Abstract
Background
There has been a gradual ‘upward-creep’ of revascularization thresholds for both Fractional Flow Reserve (FFR) and Instantaneous Wave-Free Ratio (iFR), prior to the clinical outcome trials for both indices. The increase in revascularization that has potentially resulted is at odds with increasing evidence questioning the benefits of revascularizing stable coronary disease. Using an independent invasive reference standard, this study primarily aimed to define optimal thresholds for FFR and iFR and also aimed to compare the performance of iFR, FFR and resting Pd/Pa.
Methods and Results
Distal coronary (Pd) and aortic pressure (Pa) were measured in 75 patients undergoing coronary angiography+/-PCI with resting Pd/Pa, iFR and FFR calculated. Doppler Average Peak Flow Velocity (APV) was simultaneously measured and Hyperemic Stenosis Resistance calculated as hSR=Pa-Pd/APV (using hSR >0.80mmHg.cm-1.s as invasive reference standard). An FFR threshold of 0.75 had optimum diagnostic accuracy (84%) whereas for iFR this was 0.86 (76%). At these thresholds, the discordance in classification between indices was 11%. The accuracy of contemporary thresholds (FFR 0.80; iFR 0.89) was significantly lower (78.7% and 65.3% respectively) with a 25% rate of discordance. The optimal threshold for Pd/Pa was 0.88 (77.3% accuracy). When comparing indices at optimal thresholds, FFR showed best diagnostic performance (AUC 0.91 FFR vs. 0.79 iFR and 0.77 Pd/Pa, p=0.002).
Conclusions
Contemporary thresholds provide suboptimal diagnostic accuracy compared to an FFR threshold of 0.75 and iFR threshold of 0.86 (cut-offs in derivation studies). Whether more rigorous thresholds would result in selecting populations gaining greater symptom and prognostic benefit needs assessing in future trials of physiology-guided revascularization.
Keywords: Stable Coronary Artery Disease, FFR, iFR, PCI, Ischemia
Introduction
The benefit of revascularization in stable coronary artery disease (CAD) is a contentious issue, with mounting evidence suggesting that patients on optimal medical therapy alone have an excellent prognosis with significantly improved symptoms, as suggested by the COURAGE trial1 and recently the Sham-controlled ORBITA trial2. On the other hand, we have evidence suggesting physiology-guided revascularization, whether it be in the form of Fractional Flow Reserve (FFR)3–5 or Instantaneous Wave Free Ratio (iFR)6,7, is associated with significantly improved symptoms and patient outcomes, compared to revascularization based on angiographic appearances alone. The benefits of revascularization seem to be greatest in those with a high burden of ischemia8,9.
The initial derivation studies of FFR were performed against a combination of non-invasive tests including SPECT and stress echocardiography and showed an optimum threshold of 0.753,10. Following this, subsequent evidence of improved clinical outcomes was demonstrated in the FAME 1 trial (showing a significant reduction in death and MI within FFR-guided PCI arm)4 and the FAME 2 trial (driven by a reduction in repeat or urgent Revascularization)4,5, using the higher threshold of FFR≤0.80, whereby clinicians were provided with the “safety net” of improved negative predictive value. FFR≤0.80 has gained such wide acceptance as the dichotomous threshold to detect significant coronary disease that many novel physiological indices, including some non-invasive measures11, have been validated against it. The invasive physiological index of iFR was also originally derived and validated against an FFR≤0.80 threshold. The ADVISE and CLARIFY studies suggested that iFR thresholds of 0.83 and 0.86 provided optimal agreement with the FFR threshold of 0.8, the latter using the ischemic arbiter of Hyperemic Stenosis Resistance (hSR). Subsequent studies demonstrated discordance of up to 40% between FFR and iFR12 and therefore an iFR grey-zone of 0.86-0.93 was suggested to allow clinicians to use iFR before clinical outcome data as this resulted in an improvement in concordance with FFR13. A drive for a discrete threshold led to further analyses of discordance14,15 and eventual adoption of a higher iFR threshold of ≤0.89 for the major clinical trials of iFR-guided revascularization. Management of stable CAD using FFR≤0.80 or iFR≤0.89 has been shown to result in equivalent outcomes in recent trials in patients who generally have a good prognosis6,7. Whether the loss of specificity inherent in this ‘upward creep’ of the diagnostic FFR and iFR thresholds results in patients being inappropriately revascularized, in the absence of a substrate for ischemia, remains unclear.
This ‘upward creep’ in thresholds has resulted in a ‘physiological greyzone’ between the FFR 0.75 and 0.8 thresholds. Recent data from the IRIS-FFR registry by Kang et al has suggested corornary Revascularization of greyzone-FFR cases is not associated with improved outcomes16. This ‘greyzone’ has also existed for iFR (0.86 - 0.93), with a hybrid iFR-FFR guided strategy sometimes used to resolve the uncertainty13. However, recent randomized trials have used a single rule-in and rule-out iFR threshold of 0.896,7.
This study aimed to assess the optimal diagnostic thresholds for these invasive physiological indices against an independent reference standard and in doing so, sought to also compare the diagnostic accuracy of these indices at both the contemporary and optimal thresholds. In the absence of a true ischemic gold standard, several noninvasive imaging methods have previously been used to further evaluate invasive physiological methods, with the major hurdle being these methods only isolate ischemia in a myocardial territory rather than a specific vessel. In this study we therefore used Hyperemic Stenosis Resistance (hSR) as an invasive reference standard of physiological significance. hSR is an invasive index calculated by measurement of both intra-coronary pressure and Doppler flow velocity and so overcomes many of the limitations of flow-only and pressure-only based indices17–19. Some have suggested it as being more stenosis specific, having previously been used as an invasive reference standard to compare pressure-derived indices, for example in the CLARIFY study20. It is considered to be independent of resting or Hyperemic conditions within the vessel20.
Methods
Study Population
Patients who were scheduled to undergo coronary angiography with a view to proceeding to PCI for suspected or confirmed stable ischemic heart disease were eligible for inclusion. Exclusion criteria were significant valvular heart disease, an unstable coronary presentation (myocardial in the prior 4 weeks or CCS IV angina), coronary disease that was not suitable for instrumentation (as below), previous CABG and contraindications to pharmacological hyperemia with adenosine. The study complies with the Declaration of Helsinki and study protocols were approved by a national and locally appointed research ethics committee. All patients provided written informed consent. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Cardiac Catheterization
Dual antiplatelet therapy was initiated in all participants prior to the procedure. Diagnostic angiography was performed via the right radial artery or right femoral artery using 5 or 6Fr catheters. Following diagnostic angiography, intracoronary pressure measurements were made with 6Fr guiding catheters. Bolus unfractionated heparin was administered to maintain an activated clotting time >250 seconds. Following diagnostic coronary angiography intracoronary nitroglycerin was administered and a 0.014” dual pressure-Doppler sensor guide wire (ComboWire, Volcano-Philips, California) was calibrated to aortic pressure and the tip delivered to the distal epicardial vessel. Aortic pressure was measured via the fluid-filled guide catheter. The Doppler signal was optimized and recording commenced. Hyperemia was achieved using an intravenous (IV) infusion (dose 140mcg/kg/min) or intracoronary (IC) boluses (dose 60mcg repeated three times) 21. After completion of the infusion or each intracoronary bolus, we waited for baseline hemodynamic parameters (Heart Rate, Blood Pressure and Doppler Flow velocity) to return to baseline before the next measurement. At the end of physiological measurements, the pressure sensor was returned to the aorta, with measurements repeated if any pressure-drift was found. Offline calculation was subsequently performed of Resting Pd/Pa, FFR, iFR, and hSR using Cardiac Waves Software (Kings College London, London, United Kingdom). Coronary angioplasty was then performed if indicated.
Data Recording and Analysis
Pressure data
For Pd/Pa and iFR, analyses were performed in a fully automated manner without manual selection of data time points. iFR was calculated using the method descried by Sen et al22, using a dedicated software package (CardiacWaves, King’s College London, London, UK) that was designed with Matlab (Mathworks, Natick, Massachusetts) and has been previously validated against propriatery iFR measurements23,24. In addition, we found our iFR data showed strong agreement with measurements made using proprietary software for a selection of cases from this study that had additional validation measurements with the Volcano Veratta wire data. FFR was calculated as the mean distal coronary pressure (measured with the pressure-wire) divided by the mean aortic pressure (measured simultaneously with the guiding catheter) during maximal hyperemia. In cases where there was a phasic response to IV adenosine, the value recorded was the lower 5 beat average, as per the ‘smart minimum’ method25. Where IC adenosine was used, the lowest of 3 beat average reading was taken.
Pressure/Doppler data
Peak coronary flow velocity, distal coronary pressure (Pd) and central aortic pressure (Pa) were ECG-gated and recorded continuously at a sampling frequency of 200Hz and data exported into a custom-made StudyManager program (Academic Medical Center, University of Amsterdam, Netherlands). The raw data was transferred to optical media and hSR calculated using the dedicated MATLAB software (CardiacWaves, King’s College London, London, UK). In order to account for beat-to-beat variability and reduce the effect of noise, signals were ensemble averaged over five consecutive cardiac cycles during stable hyperemia. Premature ectopic beats and the beat preceding were excluded from analysis. hSR was calculated as Pa-Pd/APV, with APV being average peak coronary flow velocity. Significant disease was defined by hSR≥0.80.
Statistical Analysis
All data are expressed as medians [1st quartile; 3rd quartile] or means (standard deviation) for continuous variables (compared using a t-test or ANOVA for continuous normal distributed variables, and Kruskal-Wallis test if continuous non-normal distributed); categorical variables are expressed as absolute and relative frequencies (compared using a Pearson chi-square test). Hypothesis testing was two-tailed and p values <0.05 were considered statistically significant.
Diagnostic accuracy was quantified by the area under receiver-operating curve (AUC (95% confidence interval) against hSR≥0.8). Bootstrapping was used to calculate Confidence Intervals (CI), using 1,000 stratified bootstrap replicates, to compare the AUC between indices and calculate the classification function. Diagnostic accuracy was calculated as Σ True positive + Σ True negative / Σ Total population for each threshold. Correlation was assessed with Pearsons’s R and adjusted R2 by fitting a linear regression model. All statistical analyses were performed using R, version 3.4.3 GUI 1.70 (The R Foundation for Statistical Computing), using packages ggplot2, RMarkdown, pROC and the tidyverse.
Results
Following exclusion of 6 cases due to poor Doppler flow signals, 75 consecutive patients between January 2015 – November 2017 were included in the study, with baseline demographics summarized in Table 1. Mean age was 62 with 56 % male. One vessel was studied per patient, where FFR was 0.78 +/- 0.12 and iFR was 0.85 +/- 0.10.
Table 1. Summary of patient demographics.
| Mean Age (years) [IQR] | 62 [53;70] |
| Gender, n (%): Male | 42 (56%) |
| Hypertension, n (%) | 45 (60%) |
| Hyperlipidemia, n (%) | 53 (71%) |
| Diabetes mellitus, n (%) | 23 (31%) |
| Smoking, n (%) | 20 (27%) |
| Previous PCI, n (%) | 22 (29%) |
| Interrogated vessel, n (%): | |
| Left Anterior Descending, LAD | 63 (84%) |
| Circumflex, LCx | 3 (4%) |
| Right Coronary Artery, RCA | 8 (11%) |
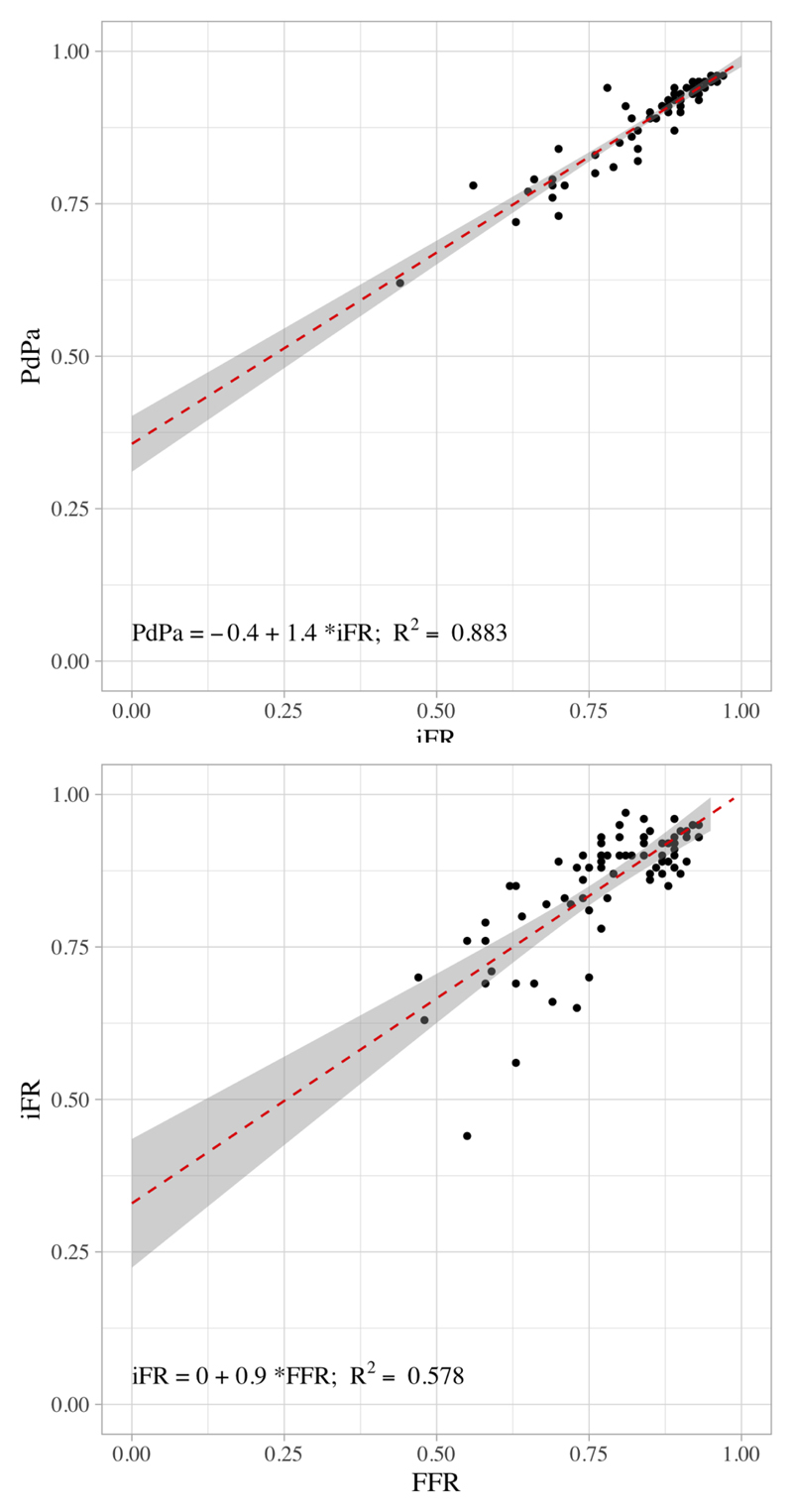
The median (interquartile range) of FFR, iFR and Pd/Pa in our study population was 0.8 (0.72-0.87), 0.89 (0.82-0.92) and 0.92 (0.88-0.94) respectively. 80% of cases included in the analysis were in the diagnostically challenging range between FFR 0.6 – 0.9. Scatter plots of the correlation between FFR vs. iFR and PdPa vs. iFR are shown in Figure 1. The Pearson’s correlation coefficients were 0.76 (FFR versus iFR) and 0.94 (for iFR versus Pd/Pa).
Figure 1. Scatterplots of PdPa versus iFR and FFR versus iFR.
Top: Scatterplot of iFR versus PdPa. Grey shaded area represents 95% confidence interval of trend line. Pearson’s R value 0.94, R2 0.88 (P<0.05).
Bottom: Scatterplot of FFR versus iFR. Grey shaded area represents 95% confidence interval of trend line. Pearson’s R value 0.76, R2 0.58 (P<0.05).
Diagnostic Performance of Invasive Resting and Hyperemic Indices
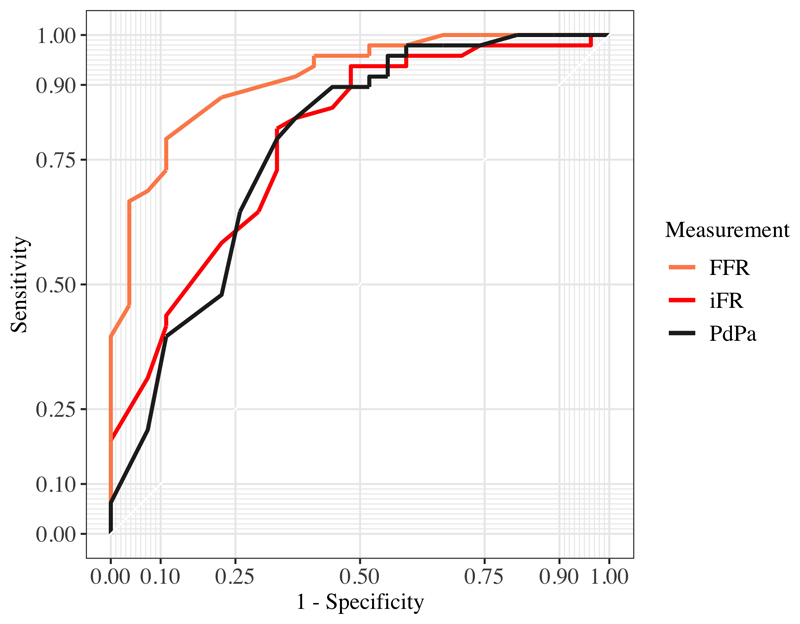
Receiver Operator Curves (ROC) for the performance of iFR, FFR and Pd/Pa in detecting a hSR value ≥ 0.8 are shown in Figure 2. FFR had the best diagnostic accuracy (p=0.002 for comparison of AUC of FFR versus iFR and Pd/Pa). The AUC to predict hSR≥0.8 was 0.92 (95% CI 0.84-0.87), 0.79 (95% CI 0.66-0.90) and 0.78 (95% CI 0.64 – 0.9) for FFR, iFR and Resting Pd/Pa respectively.
Figure 2. ROC curves for FFR, iFR and PdPa.
ROC curves with comparison made against hSR>0.8 to identify ischemia. AUC for FFR 0.92 (0.85-0.97 95% CI), iFR 0.79 (0.67-0.89 95% CI) and PdPa 0.77 (0.65-0.89 95% CI). FFR AUC was significantly different (P=0.003) compared to iFR and PdPa AUCs.
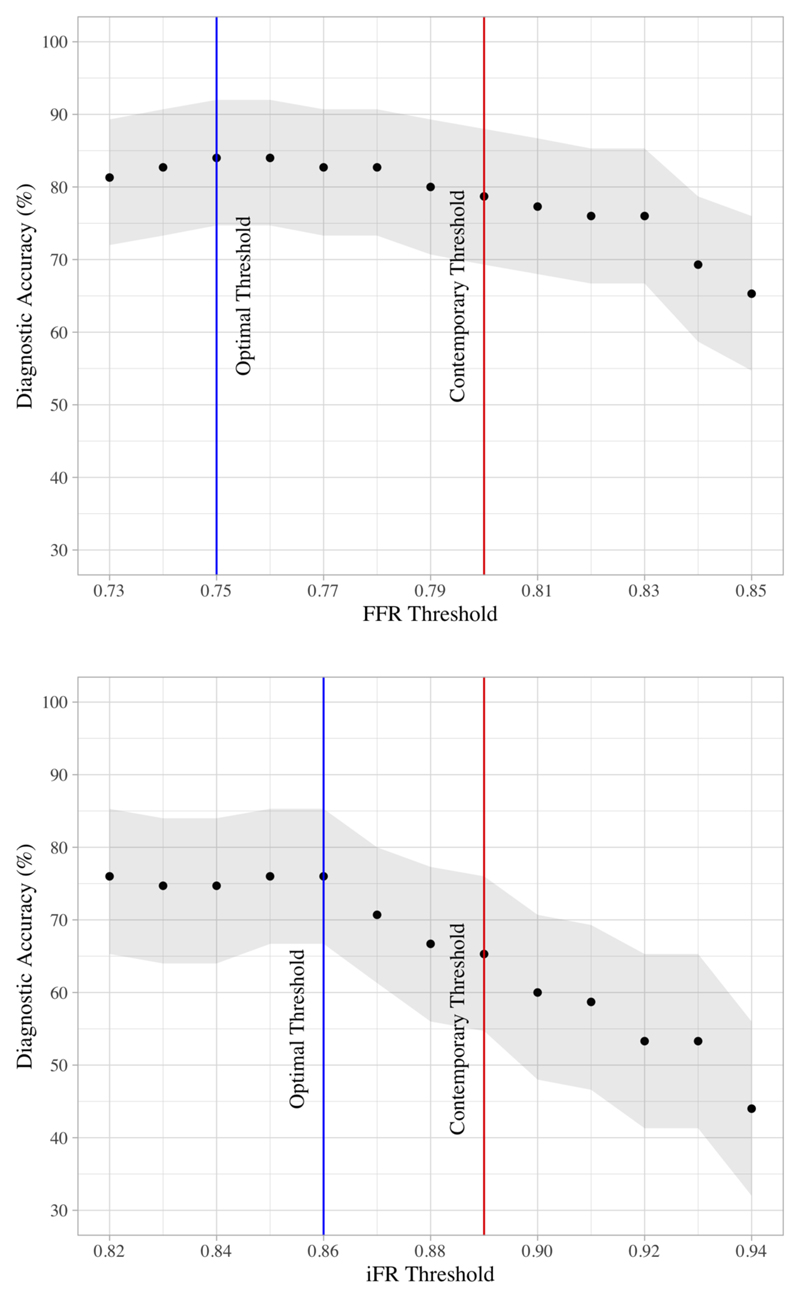
Sensitivity, specificity, NPV, and PPV for the contemporary FFR threshold (≤0.80) were 89.3%, 73.5%, 92.5% and 65.7% respectively, with an overall diagnostic accuracy of 78.7%. Peak diagnostic accuracy was found at FFR thresholds of 0.75 - 0.76 (diagnostic accuracy 84%). Sensitivity, specificity, NPV, and PPV for the contemporary iFR threshold (≤0.89) were 78.3%, 58%, 82.9% and 51.2% respectively, with an overall diagnostic accuracy of 65.3%. Peak diagnostic accuracy was found at iFR thresholds of 0.85 - 0.86 (76%). Peak diagnostic accuracy for resting Pd/Pa was found at a threshold of 0.88 (55.6% Sensitivity, 89.8% specificity, 78.7% NPV, 76% PPV and an overall diagnostic accuracy of 77.3%). A graphical representation of the diagnostic accuracy of different thresholds of FFR and iFR is shown in Figure 3.
Figure 3. Graph Showing Diagnostic Accuracy of Varying FFR and iFR Thresholds.
Graphs comparing diagnostic accuracy of varying thresholds of FFR (Top) and iFR (Bottom). Greyed area represents 95% confidence interval. Blue Line represents the optimal threshold identified in this study versus an invasive reference standard. Red line identifies the contemporary threshold used for each index in current clinical practice.
Using contemporary thresholds, there was discordance between FFR and iFR in 25.3% of cases with an equal number of cases where FFR +ve / iFR –ve and iFR +ve / FFR –ve. There was no significant difference (p>0.05) in baseline characteristics or coronary flow reserve (CFR) between these discordant groups. When adopting the optimal iFR and FFR thresholds from this study, the discordance between FFR and iFR fell to 10.7%.
Discussion
The main findings of this study are:
-
1)
The optimal diagnostic thresholds for FFR and iFR were found to be 0.75 and 0.86 respectively, which are in keeping with the thresholds identified when each index was originally derived. These thresholds provide superior diagnostic accuracy than contemporary thresholds for FFR (≤0.80) and iFR (≤0.89).
-
2)
When comparing indices at optimal thresholds, FFR showed better diagnostic performance compared to the resting indices, iFR and Pd/Pa, whilst the diagnostic accuracies of the latter are comparable.
Our results suggest that contemporary iFR (0.89) and FFR (0.80) thresholds provide suboptimal diagnostic performance compared to the original thresholds from derivation studies. With evidence to suggest the benefits of revascularizing stable CAD may not be as significant as originally anticipated and many questioning the role of PCI in stable CAD2, the ‘safety net’ of adopting higher thresholds with higher NPVs in exchange for lower overall diagnostic accuracy may be unjustified, particularly given the low event rate often seen in large trials of stable CAD4–7.
Adopting more rigorous thresholds means more targeted Revascularization of those patients with a greater burden of ischemia: with significant evidence existing to suggest this may be of greater clinical benefit. The ESC Guidelines and recent ACC/AHA Appropriate Use Criteria recognize that patients with a greater burden of ischemia are higher risk and more likely to benefit from Revascularization26, 27. The Hachamovitch et al cohort study of over 10,000 patients showed Revascularization compared with medical therapy had greater survival benefit (absolute and relative) in patients with moderate to large amounts of inducible ischemia8 and this evidence was supported by the nuclear sub-study of COURAGE, demonstrating that patients with a ≥5% reduction of ischemia on MPS had better outcomes from Revascularization compared to medical therapy28. In addition, in recent years, meta-analysis of FFR data has suggested lesions with lower FFR values receive larger absolute benefits from Revascularization29, with the analysis suggesting that an FFR as low as 0.67 provides optimal benefit for a composite involving death, MI, and Revascularization. Despite this weight of observational data, and data showing improved clinical outcomes out at 5 years using FFR-guided revascularization30, definitive evidence, in the form of adequately powered randomized trials, is still lacking to suggest better ischemic detection at lower thresholds translates to better clinical outcomes. Further evidence that revascularizing patients with greater ischemic burden yields greater patient benefit may come in the form of the ongoing ISCHEMIA trial (NCT01471522). Until this trial reports, there exists substantial observational data to suggest patients with the greatest ischemic burden are likely to experience most benefit from Revascularization and therefore the more rigorous iFR and FFR thresholds that this study supports, may well translate into improved clinical outcomes.
Whilst our study is not the first to demonstrate significant discordance between FFR and iFR, it is the first to do so for modern thresholds using an invasive reference standard for ischemia resulting from epicardial CAD. Very few studies have correlated iFR and FFR versus an independent reference standard. Petraco et al used coronary flow reserve (CFR) as an independent reference to show that an iFR threshold of 0.9 had better diagnostic discrimination than an FFR of 0.8 (iFR AUC 0.82; FFR AUC 0.72; p <0.001)31. Using CFR as a gold standard for invasive physiological measures of epicardial coronary disease may be significantly confounded by its dependence on external hemodynamics, including aortic pressure, heart rate and resting microvascular resistance. FFR is generally accepted to be less dependent on external factors, with De Bruyne et al 32 demonstrating no significant change in values when aortic pressure, myocardial contractility and heart rate were manipulated in vivo across normal physiological ranges. However subsequent modelling and bench work by Siebes et al19 suggested that pressure-derived indices may be somewhat more dependent on aortic pressure, particularly in circumstances where Pa is low in patients with high coronary outflow pressure (Pb). It is therefore important to realise that all currently available indices of stenosis severity, not just CFR, may be dependent on microvascular resistance and external haemodynamic factors to varying degrees, particularly around the the clinically-relevant “grey-zone”.
Other studies to compare iFR and FFR (at contemporary thresholds) versus invasive reference standard for ischemia include the study by Hwang et al, who used PET Relative Flow Reserve to show no significant difference in AUC between iFR and FFR (iFR AUC 0.77; FFR AUC 0.83; P=0.05) but with FFR showing better discriminatory ability when compared to an iFR threshold of 0.933, in keeping with the findings of our study. Sen et al previously also used hSR, to show similar diagnostic performance of iFR and FFR in 51 patients (iFR AUC 0.93; FFR AUC 0.96; P=0.48), although this study included patients with a milder spectrum of disease; less than 2/3rd were within the diagnostically challenging range 0.60-0.9020. In comparison to these studies we present the largest cohort (n=75) in which an invasive reference standard for ischemia has been applied17,18 with 80% of patients in our study within the diagnostically uncertain FFR range between 0.6 and 0.9.
The implications of this work are wide-ranging, including support for those who recommend reverting away from binary thresholds and instead using a grey zone approach: using clinical judgment for revascularization when the value falls in the grey zone 34. For example, there is some evidence to suggest PCI of lesions with FFR between 0.75 and 0.80 is associated with greater clinical benefit for proximal lesions 35. A grey-zone approach to resting indices may be of even further value given the narrower band-width at which these indices are commonly used. The results of our study also support moving away from a grey-zone approach in favour of considering using the original and more stringent thresholds in future studies of both FFR and iFR: further supported by the recent data by Kang et al suggesting PCI of greyzone-FFR cases do not derive net clinical benefit16.
If future trials for physiology-guided Revascularization did use more stringent thresholds, this would mean Revascularization is reserved for a higher risk cohort which may yield greater benefit than the surprisingly modest outcomes and weak symptom benefit suggested by some recent trials of PCI for stable CAD1,2.
Study Limitations
We recognize there is currently no gold standard ischemia test, and this is reflected by the fact previous studies have used PET, CFR and hSR as a reference standard, without consensus. We used hSR, as this the most theoretically robust invasive vessel-specific physiological test, which is known to have greater accuracy in detecting inducible ischemia17 and has previously been used in derivation work of pressure-derived indices of stenosis severity20. However, hSR lacks the prognostic evidence base that the pressure derived indices have and given the steep learning curve, its use is limited to the research arena.
We acknowledge that stenosis resistance can also be measured in resting conditions, but small errors in pressure and flow velocity may result in relatively large errors, making this inappropriate for reference-standard. Therefore a potential explanation for our additional finding of FFR superiority over iFR is the use of a hyperemic index as the reference standard test. That said, hyperemic conditions continue to be widely recognized as the reference standard against which novel invasive indices are often adjudged, including in the recent derivation work of resting indices20.
We also recognize that this study has a relatively small sample size from a single centre. Whilst it is certainly the largest study of its kind, lend support to a large multi-centre randomized study, rather than being practice changing per se.
Conclusions
Modern FFR and iFR thresholds provide suboptimal diagnostic accuracy compared to a FFR threshold of 0.75 or an iFR threshold of 0.86 respectively, which are also the binary cut-offs from the original derivation studies. Fractional flow reserve had better diagnostic accuracy than either of the resting indices, iFR and Pd/Pa. When used to guide treatment, the less specific contemporary thresholds could lead to inappropriate treatment of vessels. Whether the use of the more rigorous thresholds would result in selection of a population gaining greater symptom and prognostic benefit needs to be assessed in future studies of physiology-guided Revascularization.
What is Known
Evidence is mounting to suggest that patients on optimal medical therapy alone have an excellent prognosis with significantly improved symptoms, as suggested by the COURAGE trial and recently the Sham-controlled ORBITA trial. In light of this, it has become even more important to ensure we carefully select patients for revascularization.
Evidence suggesting physiology-guided revascularization, whether it be in the form of Fractional Flow Reserve (FFR) or Instantaneous Wave Free Ratio (iFR) is associated with significantly improved symptoms and patient outcomes, compared to revascularization based on angiographic appearances alone.
The benefits of revascularization seem to be greatest in those with a high burden of ischemia
What the Study Adds
Contemporary thresholds of invasive physiological indices provide suboptimal diagnostic accuracy, compared to the original more stringent thresholds described in derivation studies. This may be leading to inappropriate treatment of vessels, which are not capable of causing ischemia.
The findings of this study would also support using more robust and stringent thresholds in future trials comparing revascularization modalities and when assessing the efficacy of revascularization.
Sources of Funding
Authors BM and HR are funded by British Heart Foundation Clinical Research Training Fellowships (FS/15/78/31678 and FS/16/49/32320)
Footnotes
Conflicts of Interest Disclosures:
None of the authors have any conflict of interest or relationships with industry that could have influenced this manuscript.
References
- 1.Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 2.Al-Lamee R, Thompson D, Dehbi H-M, Sen S, Tang K, Davies J, Keeble T, Mielewczik M, Kaprielian R, Malik IS, Nijjer SS, et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet. 2017;391:31–40. doi: 10.1016/S0140-6736(17)32714-9. [DOI] [PubMed] [Google Scholar]
- 3.Bech GJW, De Bruyne B, Pijls NHJ, de Muinck ED, Hoorntje JCA, Escaned J, Stella PR, Boersma E, Bartunek J, Koolen JJ, Wijns W. Fractional Flow Reserve to Determine the Appropriateness of Angioplasty in Moderate Coronary Stenosis : A Randomized Trial. Circulation. 2001;103:2928–2934. doi: 10.1161/01.cir.103.24.2928. [DOI] [PubMed] [Google Scholar]
- 4.Tonino PAL, De Bruyne B, Pijls NHJ, Siebert U, Ikeno F, van t Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, Ver Lee PN, et al. Fractional Flow Reserve versus Angiography for Guiding Percutaneous Coronary Intervention. N Engl J Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 5.De Bruyne B, Pijls NHJ, Kalesan B, Barbato E, Tonino PAL, Piroth Z, Jagic N, Möbius-Winkler S, Rioufol G, Witt N, Kala P, et al. Fractional Flow Reserve–Guided PCI versus Medical Therapy in Stable Coronary Disease. New England Journal of Medicine. 2012;367:991–1001. doi: 10.1056/NEJMoa1205361. [DOI] [PubMed] [Google Scholar]
- 6.Davies JE, Sen S, Dehbi H-M, Al-Lamee R, Petraco R, Nijjer SS, Bhindi R, Lehman SJ, Walters D, Sapontis J, Janssens L, et al. Use of the Instantaneous Wave-free Ratio or Fractional Flow Reserve in PCI. N Engl J Med. 2017 doi: 10.1056/NEJMoa1700445. NEJMoa1700445. [DOI] [PubMed] [Google Scholar]
- 7.Götberg M, Christiansen EH, Gudmundsdottir IJ, Sandhall L, Danielewicz M, Jakobsen L, Olsson S-E, Öhagen P, Olsson H, Omerovic E, Calais F, et al. Instantaneous Wave-free Ratio versus Fractional Flow Reserve to Guide PCI. N Engl J Med. 2017 doi: 10.1056/NEJMoa1616540. NEJMoa1616540. [DOI] [PubMed] [Google Scholar]
- 8.Hachamovitch R. Comparison of the Short-Term Survival Benefit Associated With Revascularization Compared With Medical Therapy in Patients With No Prior Coronary Artery Disease Undergoing Stress Myocardial Perfusion Single Photon Emission Computed Tomography. Circulation. 2003;107:2900–2907. doi: 10.1161/01.CIR.0000072790.23090.41. [DOI] [PubMed] [Google Scholar]
- 9.Johnson NP, Toth GG, Lai D, Zhu H, Açar G, Agostoni P, Appelman Y, Arslan F, Barbato E, Chen S-L, Di Serafino L, et al. Prognostic Value of Fractional Flow Reserve. Journal of the American College of Cardiology. 2014;64:1641–1654. doi: 10.1016/j.jacc.2014.07.973. [DOI] [PubMed] [Google Scholar]
- 10.Pijls NHJ, De Bruyne B, Peels K, van der Voort PH, Bonnier HJRM, Bartunek J, Koolen JJ. Measurement of Fractional Flow Reserve to Assess the Functional Severity of Coronary-Artery Stenoses. New England Journal of Medicine. 1996;334:1703–1708. doi: 10.1056/NEJM199606273342604. [DOI] [PubMed] [Google Scholar]
- 11.Lockie T, Ishida M, Perera D, Chiribiri A, De Silva K, Kozerke S, Marber M, Nagel E, Rezavi R, Redwood S, Plein S. High-resolution magnetic resonance myocardial perfusion imaging at 3.0-Tesla to detect hemodynamically significant coronary stenoses as determined by fractional flow reserve. Journal of the American College of Cardiology. 2011;57:70–75. doi: 10.1016/j.jacc.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Johnson NP, Kirkeeide RL, Asrress KN, Fearon WF, Lockie T, Marques KMJ, Pyxaras SA, Rolandi MC, van t Veer M, De Bruyne B, Piek JJ, et al. Does the Instantaneous Wave-Free Ratio Approximate the Fractional Flow Reserve? Journal of the American College of Cardiology. 2013;61:1428–1435. doi: 10.1016/j.jacc.2012.09.064. [DOI] [PubMed] [Google Scholar]
- 13.Petraco R, Escaned J, Sen S, Nijjer S, Asrress KN, Echavarria-Pinto M, Lockie T, Khawaja MZ, Cuevas C, Foin N, Broyd C, et al. Classification performance of instantaneous wave-free ratio (iFR) and fractional flow reserve in a clinical population of intermediate coronary stenoses: results of the ADVISE registry. EuroIntervention. 2013;9:91–101. doi: 10.4244/EIJV9I1A14. [DOI] [PubMed] [Google Scholar]
- 14.Escaned J, Echavarria-Pinto M, Garcia-Garcia HM, van de Hoef TP, de Vries T, Kaul P, Raveendran G, Altman JD, Kurz HI, Brechtken J, Tulli M, et al. Prospective Assessment of the Diagnostic Accuracy of Instantaneous Wave-Free Ratio to Assess Coronary Stenosis Relevance: Results of ADVISE II International, Multicenter Study (ADenosine Vasodilator Independent Stenosis Evaluation II) JACC Cardiovasc Interv. 2015;8:824–833. doi: 10.1016/j.jcin.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 15.Jeremias A, Maehara A, Généreux P, Asrress KN, Berry C, De Bruyne B, Davies JE, Escaned J, Fearon WF, Gould KL, Johnson NP, et al. Multicenter Core Laboratory Comparison of the Instantaneous Wave-Free Ratio and Resting Pd/Pa With Fractional Flow Reserve. Journal of the American College of Cardiology. 2014;63:1253–1261. doi: 10.1016/j.jacc.2013.09.060. [DOI] [PubMed] [Google Scholar]
- 16.Kang D-Y, Ahn J-M, Lee CH, Lee PH, Park D-W, Kang S-J, Lee S-W, Kim Y-H, Lee CW, Park S-W, Park S-J. Deferred vs. performed revascularization for coronary stenosis with grey-zone fractional flow reserve values: data from the IRIS-FFR registry. Eur Heart J. 2018;360:213. doi: 10.1093/eurheartj/ehy079. [DOI] [PubMed] [Google Scholar]
- 17.Meuwissen M, Siebes M, Chamuleau SAJ, van Eck-Smit BLF, Koch KT, de Winter RJ, Tijssen JGP, Spaan JAE, Piek JJ. Hyperemic stenosis resistance index for evaluation of functional coronary lesion severity. Circulation. 2002;106:441–446. doi: 10.1161/01.cir.0000023041.26199.29. [DOI] [PubMed] [Google Scholar]
- 18.Spaan JAE. Physiological Basis of Clinically Used Coronary Hemodynamic Indices. Circulation. 2006;113:446–455. doi: 10.1161/CIRCULATIONAHA.105.587196. [DOI] [PubMed] [Google Scholar]
- 19.Siebes M, Chamuleau SAJ, Meuwissen M, Piek JJ, Spaan JAE. Influence of hemodynamic conditions on fractional flow reserve: parametric analysis of underlying model. AJP: Heart and Circulatory Physiology. 2002;283:H1462–70. doi: 10.1152/ajpheart.00165.2002. [DOI] [PubMed] [Google Scholar]
- 20.Sen S, Asrress KN, Nijjer S, Petraco R, Malik IS, Foale RA, Mikhail GW, Foin N, Broyd C, Hadjiloizou N, Sethi A, et al. Diagnostic classification of the instantaneous wave-free ratio is equivalent to fractional flow reserve and is not improved with adenosine administration. Results of CLARIFY (Classification Accuracy of Pressure-Only Ratios Against Indices Using Flow Study) Journal of the American College of Cardiology. 2013;61:1409–1420. doi: 10.1016/j.jacc.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 21.Adjedj J, Toth GG, Johnson NP, Pellicano M, Ferrara A, Floré V, Di Gioia G, Barbato E, Muller O, De Bruyne B. Intracoronary Adenosine: Dose-Response Relationship With Hyperemia. JACC Cardiovasc Interv. 2015;8:1422–1430. doi: 10.1016/j.jcin.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 22.Sen S, Escaned J, Malik IS, Mikhail GW, Foale RA, Mila R, Tarkin J, Petraco R, Broyd C, Jabbour R, Sethi A, et al. Development and Validation of a New Adenosine-Independent Index of Stenosis Severity From Coronary Wave–Intensity Analysis. Journal of the American College of Cardiology. 2012;59:1392–1402. doi: 10.1016/j.jacc.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Johnson NP, Jeremias A, Zimmermann FM, Adjedj J, Witt N, Hennigan B, Koo B-K, Maehara A, Matsumura M, Barbato E, Esposito G, et al. Continuum of Vasodilator Stress From Rest to Contrast Medium to Adenosine Hyperemia for Fractional Flow Reserve Assessment. JACC Cardiovasc Interv. 2016;9:757–767. doi: 10.1016/j.jcin.2015.12.273. [DOI] [PubMed] [Google Scholar]
- 24.van’t Veer M, Pijls NHJ, Hennigan B, Watkins S, Ali ZA, De Bruyne B, Zimmermann FM, van Nunen LX, Barbato E, Berry C, Oldroyd KG. Comparison of Different Diastolic Resting Indexes to iFR: Are They All Equal? Journal of the American College of Cardiology. 2017;70:3088–3096. doi: 10.1016/j.jacc.2017.10.066. [DOI] [PubMed] [Google Scholar]
- 25.Johnson NP, Johnson DT, Kirkeeide RL, Berry C, De Bruyne B, Fearon WF, Oldroyd KG, Pijls NHJ, Gould KL. Repeatability of Fractional Flow Reserve Despite Variations in Systemic and Coronary Hemodynamics. JACC Cardiovasc Interv. 2015;8:1018–1027. doi: 10.1016/j.jcin.2015.01.039. [DOI] [PubMed] [Google Scholar]
- 26.Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, Smith PK. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 Appropriate Use Criteria for Coronary Revascularization in Patients With Stable Ischemic Heart Disease: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. Journal of the American College of Cardiology. 2017;69:2212–2241. doi: 10.1016/j.jacc.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 27.2013 ESC guidelines on the management of stable coronary artery disease. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 28.Shaw LJ, Berman DS, Maron DJ, Mancini GBJ, Hayes SW, Hartigan PM, Weintraub WS, O'Rourke RA, Dada M, Spertus JA, Chaitman BR, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283–1291. doi: 10.1161/CIRCULATIONAHA.107.743963. [DOI] [PubMed] [Google Scholar]
- 29.Johnson NP, Toth GG, Lai D, Zhu H, Açar G, Agostoni P, Appelman Y, Arslan F, Barbato E, Chen S-L, Di Serafino L, et al. Prognostic Value of Fractional Flow Reserve. Journal of the American College of Cardiology. 2014;64:1641–1654. doi: 10.1016/j.jacc.2014.07.973. [DOI] [PubMed] [Google Scholar]
- 30.Xaplanteris P, Fournier S, Pijls NHJ, Fearon WF, Barbato E, Tonino PAL, Engstrøm T, Kääb S, Dambrink J-H, Rioufol G, Toth GG, et al. Five-Year Outcomes with PCI Guided by Fractional Flow Reserve. N Engl J Med. 2018;379:250–259. doi: 10.1056/NEJMoa1803538. [DOI] [PubMed] [Google Scholar]
- 31.Petraco R, van de Hoef TP, Nijjer S, Sen S, van Lavieren MA, Foale RA, Meuwissen M, Broyd C, Echavarria-Pinto M, Foin N, Malik IS, et al. Baseline instantaneous wave-free ratio as a pressure-only estimation of underlying coronary flow reserve: results of the JUSTIFY-CFR Study (Joined Coronary Pressure and Flow Analysis to Determine Diagnostic Characteristics of Basal and Hyperemic Indices of Functional Lesion Severity-Coronary Flow Reserve) Circulation: Cardiovascular Interventions. 2014;7:492–502. doi: 10.1161/CIRCINTERVENTIONS.113.000926. [DOI] [PubMed] [Google Scholar]
- 32.De Bruyne B, Bartunek J, Sys SU, Pijls NH, Heyndrickx GR, Wijns W. Simultaneous coronary pressure and flow velocity measurements in humans. Feasibility, reproducibility, and hemodynamic dependence of coronary flow velocity reserve, hyperemic flow versus pressure slope index, and fractional flow reserve. Circulation. 1996;94:1842–1849. doi: 10.1161/01.cir.94.8.1842. [DOI] [PubMed] [Google Scholar]
- 33.Hwang D, Jeon K-H, Lee JM, Park J, Kim CH, Tong Y, Zhang J, Bang J-I, Suh M, Paeng JC, Na S-H, et al. Diagnostic Performance of Resting and Hyperemic Invasive Physiological Indices to Define Myocardial Ischemia: Validation With (13)N-Ammonia Positron Emission Tomography. JACC Cardiovasc Interv. 2017;10:751–760. doi: 10.1016/j.jcin.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 34.Fearon WF. Invasive Coronary Physiology for Assessing Intermediate Lesions. Circulation: Cardiovascular Interventions. 2015;8:e001942–e001942. doi: 10.1161/CIRCINTERVENTIONS.114.001942. [DOI] [PubMed] [Google Scholar]
- 35.Adjedj J, De Bruyne B, Floré V, Di Gioia G, Ferrara A, Pellicano M, Toth GG, Bartunek J, Vanderheyden M, Heyndrickx GR, Wijns W, et al. Significance of Intermediate Values of Fractional Flow Reserve in Patients With Coronary Artery Disease. Circulation. 2016;133:502–508. doi: 10.1161/CIRCULATIONAHA.115.018747. [DOI] [PubMed] [Google Scholar]