Abstract
A plasma membrane amino acid transporter B0,+ (ATB0,+), encoded by the SLC6A14 gene, is specific for neutral and basic amino acids. It is up-regulated in several types of malignant cancers. Neurotransmitter transporters of the SLC6 family interact with specific SEC24 proteins of the COPII complex along their pathway from the endoplasmic reticulum (ER) to Golgi. This study focused on the possible role of SEC24 proteins in ATB0,+ trafficking. Rat ATB0,+ was expressed in HEK293 cells, its localization and trafficking were examined by Western blot, deglycosylation, immunofluorescence (co-localization with ER and trans-Golgi markers) and biotinylation. The expression of ATB0,+ at the plasma membrane was decreased by dominant negative mutants of SAR1, a GTPase, whose activity triggers the formation of the COPII complex. ATB0,+ co-precipitated with SEC24C (but not with the remaining isoforms A, B and D). This interaction was confirmed by immunocytochemistry and the proximity ligation assay. Co-localization of SEC24C with endogenous ATB0,+ was also observed in MCF-7 breast cancer cells. Contrary to the endogenous transporter, part of the overexpressed ATB0,+ is directed to proteolysis, a process significantly reversed by a proteasome inhibitor bortezomib. Co-transfection with a SEC24C dominant negative mutant attenuated ATB0,+ expression at the plasma membrane, due to proteolytic degradation. These results support a hypothesis that lysine at position +2 downstream of the ER export “RI” motif on the cargo protein is crucial for SEC24C binding and for further trafficking to the Golgi. Moreover, there is an equilibrium between ER export and degradation mechanisms in case of overexpressed transporter.
Keywords: Amino acid transporter, SLC6A14, SEC24 proteins, SAR1, ER export
1. Introduction
Essential nutrients enter the cells via specialized transport systems located at the plasma membrane. Amino acids are used as both, energetic substrates and protein building blocks, and can be taken up by the cells due to the activity of transporters, which differ in substrate specificity, energy dependence as well as regulation of their cellular expression. The amino acid transporter B0,+ (ATB0,+), encoded by the SLC6A14 gene, belongs to a superfamily of neurotransmitter transporters of amino acids and osmolytes, which co-transport their substrates along with Na+ and Cl− ions (for reviews see, [1,2]). Indices “0” and “+” refer to ATB0,+ substrate specificity, namely neutral and basic amino acids, respectively. ATB0,+ was originally cloned from human mammary gland and its mRNA was detected in hippocampus, salivary gland, lungs and trachea [3], but it was also found to be expressed in the brain, particularly in astrocytes and brain capillary endothelial cells forming the blood-brain barrier [4–6]. Due to its broad substrate specificity, ATB0,+ appears to play a key role in amino acid delivery to cancer cells. ATB0,+ was found to be up-regulated in malignant estrogen-receptor positive breast cancer lines [7,8]. Apart from the possibility of SLC6A14 regulation at transcriptional level, ATB0,+ is known to be regulated post-translationally as well. It has seven potential glycosylation sites in its second extracellular loop and one in its third extracellular loop [3]. When overexpressed, its electrophoretic mobility is lower than predicted from its amino acid sequence [9], suggestive of a highly glycosylated form in vivo. Moreover, activation of protein kinase C resulted in increased transporter phosphorylation of a serine moiety and increased amount of ATB0,+ in the plasma membrane, as well as in an augmented transport activity catalyzed by ATB0,+ [5,9]. These observations hinted toward a possible regulation of ATB0,+ during the course of its trafficking to the cell surface.
Trafficking of many transporters has been reported to play a significant role in their regulation and their expression at the plasma membrane. Several studies revealed that protein kinase C (PKC) can regulate SLC6 family members. Transporters for dopamine [10,11], serotonin [12], noradrenaline [13,14] and glycine [15–17] were reported to undergo internalization upon PKC stimulation, which correlated with decreased transport activity. We observed an opposite phenomenon in the case of ATB0,+ whose activity and surface expression were increased upon PKC activation [5,9].
ATB0,+ is a highly hydrophobic protein comprised of twelve transmembrane domains. It is delivered to the plasma membrane following several steps of vesicle budding, movement and fusion to different membranous compartments. The first step of transporter trafficking is the export from the endoplasmic reticulum (ER) compartment to cis-Golgi. This anterograde transport relies on coatomer II (COPII) proteins. The assembly of COPII begins with the recruitment of SEC23/SEC24 proteins by the GTPase SAR1, after GDP-GTP exchange is catalyzed by SEC12, an ER-resident guanine nucleotide exchange factor. Subsequently, the SEC13/SEC31 complex is also recruited. The hydrolysis of GTP on SAR1 results in vesicle fission (for review, see [18]). SLC6 transporters were shown to depend on a direct interaction with SEC24 proteins, and that this interaction relies on specific SEC24 paralogs, on ER export motifs localized on the cargo protein C-terminal regions. A hydrophobic residue at the +2 position downstream from the ER export motif correlated with a preference for SEC24 isoform D, while a hydrophilic residue at the same site preferentially recruited SEC24 isoform C [19]. Similar to the serotonin transporter, the C-terminal of ATB0,+ also contains an ER export “RI” motif, with a lysine moiety at the +2 position, qualifying for an interaction with SEC24C [19,20]. Therefore, the present study focused on a putative role of SEC24 proteins in ATB0,+ trafficking between the ER and Golgi, the initial step in transporter delivery to its site of action at the plasma membrane.
2. Materials and Methods
2.1. Materials
Polyclonal antibodies against SEC24 A, B, C and D isoforms used in immunoprecipitation experiments were purchased from Cell Signaling Technology (Denver, Massachusetts, USA), rabbit anti-SEC24C antibody (NBP1-81550) and rabbit polyclonal anti-calnexin antibody were from Novus Biologicals (Cambridge, UK). Biotin rabbit anti-SLC6A14 antibody was from USBiological Life Sciences (VWR International, Gdańsk, Poland). TGN (trans-Golgi network protein 2) goat polyclonal antibody (C-15) was from Santa Cruz Biotechnology, Inc. (AMX, Łódz, Poland). Alexa Fluor 568® goat anti-mouse, Alexa Fluor 488® goat antirabbit, Alexa Fluor 568® donkey anti-rabbit, Alexa Fluor 488® donkey anti-mouse, Alexa Fluor 647® donkey anti-goat antibodies, Alexa Fluor 568® conjugated streptavidin, ProLong® Diamond Antifade Mountant with DAPI and TO-PRO®-3 were from Thermo Fisher Scientific (Life Technologies Polska, Warsaw, Poland). Alexa Fluor® 488-conjugated AffinityPure Fab Fragment Goat Anti-Rabbit IgG (H + L) was from Jackson ImmunoResearch Laboratories, Inc. (Ely, Cambridgeshire, UK). EZ-Link® Sulfo-NHS-LC-Biotin [Sulfosuccinimidyl-6-(biotinamido) hexanoate] and Pierce® Avidin Agarose Resin were from Pierce (Rockford IL, USA), jetPRIME® transfection reagent was from Polypus Transfection (VWR International, Gdańsk, Poland). Peptide-N4-(acetyl-β-glucosaminyl)-asparagine amidase (PNGase F, EC 3.5.1.52), endoglycosidase H (Endo H, EC 3.2.1.96) and plasmid pCI-neo were from PROMEGA (Warsaw, Poland). Bortezomib (PS-341) was from Selleckchem (STI, Poznań, Poland). Geneticin (G418) was from BioShop (Burlington, Canada). Duolink in situ detection kit, monoclonal anti-FLAG M2 antibody, anti-FLAG M2 affinity agarose gel, rabbit serum, IGEPAL CA-630 (octylphenoxypolyethoxyethanol, nonidet P-40) and all other reagents were from Sigma (Poznań, Poland).
2.2. Vectors
Rat ATB0,+ cDNA was cloned into the p3xFLAG-CMV14 vector (p3xFLAG-CMV14/B0,+), as described [9]. Vectors encoding dominant negative mutants of Sar1 GTPase (Sar1a-T39N and Sar1b-T39N) were obtained as given in [21]. Vectors pCI-neo encoding dominant negative mutants of SEC24 isoforms - Sec24C-D796V/D797N (Sec24C-VN) and Sec24D-D733V/N734N (Sec24D-VN) were obtained and used as given in [20,21].
2.3. Cell culture and treatment
Epithelial adenocarcinoma MCF-7 cells (kindly provided by prof. Bożena Kamińska-Kaczmarek, Nencki Institute of Experimental Biology, Warsaw, Poland) were cultured in 10% fetal bovine serum, 90% Dulbecco's modified Eagle's medium supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml) and fungizone (0.25 μg/ml). HEK293 cells (ATCC, Manassas, VA, USA) were cultured in 10% fetal bovine serum, 90% Eagle's minimum essential medium supplemented with penicillin, streptomycin and fungizone, as given above. Cells were cultured at 37 °C in a humid atmosphere of 5% CO2. Transfection of HEK293 cells was performed with jetPRIME® according to the supplier protocol. The cells were either taken for experiments after 24 h or 48 h (transient transfection) or grown in the culture medium supplemented with 300 μg/ml geneticin (stable transfection), as described in [9]. Transfection with the dominant negative mutants Sar1a-T39N and Sar1b-T39N [19,21,22] was done simultaneously with p3xFLAG-CMV14/B0,+. Each plasmid encoding dominant negative Sar1 mutants (Sar1a-T39N and Sar1b-T39N) was used in 3:1 excess over p3xFLAG-CMV14/B0,+ [23], plasmids encoding Sec24C-VN or Sec24D-VN were used in excess over p3xFLAG-CMV14/B0,+, as indicated in the figure legends [24]. In experiments with protein kinase C activations the cells were washed with 138 mM NaCl, 10 mM Tris, pH 7.4 and treated for 30 min either with 0.1% dimethylsulfoxide (DMSO) as a vehicle or with 200 nM phorbol 12-myristate 13-acetate (PMA). Cell viability was assayed using 0.4% trypan blue staining and a hemocytometer chamber.
2.4. Immunoblotting and immunoprecipitation
After the pretreatment outlined in the pertinent figure legends, cells were washed with PBS and lysed in 150 mM NaCl, 10 mM EDTA, 1% IGEPAL CA-630 (nonidet P-40), 50 mM Tris, pH 7.4 supplemented with protease inhibitor cocktail. After estimation of protein content the samples containing an equal amount of protein were subjected either to immunoblotting or to immunoprecipitation with anti-FLAG M2 affinity agarose gel. Elution was performed with 3xFLAG® PEPTIDE (3 μg/100 μg protein). The blots were analyzed with antibodies indicated in figure legends.
2.5. Immunocytochemistry analysis
HEK293 cells were washed three times with phosphate buffered saline (PBS) and fixed with methanol precooled at −20 °C. The cells were kept at −20 °C for 15 min, followed by three consecutive washes with PBS at room temperature. The unspecific binding sites were blocked for 2 h at room temperature with 5% goat serum in PBS. The cells were treated for 1 h at 4 °C with anti-FLAG antibody (1:1000) and one of the following antibodies: anti-calnexin antibody (1:500), anti-TGN38 (1:100), anti-SEC24C (1:200), anti-SEC24D (1:100). The following antibodies (1:500 each) were used for detection of primary antibodies: Alexa Fluor 568 conjugated goat anti-mouse and Alexa Fluor 488 conjugated goat anti-rabbit antibodies in double labeling, Alexa Fluor 568® donkey anti-rabbit, Alexa Fluor 488® donkey anti-mouse, Alexa Fluor 647® donkey anti-goat antibodies in triple labeling experiment. MCF-7 cells were fixed with methanol, as described above and after blocking with goat serum were incubated with either SEC24C (1:200) or SEC24D (1:100) antibodies and, after washing, with the Alexa Fluor® 488-conjugated AffinityPure Fab Fragment Goat Anti-Rabbit IgG (H + L) (1:400). The cells were next blocked overnight with 5% goat serum in PBS, then washed 3 times with PBS and additionally fixed for 10 min at room temperature with 1% paraformaldehyde. After 3 washes with PBS, they were incubated for 20 min with 20 mM NH4Cl in PBS, washed 3 times with PBS and blocked for 2 h with 5% goat serum. They were subsequently washed 3 times with PBS and blocked for 2 h at room temperature with 20% rabbit serum in PBS. After washing with PBS, the cells were next incubated with the anti-SLC6A14-biotin conjugated antibody (1:250), followed by incubation with Alexa Fluor 568® conjugated streptavidin (1:500). The cells were next rinsed 3 times with PBS and the samples were mounted in ProLong® Diamond Antifade Mountant with DAPI. The cells were examined with Confocal Microscopes Zeiss LSM 780 or Zeiss LSM 800 with Airyscan Detector (in both cases using 63× oil immersion objective), with excitation at 488 nm and emission at 495–550 nm for Alexa Fluor 488, excitation at 561 nm and emission at 580–640 nm for Alexa Fluor 568 and excitation at 647 nm and emission at 645–700 nm for Alexa Fluor 647. Excitation at 405 nm and emission at 414–471 nm were used for detection of DAPI.
2.6. Proximity ligation assay
A possibility of a direct interaction of ATB0,+ with the SEC24 proteins was analyzed by proximity ligation assay. HEK293 cells were transfected with either p3xFLAG-CMV14 or p3xFLAG-CMV14/B0,+. The cells were subsequently washed and fixed with methanol, as for immunocytochemistry experiments, followed by incubation with two primary antibodies: anti-FLAG (1:1000) and either anti-SEC24C (1:200) or anti-SEC24D (1:100), as indicated in the figure legend. The next steps: washing, incubation with assay probes, ligation with the ligase and amplification with polymerase followed the supplier protocol. The analysis, after mounting in a medium with DAPI, was performed with the Zeiss LSM 800 spectral confocal microscope (using 40× oil immersion objective), with excitation at 400 nm and emission at 405–490 nm for DAPI (nuclei) and excitation at 561 nm and emission at 568–712 nm for Reagents Orange.
2.7. Biotinylation
After experimental treatment indicated in the figure legends, HEK293 cells were washed with ice-cold PBS containing 0.1 mM CaCl2 and 1 mM MgCl2 (PBS/Ca/Mg) and incubated in the same solution supplemented with 1 mg/ml EZ-Link® Sulfo-NHS-LC-Biotin for 30 min at 4 °C. The free sulfo-NHS-biotin was removed by incubation with 100 mM glycine in PBS/Ca/Mg for 15 min at 4 °C with shaking and two washes with ice-cold PBS/Ca/Mg. The cells were collected in RIPA buffer (150 mM NaCl, 10 mM EDTA, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris, pH 7.4) supplemented with proteases inhibitors. Homogenization was performed on ice by passing through a 25G needle. The lysates were centrifuged at 1,000 ×g for 15 min at 4 °C. The supernatants were collected and, after estimation of protein content, the samples containing equal amounts of protein (100 μg) were subjected to binding to Pierce® Avidin Agarose Resin according to the supplier protocols. Biotinylated proteins were eluted from Avidin Agarose Resin with sample buffer and subjected to electrophoretic separation and Western blot analysis.
2.8. Deglycosylation
HEK293 cells were transfected with p3xFLAG-CMV14/B0,+ vector. After washing the cells were lysed, as described in the Section 2.4. For treatment with PNGase F samples containing 100 μg protein in 35 μl were supplemented with 2.5 μl 2% SDS with 1 M 2-mercaptoethanol, according to the protocol provided by the PNGase F supplier. Denaturation by heating was omitted to avoid protein aggregation. The sample was supplemented with 2.5 μl of 15% Triton X-100, 10 units PNGase F and incubated for 24 h at 37 °C. For treatment with Endo H the samples containing 100 μg protein in 22 μl were supplemented with 1 μl of 10× denaturating solution, heated for 5 min at 95 °C and subsequently cooled to room temperature. Next 2 μl of 10× Endo H reaction buffer was added to the samples, followed by addition of 5 μl of Endo H (2500 U) and water to increase total volume to 40 μl. The reaction was allowed to proceed for 18 h at 37 °C. The reactions were terminated by adding 5× concentrated sample buffer, electrophoresis and Western blot analysis.
2.9. Statistical analysis
At least 3 experiments were performed for each Western blot analysis, and, where indicated, the quantitative analysis was performed with use of the gel analysis tools in Fiji [25]. Where applicable, the mean ± SD was calculated for each set of experiments. The statistical analysis was performed with the software package GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA) using an unpaired t-test with Welch's correction and ANOVA with Tuckey's multiple comparison test for the comparison of two and more than two samples, respectively. The threshold of statistical significance was set at P < 0.05.
3. Results
3.1. COPII is necessary for ATB0,+ trafficking to the plasma membrane
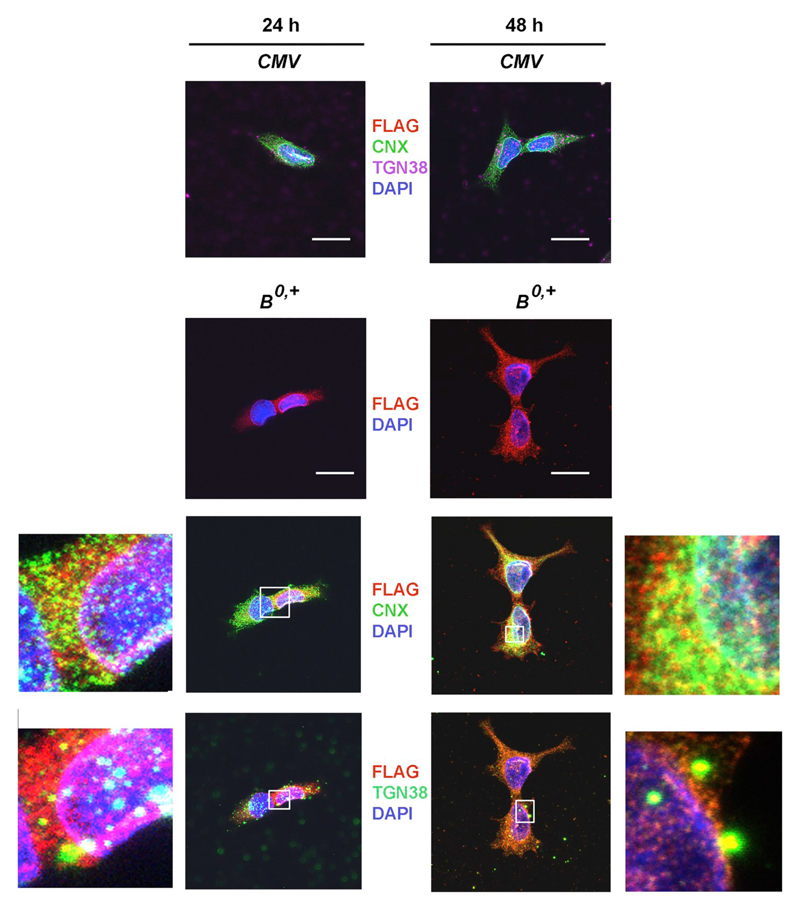
In order to study the mechanism of transporter exit from the ER, we aimed at identifying the time points when ATB0,+ was synthesized and when its trafficking to the Golgi commenced. We analyzed the cellular localization of the transporter using an antibody against calnexin, an ER resident protein responsible for the folding of newly synthesized N-linked glycoproteins and an ER lectin chaperone [26,27] and antibodies against trans-Golgi network protein 2 (TGN38) [28]. As shown in Fig. 1, there was more transporter 48 h after transfection. In addition, after 24 h ATB0,+ was mainly confined to the perinuclear region, while 48 h after transfection was also visible in cell protrusions and on the cell surface. At both time points, immunostaining for ATB0,+ was co-localized with calnexin indicating the presence of ATB0,+ in the ER. In contrast co-localization with the trans-Golgi marker (TGN38) was very sporadic 24 h after transfection and became more pronounced after 48 h. These observations indicate that more ATB0,+ leaves the ER after 48 h.
Fig. 1.
Localization of ATB0,+ 24 h or 48 h after transfection of HEK293 cells with the p3xFLAG-CMV14/B0,+ vector (B0,+) or p3xFLAG-CMV14 (CMV). Detection was performed, as described in the Materials and methods Section 2.5 with anti-FLAG antibody (red), ER was visualized with anti-calnexin antibody CNX (green), nuclei with DAPI (blue), trans-Golgi network with anti TGN38 (either green or magenta) The selected areas were magnified and shown either to the left or to the right of the respective panels. Bar 20 μm.
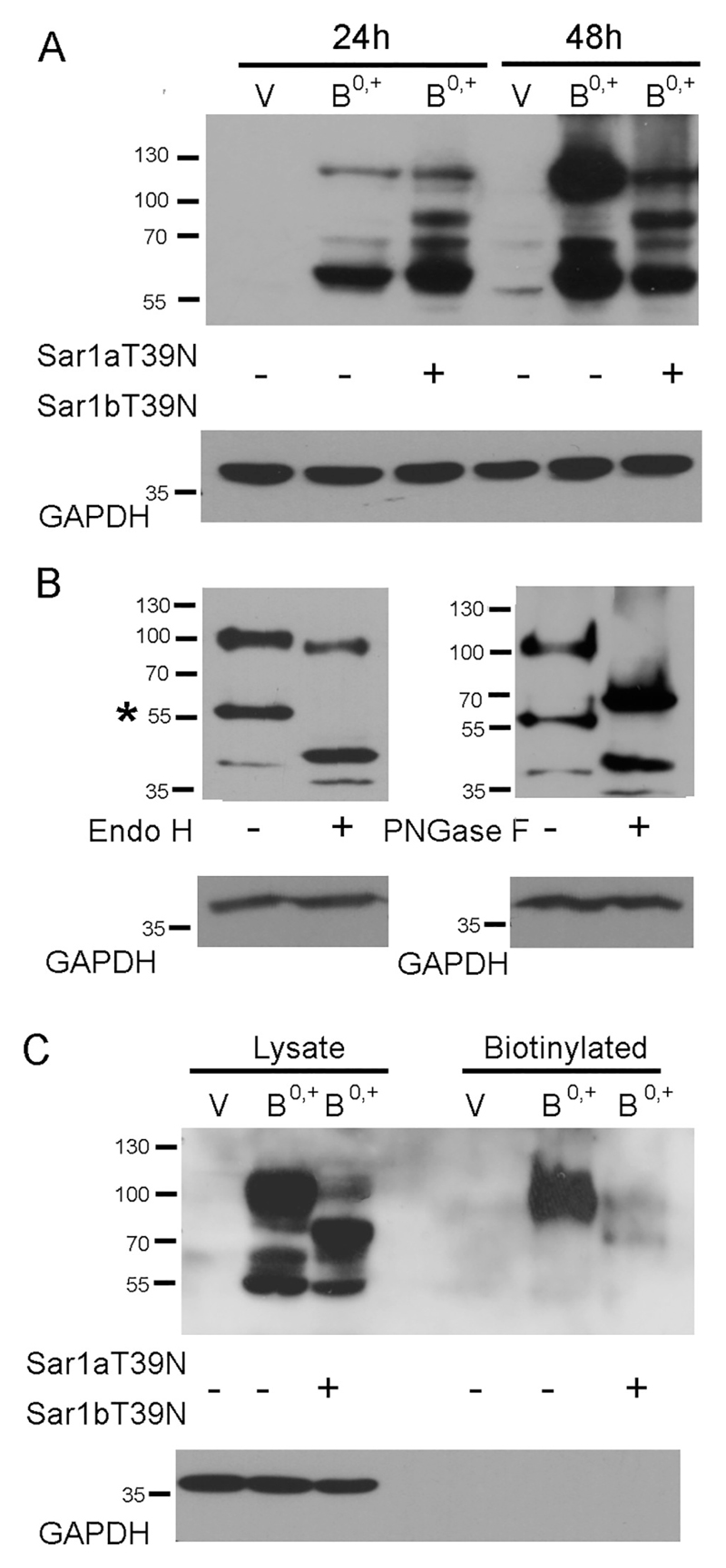
In order to elucidate the mechanism of transporter exit from the ER, we studied the effects of negative dominant mutants of Sar1, a GTP-binding protein, which initiates the formation of COPII vesicles. As shown in Fig. 2A, 24 h after transfection the major fraction of the transporter can be seen as non-glycosylated band migrating with molecular mass of about 55,000–60,000, while the fully glycosylated band can be seen as a species of low electrophoretic mobility. In the presence of Sar1 dominant negative mutants, the additional intermediate bands can be seen, representing core glycosylation products. The Western blot data indicate that a majority of the transporter still resides in the ER after 24 h, suggesting this time point unsuitable for studies of possible inhibition of transporter exit from ER. When a similar analysis was performed 48 h after ATB0,+ transfection, 65% of ATB0,+ was detected as a fully glycosylated, high molecular weight species. Co-expression of the dominant negative Sar1 mutants attenuated the amount of the high molecular weight transporter species and resulted in the formation of additional bands representing intermediate glycosylation products. In order to verify that the high molecular weight and intermediate bands represents the glycosylated forms of the transporter, the cell extract was treated either with Endo H or with PNGase F. After incubation in the presence of Endo H, the band migrating slightly above 55 kDa was shifted in a quantitative manner to higher mobility (left-hand panel, Fig. 2B). Hence this band represents the core-glycosylated species (marked by an asterisk in Fig. 2B). In contrast, the high molecular weight band (migrating at 100 kDa) disappeared after digestion with PNGase F (Fig. 2B, right-hand panel). Hence this species corresponds to the mature glycosylated species. We note that after digestion with PNGase F, we observed two bands, i.e. the fully deglycosylated species, which was also produced by Endo H, and a second band migrating at about 60 kDa. This band presumably corresponds to incompletely cleaved intermediates. ATB0,+ has eight potential N-linked glycosylation sites [3], hence it is not surprising that complete deglycosylation is not readily achieved. Similarly, we note that incubation with Endo H produced a subtle shift of the high molecular band to higher mobility (Fig. 2B, left-hand panel). This may reflect the fact that with so many potential glycosylation sites, not all core glycans are subjected to complex glycosylation in the Golgi apparatus and are thus successible to cleavage by Endo H. We relied on cell surface biotinylation to verify by an independent approach that the band migrating at 100 kDa was localized at the cell surface: cells were treated with a membrane-impermeable biotinylation reagent sulfo-NHS-biotin and the biotinylated proteins were enriched by affinity purification. Only the high molecular weight species of ATB0,+ was seen (Fig. 2C); biotinylation of this high molecular form was almost completely absent after co-transfection with dominant negative Sar1 mutants (last lane in Fig. 2C). We further analyzed the trafficking direction of ATB0,+ containing vesicles looking at co-localization with various Rab GTPases, known to be involved in various steps of vesicular transport [29,30]. As shown in the supplementary material (Fig. S1), after 24 h we found co-localization with Rab1, a protein playing a role in the ER to Golgi trafficking. Co-localization with Rab11, which is important in the traffic to the plasma membrane was detected after 24 and 48 h time points (Fig. S1). No co-localization occurred between ATB0,+ and Rab5, known to be involved in trafficking of endocytic vesicles (Fig. S1), pointing to a low turn-over of the transporter. These data led us to select 48 h as a time point for further studies of ATB0,+ transporter trafficking to the cell surface, i.e. the time at which a portion of the transporter already reaches the plasma membrane and is present there in its mature, fully glycosylated form.
Fig. 2.
COPII dependent exit of ATB0,+ from ER. (A) The amount of ATB0,+ was analyzed by Western blot 24 h or 48 h after transfection either under control conditions or after transfection with Sar1 dominant negative mutants Sar1aT39N and Sar1bT39N. V – transfection with CMV vector without insert (p3xFLAG-CMV14). (B) Deglycosylation of ATB0,+ performed 48 h after transfection. The core-glycosylated band (Endo H –sensitive) is indicated by asterisk. (C) Biotinylation of cell surface proteins with a membrane impermeable reagent performed 48 h after transfection. GAPDH was detected, as loading control.
3.2. Out of four SEC24 paralogs, only SEC24C isoform co-precipitates with ATB0,+
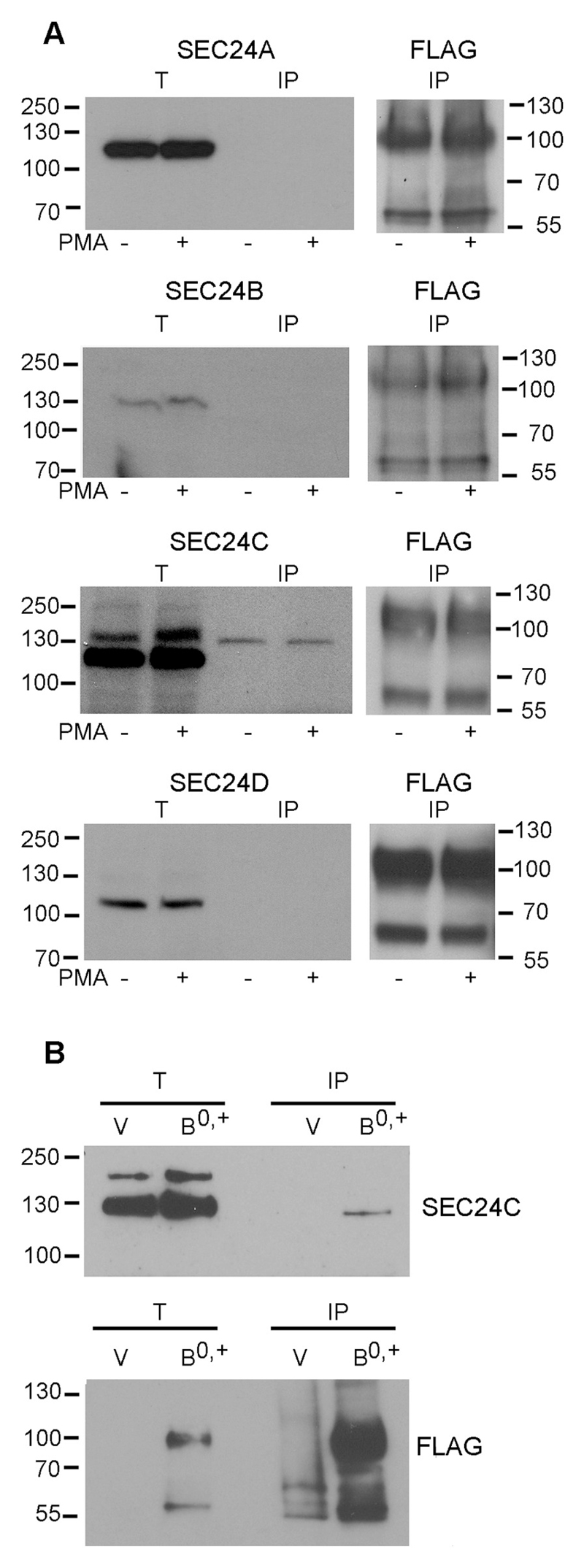
Cargo proteins bind different SEC24 isoforms in a selective manner. They preferentially recruit either A/B, e.g. type I membrane proteins (vesicular stomatitis virus glycoprotein, ERGIC53), or C/D isoforms, e.g. glycosylphosphatidylinositol-anchored proteins [31]. Members of the SCL6 family were shown to interact either with SEC24C or SEC24D proteins to allow for their exit from the ER. This preference toward a particular SEC24 isoform was found to depend on the nature of the amino acid at the +2 position downstream from the ER export motif on transporter C-terminal regions. Alignment of C-terminal amino acid sequences of three SLC family members [32] shows that the C-terminus of ATB0,+ contains, like SERT, a lysine residue at the +2 position downstream from the ER export RI motif (Table 1). In the family of neurotransmitter transporters, this hydrophilic lysine residue was shown to be crucial for recognizing the SEC24C isoform. In contrast, a hydrophobic residue (e.g. valine) in GAT-1 was shown to be responsible for an interaction with the D isoform [19]. Our aim was to verify that this rule also applied to the amino acid subfamily of SLC6 proteins. As is evident from Fig. 3, only SEC24C was detected as the isoform co-precipitating with ATB0,+. Activation of protein kinase C (PKC) by PMA (phorbol 12-myristate 13-acetate) increased surface levels of ATB0,+ [5,9]. However, the amount of SEC24C, which was co-precipitated with ATB0,+ was not affected by preincubation of the cells with PMA, indicating that this kinase is not involved in ATB0,+ exit from the ER, but rather in further steps of trafficking to the plasma membrane. It is worth noting that SEC24C was detected as bands of different electrophoretic mobility, resulting possibly from posttranslational modifications, most probably phosphorylation, since it contains several phosphorylation sites (https://www.phosphosite.org/proteinAction?id=5736&showAllSites=true) and there are reports on SEC24C phosphorylation, when co-expressed with AKT kinase [33]. We wanted to further verify, if SEC24C can be detected with other antibody (Novus Biologicals) and to establish which band co-precipitates with ATB0,+. As shown in Fig. 3B, SEC24C co-precipitates with the FLAG-tagged transporter and is not detected when the cells are transfected with the vector without an insert. Moreover, the species of SEC24C co-precipitating with the transporter migrates with higher mobility. This observation would suggest that it is a non-phosphorylated or less phosphorylated SEC24C interacting with ATB0,+, however, this hypothesis should be verified experimentally.
Table 1. Amino acid sequence alignment of C-termini of selected SLC6 transporters.
| Transporter | Amino acid sequence of C-terminus |
|---|---|
| rATB0,+ | KIVRAEG-NILQRIIKCCRPASNWGPYLEKHRGERYKDMA----EPAK---------------------ETDHEIPT-ISGTRKPE |
| hSERT | RLIITPG-TFKERIIKSITPETPTEIP-C-------------------------------------------GD------IRLNAV |
| hGAT1 | MFLTLKG-SLKQRIQVMVQPSEDIV-RPE-NGPE----------------------------------QP-Q--AGS-STSKEAYI |
The ER export motifs RI are indicated in bold, the residues proposed [19] determine binding of SEC24C (lysine) and SEC24D (valine) are indicated in bold underlined type. Multiple sequence alignment was performed with use of T-coffee programme [32]. The accession numbers for rat ATB0,+ - NM_001037544, for human SERT - NP_001036 and for human GAT1 - NP_003033.
Fig. 3.
Verification of ATB0,+ interaction with SEC24 proteins. (A) HEK293 cells stably transfected with p3xFLAG-CMV14/B0,+ vector were treated either with DMSO alone or with 200 nM PMA (PKC activator). ATB0,+ has been immunoprecipitated with anti-FLAG antibody immobilized on agarose resin, as described in Materials and methods and eluted with FLAG peptide. The eluates (IP) were analyzed with antibodies against SEC24 isoforms (Cell Signaling Technology). (B) Analysis of SEC24C co-precipitating with ATB0,+ (Novus Biologicals anti-SEC24C antibody). For better separation of SEC24C double bands, the lower acrylamide concentration (8%) was used in the separating gel. Cells were either transfected with p3xFLAG-CMV14 vector (V) or with p3xFLAG-CMV14/B0,+ vector (B0,+). T, corresponds to Western blot analysis of the total cell extract, IP for the analysis of fraction eluted with FLAG peptide. As controls, immunoprecipitates were analyzed for the presence of ATB0,+ with anti-FLAG antibodies.
3.3. SEC24C plays a key role in ATB0,+ export from the ER compartment
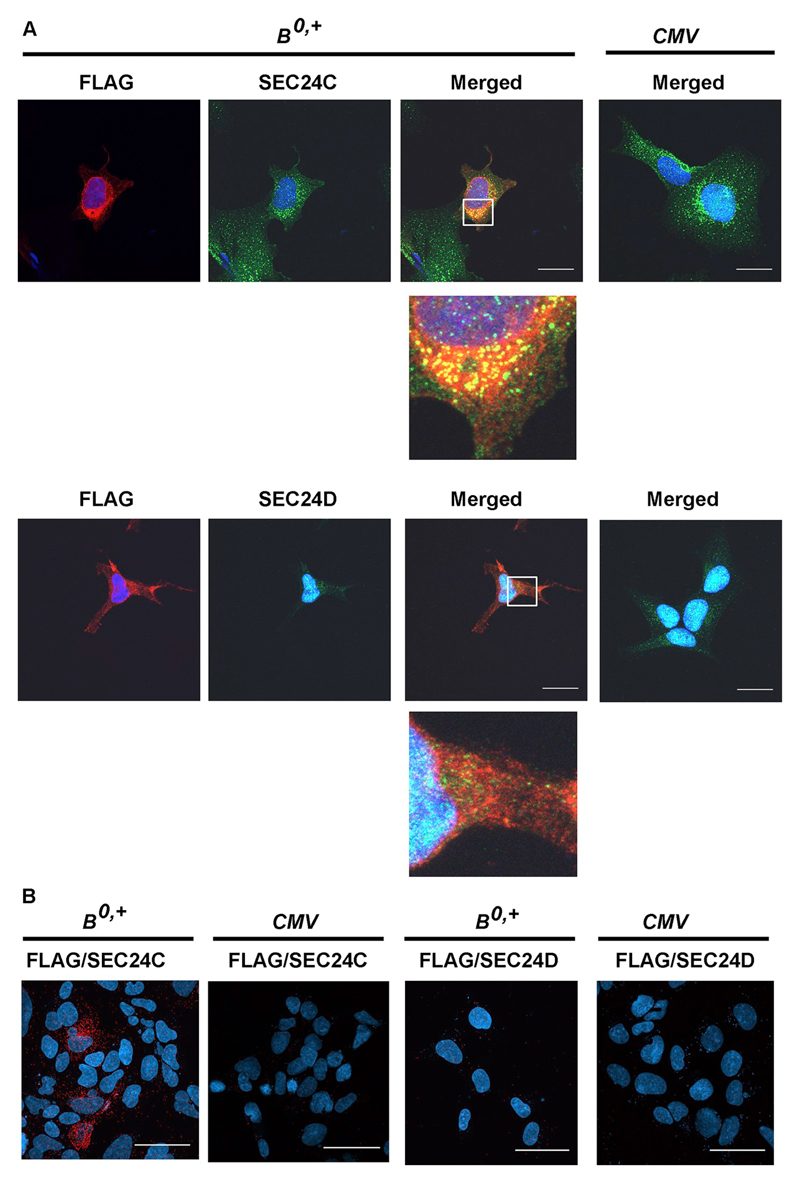
We investigated the nature of interaction between ATB0,+ and SEC24C by immunofluorescence. As shown in Fig. 4A, we detected co-localization between the two proteins, visible as yellow puncta close to the cell nuclei, which was consistent with immunoprecipitation experiments. No co-localization was detected when we used anti-SEC24D antibodies in the assays. However, co-localization only provides evidence for spatial vicinity rather than for a direct protein-protein interactions. Accordingly, we opted for using a PLA assay, which allows for detection of interactions between two proteins not farther than 40 nm from each other. As displayed in Fig. 4B, such a direct interaction could only be detected with SEC24C.
Fig. 4.
Analysis of ATB0,+ interaction with SEC24C and SEC24D. HEK293 cells were transfected for 48 h with the p3xFLAG-CMV14/B0,+ vector (B0,+) or p3xFLAG-CMV14 (CMV). (A) Localization of ATB0,+ was detected with anti-FLAG antibody (red), SEC24C and SEC24D with the corresponding antibodies (green), nuclei with DAPI (blue). Magnifications of selected areas are shown below the merged pictures. Bar 20 μm. (B) Proximity ligation assay performed with anti-FLAG and either anti-SEC24C or anti-SEC24D antibodies. Representative pictures out of 10 different images are shown. Bar 50 μm.
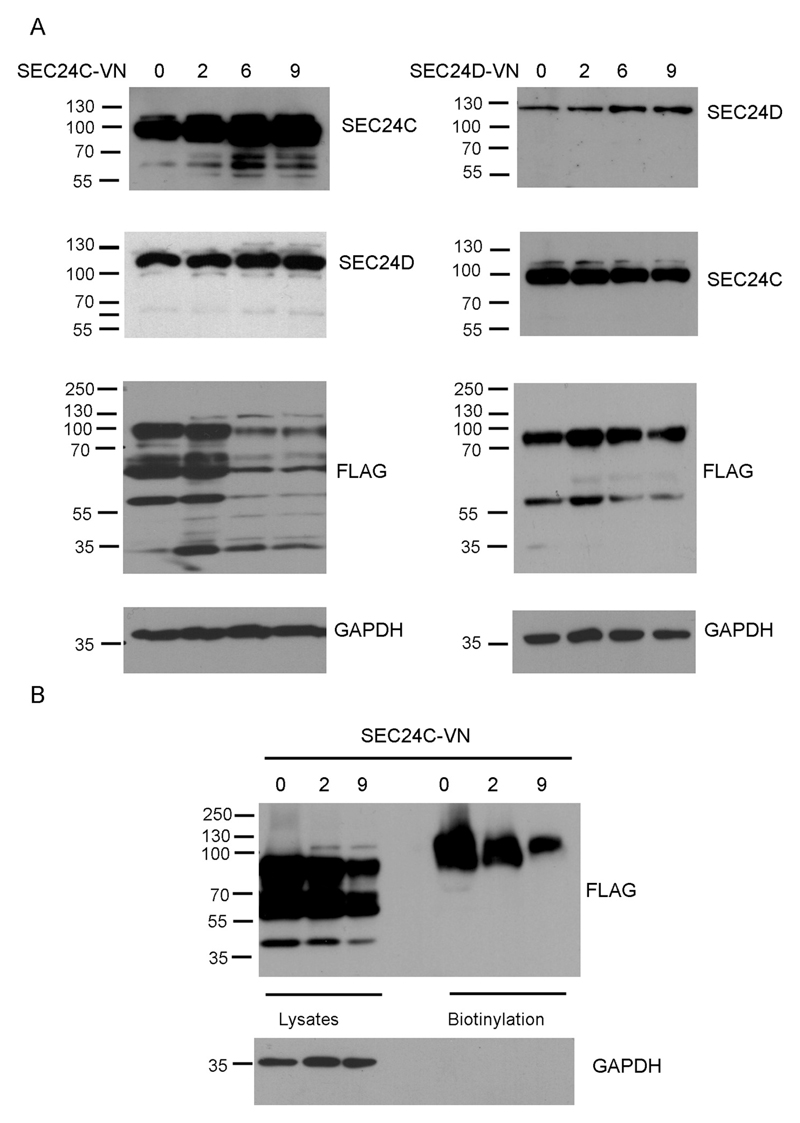
We further analyzed whether co-transfection with dominant negative mutants SEC24C-VN and SEC24D-VN affected ATB0,+ levels at the plasma membrane. These mutants were shown previously to reduce surface levels and substrate uptake by SERT and NET, respectively [20]. As shown in Fig. 5A, vectors carrying both genes had to be added in substantial excess (9 times more than ATB0,+ gene-carrying vector) in order to detect an increased level of both SEC24 proteins (150 ± 15% of SEC24C when compared to the control and 160 ± 20% in the case of SEC24D). It ought to be noted that transfection with SEC24C-VN did not affect the level of SEC24D. In contrast, the transfection with SEC24D-VN resulted in reduced SEC24C levels, by 25–30% compared to control conditions. It is worth mentioning that co-transfection with both mutants decreased viability of HEK293 cells after 48 h by 20% (Fig. S2), pointing to an important physiological role of both SEC24 proteins in proper functioning of the cell. As shown in Fig. 5A, a high excess (6× and 9×) of SEC24C-VN decreased the amount of both glycosylated and non-glycosylated bands of ATB0,+ by 51–60%. Interestingly, a two-fold excess of SEC24D-VN enhanced the expression levels of ATB0,+ by 30%. Although this phenomenon was very reproducible (observed in 6 independent transfections), there isn't any straightforward explanation for this finding. However, a 9-fold excess of SEC24D-VN, led to ATB0,+ levels comparable to those of the control, i.e. 95 ± 7% (Fig. 5A, right panels), in spite of a decreased level of endogenous SEC24C. Most probably, the level of endogenous SEC24C is still sufficient to facilitate exit of ATB0,+exit the ER.
Fig. 5.
Effect of co-transfection with dominant negative mutants of SEC24. HEK293 cells were either transfected with p3xFLAG-CMV14/B0,+ vector or co-transfected with SEC24C-VN or SEC24D-VN, at ratio indicated in the figure. (A) Level of SEC24 proteins was analyzed by Western blot with the corresponding anti-SEC24 antibodies. Level of over-expressed ATB0,+ was analyzed with anti-FLAG antibodies. (B) The cells were biotinylated with membrane impermeable reagent and the biotinylated fraction, as well as total protein content were analyzed by Western blot with anti-FLAG antibodies. GAPDH was detected, as loading control.
An analysis of ATB0,+ expression at the cell surface by biotinylation experiments after co-transfection with the SEC24C-VN mutant, demonstrated a much lower level of the transporter at the plasma membrane, corresponding to 60% and 30% of control levels for 6-fold and 9-fold excess of the SEC24C dominant negative mutant, respectively (Fig. 5B). However, this resulted from total reduced protein levels after the SEC24C-VN transfection. Since proteins in the ER are subjected to permanent quality control, this observation is indicative of ATB0,+ undergoing ER-associated degradation (ERAD), if incapable of exiting the ER. Thus, we analyzed the amount of ATB0,+ after treatment with proteasome inhibitor, bortezomib (PS-341) [34]. We used concentrations shown previously to be effective in HEK293 cells [35] but this treatment strongly affected cell viability (Fig. S3). It has to be stressed, however, that co-transfection with SEC24C-VN did not further impair cell survival. As presented in Fig. 6A, treatment with bortezomib dramatically increased the total amount of ATB0,+, although the vast majority of the transporter is present in the non-glycosylated form. Cells transfected with a dominant negative mutant of SEC24C and treated with bortezomib also contained a large amount of non-fully glycosylated ATB0,+ species, compared to control. Moreover, the core-glycosylated band accumulated, indicating the retention of the transporter in the ER. Interestingly, when bortezomib-treated cells were transfected with SEC24C-VN, the level of ATB0,+ was lower than in the cells treated with bortezomib alone. This indicates that non-functional SEC24C directs the overexpressed protein not only to the proteasome proteolytic pathway but also to another one, in all probability autophagy. This result is in agreement with previous observations that COPII components can regulate not only secretion, but also autophagy (for review, see [36]), and that the ER exit sites can initiate the formation of the phagophore [37]. A similar effect of bortezomib was observed in stably transfected cells (Fig. 6B); the fully glycosylated band is visible under all experimental conditions, suggesting a low turn-over of the transporter localized at the cell surface. This hypothesis was further confirmed when stably transfected cells were additionally transfected with dominant negative mutants of Sar1. As presented in Fig. S4, in stably transfected cells the major fraction of the transporter was detected as a high molecular weight – fully glycosylated species, the levels of which were not changed 48 h after expression of Sar1aT39N and Sar1bT39N. We further explored whether directing the transporter to proteolysis was also observed for the endogenous ATB0,+. ATB0,+ is known to be expressed in malignant breast cancer cell lines expressing the estrogen receptor α [8]. Hence, we chose the MCF7 cell line; as shown in Fig. 6C, ATB0,+ was mainly present as core-glycosylated species and its expression levels remained unaffected by bortezomib treatment (95–110% of the control).
Fig. 6.
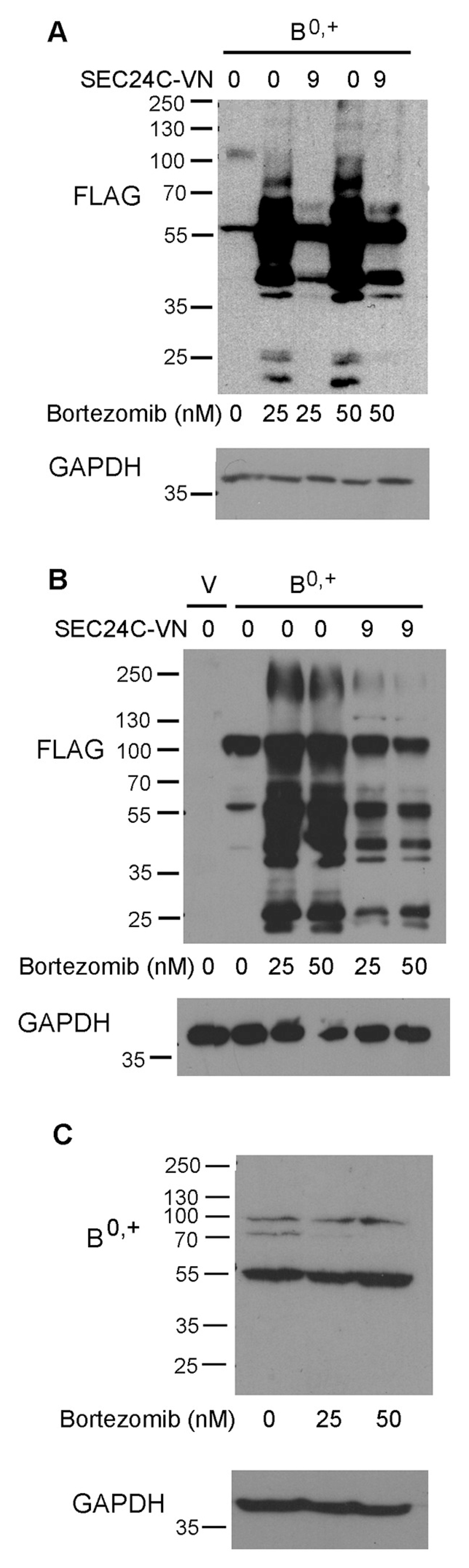
Effect of proteasome inhibitor – bortezomib on ATB0,+ level. (A) HEK293 cells were transfected for 48 h with p3xFLAG-CMV14/B0,+ vector (B0,+) and, where indicated with 9-fold excess of SEC24C-VN and an indicated concentration of bortezomib. (B) Cells were stably transfected either with p3xFLAG-CMV14 (V) or with p3xFLAG-CMV14/B0,+ (B0,+) and SEC24C-VN and bortezomib were added where indicated for the last 48 h. (C) MCF7 cells were incubated for 48 h with indicated concentration of bortezomib. In (A) and (B) ATB0,+ was detected with anti-FLAG antibodies, in (C) with anti-ATB0,+ antibodies. GAPDH was detected, as loading control.
3.4. SEC24C interacts with endogenous ATB0,+
We also examined whether ATB0,+ and SEC24C interacted when neither protein was overexpressed. Unfortunately, all commercially available antibodies against the proteins in question were raised in rabbit. Hence we resorted to using biotin-conjugated antibodies against the transporter and fluorescently labeled streptavidin for its detection. In order to avoid binding of the anti-B0,+ antibody to the secondary antibody used for SEC24 detection, we used the monovalent anti-rabbit IgG fragments conjugated to Alexa Fluor® 488 and additional fixing and blocking with the rabbit serum. As shown in Fig. 7, ATB0,+ was mainly localized in the cytoplasm, where it co-localized with SEC24C in the perinuclear region. No co-localization was detected with SEC24 isoform D.
Fig. 7.
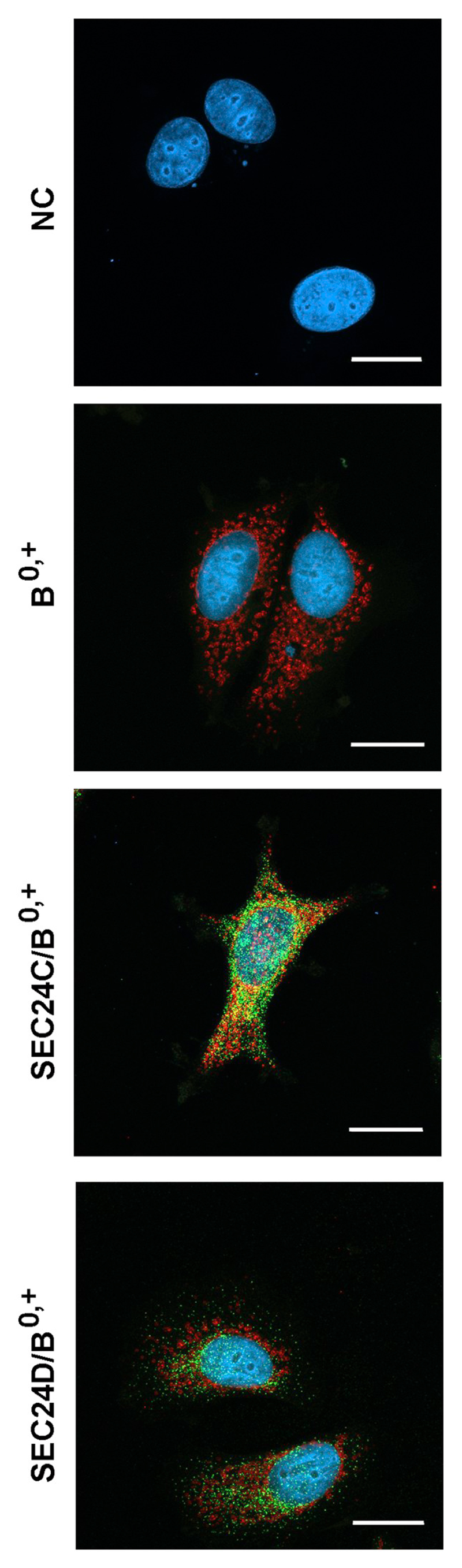
Localization of ATB0,+ and SEC24C in MCF7 cells. Cells were fixed with methanol, as described in Materials and methods. They were incubated, as indicated, with either anti-SEC24C or anti-SEC24D antibodies, detected with Alexa Fluor® 488-conjugated AffinityPure Fab Fragment Goat Anti-Rabbit IgG (H + L) (green). Further treatment was performed as described in Materials and methods Section 2.5. Next the cells were incubated with anti-ATB0,+ antibodies conjugated with biotin, followed by incubation with AlexaFluor568 conjugated streptavidin (red). Nuclei are visualized with DAPI. NC, negative control without the primary antibodies. B0,+, analysis of B0,+ localization without anti-SEC24 antibodies. Bar 20 μm.
4. Discussion
As all plasma membrane proteins, ATB0,+ is co-translationally inserted into the ER membrane. An analysis of the ATB0,+ amino acid sequence (http://sigpep.services.came.sbg.ac.at/results/tempxUDJjT/signalblast.html) revealed that it, similar to other SLC6 transporters [e.g. GAT1 (SLC6A1), DAT (SLC6A3) or SERT (SLC6A4)], does not contain any signal sequence, i.e. it is inserted into the ER membrane at the translocon via the lateral gate of the protein-conducting channel (Sec61) [38–41], beginning with the insertion of the first transmembrane helix. Our analysis of the ATB0,+ proteome by mass spectrometry indicated the presence of several ribosomal proteins as well as a signal recognition particle and its receptor (Ł. Samluk and K.A. Nałęcz, unpublished). Our experiments with non-permeabilized and permeabilized cells demonstrated that both N- and C-termini of ATB0,+ are located intracellularly [9], meaning that they are also at the cytoplasmic side of the ER membrane. The first step in trafficking of proteins to the plasma membrane is their export from the ER to cis-Golgi. A marked decrease in ATB0,+ surface expression following a co-transfection with a SAR1 dominant negative mutant indicates that ATB0,+ depends on a classical COPII pathway for its anterograde transport to the Golgi. This involves SEC24 isoform C as a cargo-recognizing protein, required for COPII vesicle formation.
Several ER export signals located at C-terminal regions of cargo proteins have been described for secreted and membrane proteins; the diacidic DXE motif [42], the dihydrophobic FF motifs, whereby the diphenylalanine can be substituted by dityrosine, dileucine, divaline or C-terminal valine [43]. In the ATB0,+ C-terminus, there is a diacidic 627DHE629 motif, albeit the additional proposed YXXΦ motif, found in numerous transmembrane proteins exiting the ER, is not present [42]. There is no C-terminal valine nor dihydrophobic motif in ATB0,+, which happen to be frequent in secreted and type I membrane proteins. Some multispanning membrane proteins, e.g. glucose transporters, some potassium channels, band 3 anion exchanger and the plasma membrane calcium ATPase, all have C-terminal valines [43]. Other transmembrane proteins, such as G-protein coupled receptors, carry a triple arginine motif [44], also not found in ATB0,+. In fact, ATB0,+ does not have any other typical ER export signal of transmembrane proteins – such as ΦXΦXΦ, shown to interact selectively with Sec24C [45]. It is worth adding that, even though these C-terminal dihydrophobic motifs were shown to interact with COPII subunits, single knock-downs of the four Sec24 isoforms demonstrated that a preference for a particular isoform was not absolute [46].
Trafficking studies of the neurotransmitter transporter branch of the SLC6 family made use of serial truncations and site-directed mutagenesis to demonstrate the importance of the ER export motif (i.e. RI in SERT; KL in DAT and GAT-3; RL in NET, GAT-1, GAT-2 and GLYT1), in binding their cognate SEC24 component in the COPII complex, allowing for concentrative ER export to take place [23]. In addition, the amino acid residue located at the +2 position C-terminal from the RI/KL/RL motif was shown to specify the SEC24 isoform preference required for that cargo in question. Hence, binding of Sec24 isoform C is determined by hydrophilic residues at this position (e.g. K in SERT and GAT-3), whereas hydrophobic residue exhibit a preference for Sec24D (e.g. V in GAT-1, Y in NET and DAT) [19,20]. The presence of a RIIK motif within the C-terminal cytosolic fragment of ATB0,+ is reminiscent of SERT, alluding to this transporter also being recognized by the C paralog of SEC24 proteins. Our results confirmed the proposed model: both the immunoprecipitation experiments and the co-localization demonstrated an exclusive interaction of SEC24C with, both the overexpressed and the endogenous, ATB0,+ transporter. These observations confirmed the SEC24 isoform preference of cargo proteins, based on the nature of the amino acid at the +2 position from the ER export motif. Our observations thus indicate that the specificity toward SEC24 paralogs, can also be expanded to the amino acid transporter family of SLC6 proteins.
Interestingly, it has been reported that different SEC24 isoforms could be recruited to the same ER exit sites, as shown for SEC24C and SEC24A [47]. Moreover, homologs of SEC24 isoforms were shown to be distributed either non-uniformly or homogenously, a phenomenon dependent on ER stress [48]. Our experiments with bortezomib showed that a substantial portion of the transporter was directed to proteolysis, most probably due to the unfolded protein response. Nevertheless, even under experimental conditions leading to ER stress, we were only able to detect an exclusive interaction of ATB0,+ with SEC24C. This observation suggests that SEC24C and SEC24D localize at different ER exit sites and are unevenly distributed to different vesicles. It should be noted that the ATB0,+ interaction with SEC24C may have physiological implications, since homozygous SEC24C deficiency is known to be lethal at the early post-implantation stage during embryonic development [49], and since ATB0,+activity is known to be required for blastocyst implantation [50]. Any inhibition of ATB0,+ exit from ER, as in case of SEC24C-VN expression, can direct the transporter to proteolytic degradation, resulting as a consequence in its lower level at the plasma membrane.
The interaction between ATB0,+ and SEC24C was not affected by treatment with PMA, a phorbol ester activating PKC. Since we previously observed that PMA treatment resulted in an increased amount of ATB0,+ at the plasma membrane [5,9], the lack of PMA influence on the ATB0,+/SEC24C interaction would point to the role of PKC, at post cargo-recognition stages of transporter trafficking. Such a notion can be justified by the fact that the Hrr25p kinase (human ortholog – casein kinase Iδ) has been generally acknowledged as the only kinase that phosphorylates SEC23p [51], thus fostering ER-Golgi traffic. Moreover, PMA was shown to induce the transfer of PKCα (the only conventional PKC isoform co-precipitating with ATB0,+) to the plasma membrane where both the kinase and the transporter were found to co-localize [9]. Therefore, it appears that, although the phosphorylation of ATB0,+ by PKC correlated with an increased amount of the transporter at the cell surface, PKC is not involved in transporter trafficking, or at least not in the exit from the ER.
The fact that SEC24C was shown to be phosphorylated by AKT/protein kinase B [33], a kinase hyperactivated in cancer cells [52], is inherently of added interest. In addition, ATB0,+ is up-regulated in several cancers [53–55], including the estrogen receptor positive breast cancer [7,8]. It should be emphasized that other amino acid transporting systems up-regulated in cancer, such as ASCT2 (SLC1A5), LAT1 (SLC7A5) and xCT (SLC7A11), all function in an exchange mode, so the uptake of one amino acid requires the removal of another from the cell, while ATB0,+ is capable of catalyzing the net uptake of a broad spectrum of amino acids. What is more, ATB0,+ supplies cells with leucine (an activator of mTOR), glutamine (required for nucleotide biosynthesis) and arginine (essential for tumor cells) [56]. Hence insights into ATB0,+ regulation, in particular the mechanism leading to ATB0,+ retention in the ER, may provide novel prospects in its pharmacological modulation and be of therapeutic relevance beneficial in cancer treatment.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbamcr.2018.11.005.
Acknowledgments
The authors would like to thank Prof. Bożena Kamińska for MCF7 cells and Dr Katarzyna Bartkowska for fluorescently-labeled streptavidin. The authors are grateful to Prof. Katarzyna Kwiatkowska for her advice on MCF7 immunocytochemistry experiments. This work was financed with Polish National Science Centre Grant No. 2012/B/NZ3/00225, with the EU Horizon 2020 Research and Innovation Programme under Marie Sklodowska-Curie Grant Agreement No. 665735 (Bio4Med) and with the funds from the Polish Ministry of Science and Higher Education as part of the 2016–2020 funds for the implementation of international projects (Agreement No. 3548/H2020/COFUND2016/2) and grants of the Austrian Science Fund FWF (P31255 and SFB35-10 to Sonja Sucic and Michael Freissmuth, respectively).
Abbreviations
- ASCT
amino acid transporter system ASC
- ATB0,+
amino acid transporter B0,+
- CFP
cyan fluorescent protein
- COPII
vesicle coat protein complex, coatomer II
- DAPI
4′,6-diamidino-2-phenylindole
- DAT
dopamine transporter
- DMSO
dimethylsulfoxide
- ER
endoplasmic reticulum
- ERAD
ER-associated degradation
- GABA
γ-aminobutyric acid
- GAT
GABA transporter
- GFP
green fluorescent protein
- LAT
amino acid transporter system L
- NET
noradrenaline transporter
- PBS
phosphate buffered saline
- PKC
protein kinase C
- PLA
proximity ligation assay
- PMA
phorbol 12-myristate 13-acetate
- Sar1
COPII-associated small GTPase
- SDS
sodium dodecyl phosphate
- SERT
serotonin transporter
- SLC
solute carrier
- xCT
amino acid transporter system xc
Footnotes
Authors' contribution
ŁS, SS, MF and KAN - conceptualization; VK, BJ, ŁS - investigation; DJ vectors validation. KAN - funding acquisition, writing -review & editing. All the authors read and approved the manuscript submitted for publication.
Conflict of interest statement
The authors declare no conflict of interest.
Transparency document
The Transparency document associated with this article can be found, in online version.
References
- [1].Broer S, Gether U. The solute carrier 6 family of transporters. Br J Pharmacol. 2012;167:256–278. doi: 10.1111/j.1476-5381.2012.01975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rudnick G, Kramer R, Blakely RD, Murphy DL, Verrey F. The SLC6 transporters: perspectives on structure, functions, regulation, and models for transporter dysfunction. Pflűgers Arch. 2014;466:25–42. doi: 10.1007/s00424-013-1410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sloan JL, Mager S. Cloning and functional expression of a human Na(+) and Cl (–)-dependent neutral and cationic amino acid transporter B(0+) J Biol Chem. 1999;274:23740–23745. doi: 10.1074/jbc.274.34.23740. [DOI] [PubMed] [Google Scholar]
- [4].Czeredys M, Mysiorek C, Kulikova N, Samluk L, Berezowski V, Cecchelli R, Nalecz K. A polarized localization of amino acid/carnitine transporter B0,+ (ATB0,+) in the blood-brain barrier. Biochem Biophys Res Commun. 2008;376:267–270. doi: 10.1016/j.bbrc.2008.08.122. [DOI] [PubMed] [Google Scholar]
- [5].Samluk L, Czeredys M, Nalecz KA. Regulation of amino acid/carnitine transporter B0,+ (ATB0,+) in astrocytes by protein kinase C: independent effects on raft and non-raft transporter subpopulations. J Neurochem. 2010;115:1386–1397. doi: 10.1111/j.1471-4159.2010.07040.x. [DOI] [PubMed] [Google Scholar]
- [6].Michalec K, Mysiorek C, Kuntz M, Berezowski V, Szczepankiewicz AA, Wilczynski GM, Cecchelli R, Nalecz KA. Protein kinase C restricts transport of carnitine by amino acid transporter ATB(0,+) apically localized in the blood-brain barrier. Arch Biochem Biophys. 2014;554:28–35. doi: 10.1016/j.abb.2014.05.006. [DOI] [PubMed] [Google Scholar]
- [7].Karunakaran S, Umapathy NS, Thangaraju M, Hatanaka T, Itagaki S, Munn DH, Prasad PD, Ganapathy V. Interaction of tryptophan derivatives with SLC6A14 (ATB0,+) reveals the potential of the transporter as a drug target for cancer chemotherapy. Biochem J. 2008;414:343–355. doi: 10.1042/BJ20080622. [DOI] [PubMed] [Google Scholar]
- [8].Karunakaran S, Ramachandran S, Coothankandaswamy V, Elangovan S, Babu E, Periyasamy-Thandavan S, Gurav A, Gnanaprakasam JP, Singh N, Schoenlein PV, Prasad PD, et al. SLC6A14 (ATB0,+) protein, a highly concentrative and broad specific amino acid transporter, is a novel and effective drug target for treatment of estrogen receptor-positive breast cancer. J Biol Chem. 2011;286:31830–31838. doi: 10.1074/jbc.M111.229518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Samluk L, Czeredys M, Skowronek K, Nalecz KA. Protein kinase C regulates amino acid transporter ATB(0,+) Biochem Biophys Res Commun. 2012;422:64–69. doi: 10.1016/j.bbrc.2012.04.106. [DOI] [PubMed] [Google Scholar]
- [10].Daniels GM, Amara SG. Regulated trafficking of the human dopamine transporter. Clathrin-mediated internalization and lysosomal degradation in response to phorbol esters. J Biol Chem. 1999;274:35794–35801. doi: 10.1074/jbc.274.50.35794. [DOI] [PubMed] [Google Scholar]
- [11].Foster JD, Adkins SD, Lever JR, Vaughan RA. Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J Neurochem. 2008;105:1683–1699. doi: 10.1111/j.1471-4159.2008.05262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Qian Y, Galli A, Ramamoorthy S, Risso S, Defelice LJ, Blakely RD. Protein kinase C activation regulates human serotonin transporters in HEK-293 cells via altered cell surface expression. J Neurosci. 1997;17:45–57. doi: 10.1523/JNEUROSCI.17-01-00045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Apparsundaram S, Schroeter S, Giovanetti E, Blakely RD. Acute regulation of norepinephrine transport: II. PKC-modulated surface expression of human norepinephrine transporter proteins. J Pharmacol Exp Ther. 1998;287:744–751. [PubMed] [Google Scholar]
- [14].Jayanthi LD, Annamalai B, Samuvel DJ, Gether U, Ramamoorthy S. Phosphorylation of the norepinephrine transporter at threonine 258 and serine 259 is linked to protein kinase C-mediated transporter internalization. J Biol Chem. 2006;281:23326–23340. doi: 10.1074/jbc.M601156200. [DOI] [PubMed] [Google Scholar]
- [15].Gomeza J, Zafra F, Olivares L, Gimenez C, Aragon C. Regulation by phorbol esters of the glycine transporter (GLYT1) in glioblastoma cells. Biochim Biophys Acta. 1995;1233:41–46. doi: 10.1016/0005-2736(94)00249-o. [DOI] [PubMed] [Google Scholar]
- [16].Sato K, Adams R, Betz H, Schloss P. Modulation of a recombinant glycine transporter (GLYT1b) by activation of protein kinase C. J Neurochem. 1995;65:1967–1973. doi: 10.1046/j.1471-4159.1995.65051967.x. [DOI] [PubMed] [Google Scholar]
- [17].Fornes A, Nunez E, Alonso-Torres P, Aragon C, Lopez-Corcuera B. Trafficking properties and activity regulation of the neuronal glycine transporter GLYT2 by protein kinase C. Biochem J. 2008;412:495–506. doi: 10.1042/BJ20071018. [DOI] [PubMed] [Google Scholar]
- [18].Lord C, Bhandari D, Menon S, Ghassemian M, Nycz D, Hay J, Ghosh P, Ferro-Novick S. Sequential interactions with Sec23 control the direction of vesicle traffic. Nature. 2011;473:181–186. doi: 10.1038/nature09969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sucic S, Koban F, El-Kasaby A, Kudlacek O, Stockner T, Sitte HH, Freissmuth M. Switching the clientele: a lysine residing in the C terminus of the serotonin transporter specifies its preference for the coat protein complex II component SEC24C. J Biol Chem. 2013;288:5330–5341. doi: 10.1074/jbc.M112.408237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sucic S, El-Kasaby A, Kudlacek O, Sarker S, Sitte HH, Marin P, Freissmuth M. The serotonin transporter is an exclusive client of the coat protein complex II (COPII) component SEC24C. J Biol Chem. 2011;286:16482–16490. doi: 10.1074/jbc.M111.230037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Farhan H, Reiterer V, Korkhov VM, Schmid JA, Freissmuth M, Sitte HH. Concentrative export from the endoplasmic reticulum of the gamma-aminobutyric acid transporter 1 requires binding to SEC24D. J Biol Chem. 2007;282:7679–7689. doi: 10.1074/jbc.M609720200. [DOI] [PubMed] [Google Scholar]
- [22].Rowe T, Balch WE. Expression and purification of mammalian Sarl. Methods Enzymol. 1995;257:49–53. doi: 10.1016/s0076-6879(95)57009-8. [DOI] [PubMed] [Google Scholar]
- [23].El-Kasaby A, Just H, Malle E, Stolt-Bergner PC, Sitte HH, Freissmuth M, Kudlacek O. Mutations in the carboxyl-terminal SEC24 binding motif of the serotonin transporter impair folding of the transporter. J Biol Chem. 2010;285:39201–39210. doi: 10.1074/jbc.M110.118000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Montgomery TR, Steinkellner T, Sucic S, Koban F, Schuchner S, Ogris E, Sitte HH, Freissmuth M. Axonal targeting of the serotonin transporter in cultured rat dorsal raphe neurons is specified by SEC24C-dependent export from the endoplasmic reticulum. J Neurosci. 2014;34:6344–6351. doi: 10.1523/JNEUROSCI.2991-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lederkremer GZ. Glycoprotein folding, quality control and ER-associated degradation. Curr Opin Struct Biol. 2009;19:515–523. doi: 10.1016/j.sbi.2009.06.004. [DOI] [PubMed] [Google Scholar]
- [27].Chevet E, Smirle J, Cameron PH, Thomas DY, Bergeron JJ. Calnexin phosphorylation: linking cytoplasmic signalling to endoplasmic reticulum lumenal functions. Semin Cell Dev Biol. 2010;21:486–490. doi: 10.1016/j.semcdb.2009.12.005. [DOI] [PubMed] [Google Scholar]
- [28].Luzio JP, Brake B, Banting G, Howell KE, Braghetta P, Stanley KK. Identification, sequencing and expression of an integral membrane protein of the trans-Golgi network (TGN38) Biochem J. 1990;270:97–102. doi: 10.1042/bj2700097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bhuin T, Roy JK. Rab proteins: the key regulators of intracellular vesicle transport. Exp Cell Res. 2014;328:1–19. doi: 10.1016/j.yexcr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- [30].Steele DF, Fedida D. Cytoskeletal roles in cardiac ion channel expression. Biochim Biophys Acta. 2014;1838:665–673. doi: 10.1016/j.bbamem.2013.05.001. [DOI] [PubMed] [Google Scholar]
- [31].Bonnon C, Wendeler MW, Paccaud JP, Hauri HP. Selective export of human GPI-anchored proteins from the endoplasmic reticulum. J Cell Sci. 2010;123:1705–1715. doi: 10.1242/jcs.062950. [DOI] [PubMed] [Google Scholar]
- [32].Notredame C, Higgins DG, Heringa J. T-coffee: a novel method for fast and accurate multiple sequence alignment. J Mol Biol. 2000;302:205–217. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- [33].Sharpe LJ, Luu W, Brown AJ. Akt phosphorylates Sec24: new clues into the regulation of ER-to-Golgi trafficking. Traffic. 2011;12:19–27. doi: 10.1111/j.1600-0854.2010.01133.x. [DOI] [PubMed] [Google Scholar]
- [34].Berkers CR, Verdoes M, Lichtman E, Fiebiger E, Kessler BM, Anderson KC, Ploegh HL, Ovaa H, Galardy PJ. Activity probe for in vivo profiling of the specificity of proteasome inhibitor bortezomib. Nat Methods. 2005;2:357–362. doi: 10.1038/nmeth759. [DOI] [PubMed] [Google Scholar]
- [35].Gelman JS, Sironi J, Berezniuk I, Dasgupta S, Castro LM, Gozzo FC, Ferro ES, Fricker LD. Alterations of the intracellular peptidome in response to the proteasome inhibitor bortezomib. PLoS One. 2013;8:e53263. doi: 10.1371/journal.pone.0053263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Farhan H, Kundu M, Ferro-Novick S. The link between autophagy and secretion: a story of multitasking proteins. Mol Biol Cell. 2017;28:1161–1164. doi: 10.1091/mbc.E16-11-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ge L, Zhang M, Kenny SJ, Liu D, Maeda M, Saito K, Mathur A, Xu K, Schekman R. Remodeling of ER-exit sites initiates a membrane supply pathway for autophagosome biogenesis. EMBO Rep. 2017;18:1586–1603. doi: 10.15252/embr.201744559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gogala M, Becker T, Beatrix B, Armache JP, Barrio-Garcia C, Berninghausen O, Beckmann R. Structures of the Sec61 complex engaged in nascent peptide translocation or membrane insertion. Nature. 2014;506:107–110. doi: 10.1038/nature12950. [DOI] [PubMed] [Google Scholar]
- [39].Martinez-Gil L, Sauri A, Marti-Renom MA, Mingarro I. Membrane protein integration into the endoplasmic reticulum. FEBS J. 2011;278:3846–3858. doi: 10.1111/j.1742-4658.2011.08185.x. [DOI] [PubMed] [Google Scholar]
- [40].Park E, Rapoport TA. Mechanisms of Sec61/SecY-mediated protein translocation across membranes. Annu Rev Biophys. 2012;41:21–40. doi: 10.1146/annurev-biophys-050511-102312. [DOI] [PubMed] [Google Scholar]
- [41].Van den Berg B, Clemons WM, Jr, Collinson I, Modis Y, Hartmann E, Harrison SC, Rapoport TA. X-ray structure of a protein-conducting channel. Nature. 2004;427:36–44. doi: 10.1038/nature02218. [DOI] [PubMed] [Google Scholar]
- [42].Nishimura N, Balch WE. A di-acidic signal required for selective export from the endoplasmic reticulum. Science. 1997;277:556–558. doi: 10.1126/science.277.5325.556. [DOI] [PubMed] [Google Scholar]
- [43].Nufer O, Guldbrandsen S, Degen M, Kappeler F, Paccaud JP, Tani K, Hauri HP. Role of cytoplasmic C-terminal amino acids of membrane proteins in ER export. J Cell Sci. 2002;115:619–628. doi: 10.1242/jcs.115.3.619. [DOI] [PubMed] [Google Scholar]
- [44].Dong C, Nichols CD, Guo J, Huang W, Lambert NA, Wu G. A triple arg motif mediates alpha(2B)-adrenergic receptor interaction with Sec24C/D and export. Traffic. 2012;13:857–868. doi: 10.1111/j.1600-0854.2012.01351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Otsu W, Kurooka T, Otsuka Y, Sato K, Inaba M. A new class of endoplasmic reticulum export signal PhiXPhiXPhi for transmembrane proteins and its selective interaction with Sec24C. J Biol Chem. 2013;288:18521–18532. doi: 10.1074/jbc.M112.443325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wendeler MW, Paccaud JP, Hauri HP. Role of Sec24 isoforms in selective export of membrane proteins from the endoplasmic reticulum. EMBO Rep. 2007;8:258–264. doi: 10.1038/sj.embor.7400893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Adolf F, Rhiel M, Reckmann I, Wieland FT. Sec24C/D-isoform-specific sorting of the preassembled ER-Golgi Q-SNARE complex. Mol Biol Cell. 2016;27:2697–2707. doi: 10.1091/mbc.E16-04-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Iwasaki H, Yorimitsu T, Sato K. Distribution of Sec24 isoforms to each ER exit site is dynamically regulated in Saccharomyces cerevisiae. FEBS Lett. 2015;589:1234–1239. doi: 10.1016/j.febslet.2015.04.006. [DOI] [PubMed] [Google Scholar]
- [49].Adams EJ, Chen XW, O'Shea KS, Ginsburg D. Mammalian COPII coat component SEC24C is required for embryonic development in mice. J Biol Chem. 2014;289:20858–20870. doi: 10.1074/jbc.M114.566687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Van Winkle LJ, Tesch JK, Shah A, Campione AL. System B0,+ amino acid transport regulates the penetration stage of blastocyst implantation with possible long-term developmental consequences through adulthood. Hum Reprod Update. 2006;12:145–157. doi: 10.1093/humupd/dmi044. [DOI] [PubMed] [Google Scholar]
- [51].Milne DM, Looby P, Meek DW. Catalytic activity of protein kinase CK1 delta (casein kinase 1delta) is essential for its normal subcellular localization. Exp Cell Res. 2001;263:43–54. doi: 10.1006/excr.2000.5100. [DOI] [PubMed] [Google Scholar]
- [52].Wang Q, Chen X, Hay N. Akt as a target for cancer therapy: more is not always better (lessons from studies in mice) Br J Cancer. 2017;117:159–163. doi: 10.1038/bjc.2017.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Penheiter AR, Erdogan S, Murphy SJ, Hart SN, Felipe Lima J, Rakhshan Rohakhtar F, O'Brien DR, Bamlet WR, Wuertz RE, Smyrk TC, Couch FJ, et al. Transcriptomic and immunohistochemical profiling of SLC6A14 in pancreatic ductal adenocarcinoma. Biomed Res Int. 2015;2015 doi: 10.1155/2015/593572. 593572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Gupta N, Prasad PD, Ghamande S, Moore-Martin P, Herdman AV, Martindale RG, Podolsky R, Mager S, Ganapathy ME, Ganapathy V. Up-regulation of the amino acid transporter ATB(0,+) (SLC6A14) in carcinoma of the cervix. Gynecol Oncol. 2006;100:8–13. doi: 10.1016/j.ygyno.2005.08.016. [DOI] [PubMed] [Google Scholar]
- [55].Sikder MOF, Yang S, Ganapathy V, Bhutia YD. The Na(+)/Cl(−)-coupled, broad-specific, amino acid transporter SLC6A14 (ATB(0,+)): emerging roles in multiple diseases and therapeutic potential for treatment and diagnosis. AAPS J. 2017;20:12. doi: 10.1208/s12248-017-0164-7. [DOI] [PubMed] [Google Scholar]
- [56].Bhutia YD, Babu E, Ramachandran S, Ganapathy V. Amino acid transporters in cancer and their relevance to “glutamine addiction”: novel targets for the design of a new class of anticancer drugs. Cancer Res. 2015;75:1782–1788. doi: 10.1158/0008-5472.CAN-14-3745. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.