Abstract
Introduction
Outcome data on radiotherapy for prostate cancer in an elderly population are sparse. The CHHiP trial provides a large, prospectively collected, contemporary dataset in which to explore outcomes by age.
Methods and Materials
CHHiP participants received 3-6 months of androgen deprivation therapy and were randomly assigned (1:1:1) to receive 74Gy in 37 fractions (conventional fractionation), 60Gy in 20 fractions or 57Gy in 19 fractions. Toxicity was assessed using clinician-reported and patient-reported outcome (CRO/PRO) questionnaires. Participants were categorised as aged less than 75 (<75) or 75 years and older (75+).Outcomes were compared by age-group.
Results
491/3216 (15%) were 75+. There was no difference in biochemical or clinical failure (BCF) rates between the <75 and the 75+ group for any of the fractionation schedules. In the 75+ group BCF-free rates favoured hypofractionation and at 5 years were 74Gy: 84.7%, 60Gy: 91%, 57Gy: 87.7%. The incidence of CRO (G3) acute bowel toxicity was 2% in both age-groups. Grade 3 acute bladder toxicity was 8% and 7%. Five year cumulative incidence of CRO grade 2+ late bowel side effects was similar in both age groups. However, in the 75+ group, there was a suggestion of a higher cumulative incidence of bowel bother (≥small) with 60Gy compared to 74Gy and 57Gy. Patient-reported bladder bother was slightly higher in the 75+ group than the <75 group and there was a suggestion of a lower cumulative incidence of bladder bother with 57Gy compared to 74Gy and 60Gy in the 75+ group which was not evident in those <75.
Conclusion
Hypofractionated radiotherapy appears to be well tolerated and effective in men over 75. The 57 Gy schedule has potential advantages in that it may moderate long term side effects without compromising treatment efficacy in this group.
Introduction
Prostate cancer (PCa) is the most common cancer in men in the UK with 46,690 new cases and 11,287 deaths in 2014 (1). Fifty-four percent of all new cases of prostate cancer are diagnosed in men aged over 70 years, with the highest incidence in men over 90 (1). Management options for localised disease include active surveillance in those with low-risk disease, external beam radiotherapy, radical prostatectomy, and watchful waiting in those not suitable for radical treatment.
The Conventional or Hypofractionated High Dose Intensity Modulated Radiotherapy in Prostate Cancer (CHHiP) trial (CRUK/06/016) compared conventional and hypofractionated high-dose intensity-modulated radiotherapy (HFRT) for prostate cancer (2). The hypofractionated regimen of 60 Gy in 20 fractions was shown to be non-inferior to the conventional fractionation of 74 Gy in 37 fractions, supporting its use as a new standard of care for external beam radiotherapy for prostate cancer.
Although age is not a factor in the likelihood of a patient completing radiotherapy (3), elderly patients are generally under-represented in clinical trials resulting in the lack of a robust evidence base (4, 5). The median age in the CHHiP trial was 69 years (range 44 to 85). This reflects the age-related incidence of prostate cancer and the appropriate use of a patient’s performance status rather than age to direct treatment decisions. In this exploratory analyses of the CHHiP data we compare treatment outcomes in terms of time to biochemical or clinical failure and treatment related toxicity in patients categorised as less than 75 (<75) or 75 years and older (75+).
Methods and Materials
Study design and randomisation
The CHHiP study design has been described elsewhere (2). Briefly, men ≥16 years with a WHO performance status of 0 or 1 and histologically proven, T1b-T3aN0M0 prostate cancer were eligible. Patients with T3 tumours and a Gleason score ≥8, or a life expectancy of less than 10 years were ineligible. Initially, men with a prostate-specific antigen (PSA) ≤40 ng/ml and a risk of pelvic lymph node involvement of < 30 % were eligible, but this was revised in August 2006 to a requirement of PSA <30 ng/ml and a risk of seminal vesicle involvement of <30% to reflect the developing consensus of a need for long-term androgen deprivation (ADT) in men with locally advanced disease. The trial was reviewed by the London Multicentre Research Ethics Committee (04/MRE02/10) and was in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
Men were randomised (1:1:1) to receive 74 Gy in 37 fractions over 7.4 weeks (conventional fractionation) or one of two hypofractionated regimens using daily fractions of 3 Gy; 60 Gy in 20 fractions over 4 weeks or 57 Gy in 19 fractions over 3.8 weeks. Randomisation was stratified for National Comprehensive Cancer Network (NCCN) risk classification and treatment centre, but not patient age. It was not possible to mask patients or clinicians to treatment allocation.
Procedures
3-6 months of ADT before and during radiotherapy was mandated in men with NCCN intermediate and high-risk disease, but was optional in those with low-risk disease. All radiotherapy was given using an intensity-modulated radiotherapy (IMRT) technique. Further details of the treatment and its quality assurance have been reported previously (2). PSA concentrations were recorded before commencing ADT and radiotherapy and then at weeks 10, 18, and 26 after radiotherapy and then at 6-month intervals for 5 years and then annually.
Acute and late toxicity was assessed using clinician-reported outcome (CRO) grading systems and patient-reported outcome (PRO) questionnaires. The Radiation Therapy Oncology Group (RTOG) system (6) was used to score toxicity every week during radiotherapy and at weeks 10, 12 and 18. Bowel, bladder and sexual function assessments were made before ADT and the start of radiotherapy and were graded according to the Late Effects on Normal Tissues: Subjective/Objective/Management (LENT/SOM) (7) and Royal Marsden Hospital (RMH) (8) scoring systems. Late toxicity was collected six monthly to 2 years and then annually to 5 years using all three toxicity scales. Men participating in a PRO substudy received questionnaires at baseline if they had not yet started ADT and all men received questionnaires pre-radiotherapy and at 10 weeks and 6, 12, 18 and 24 months after the start of radiotherapy and then annually until 5 years. Further details of the PRO substudy are presented elsewhere (9).
Outcomes
Biochemical or clinical failure (BCF) was the primary endpoint. The Phoenix consensus guidelines of a PSA concentration greater than the nadir plus 2 ng/ml (10) was used after 2007 and applied retrospectively to patients recruited before this date. Other recurrence (failure) events included recommencement of ADT, local recurrence, lymph node or pelvic recurrence and distant metastases. Acute toxicity was reported as the highest grade of bowel and bladder toxicity in the first 18 weeks from the start of radiotherapy. CRO late toxicity outcomes were reported using the time to first grade 2 or more toxic effect using the RTOG, LENT/SOM and RMH scoring systems. PROs of interest were time to first small or greater overall bowel bother and overall urinary bother reported as single items on the UCLA-PCI (11) and EPIC-50 (12) questionnaires.
Statistical considerations
All analyses presented are exploratory post-hoc subgroup analyses. As this was a non-randomised comparison, statistical comparisons were made for the baseline demographic data presented by age group (<75 and 75+) (T-tests, Mann-Whitney, chi2 and chi2 trend tests were used as appropriate). Kaplan-Meier methods were used to analyse time-to-event data. Comparisons of each hypofractionated regimen compared to 74Gy were made within each age group using the log-rank test. Hazard ratios less than 1 favoured hypofractionated radiotherapy. Acute and late toxicity data were analysed using the same methodology as previously described (2), with treatment comparisons made within each age group separately. Toxicity of grade 2 at five years from starting radiotherapy was of primary interest. Patient reported outcomes were analysed using the same methodology as previously described (9), small or greater bother was of primary interest. A significance level of 1% was used due to multiple testing. All analyses were conducted using STATA version 13.0 and based on the primary analysis data snapshot taken on 8th September 2015.
Results
Baseline demographics
Baseline demographics and medical history for patients in the <75 (n=2725) and 75+ (n=491) groups are shown in Table 1 and Supplementary Table 1. There was a significant difference (p<0.0001) in NCCN risk group distribution between the age groups with a higher proportion of intermediate-risk disease compared to low-risk disease in the 75+ group. The 75+ group had more Gleason 7 but fewer Gleason 6 cancers than the <75 group and the 75+ group had a larger maximum length of biopsy core involvement. Median PSA levels were higher in the 75+ group (11.4ng/ml) compared with the < 75 group (9.8ng/ml p < 0.0001) but pre-hormone testosterone levels were similar. Prostate volume was larger in the 75+ group (median 42.7 cm3) compared to the < 75 group (median 37.0 cm3, p = 0.001). More patients in the 75+ group compared with <75 had a previous transurethral resection of prostate (13% vs 7% respectively, p < 0.0001, see Supplementary Table 1). IGRT use was similar in the two groups but more men in the <75 group received bicalutamide alone (p=0.014).
Table 1. Baseline demographics for patients <75 and ≥75 years old.
| <75 (N=2725) | 75+ (N=491) | P-value | ||||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| Age | ||||||
| Median (IQR) | 67 (63-71) | 76 (75-78) | <0.0001 | |||
| Treatment group | ||||||
| 74Gy | 898 | 33 | 167 | 34 | 0.709 | |
| 60Gy | 925 | 34 | 149 | 30 | ||
| 57Gy | 902 | 33 | 175 | 36 | ||
| NCCN risk group | ||||||
| High Risk | 321 | 12 | 64 | 13 | <0.0001 | |
| Intermediate Risk | 1956 | 72 | 391 | 80 | ||
| Low Risk | 448 | 16 | 36 | 7 | ||
| Intended hormone therapy | ||||||
| LHRH+ short term AA | 2264 | 84 | 436 | 89 | 0.014 | |
| 150mg Bicalutamide | 357 | 13 | 46 | 9 | ||
| MAB | 3 | <1 | 2 | <1 | ||
| Bicalutamide-other | 2 | <1 | 0 | 0 | ||
| LHRH alone | 0 | 0 | 2 | <1 | ||
| None | 86 | 3 | 4 | <1 | ||
| Gleason score | ||||||
| ≤6 | 975 | 36 | 147 | 29 | 0.018 | |
| 7 | 1668 | 61 | 327 | 67 | ||
| 8 | 82 | 3 | 17 | 4 | ||
| Clinical T stage | ||||||
| T1 | 1034 | 38 | 136 | 28 | <0.0001 | |
| T2 | 1452 | 53 | 314 | 64 | ||
| T3 | 236 | 9 | 41 | 8 | ||
| TX | 1 | <1 | 0 | 0 | ||
| Missing/Not done | 1 | <1 | 0 | 0 | ||
| Pre-hormone PSA (ng/ml) | ||||||
| No. with data | 2724 | 490 | ||||
| Median (IQR) | 9.8 (7.0, 14.2) | 11.4 (8.6, 14.8) | <0.0001 | |||
| Pre-hormone testosterone (nmol/L) | 0.883 | |||||
| No. with data | 1114 | 146 | ||||
| Median (IQR) | 12.6 (9.5, 16.2) | 12.3 (9.5, 16.4) | ||||
| Pre-hormone LH (iu/L) | ||||||
| No. with data | 1033 | 123 | 0.024 | |||
| Median (IQR), range | 4 (3, 6) | 1, 56 | 5 (3, 7) | |||
| IGRT used | ||||||
| Yes | 825 | 33 | 148 | 33 | 0.963 | |
| No | 1686 | 67 | 304 | 67 | ||
| Prostate volume (cm3) | ||||||
| No. with data | 936 | 217 | 0.001 | |||
| Median (IQR) | 37.0 (28.0, 50.0) | 42.7 (30.3, 54.8) | ||||
| Maximum length of core involvement (%) | ||||||
| No. with data | 1451 | 289 | 0.007 | |||
| Median (IQR) | 35 (15, 60) | 40 (20, 70) | ||||
| Maximum length of core involvement (mm) | ||||||
| No. with data | 452 | 92 | 0.007 | |||
| Median (IQR) | 9 (4, 17) | 12 (7, 20) | ||||
Time to biochemical or clinical failure
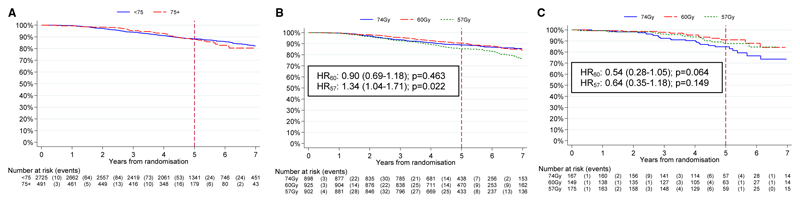
There was no evidence of a difference in BCF between the two age groups (p=0.909) (Figure 1A). In the <75 age group, 5 year BCF-free rates were 88.9% (95% CI 86.5-90.9), 90.5% (88.3-92.3) and 85.5% (82.8-87.8) in the 74 Gy, 60 Gy and 57 Gy groups respectively (Figure 1B). In the 75+ age group, 5 year BCF-free rates were 84.7% (77.3-89.9), 91.0% (83.7-95.1) and 87.7% (80.2-92.4) in the 74 Gy, 60 Gy and 57 Gy groups respectively (Figure 1C). BCR-free rates for the 74Gy group were slightly better in ≤75yr group compared with 75+ group (in keeping with less favourable presenting features in 75+ group) which seemed to be favourably modified by hypofractionation. (Figure 1C).
Figure 1.
Time to biochemical failure or prostate cancer recurrence (A) for <75 and ≥75 years old (B) <75 years old by treatment group (C) ≥75 years old by treatment group
Acute toxicity
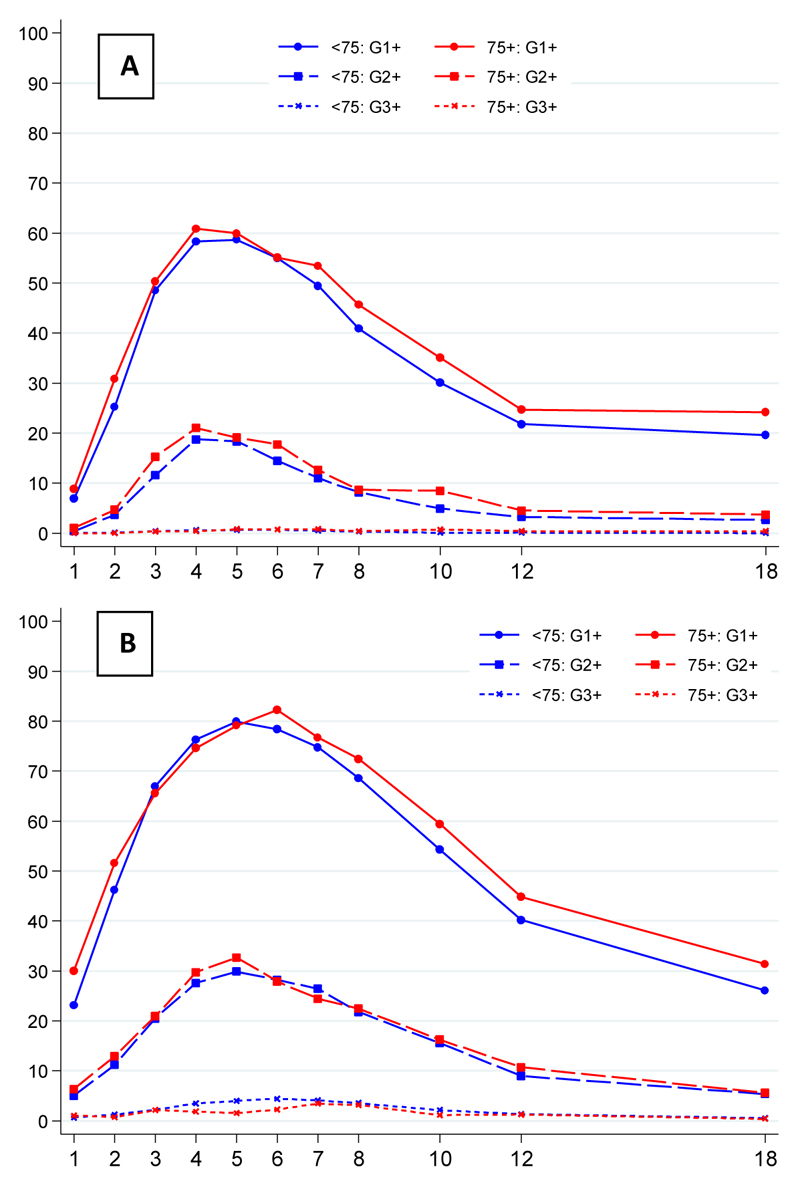
The prevalence of clinician assessed bowel (Figure 2A) and bladder (Figure 2B) toxicity from week 1 to week 18 was similar in the two age groups.
Figure 2.
Prevalence of clinician assessed RTOG (A) bowel and (B) bladder toxicity during week 1 to 18 from start of radiotherapy for <75 and ≥75 years old by toxicity grade
There was no evidence of a difference in peak acute RTOG bowel toxicity (Figure 2A), between the age groups (p = 0.561) with 34/1859 (2%) and 5/289 (2%) of the <75 and 75+ groups experiencing grade 3 bowel toxicity, with no reported grade 4 bowel toxicity. Within the <75 age group, there was a significant difference in peak acute bowel toxicity between the control and both hypofractionated groups (p < 0.0001 for both 60 Gy and 57 Gy comparisons) although this did not reach statistical significance in the 75+ group (p = 0.097 and p = 0.054 for 60 Gy and 57 Gy); Supplementary Table 2. At 18 weeks, there was no significant difference in the distribution of the grade of acute bowel toxicity between age groups (p=0.274).
There was no evidence of a difference in peak acute RTOG bladder toxicity (Figure 2B), between the age groups (p = 0.920). Grade 3 and 4 toxicity was recorded in 147/1859 (8%) and 21/1859 (1%) with 20/289 (7%) and 2/289 (1%) in the <75 and 75+ groups respectively. Within the <75 age group, there was no significant difference in acute bladder toxicity noted between the control and either hypofractionated group (p=0.969 and p=0.569 for 60 Gy and 57 Gy groups respectively). However, within the 75+ age group there was more acute bladder toxicity in the control group than the 60 Gy group (p=0.004) but not 57 Gy (p=0.083); Supplementary Table 2. Differences had disappeared by 18 weeks.
Late toxicity
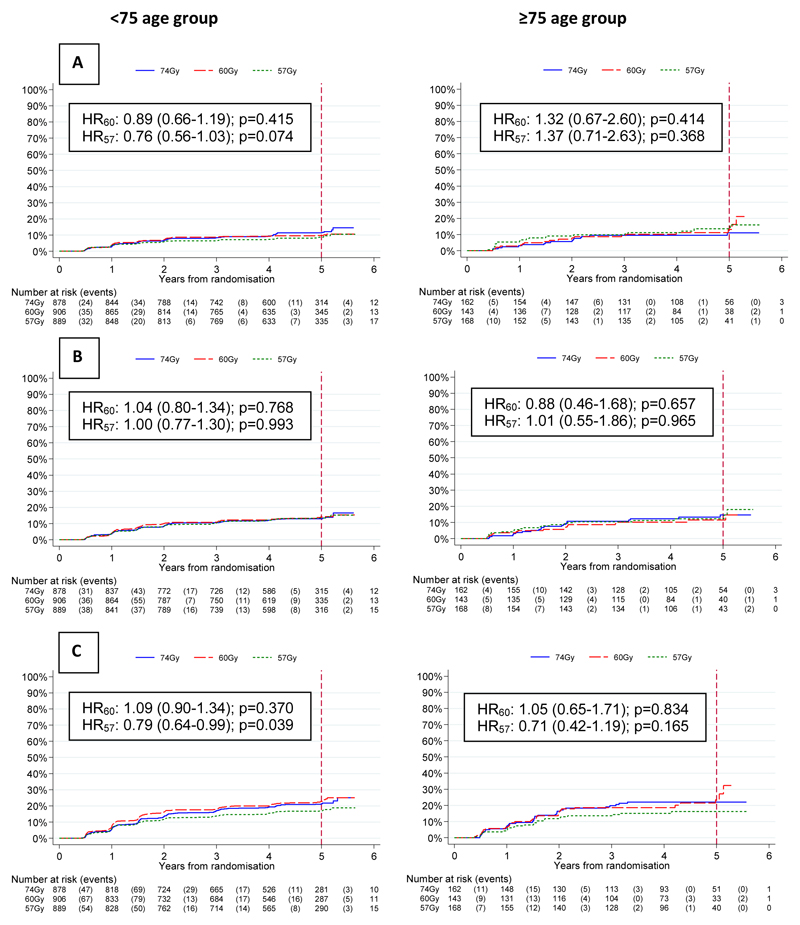
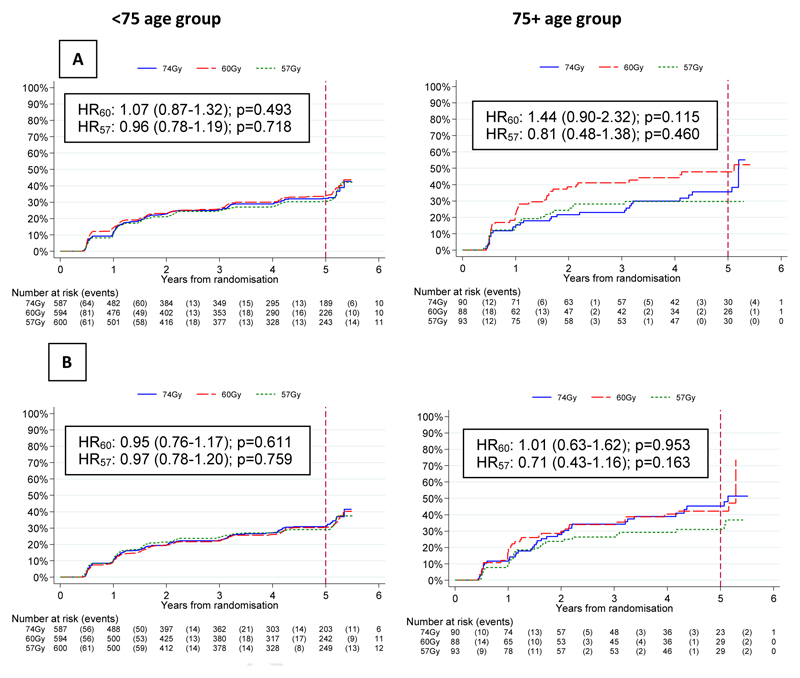
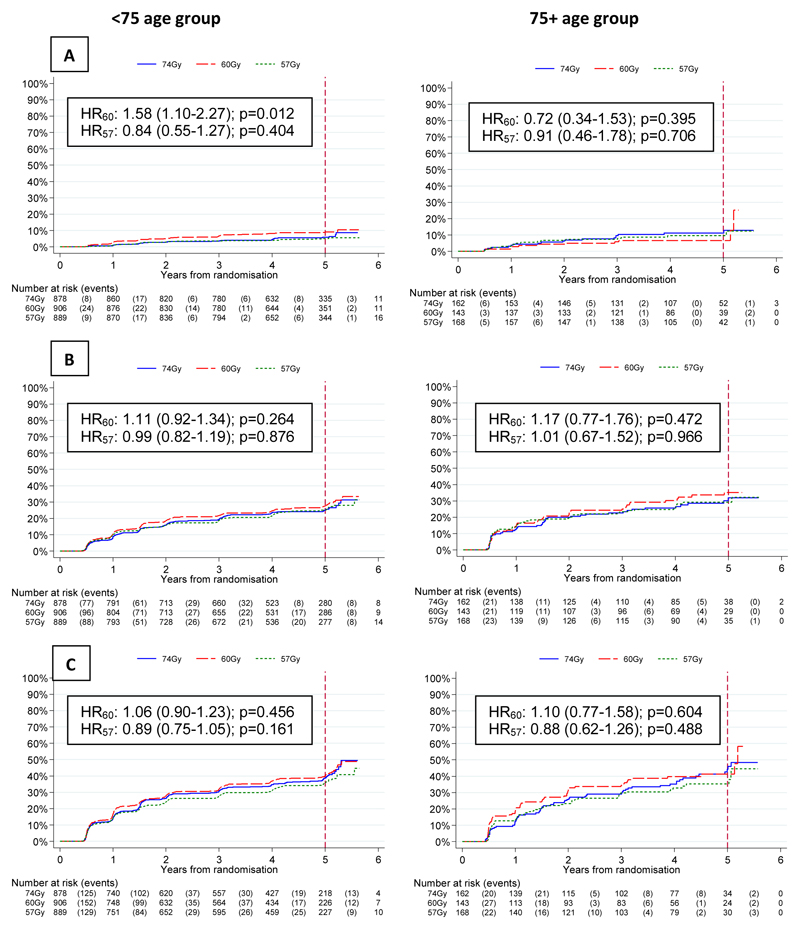
There was no evidence of a difference in time to first grade 2+ bowel toxicity using any CRO scale for either hypofractionated group compared to the control group in either age group (Figure 3). The 5 year cumulative incidences of grade2+ RTOG, RMH, and LENT-SOM bowel late side effects were similar with rates of 9.9% (95% CI: 8.8-11.2) / 12.5% (9.5-16.3), 13.5% (12.2-14.9) / 12.9% (10.0-16.6), 20.4% (18.8-22.1) / 20.4% (16.8-24.7) for the <75 and 75+ groups respectively (Figure 3). The prevalence of CRO late side-effects was stable over time from 1-5yrs with 2 year grade2+ RTOG, RMH and LENT-SOM bowel toxicity of 68/2430 (3%), 87/2413 (4%) and 131/2352 (6%) in the <75 group compared with 12/413 (3%), 21/412(5%) and 29/401 (7%) in the 75+ group for the 74 Gy, 60 Gy and 57 Gy schedules. (Supplementary Figures 1-3). Patient-reported small+ bowel bother peaked at 10 weeks after the start of radiotherapy and was similar in both age groups (Supplementary Figures 9-10). At 2 years, the prevalence of small+ bowel bother was 146/1159 (13%) and 28/153 (18%) in the <75 and 75+ groups respectively remaining slightly higher in the 75+ group at all time points to 5 years (Supplementary Figures 9-10) when the cumulative incidences of small+ bowel bother were 32% (95% CI: 30-35) and 38% (32-44) in the <75 and 75+ groups. However although there was no evidence of a difference between the fractionation schedules in the <75 group, in the 75+ group there was a suggestion of higher cumulative incidence of small+ bowel bother with 60Gy compared to 74Gy (HR 1.44, CI 0.90-2.32, p=0.115) or 57Gy (HR 0.81, CI0.48-1.38), p=0.460) although this did not reach conventional statistical significance (Figure 5A).
Figure 3.
Time to first grade 2+ bowel toxicity assessed by (A) RTOG, (B) RMH and (C) LENTSOM scales, for <75 and ≥75 year olds by treatment group
Figure 5.
Time to first small+ (A) bowel and (B) urinary bother, for <75 and ≥75 year olds by treatment group
There was no certain evidence of a difference in time to first grade 2+ bladder toxicity using CROs for either hypofractionated group compared to the control group in either age group (Figure 4). However, there was a suggestion of increased RTOG toxicity with 60Gy in the <75 group (p=0.012). The 5 year cumulative incidences of grade2+ RTOG, RMH, and LENT-SOM bladder late side effects were similar with rates of 6.6 (95% CI 5.7-7.7) % / 9.2 (6.9-12.3) %, 25.9 (24.2-27.7) % / 32.1 (27.6-37.0) % and 38.1 (36.1-40.1) % / 40.5 (35.7-45.7) % for the <75 and 75+ groups respectively (Figure 4). The 2-year prevalence of grade2+ RTOG, RMH, and LENT-SOM bladder toxicity was 32/2430 (1%), 193/2417 (8%) and 287/2346 (12%) in the <75 group compared with 8/413 (2%), 39/410 (10%) and 54/399 (14%) in the 75+ group and were stable over time (Supplementary Figures 4-6). Grade 1 RMH bladder symptoms were persistently greater in the 75+ group both pre and post treatment (Supplementary Figure 5A). Patient-reported small+ bladder bother peaked at 10 weeks after the start of radiotherapy and was similar in both age groups (Supplementary Figures 11-12). At 2 years, the prevalence of small+ bladder bother was 140/1154 (12%) and 33/149 (22%) in the <75 and 75+ groups respectively remaining slightly higher in the 75+ group at all time points to 5 years (Supplementary Figure 11-12) when the cumulative incidences of small+ bladder bother were 30% (95% CI: 28-33) and 39% (33-46) in the <75 and 75+ groups. However, although there was no difference between the fractionation schedules in the <75 group there was a suggestion of a lower cumulative incidence of small+ bladder toxicity with 57Gy compared to 74 Gy (HR 0.71, CI 0.43-1.16: p=0.163) or 60Gy in the 75+ group (HR 1.01, CI 0.63-1.62, p=0.953 (Figure 5B).
Figure 4.
Time to first grade 2+ bladder toxicity assessed by (A) RTOG, (B) RMH and (C) LENTSOM scales, for <75 and ≥75 year olds by treatment group
At 2 years, the incidence of LENTSOM sexual dysfunction grade2+ was 1402/2189 (64%) and 262/360 (73%) in the <75 and 75+ groups and 825/1255 (66%) and 109/161 (68%) respectively at 5 years. The increased incidence of erectile dysfunction in the 75+ group pre-dated hormone and radiotherapy and persisted for the 5 years of follow-up (Supplementary Figure 7). There was no evidence of a difference in time to grade 2+ erectile dysfunction between the fractionation schedules in either age group (Supplementary Figure 8).
Discussion
The poor recruitment of older adults in to clinical trials is thought to be due to decline in functional reserve, increased comorbid conditions, lack of social support and the increased concomitant medications in elderly patients (13). When making decisions about their cancer treatment, older patients also have concerns about treatment-related discomfort, fear of side effects and transport issues (14). In an elderly population, the patient’s functional status and the presence of ‘geriatric syndromes’ such as dementia, depression, osteoporosis or falls are associated with increased chemotherapy toxicity (15). Data on radiotherapy outcomes and toxicity in an elderly population are sparse.
In this post-hoc subgroup analysis of the CHHiP trial, there was no evidence of a difference in BCF in ≤75 and 75+ groups. Results in the ≤75 group mirrored our previous report (2) with higher BCF rates in the 57Gy randomised trial arm. However, in the 75+ group both 60Gy and 57Gy showed higher (91.0% and 87.7% respectively) 5 year BCF-free outcome than 74Gy (84.7%) although this was not statistically significant. Equivalent results were seen in the 75+ group to ≤75 group despite less favourable features at presentation. This imbalance of prognostic factors between age groups may relate to clinician or patient preference for an active surveillance strategy with increasing age as observed previously In a Canadian population-based study (16). We are not aware of any previous evidence of a relatively beneficial effect of hypofractionated RT in older patients with PCa. This could have resulted from an imbalance of other unmeasured prognostic factors or perhaps slower or incomplete testosterone recovery. Alternatively, it may be a chance finding due to the relatively small proportion of elderly patients (15% of the overall trial population). Although non-inferiority of 57Gy compared with 74Gy could not be claimed formally in the whole trial population (5-year control rates of 85.9% vs 88.3%) the 57 Gy schedule has potential advantages in that it may moderate long term side effects without a meaningful compromise of treatment efficacy in elderly patients. The 57Gy schedule has been recently endorsed by NHS England Guidance for consideration in frail elderly patients (17).
Previously one study in a mixed cohort of patients aged over 70 showed no increase in Grade 3 to 4 toxicity in more vulnerable or frail patients (5). To our knowledge, this is the first assessment of both CRO and PRO in elderly patients with PCa treated with HFRT. Whilst this was not a pre-planned analysis and results must be regarded as exploratory, the large number of patients recruited to the CHHiP trial permit some observations. There was no increase in peak acute bowel or bladder toxicity in the 75+ group compared with the ≤75 group and HFRT appeared well tolerated in elderly patients.
The difference in acute bowel toxicity between the control and HFRT groups seen in the < 75 age group (p < 0.0001 for both 60 Gy and 57 Gy comparisons) failed to reach statistical significance in the 75+ group. This is reassuring but most likely relates to the smaller sample size in the 75+ group. Importantly 18 weeks after radiotherapy acute bowel toxicity had settled satisfactorily in both age cohorts with no differences between the fractionation schedules. With respect to acute bladder toxicity, there was a significant increase in RTOG grade 2+ toxicity between the control and 60 Gy group (p=0.004) but not the 57 Gy cohort (p=0.083) in the 75+ group. This difference was not seen for the <75 group (see Supplementary Table 2). This might reflect a higher incidence of pre-treatment bladder dysfunction and support use of the 57 Gy in 19 fractions regimen in older men, particularly as this schedule was not associated with a decrease in treatment efficacy compared to 74Gy or 60Gy.
There were no consistent differences in prevalence or cumulative incidence of CRO late bowel toxicity up to 5 years after radiotherapy between the ≤75 and 75+ groups. Similar findings were seen using conventional or HFRT and assessments with RTOG, RMH or LENT-SOM instruments. However, using PRO there was consistent increase in reporting of “bowel bother” in the 75+ group and this appeared to be most pronounced in the 60Gy group rather than 74Gy or 57Gy cohorts. Fractionation schedule was not related to bowel bother in the <75 group.
There appeared to be more bladder symptoms in the 75+ group compared to the <75 group at 5yrs measured using the CRO instruments. This was confirmed using PRO and all degrees of “bladder bother” were increased in the 75+ group. Fractionation schedule appeared unrelated to “bladder bother” in the ≤75 group but 57Gy appeared to be associated with reduced “bother” scores in the 75+ group rather than those treated by 74Gy and 60 Gy although this failed to reach statistical significance. Although it is difficult to separate treatment effects from an increase in urinary symptoms in an elderly population this might sound a cautionary note against dose escalation in more aged patients.
Erectile dysfunction was increased post- treatment in the 75+ group. This was expected as increasing age has previously been identified as a risk factor for erectile dysfunction following ADT and radiotherapy for prostate cancer (18). Higher levels of dysfunction were scored using the LENT-SOM instrument than PRO assessing “bother” perhaps reflecting the change in importance of erectile dysfunction with increasing age. However post-ADT testosterone recovery may be delayed and incomplete in older patients. As having a normal testosterone level is important in the recovery of erectile dysfunction as well as other health issues, it is recommended that this should be assessed post-treatment (19).
Conclusions
HFRT using 60 Gy or 57 Gy delivered in 3 Gy fractions appears to be well tolerated and effective in more elderly men and age should not be a barrier to implementing shorter radiotherapy schedules. The 57 Gy schedule has potential advantages in moderating long term bowel and bladder side effects whilst maintain satisfactory PCa control.
Supplementary Material
Summary.
The efficacy and toxicity of radiotherapy for localised prostate cancer in CHHiP trial participants aged 75 and over was compared with patients younger than 75. There was no evidence of a difference in biochemical or clinical recurrence free survival or clinically significant toxicity between the older and younger patient groups. Hypofractionated radiotherapy is an effective and well tolerated treatment for localised prostate cancer in an elderly population with good performance status.
Acknowledgments
We thank the patients and all investigators and research support staff at the participating centres. We acknowledge statistical support from Jo Haviland (The Institute of Cancer Research). We acknowledge support of Cancer Research UK (C8262/A7253, C1491/A9895, C1491/A15955, SP2312/021), the Department of Health, the National Institute for Health Research Cancer Research Network and NHS funding to the NIHR Biomedical Research Centre at the Royal Marsden NHS Foundation Trust and The Institute of Cancer Research, London.
Footnotes
Declaration of interests
Dr Khoo reports Honoraria for Speakers Bureaus with Accuray, Astellas, Bayer, Ipsen, Janssen, Takeda and Tolmar. Professor Hall reports grants from Cancer Research UK during the conduct of the study; grants from Accuray Inc, outside the submitted work. All other authors declared no conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cancer Research UK. Prostate cancer statistics. [accessed 1st Nov 2017]; https://www.cancerresearchuk.org/health-%20professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer.
- 2.XXXXXXXXXXX
- 3.Spyropoulou D, Pallis AG, Leotsinidis M, Kardamakis D. Completion of radiotherapy is associated with the Vulnerable Elders Survey-13 score in elderly patients with cancer. J Geriatr Oncol. 2014;5(1):20–5. doi: 10.1016/j.jgo.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Scher KS, Hurria A. Under-representation of older adults in cancer registration trials: Known problem, little progress. J Clin Oncol. 2012;30(17):2036–8. doi: 10.1200/JCO.2012.41.6727. [DOI] [PubMed] [Google Scholar]
- 5.Keenan LG, O'Brien M, Ryan T, Dunne M, McArdle O. Assessment of older patients with cancer: Edmonton Frail Scale (EFS) as a predictor of adverse outcomes in older patients undergoing radiotherapy. J Geriatr Oncol. 2016 doi: 10.1016/j.jgo.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31(5):1341–6. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 7.LENT SOMA tables. Radiother Oncol. 1995;35(1):17–60. [PubMed] [Google Scholar]
- 8.Dearnaley DP, Sydes MR, Langley RE, Graham JD, Huddart RA, Syndikus I, et al. The early toxicity of escalated versus standard dose conformal radiotherapy with neo-adjuvant androgen suppression for patients with localised prostate cancer: results from the MRC RT01 trial (ISRCTN47772397) Radiother Oncol. 2007;83(1):31–41. doi: 10.1016/j.radonc.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 9.XXXXXXXXXXX
- 10.Roach M, Hanks G, 3rd, Thames H, Schellhammer P, Jr, Shipley WU, Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965–74. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 11.Litwin MS, Hays RD, Fink A, Ganz PA, Leake B, Brook RH. The UCLA Prostate Cancer Index: development, reliability, and validity of a health-related quality of life measure. Med Care. 1998;36:1002–12. doi: 10.1097/00005650-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 13.Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: A 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22(22):4626–31. doi: 10.1200/JCO.2004.02.175. [DOI] [PubMed] [Google Scholar]
- 14.Puts MT, Tapscott B, Fitch M, Howell D, Monette J, Wan-Chow-Wah D, et al. A systematic review of factors influencing older adults' decision to accept or decline cancer treatment. Cancer Treat Rev. 2015;41(2):197–215. doi: 10.1016/j.ctrv.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Ramjaun A, Nassif MO, Krotneva S, Huang AR, Meguerditchian AN. Improved targeting of cancer care for older patients: A systematic review of the utility of comprehensive geriatric assessment. J Geriatr Oncol. 2013;4(3):271–81. doi: 10.1016/j.jgo.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Richard PO, Alibhai SMH, Panzarella T, Klotz L, Komisarenko M, Fleshner NE, et al. The uptake of active surveillance for the management of prostate cancer: A population-based analysis. Canadian Urological Association Journal. 2016;10(9–10):333–8. doi: 10.5489/cuaj.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clinical Commissioning Policy: Hypofractionated external beam radiotherapy in the treatment of localised prostate cancer (adults) Prepared by NHS England Specialised Services Clinical Reference Group for Radiotherapy. Published by NHS England in electronic format only; First published: October 2017. [Google Scholar]
- 18.DiBlasio CJ, Malcolm JB, Derweesh IH, Womack JH, Kincade MC, Mancini JG, et al. Patterns of sexual and erectile dysfunction and response to treatment in patients receiving androgen deprivation therapy for prostate cancer. BJU Int. 2008;102(1):39–43. doi: 10.1111/j.1464-410X.2008.07505.x. [DOI] [PubMed] [Google Scholar]
- 19.White ID, Wilson J, Aslet P, Baxter AB, Birtle A, Challacombe B, et al. Development of UK guidance on the management of erectile dysfunction resulting from radical radiotherapy and androgen deprivation therapy for prostate cancer. Int J Clin Pract. 2015;69(1):106–23. doi: 10.1111/ijcp.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.