ABSTRACT
Henoch Schönlein purpura (HSP) following vaccine administration has been described in case reports and in a small number of observational studies. We herein reported a case of HSP occurring in an otherwise healthy 37-year-old male after immunization with lyophilized purified vero cell rabies vaccine (PVRV). After the anti-allergy therapy with hormone, the purpuric lesions gradually disappeared. After evaluating, another PVRV with different dose (0.5 ml), strains, excipient and without residues was chosen for the new anti-rabies immunization program, and the patient has had no recurrence of allergic symptoms. Although significant lower than the levels of normal 20–50 year population at day 21, the neutralizing antibody (RVNA) titers of this boy showed adequate protective antibody (3.23 vs 7.15 IU/ml). This case report emphasizes the importance that clinicians should be aware of HSP as a potential adverse event associated with PVRV vaccination. And adverse events (AEs) after immunization should be carefully treated, changing immunization program in time is necessary. While enrolling a new anti-rabies immunization program, the properties of different rabies vaccines taking with special emphasis on strains, excipient and residues is imperative before vaccination so that an appropriate immune program can be managed to be initiated.
Keywords: allergic reaction, neutralizing antibody (RVNA) titer, purified vero cell rabies vaccine (PVRV), rabies virus neutralizing antibody (RVNA), Henoch Schönlein purpura (HSP)
Introduction
Rabies is a serious public health problem throughout the world. There are approximately 55,000 deaths of rabies per year in the world, 84% of which occur in rural areas of developing countries.1 Because of very high case-fatality ratio (nearly 100%) of rabies, prophylaxis by vaccination is the only protection against rabies. Although rabies vaccines in the market had been carefully evaluated,2 there are still some reports that severe allergic reaction that had to be stopped or change the immunization program following rabies vaccination such as acute allergic purpura.3,4 Once these allergic reactions occur, high dose steroid therapy is very essential to the patients, whereas glucocorticoids are considered to suppress patients’ immune system, thereby may leading to the failure of rabies vaccination. In this study, we reported a case of severe allergic purpura following rabies vaccination.
Patient presentation
A 37-year-old male had suffered a dog bite on the right lower ankle 1 day prior to admission, and the level of exposure was identified as Category III according to World Health Organization recommendations. He never had been hospitalized because of any diseases over 10 years, and he received no blood transfusion, surgery, or long-term medication. And there was no special medical history but occasional colds in the past. After wound cleaning, an Essen regimen (1-1-1-1-1, consisting of 5 doses of rabies vaccine in 5 visits on days 0, 3, 7, 14 and 21) and lyophilized purified vero cell rabies vaccine (PVRV) was used for the immunization program. He had fever (37.5°C) within 5 hours after first dose immunization with vaccine A, and fever relieved within 12 to 24 hours without treatment. 3 days later after second dose immunization, he had fever (38.2°C) within 3 hours and relieved within 24 to 48 hours without treatment. 7 days after third immunization, he had fever (38.9°C at 2 hours, 39.1°C at 4 hours), and he presented with a palpable purpuric rash in an extensor distribution affecting both lower legs. He was then hospitalized for severe Henoch Schönlein purpura (HSP) (Table 1). His knee and ankle joints were tender and painful. A physical examination revealed moderate pitting edema and palpable purpura on both lower legs. Blood test results showed a white blood cell count of 15,600/mm3, a significant high level of Creactive protein (CRP) concentration of 17.61 mg/dL, and a platelet count of 33.3 × 104 /mm3. The plasma coagulation factor XIII (F XIII) activity was 47% (normal 70–140%). Laboratory studies revealed normal values for the following: complete blood cell count, PT, PTT, and urinalysis. We made a diagnosis of HSP in conjunction with the clinical course. In view of the clinical and pathological severity, he was started on 8 mg Dexamethasone Sodium Phosphate for 3 days, with plus 5 mg oral Cetirizine hydrochloride tables for 2 times in 2 day and 7.5 mg for one time per day in the following 2 day. During the steroid therapy, his allergy lesions alleviated. 1 day after the steroid therapy discontinued, the immunogenicity analysis and allergy testing were conducted. Rabies virus neutralizing antibody (RVNA) titers in the serum were measured by rapid fluorescent focus inhibition test (RFFIT) in the virology laboratory of Wuhan Centers for Disease Prevention and Control (Wuhan CDC). Allergy testing was performed with UniCAP systems (Pharmacia Biotech, Uppsala, Sweden) by Wuhan Institute of Dermatology and Venereology. Test result indicated that the patient was not sensitive to allergen exposure (Table 2).
Table 1.
Patient presentation of vaccine, adverse events and treatment.
| Days post vaccination | Vaccine | Dose | AEs | Treatment |
|---|---|---|---|---|
| 0 | PVCV vaccine A | 1 Dose | Fever (37.5℃) within 5 hours after vaccination | Fever relieved within 12 to 24 hours without treatment |
| 3 | PVCV vaccine A | 1 Dose | Fever (38.2℃) within 3 hours | Fever relieved within 24 to 48 hours without treatment |
| 7 | PVCV vaccine A | 1 Dose | Allergic purpura and fever (38.9℃ at 2 hours, 39.1℃ at 4 hours) | Steroid therapy |
| 14 | — | — | Allergy testing | |
| 15 | PVCV vaccine B | 2 Dose | No new AEs occur | — |
| 21 | PVCV vaccine B | 1 Dose | No new AEs occur | — |
| 35 | PVCV vaccine B | 1 Dose | No new AEs occur | — |
Table 2.
The results of allergy testing.
| Allergen Name | Concentration (kU/L) | Classification |
|---|---|---|
| Inhalant allergen screening | < 0.35 | 0 |
| House dust | < 0.35 | 0 |
| House dust mite | 0.39 | 1 |
| Dust mite | < 0.35 | 0 |
| Cat | < 0.35 | 0 |
| Oak pollen | < 0.35 | 0 |
| Elm pollen | < 0.35 | 0 |
| Plane pollen | < 0.35 | 0 |
| Willow pollen | < 0.35 | 0 |
| Black poplar pollen | 0.40 | 1 |
| Penicillium | < 0.35 | 0 |
| Bud cladosporium | < 0.35 | 0 |
| Aspergillus fumigates | < 0.35 | 0 |
| Candida albicans | < 0.35 | 0 |
| Alternaria mold | < 0.35 | 0 |
| Helminthosporium mold | < 0.35 | 0 |
| Food allergen | < 0.35 | 0 |
| Agg | < 0.35 | 0 |
| Milk | < 0.35 | 0 |
Notes. Food allergen includes egg, milk, wheat, cod, soybean, peanut.
Classification of specific IgE (sIgE): Phl p 0, < 0.35kU/L; Phl p 1, ≧0.35kU/L; Phl p 2, ≧0.70kU/L; Phl p 3, ≧3.5kU/L; Phl p 4, ≧17.5kU/L; Phl p 5, ≧50kU/L; Phlp 6, ≧100kU/L.
Vaccine change program
According to the neutralizing antibody titers showed in Figure 1, 15 days after immunization program with vaccine A, the result of RFFIT indicated lower level of antibodies. As anti-rabies immunization was delayed by anti-allergy therapy, it was necessary to continue the administration with a selected rabies vaccine and regimen, and both safety and immunogenicity for the clinical vaccination need to be viewed cautiously. To conduct a new anti-rabies immunization program, another PVRV (0.5 ml/dose) without antibiotic residues (Table 3), and Zagreb regimen (2-1-1, consisting of 4 doses in 3 visits on days 0, 7 and 21, 2-site intradermal injections on day 0) was enrolled. Side effects were observed for 30 min after each vaccination, and proactive visit by clinicians was conducted at 24 h, 48 h, 72 h, and 14 d post-immunization, to record any adverse reactions, according to the “Preventive vaccine clinical trials, adverse events grading guidelines” issued by the China Food and Drug Administration. The patient had not occur any AEs and allergic purpura was not aggravated during the following immunization.
Figure 1.
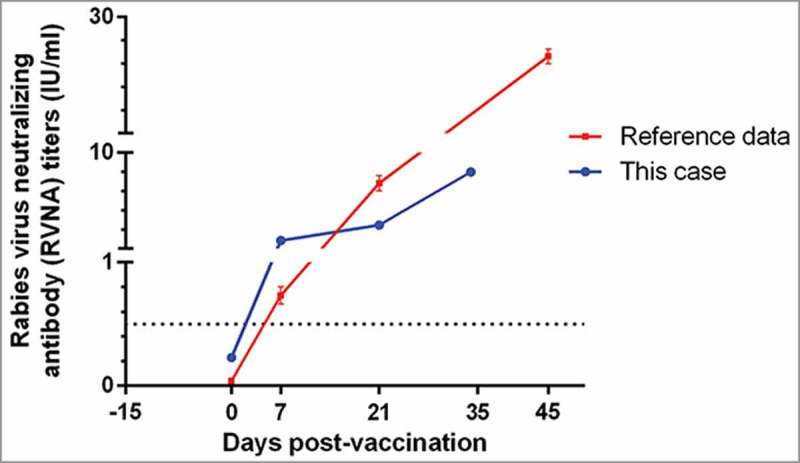
The rabies virus neutralizing antibody (RVNA) titers of a male with severe HSP following rabies vaccination, and received a new anti-rabies programe 15 days later.
Table 3.
Properties of 2 different rabies vaccines.
| Vaccine A | Vaccine B | |
|---|---|---|
| Cell types | Vero cell | Vero cell |
| Dosage(ml) | 1.0 | 0.5 |
| Formation | lyophilized | lyophilized |
| Production | bioreactor | bioreactor |
| Strains | aG | PV2061 |
| Inactivated form | β-propiolactone | β-propiolactone |
| Excipient | Saccharose | Dextran |
| Residues | gentamycin | — |
Immunogenicity results
The immunogenicity analysis was completed using the homologous rapid fluorescent focus inhibition test (RFFIT) results. Before the new immunization program, his antibody titers indicated lower level of antibodies (0.23 IU/ml). In order to achieve adequate immune response as soon as possible, Zagreb regimen (2-1-1, consisting of 4 doses in 3 visits on days 0, 7 and 21, 2-site intradermal injections on day 0) was used for the new immunization program. No further allergy was reported, and immunogenicity analysis indicated successful protection (RVNA titer >0.5 IU/ml, an indicator of an adequate adaptive immune response used by the World Health Organization [WHO]5) was deduced by vaccine B. RVNA of this case at day 21 (3.23 vs 7.15 IU/ml) remained significant lower than that of normal male group who were 20–50 years old in our previous study.6
Discussion
Henoch Schönlein purpura (HSP), also known as IgA vasculitis (IgAV), is a systemic small-vessel vasculitis that predominantly affects adolescents and is rare in adults.7 HSP following vaccine administration has been described in case reports and in a small number of observational studies conducted during vaccination campaigns, including those vaccines against influenza, pneumococcal disease, hepatitis virus, and meningococcal disease.8–11 We encountered a case of HSP following PVRV immunization for anti-rabies. It is possible that after administration of the PVRV, immune complexes may form between vaccine antigens and native antibodies, thereby initiate a vasculitic process. The temporal association between PVRV and the development of HSP in this patient is notable. It is important for all clinicians to recognize that PVRV has this rare but potentially serious systemic complication.
With respect to the development of local reactions, we speculated that HSP was caused by PVRV. Although a causal relationship cannot be established, this hypothesis remains likely. The symptoms of fever grow progressively with onset of vaccination, till HSP occurred. After that vaccination stopped, a steroid therapy was included, and the purpuric lesions gradually disappeared. However, it was necessary to conduct a new immunization program, although it was challenged to choose suitable rabies vaccines. Allergic reaction may caused by strains, excipient, or gentamicin, although the patient denied any known antibiotic allergies. In addition, the volume of primary immunized vaccine should be also taken into account, as administrated with a small dose have comparative high safety, based on our previous study.2 After evaluating, another PVRV with different dose (0.5 ml), strains, excipient and without residues was chosen for the new anti-rabies immunization program, and the patient has had no recurrence of allergic symptoms.
It has been suggested by Pharmacopoeia of the People's Republic of China (PPRC, 2010 Edition) that the using of PVRV should not company with corticosteroids, but there are currently no clear guidelines for what is the most appropriate management. In this 37 years old male with HSP, his antibody titers indicated lower level of antibodies (<0.5 IU/ml) after steroid therapy. So it was necessary to continue the administration with a selected rabies vaccine for this suspected male. After the new immunization treatment, RVNA results in this study indicated protective antibody titers (>0.5 IU/ml) induced by the new vaccine. The neutralization antibody titers analysis indicated that RVNA titers of this male remained lower than that of normal 20–50 years old male we reported before, which might be affected by the hormone during the anti-allergy therapy, as previously we also observed negative effect of the hormone on RVNA titers in a case with acute disseminated encephalomyelitis and a case with severe allergic reaction to rabies vaccine.3,4
In summary, we report a case of HSP occurring in an otherwise healthy 37-year-old male after immunization with PVRV. This case report emphasizes again the importance to discontinue immunization program once adverse events following immunization (AEFI) onset. Clinicians should be aware of HSP as a potential adverse event associated with PVRV vaccination. And AEs after immunization should be carefully treated, changing immunization program in time. While enrolling a new anti-rabies immunization program, the properties of different rabies vaccines taking with special emphasis on strains, excipient and residues is imperative before vaccination so that an appropriate immune program can be managed to be initiated. In addition, immunogenicity of PVRV might be affected by the hormone during the anti-allergy therapy, but it can still induce protective antibody titers (>0.5 IU/ml).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Organization WH. WHO Expert Consultation on Rabies. Second report. World Health Organization Technical Report. 2013;982:1. [PubMed] [Google Scholar]
- 2.Peng J, Sha L, Zhu Z, Man Z, Quan H, Yuan F. Safety comparison of four types of rabies vaccines in patients with WHO category II animal exposure: An observation based on different age groups. Medicine. 2016;95:e5049. doi: 10.1097/MD.0000000000005049. PMID:27893654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peng J, Chen L, Zhu ZG, Zhu ZR, Hu Q, Fang Y. Effect of Corticosteroids on RVNA production of a patient with acute disseminated encephalomyelitis following rabies vaccination as well as administration of HRIG. Hum Vaccin Immunother. 2014;10:3622–6. doi: 10.4161/21645515.2014.979621. PMID:25668669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang Y, Liu MQ, Chen L, Zhu ZG, Zhu ZR, Hu Q. Rabies post-exposure prophylaxis for a child with severe allergic reaction to rabies vaccine. Hum Vaccin. 2016;12:1802–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manning SE, Rupprecht CE, Fishbein D, Hanlon CA, Lumlertdacha B, Guerra M, Meltzer MI, Dhankhar P, Vaidya SA, Jenkins SR. et al. . Human rabies prevention–United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2008;57:1–27. PMID:18185492 [PubMed] [Google Scholar]
- 6.Fang Y, Chen L, Liu MQ, Zhu ZG, Zhu ZR, Hu Q. Comparison of safety and immunogenicity of PVRV and PCECV immunized in patients with WHO category II animal exposure: a study based on different age groups. Plos Negl Trop Dis. 2014;8:e3412–e. doi: 10.1371/journal.pntd.0003412. PMID:25522244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peláez Ballesta A. Henoch-Schönlein purpura. [Google Scholar]
- 8.Fox BC, Peterson A. Leukocytoclastic vasculitis after pneumococcal vaccination. Am J Infect Control. 1998;26:365. doi: 10.1016/S0196-6553(98)80021-7. PMID:9638297 [DOI] [PubMed] [Google Scholar]
- 9.Le HC, Cohen P, Bousser MG, Letellier P, Guillevin L. Suspected hepatitis B vaccination related vasculitis. J Rheumatol 1999;26:191. PMID:9918261 [PubMed] [Google Scholar]
- 10.Watanabe T, Onda H. Henoch-Schonlein purpura with antiphospholipid antibodies following an influenza vaccination. Pediatr Nephrol 2001;16:458–9. doi: 10.1007/s004670100569. PMID:11405122 [DOI] [PubMed] [Google Scholar]
- 11.Sunit J, Natalia V, Jenny S. Henoch-Sch?nlein purpura after hepatitis A vaccination. Annals of Allergy Asthma & Immunology Official Publication of the American College of Allergy Asthma & Immunology 2011;107:180–1. doi: 10.1016/j.anai.2011.05.006. [DOI] [PubMed] [Google Scholar]


