ABSTRACT
A 2018 manufacturer post-licensure safety study identified a possible association between Rotarix (RV1) rotavirus vaccine and lower respiratory tract infections (LRTI) in infants within 0–6 days following receipt of RV1 dose 1.
We reviewed reports to the Vaccine Adverse Event Reporting System (VAERS) of LRTI occurring 0–6 days and 0–29 days post vaccination following RotaTeq (RV5) or Rotarix (RV1) vaccinations in conjunction with either Prevnar (PCV7) or Prevnar 13 (PCV13), in infants aged 6 to 15 weeks. There was no significant difference in LRTI reports to VAERS in the 0–6 days and 0–29 days following receipt of either RV5 or RV1 given with either pneumococcal vaccine.
KEYWORDS: Adverse event, passive surveillance, rotavirus, Rotavirus vaccine, Vaccine safety, VAERS
Introduction
There are two live oral rotavirus vaccines in use in the United States: pentavalent human-bovine reassortant vaccine (RV5, RotaTeq, Merck) and human monovalent vaccine (RV1, Rotarix, GlaxoSmithKline [GSK]) licensed in 2006 and 2008, respectively.1,2 RV5 vaccine is recommended as a 3-dose series administered at 2, 4 and 6 months of age3 and RV1 as a 2-dose series administered at 2 and 4 months; the minimum age for the first dose for both vaccines is 6 weeks and maximum age is 14 weeks.4 These vaccines are frequently co-administered with other childhood schedule vaccines (e.g., DTaP, IPV, pneumococcal vaccines).5
Recently, results of a manufacturer RV1 post-licensure safety study documented an elevated risk of acute lower respiratory infections (LRTI) among recipients of RV1 compared to recipients of concurrent inactivated poliovirus vaccine (cIPV) within 0–6 days following dose 1.6 This is the first published study to identify an increased risk of acute LRTI following RV1. In follow up, we reviewed VAERS reports of LRTI in infants aged 6 to 15 weeks occurring 0–29 days following receipt of dose 1 of either RV5 or RV1 vaccines. Since concurrent receipt of 7-valent pneumococcal conjugate vaccine (PCV7, Prevnar, Pfizer, Inc.) and 13-valent pneumococcal, conjugate vaccine (PCV13, Prevnar13, Pfizer, Inc.) may be expected to attenuate the risk of a child developing a LRTI we restricted reports to only those infants who received either of the rotavirus vaccines plus either of these pneumococcal conjugate vaccines.7,8
Results
From January, 2008 through December 2016, VAERS received a total of 546 reports among infants aged 6 to 15 weeks who reported any AEs within 0–29 days following RV1 vaccination and 2,111 following RV5 (Fig. 1). A total of 35 reports met the initial LRTI diagnosis with onset 0–29 days, seven reports in the RV1 group and 28 in the RV5 group. However, due to insufficient information in some of the LRTI reports, only 18 (51%) reports within 0–29 days were confirmed with this diagnosis and of these 15 (83%) reports were within 0–6 days (Fig. 1). There were four deaths and 11 non-death serious reports in RV5 group and three non-death serious reports in RV1 group (Fig. 1). Most reports indicated infants also received diphtheria-tetanus-acellular pertussis vaccine (DTaP), inactivated polio vaccine (IPV), Haemophilus influenzae type b vaccine (Hib) and hepatitis b vaccine (HepB) administered concomitantly at the same visit (Tables 1, 2 ).
Figure 1.
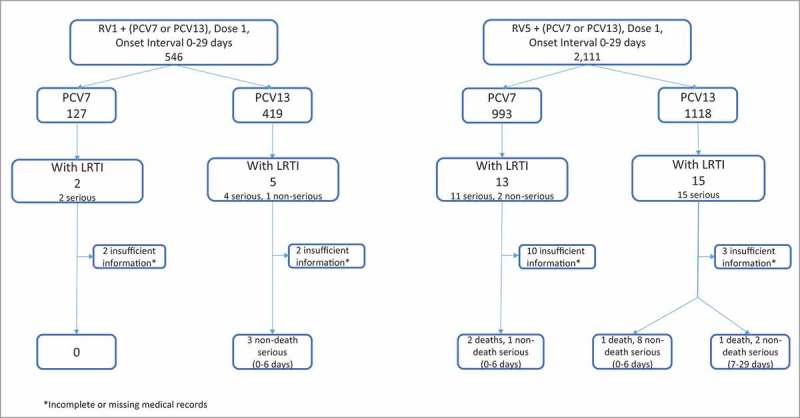
LRTI Reports Following Rotavirus Vaccines, VAERS 2008–2016.
Table 1.
Clinical Reviews of LRTI Death Reports Following RV1 or RV5 Vaccination, VAERS 2008–2016.
| No. | Age (weeks) | Sex | Year | Vaccine Group | Other vaccines | Onset Interval (days) | Past Medical History/ Family History | Pre-existing Conditions | Cause of Death |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 9 | F | 2008 | RV5 and PCV7 | DTaP HepB IPV Hib | 3 | Sick contacts at home, tobacco smoking at home | RSV bronchiolitis, tinea corporis | Viral pneumonia |
| 2 | 8 | M | 2012 | RV5 and PCV7 | DTaP HepB IPV Hib | 1 | None | Congestion, cough 3 days prior to vaccination | Sudden unexplained infant death. Other findings: parainfluenza (type 3) bronchiolitis |
| 3 | 7 | M | 2012 | RV5 and PCV13 | DTaP HepB IPV Hib | 1 | None | None | Bilateral pneumonia (Group A, Streptococcus pyogenes) |
| 4 | 11 | F | 2014 | RV5 and PCV13 | DTaP HepB IPV Hib | 13 | Unknown | Unknown | Respiratory insufficiency secondary to early bronchopneumonia and chronic interstitial pneumonitis, SIDS |
RSV, respiratory syncytial virus; SIDS, sudden infant death syndrome
Table 2.
Clinical Review of LRTI Non-Death Serious Reports Following RV1 or RV5 Vaccination, VAERS 2008–2016.
| RV1 and PCV13 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Age (weeks) | Sex | Year | Other Vaccines | Onset Interval (days) | Past Medical History/Family History | Pre-existing Conditions | Primary Diagnosis | Outcome |
| 1 | 8 | F | 2014 | DTaP HepB IPV Hib | 0 | None | URTI at the time of vaccination | Non-RSV bronchiolitis, gastroesophageal reflux | Recovered |
| 2 | 8 | M | 2015 | DTaP HepB IPV Hib | 2 | Neonatal transient tachypnea | Influenza-like symptoms for 4 days PTA | RSV bronchiolitis, acute otitis media | Recovered |
| 3 |
9 |
M |
2016 |
DTaP HepB IPV Hib |
0 |
None |
Rhinorrhea, cough, congestion for 3 past days |
Viral bronchitis |
Recovered |
| RV5 and PCV7 | |||||||||
| No. |
Age (weeks) |
Sex |
Year |
Vaccines |
Onset Interval (days) |
Past Medical History/Family History |
Pre-existing Conditions |
Primary Diagnosis |
Outcome |
| 1 |
8 |
M |
2008 |
DTaP HepB IPV |
1 |
Premature birth at 32 weeks |
None |
Pneumonia |
Recovered |
| RV5 and PCV13 | |||||||||
| No. |
Age (weeks) |
Sex |
Year |
Vaccines |
Onset Interval (days) |
Past Medical History/Family History |
Pre-existing Conditions |
Primary Diagnosis |
Outcome |
| 1 | 9 | F | 2011 | DTaP IPV Hib HepB | 1 | Down Syndrome, congenital heart disease | Rhinorrhea, nasal congestion, wheezing 3 days PTA. Several family members had respiratory illness | Bronchiolitis | Recovered |
| 2 | 7 | F | 2011 | DTaP HepB IPV Hib | 0 | None | None | Pneumonia, viral URTI | Recovered |
| 3 | 9 | M | 2011 | DTaP HepB IPV Hib | 14 | Sickle cell trait | Rhinorrhea | Pneumonia, Sweet Syndrome | Recovered |
| 4 | 8 | M | 2012 | DTaP IPV Hib | 5 | Neonatal jaundice FH: Graves’ disease | Mother has had URTI symptoms | Acute bronchiolitis | Recovered |
| 5 | 7 | F | 2012 | DTaP IPV Hib HepB | 2 | Eczema | Sibling with congestion at home | Bronchiolitis, enterococcal bacteremia | Recovered |
| 6 | 8 | M | 2013 | DTaP IPV Hib | 23 | None | Rhinorrhea for 1–2 weeks PTA | RSV bronchiolitis | Recovered |
| 7 | 9 | M | 2015 | DTaP HepB IPV Hib | 0 | FH: asthma, allergies | Bronchiolitis (cough, congestion and wheezing) 1 week PTA | Acute bronchitis due to rhinovirus | Recovered |
| 8 | 8 | F | 2015 | DTaP HepB IPV Hib | 1 | Gastroesophageal reflux and prior ED visit for acute life-threatening event due to gastroesophageal reflux, ‘noisy breathing” noted since birth | Nasal congestion for 4 days PTA | Viral bronchiolitis | Recovered |
| 9 | 8 | M | 2015 | DTaP IPV Hib | 0 | FH: diabetes mellitus | Nasal congestion 2 days PTA | Aspiration pneumonia, bilateral pneumothorax, upper GI bleed, | Recovered |
| 10 | 8 | M | 2016 | DTaP IPV Hib | 2 | Premature birth at 30 weeks. NICU inpatient from birth for 20 days Maternal history of hepatitis C, IVDU | None | Bronchiolitis due to rhinovirus | Recovered |
URTI, upper respiratory tract infection; RSV, respiratory syncytial virus; PTA, prior to admission; FH, family history; ED, emergency department; GI, gastrointestinal tract; NICU neonatal intensive care unit; IVDU, intravenous drug use.
We did not observe any trends in the number of reports with either vaccine within the 0–6 day or 0–29 day onset intervals. The proportion of infants aged 6 to 15 weeks reported with a LRTI within 0–29 days post vaccination who received RV5-PCV13 vs. RV1-PCV13 was (15/1,118) 1.3% vs. (5/419) 1.2%, respectively; the proportion of infants who received RV5-PCV7 vs. RV1-PCV7 was (13/993) 1.3% vs. (2/127) 1.6%, respectively (Fig. 1). Both comparisons yielded non-significant differences (p-value = 0.9 and 0.8, respectively). The same analysis for the 0–6 day onset interval yielded for RV5-PCV13 vs. RV1-PCV13 (11/1042) 1.1% vs. (4/388) 1.0%, respectively, and the proportions of infants who received RV5-PCV7 vs. RV1-PCV7 was (9/927) 0.9% vs. (1/113) 0.9%, respectively (data not shown). Both comparisons yielded non-significant differences (p-value = 1.0 and 1.0, respectively). When we searched reports among infants aged 6 to 15 weeks who received either PCV7 or PCV13 (without any RV) and an onset interval of 0–29 days, we identified only two serious reports of LRTI of a total 97 serious reports with these two vaccines. We did not observe any seasonal variation among the reports for either of the RV vaccine groups within the two risk windows of 0–6, 0–29 days; however, the numbers were small (data not shown).
Clinical reviews
Death reports
We identified four deaths: two reports in the RV5-PCV7 group and two reports in RV5-PCV13 (Fig. 1,Table 1). For three of these reports, the cause of death was pneumonia, and the fourth had parainfluenza virus (type 3) bronchiolitis at the time of death. The onset intervals ranged between 1–13 days. Detailed information on each of these reports is summarized in Table 1.
Non-death serious reports
We identified 14 non-death serious reports after immunization with the vaccines of interest. All were hospitalized. Twelve (86%) had an onset interval of 0–6 days post-vaccination, and for the remaining two infants the onset interval was within 7–29 days. All of these infants recovered and were discharged from the hospital in stable condition (Table 2).
Discussion
Our analysis was conducted as a follow-up to a recent manufacturer post-licensure safety study, which identified a potential association between LRTI in infants within 0–6 days following receipt of RV1 dose 1. During our study period, VAERS received only seven LRTI reports out of a total 546 reports following RV1 dose 1 with PCV7 or PCV13 compared to 28 LRTI reports out of a total of 2,111 reports following RV5 dose 1 with PCV7 or PCV13.
Our study did not observe differences in reports of LRTI to VAERS in infants aged 6 to 15 weeks who received dose 1 of RV5 or RV1 with PCV13 or PCV7 and other recommended childhood vaccines (e.g., IPV, Hib) within 0–29 days post vaccination and 0–6 days post-vaccination. In addition, our study did not find any unexpected causes of death or adverse effects (AEs) for non-death serious reports. During 2008–2016, approximately 15.4 million doses of RV1 and 84.7 million doses of RV5 were distributed in the United States (data shown with permission of GSK and Merck). However, we were unable to calculate reporting rates because data on doses distributed by age group for the different vaccine combinations was not available.
As pneumococcal conjugate vaccine (PCV7 or PCV13), concomitantly administered with RV, may be expected to trigger a protective immune response within 0–29 days and thus attenuate the risk of a child developing a LRTI, we restricted our study to only those infants who received RV and either of these two vaccines.6–7 In addition, all the verified LRTI serious reports had co-administration of several other vaccines (e.g., DTaP, IPV, HepB, Hib) at the same visit and some were noted with comorbidities (e.g., prematurity, Down Syndrome, gastroesophageal reflux) (Tables 1,2).
Although the numbers were small, we did not identify any seasonal pattern for the cases within either of the risk windows (0-6 and 0–29 days). In infants aged 6 to 15 weeks who received PCV13 or PCV7, but not RV, VAERS received only two serious LRTI reports (out of a total of 490 reports including 97 serious reports) within 0–29 days indicating that LRTI is not commonly reported to VAERS following these pneumococcal conjugate vaccines in this age-group. In 2016, the most recent year with survey data, the National Immunization Survey-Child at https://www.cdc.gov/vaccines/imz-managers/nis/about.html found 97.8% of children receiving their first RV vaccine between the ages of 6 and 14 weeks of age also received a first PCV between 6 and 14 weeks of age (CDC unpublished data). VAERS received a total of seven LRTI reports for RV administered without PCV; four following RV5 (of which two were serious: one following RV5 alone and one RV5 with HepB and DTaP-Hib-IPV, and two non-serious reports following RV5 alone) and three reports following RV1 (all were serious reports and followed co-administration with other vaccines).
Our study is subject to several limitations including those associated with passive surveillance in VAERS, such as underreporting and stimulated reporting, incomplete information, varying quality of reports, and lack of an unvaccinated comparison group.9 Reports of LRTI following RV vaccines compared with other vaccines (e.g., PCV7, PCV13) may be an example of stimulated reporting indirectly due to provider awareness of the vaccines’ known rare causal association with intussusception. In summary, because of these limitations it is usually not possible to assess for causal associations between a vaccine and an AE(s) using VAERS data. However, as a national surveillance system, VAERS can rapidly detect rare AEs and potential vaccine safety problems, which can be further explored in carefully designed epidemiological studies.9
Overall, the ad hoc nature of the LRTI analysis in the Hoffman et al study and the findings from our VAERS analysis do not fully evaluate the potential association of LRTI and RV. Therefore, a service to the field would be to have a more definitive study powered to detect LRTI risk in the week following RV vaccination with the appropriate comparison group. In conclusion, we found there was no significant difference in LRTI reports to VAERS in 0–6 and 0–29 days following receipt of either RV5 or RV1 with pneumococcal conjugate vaccines.
Methods
Data source
VAERS is a national spontaneous reporting system, established in 1990 and co-managed by the Centers for Disease Control and Prevention (CDC) and FDA that accepts reports of AEs following immunization.9 VAERS accepts reports from vaccine manufacturers, healthcare providers, and vaccine recipients (or their parents/guardians). The VAERS report form obtains information on the demographic characteristics of the vaccine recipient, type of vaccine(s) received and timing and description of AEs experienced.9 Signs and symptoms of AEs documented in the reports are coded using the Medical Dictionary for Regulatory Activities (MedDRA) Preferred Terms (PTs)10; a VAERS report may be assigned one or more PTs.
Reports are classified as serious AEs according to the FDA regulatory definition if one or more of the following are reported: death, life-threatening illness, hospitalization or prolongation of existing hospitalization, or permanent disability.11 Follow-up is conducted for all serious VAERS reports to obtain relevant medical information including a hospital discharge summary (if available) and relevant laboratory data.9
The VAERS database was searched for all primary, domestic VAERS reports for infants vaccinated between February 1, 2008 and December 31, 2016 who received the first dose of either RV1 or RV5 in combination with either PCV7 or PCV13. Other childhood vaccines recommended for this age-group were included.5 Since vaccine dose information often is missing in VAERS reports or cannot be verified, we used the child's reported age information as a proxy to estimate the dose number he/she received. We estimated the first dose by limiting our data search to infants aged 6–14 weeks and 6 days (the recommended age for the first dose of RV5 or RV1) and AEs occurring within 0–29 days post vaccination.
We searched separately for reports of any AE(s) and reports which identified a LRTI diagnosis with any of the following PTs: Bronchiolitis, Bronchitis, Bronchitis Acute, Pneumonia and Pneumonia Influenza. Our study was focused on serious LRTI, which included the relevant medical information to validate a LRTI diagnosis. We searched for all reports (serious and non-serious); however, we were only able to verify a LRTI diagnosis for serious reports11 in which follow-up medical records were obtained.9 Onset interval was defined as the interval from the vaccination date (Day 0) to the reported date of onset of the first symptom(s) within 29 days. In order to assess a possible effect of seasonality or a reporting trend due to other circulating viruses (e.g., respiratory syncytial virus), we further examined these reports by each month, year and vaccine type.
We compared the proportion of reports in infants aged 6 to 15 weeks with onset intervals of 0–6 days and 0–29 days with a diagnosed LRTI who received either RV5-PCV7 vs. RV1-PCV7 and RV5-PCV13 vs. RV1-PCV13 in infants aged 6 to 15 weeks who were reported with any AE within the same onset intervals. In addition, we searched for LRTI reports in infants aged 6 to 15 weeks with symptom onset 0–29 days who received PCV7 or PCV13 with other recommended vaccines but not RV5 or RV1. To assess the statistical significance of the difference between the proportions, we used SAS exact test (SAS version 9.4).
Clinical reviews
CDC investigators (MA and MM) reviewed available medical records of serious reports. For death reports, the cause of death was obtained from the death certificate and/or autopsy report. For non-death serious reports, we determined the primary AE based on medical record documentation including the hospital discharge summary and other diagnostic test results, if available.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.ROTARIX (Rotavirus Vaccine, Live, Oral) oral suspension. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM133539.pdf
- 2.RotaTeq (Rotavirus Vaccine, Live, Oral, Pentavalent) http://www.merck.com/product/usa/pi_circulars/r/rotateq/rotateq_pi.pdf
- 3.Parashar UD, Alexander JP, Glass RI. Prevention of rotavirus gastroenteritis among infants and children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(RR-12):1−13. [PubMed] [Google Scholar]
- 4.Cortese MM, Parashar UD. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2009;58(RR-2):1−25. [PubMed] [Google Scholar]
- 5.Recommended immunization schedule for children and adolescents aged 18 years or younger. United States, 2018. https://www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-combined-schedule.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffman V, Abu-Elyazeed R, Enger C, Esposito DB, Doherty MC, Quinlan SC, Skerry K, Holick CN, Basile P, Friedland LR, et al. Safety study of live, oral human rotavirus vaccine: A cohort study in United States health insurance plans. Hum Vaccin Immunother. 2018;Mar 13:1–9. doi: 10.1080/21645515.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Updated recommendation from the Advisory Committee on Immunization Practices (ACIP) for use of 7-valent pneumococcal conjugate vaccine (PCV7) in children aged 24–59 months who are not completely vaccinated Centers for Disease Control and Prevention (CDC). MMWR Morb Mortal Wkly Rep. 2008;57:343−4. [PubMed] [Google Scholar]
- 8.Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children — Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59;258−61. [PubMed] [Google Scholar]
- 9.Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33:4398−405. doi: 10.1016/j.vaccine.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medical Dictionary for Regulatory Activities Support Documentation. http://www.meddra.org/how-to-use/support-documentation; Accessed May 20, 2018
- 11.U.S. Food & Drug Administration. CFR – Code of Federal Regulations. 21 CFR §600.80. Revised April 1, 2017. Available at http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=600.80


