] [Google Scholar]
- 163.Saruta M, Yu QT, Fleshner PR, Mantel PY, Schmidt-Weber CB, Banham AH, Papadakis KA. Characterization of FOXP3+CD4+ regulatory T cells in Crohn's disease. Clinical immunology (Orlando, Fla). 2007;125:281–90. doi: 10.1016/j.clim.2007.08.003. PMID:17897887. [DOI] [PubMed] [Google Scholar]
- 164.Takahashi M, Nakamura K, Honda K, Kitamura Y, Mizutani T, Araki Y, Kabemura T, Chijiiwa Y, Harada N, Nawata H. An inverse correlation of human peripheral blood regulatory T cell frequency with the disease activity of ulcerative colitis. Dig Dis Sci. 2006;51:677–86. doi: 10.1007/s10620-006-3191-2. PMID:16614988. [DOI] [PubMed] [Google Scholar]
- 165.Fantini MC, Rizzo A, Fina D, Caruso R, Sarra M, Stolfi C, Becker C, Macdonald TT, Pallone F, Neurath MF, et al.. Smad7 controls resistance of colitogenic T cells to regulatory T cell-mediated suppression. Gastroenterology. 2009;136:1308–16, e1-3. doi: 10.1053/j.gastro.2008.12.053. PMID:19192480. [DOI] [PubMed] [Google Scholar]
- 166.Trzonkowski P, Bieniaszewska M, Juscinska J, Dobyszuk A, Krzystyniak A, Marek N, Mysliwska J, Hellmann A. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clinical immunology (Orlando, Fla). 2009;133:22–6. doi: 10.1016/j.clim.2009.06.001. PMID:19559653. [DOI] [PubMed] [Google Scholar]
- 167.Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Techmanska I, Juscinska J, Wujtewicz MA, Witkowski P, Mlynarski W, Balcerska A, et al.. Administration of CD4+CD25highCD127- regulatory T cells preserves beta-cell function in type 1 diabetes in children. Diabetes Care. 2012;35:1817–20. doi: 10.2337/dc12-0038. PMID:22723342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Desreumaux P, Foussat A, Allez M, Beaugerie L, Hebuterne X, Bouhnik Y, Nachury M, Brun V, Bastian H, Belmonte N, et al.. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn's disease. Gastroenterology. 2012;143:1207–17.e1-2. doi: 10.1053/j.gastro.2012.07.116. PMID:22885333. [DOI] [PubMed] [Google Scholar]
- 169.Canavan JB, Scotta C, Vossenkamper A, Goldberg R, Elder MJ, Shoval I, Marks E, Stolarczyk E, Lo JW, Powell N, et al.. Developing in vitro expanded CD45RA+ regulatory T cells as an adoptive cell therapy for Crohn's disease. Gut. 2016;65:584–94. doi: 10.1136/gutjnl-2014-306919. PMID:25715355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Inagaki-Ohara K, Chinen T, Matsuzaki G, Sasaki A, Sakamoto Y, Hiromatsu K, Nakamura-Uchiyama F, Nawa Y, Yoshimura A. Mucosal T cells bearing TCRgammadelta play a protective role in intestinal inflammation. Journal of immunology (Baltimore, Md : 1950). 2004;173:1390–8. doi: 10.4049/jimmunol.173.2.1390. PMID:15240735. [DOI] [PubMed] [Google Scholar]
- 171.Hoffmann JC, Pawlowski NN, Grollich K, Loddenkemper C, Zeitz M, Kuhl AA. Gammadelta T lymphocytes: a new type of regulatory T cells suppressing murine 2,4,6-trinitrobenzene sulphonic acid (TNBS)-induced colitis. Int J Colorectal Dis. 2008;23:909–20. [DOI] [PubMed] [Google Scholar]
- 172.Fisher JP, Heuijerjans J, Yan M, Gustafsson K, Anderson J. gammadelta T cells for cancer immunotherapy: A systematic review of clinical trials. Oncoimmunology. 2014;3:e27572. doi: 10.4161/onci.27572. PMID:24734216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gallo A, Passaro G, Gasbarrini A, Landolfi R, Montalto M. Modulation of microbiota as treatment for intestinal inflammatory disorders: An uptodate. World J Gastroenterol. 2016;22:7186–202. doi: 10.3748/wjg.v22.i32.7186. PMID:27621567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Narula N, Kassam Z, Yuan Y, Colombel JF, Ponsioen C, Reinisch W, Moayyedi P. Systematic Review and Meta-analysis: Fecal Microbiota Transplantation for Treatment of Active Ulcerative Colitis. Inflamm Bowel Dis. 2017;23:1702–9. doi: 10.1097/MIB.0000000000001228. PMID:28906291. [DOI] [PubMed] [Google Scholar]
- 175.Arnold GL, Beaves MR, Pryjdun VO, Mook WJ. Preliminary study of ciprofloxacin in active Crohn's disease. Inflamm Bowel Dis. 2002;8:10–5. doi: 10.1097/00054725-200201000-00002. PMID:11837933. [DOI] [PubMed] [Google Scholar]
- 176.Sutherland L, Singleton J, Sessions J, Hanauer S, Krawitt E, Rankin G, Summers R, Mekhjian H, Greenberger N, Kelly M, et al.. Double blind, placebo controlled trial of metronidazole in Crohn's disease. Gut 1991;32:1071–5. doi: 10.1136/gut.32.9.1071. PMID:1916494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Prantera C, Zannoni F, Scribano ML, Berto E, Andreoli A, Kohn A, Luzi C. An antibiotic regimen for the treatment of active Crohn's disease: a randomized, controlled clinical trial of metronidazole plus ciprofloxacin. Am J Gastroenterol 1996;91:328–32. PMID:8607501. [PubMed] [Google Scholar]
- 178.Prantera C, Lochs H, Grimaldi M, Danese S, Scribano ML, Gionchetti P. Rifaximin-extended intestinal release induces remission in patients with moderately active Crohn's disease. Gastroenterology. 2012;142:473–81.e4. doi: 10.1053/j.gastro.2011.11.032. PMID:22155172. [DOI] [PubMed] [Google Scholar]
- 179.Sandborn WJ, Elliott DE, Weinstock J, Summers RW, Landry-Wheeler A, Silver N, Harnett MD, Hanauer SB. Randomised clinical trial: the safety and tolerability of Trichuris suis ova in patients with Crohn's disease. Aliment Pharmacol Ther. 2013;38:255–63. doi: 10.1111/apt.12366. PMID:23730956. [DOI] [PubMed] [Google Scholar]
- 180.Weinstock JV. Helminths and mucosal immune modulation. Ann N Y Acad Sci. 2006;1072:356–64. doi: 10.1196/annals.1326.033. PMID:17057216. [DOI] [PubMed] [Google Scholar]
- 181.Wong C, Harris PJ, Ferguson LR. Potential Benefits of Dietary Fibre Intervention in Inflammatory Bowel Disease. Int J Mol Sci. 2016;17:919. doi: 10.3390/ijms17060919.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nie Y, Lin Q, Luo F. Effects of Non-Starch Polysaccharides on Inflammatory Bowel Disease. Int J Mol Sci. 2017;18:1372. doi: 10.3390/ijms18071372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mitsuyama K, Saiki T, Kanauchi O, Iwanaga T, Tomiyasu N, Nishiyama T, Tateishi H, Shirachi A, Ide M, Suzuki A, et al.. Treatment of ulcerative colitis with germinated barley foodstuff feeding: a pilot study. Aliment Pharmacol Ther. 1998;12:1225–30. doi: 10.1046/j.1365-2036.1998.00432.x. PMID:9882030. [DOI] [PubMed] [Google Scholar]
- 184.Karner M, Kocjan A, Stein J, Schreiber S, von Boyen G, Uebel P, Schmidt C, Kupcinskas L, Dina I, Zuelch F, et al.. First multicenter study of modified release phosphatidylcholine “LT-02” in ulcerative colitis: a randomized, placebo-controlled trial in mesalazine-refractory courses. Am J Gastroenterol. 2014;109:1041–51. doi: 10.1038/ajg.2014.104. PMID:24796768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Langhorst J, Wulfert H, Lauche R, Klose P, Cramer H, Dobos GJ, Korzenik J. Systematic review of complementary and alternative medicine treatments in inflammatory bowel diseases. Journal of Crohn's & colitis. 2015;9:86–106. doi: 10.1093/ecco-jcc/jju007. [DOI] [PubMed] [Google Scholar]


