Abstract
Introduction
The role of the anterior cingulate cortex (ACC) is still controversial. The ACC has been implicated in such diverse functions as cognition, arousal and emotion in addition to motor and autonomic control. Therefore the ACC is the ideal candidate to orchestrate cardiovascular performance in anticipation of perceived skeletal activity. The aim of this experiment was to investigate whether the ACC forms part of the neural network of central command whereby cardiovascular performance is governed by a top-down mechanism.
Methods & Results
Direct local field potential (LFP) recordings were made using intraparenchymal electrodes in six human ACC’s to measure changes in neuronal activity during performance of a motor task in which anticipation of exercise was uncoupled from skeletal activity itself. Parallel cardiovascular arousal was indexed by electrocardiographic changes in heart rate. During anticipation of exercise, ACC LFP power within the 25-60Hz frequency band increased significantly by 21% compared to rest (from 62.7μV2/Hz (+/-SE 4.94) to 76.0μV2/Hz (+/-SE 7.24); p=0.004). This 25-60Hz activity increase correlated with a simultaneous heart rate increase during anticipation (Pearson’s r=0.417, p=0.016).
Conclusions/Significance
We provide the first invasive electrophysiological evidence to support the role of the ACC in both motor preparation and the top-down control of cardiovascular function in exercise. This further implicates the ACC in the body’s response to the outside world and its possible involvement in such extreme responses as emotional syncope and hyperventilation. In addition we describe the frequency at which the neuronal ACC populations perform these tasks in the human.
Keywords: Anterior cingulate cortex, Motor system, Cardiovascular system, Deep brain stimulation, Local field potentials, Electrophysiology
Introduction
The dorsal anterior cingulate cortex located in the medial prefrontal cortex has been associated with a broad range of functions collectively referred to as ‘cognitive control’ (1). However, clinically, dorsal anterior cingulate stimulation and cingulotomy is associated with pain control arguing that this region of the cingulate cortex is involved in enteroceptive function (2). Anatomically this region is densely interconnected to motor cortex, spinal cord prefrontal cortex, thalamus and brain stem, suggesting this area may act as a nexus of enteroceptive input and motor action. Evidence from human sources further argues that in addition to cognitive functions, this part of the ACC more specifically is involved in autonomic function, namely the ‘central command’ of autonomic function (3).
The term ‘central command’ first coined in 1913 by Krogh & Lindhard (4) refers to the ‘top down’ mechanism by which higher brain areas influence cardiorespiratory variables particularly during exercise and is thought to be the mechanism that mediates other phenomena such as white coat hypertension and emotional syncope. Central command is proposed to be a feed forward mechanism involving parallel activation of motor and cardiovascular centres (5). Together with peripheral receptors such as baroceptors, nociceptors, and chemoceptors, central command represents one of the chief short term mechchanisms by which cardiovascular performance is influenced, particularly when exercise is anticipated (6). The ability to plan and execute the necessary alteration in cardiorespiratory function to facilitate sufficient local tissue perfusion, oxygen intake and carbon dioxide venting in proportion to the anticipated task to be undertaken is crucial to create and maintain optimal conditions for exercise.
The cardiovascular component of central command has been shown to be functionally-independent of the motor task itself, supporting the conjecture that central command is a feed forward mechanism not primarily driven by sensory input; hypnotised subjects imagining the performance of exercise of different magnitudes produced changes in cardiovascular variables such as heart rate and blood pressure correlating to the degree of imagined exercise, for example increasing whilst ‘cycling’ uphill and decreasing downhill (7).
Interrogation of the neurocircuitry of central command in the human has been limited, until recently, to non-invasive neuro-imaging studies demonstrating changes in metabolic or vascular activity in areas of the brain proposed to be involved in central command, rather than directly evaluating neuronal activity electrophysiologically. The insula, ACC, other medial prefrontal areas and the thalamus have been implicated by functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) studies (8–12). The first invasive neurophysiological evidence supporting specific brain areas involved in central command implicated two sites within its subcortical network: the subthalamic nucleus (STN) of the diencephalon and the periaqueductal grey (PAG) of the midbrain. Recordings from deep brain electrodes within the STN and PAG demonstrated changes in local field potential power during exercise anticipation and exercise performance in awake humans in parallel with increasing cardiorespiratory variables (13). In contrast, there was no such change in the activity of the globus pallidus internus, used as a control in that study.
We used the unique opportunity offered by DBS surgery to explore the electrophysiology of the dorsal ACC in an exercise task where exercise was first anticipated after a rest period then actually performed. We used a bipolar recording technique to study the ACC directly without contamination of signal from volume conduction effects, in essence recording a ‘microEEG’ from the dACC which is spatially precise (14). We studied the (d)ACC because this is a good candidate region to be involved in central command of autonomic variables because of fMRI evidence alluded to above, and a pre-requisite property of the brain areas within the central command network is the ability to influence the autonomic nervous system; the ACC satisfies this as several lines of evidence has implicated it as part of a network of structures involved in autonomic control. Firstly, pyramidal neurons of the ACC project directly and indirectly to subcortical sites that confer autonomic control such as the hypothalamus (15) and PAG (16). Secondly, increased signal in the ACC on functional neuro-imaging is seen during activities known to stimulate the autonomic nervous system such as exercise and mental stressor tasks producing increased heart rate and arterial blood pressure (3, 9, 17, 18) Thirdly, changes in autonomic variables such as heart rate and blood pressure have been demonstrated during ACC electrical stimulation in animal studies (19–22) and humans (23, 24). Fourthly, cingulate lesion studies in humans have demonstrated deranged autonomic, particularly sympathetic, drive (17, 25).
Methods & Materials
Patients with dorsal ACC deep brain stimulators in situ for chronic pain syndromes participated. The study was approved by the local research ethics committee (Oxfordshire REC C: 05/Q1605/47) and conformed to the Declaration of Helsinki. Informed written consent was obtained from all patients.
Surgical Procedure
A Cosman-Roberts-Wells base ring was applied to the patient’s head under local anaesthesia. A stereotactic computed tomography (CT) scan was performed and fused with pre-operative magnetic resonance brain imaging using the Radionics Image Fusion® and Stereoplan® software (Integra Radionics, Burlington, MA, USA). The coordinates for the target in ACC were calculated as follows. The anteroposterior (x) coordinate was selected at 2cm posterior to the tip of the frontal horn of the ipsilateral lateral ventricle In the lateral direction (y coordinate), the electrode was targeted at the interface between grey and white matter and the tip of the electrode was just touching the corpus callosum, so that all four contacts straddled the dorsal ACC. This area corresponds to Brodmann’s areas 24a’/b’ and 24c’ of the dorsal portion of the ACC. After a frontal skin incision, a 2.7mm twist-drill craniostomy was performed. A Radionics™ electrode of 1.8 mm diameter and 2.0 mm exposed tip was slowly passed transcortically to the target. The Radionics™ electrode was withdrawn and the Medtronic 3387® electrode (Medtronic Inc., MN, USA) with 4 contacts along a distance of 10.6mm was implanted. Once clinical efficacy, i.e. pain relief, had been established, the electrode was fixed to the skull using a titanium bioplate™ and externalised for clinical evaluation during the subsequent week. The wounds were then sutured.
Experimental Measurements
Heart rate (beats per minute (bpm)) was derived from a 3-lead electrocardiogram using disposable adhesive Ag/AgCl electrodes (H207PT, Kendall-LTP, MA, USA) and amplified 1000x (CED 1902, Cambridge Electronic Design, Cambridge, UK). Systolic/diastolic blood pressure (sBP/dBP) was recorded continuously, non-invasively by a Portapres (Finapres Medical Systems, Amsterdam, Netherlands) portable blood pressure monitor. The finger pressure and electrocardiogram were digitised at 4kHz with 16-bit resolution (CED 1401 Mark II, Cambridge Electronic Design, Cambridge, UK) using Spike II software (version 5.0, Cambridge Electronic Design). Local field potentials (LFPs) were simultaneously recorded with bipolar configuration from the adjacent four circumferential 1.5mm contacts of each deep brain macroelectrode. Signals were filtered at 0.5-500Hz and amplified (10000x) using isolated CED 1902 amplifiers and digitised using CED 1401 Mark II at a rate of 2.5kHz (Cambridge Electronic Design). LFPs were displayed online and saved onto hard disk using Spike II and subsequently analysed off-line.
Protocol
The experimental protocol was performed 2-5 days after the first surgical procedure whilst the electrodes were externalised. Patients sat in an upright position at rest for 3 minutes. The chair had two arm rests and in this way the right arm was supported during rest and anticipation and the left arm with the Portapres cuff was supported with the pressure transducer and finger cuff at heart level. In addition, a height correction unit was used to optimise accuracy. Patients were given a ten-second countdown before beginning right upper limb bicep flexesagainst a 2kg weight attached to the wrist at a rate of 30-60 per minute for 2 minutes. This was followed by a further three minutes of rest and the protocol repeated. Each patient performed the trial protocol 6 times. LFPs were compared during the last ten seconds of rest, the ten seconds of countdown to exercise (anticipation) and the first ten seconds of exercise in each trial. The power spectra from each of the three electrode channels were averaged for each trial.
Data Analysis
Transformation to identify the fundamental spectral frequencies of each of the 10-second epochs for Rest, Anticipation and Exercise was performed from the time domain into the frequency domain by applying a fast Fourier Transform (FFT) algorithm offline using a digital spectrum analyser (Matlab, version 6.5 MathWorks Inc., Natick, MA, USA). Signals were re-sampled at a rate of 1000Hz. A 50Hz notch filter was applied to avoid the inclusion of mains artefact. A bandpass filter of 4Hz to 90Hz was also applied. A 4A Hann window of 2s in width was selected so that the signal could be carefully examined. The area under each power spectrum for each condition was calculated for the following frequency bands: 4-8Hz (θ), 8-12Hz (α), 12-25Hz (β), 25-60Hz (low γ) and 60-90Hz (high γ) (26, 27).
The R-peaks of ECG signals were automatically detected by using the Pan-Tompkins algorithm (28). Incorrectly identified peaks were corrected, based on visual inspection. Heart rate variability (HRV) can be evaluated by many measures. Here a time-domain measurement, standard deviation of the NN interval (SDNN), was used. Due to the short time period of Anticipation condition, HRV analysis was only performed in Rest and Exercise conditions using 2-min data. The relationship between autonomic measures (heart rate, SDNN) and LFPs (power spectra in frequency bands) across the conditions was quantified by linear correlation analysis. The correlation between changes in autonomic measures and changes in power spectra of LFPs during conditions was also calculated.
Statistical Analysis
Statistical analyses were performed using SPSS version 16 (SPSS Inc.). Student’s paired samples t-tests were used to compare the states of Rest to Anticipation, Rest to Exercise and Anticipation to Exercise. Statistical significance within the LFP analysis was set at p<0.01 after adjustment was made for multiple comparisons between the five frequency bands. Heart rate was used as the chief index of cardiovascular activity. The relationship between LFP power spectral density change and heart rate change between Rest and Anticipation was investigated using a two-tailed Pearson’s correlation with a confidence level of 95%.
Electrode Mapping
Coordinates of each electrode from post-operative head computerized tomograms (CT) were transformed onto the Montreal Neurological Institute (MNI) standard and structural brain template of 152 averaged brains using the fMRIB Software Library (FSL) (29, 30).
Results
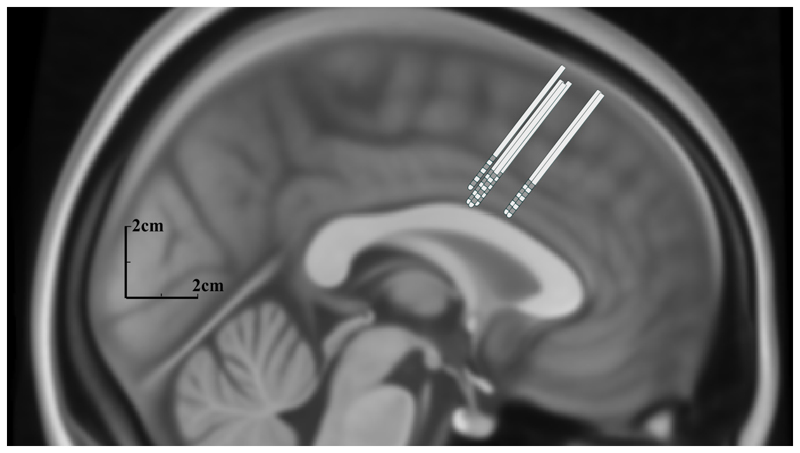
Recordings were made from six dorsal ACCs, left and right, in four patients (Figure 1). Three patients were male, one was female and mean age was 55.75 years (SD +13.5, range 42-74). Two patients suffered pain syndromes after post-stroke pain, one after cervical myelopathy and a brachial plexus injury and the other after trauma to the conus medullaris. Two patients had bilateral stimulators and two had unilateral left-sided electrodes. Local field potentials were recorded from 4 left-sided and 2 right-sided electrodes with 4 monopolar channels yielding 18 bipolar channels during 34 trials (one trial was rejected due to movement artefact in a patient with bilateral electrodes).
Figure 1.
Schematic of deep brain electrodes within the dorsal anterior cingulate cortex mapped onto a midline sagittal Montreal Neurological Institute brain template. Electrodes are scaled to size and electrode-neural interface contacts are shown in dark grey.
Cardiovascular Parameters
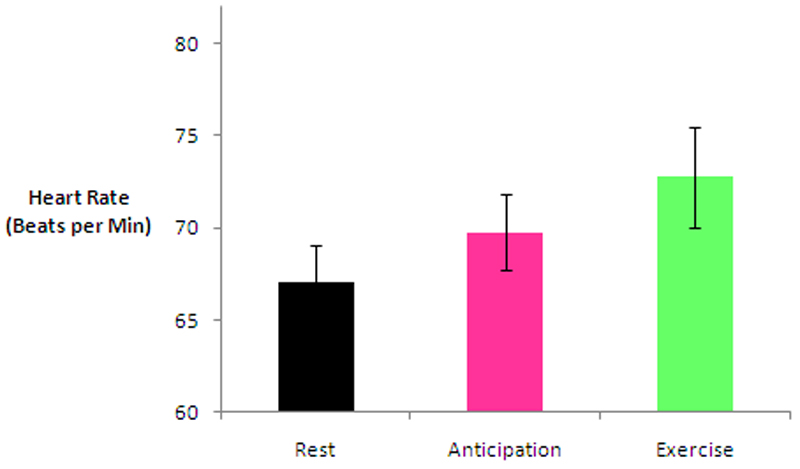
Cardiovascular variables across all four patients increased step-wise from Rest to Anticipation and Exercise. Changes in heart rate between each state are shown in Figure 2. Heart rate data was normally distributed and paired-samples t-tests were applied whereas blood pressure data was not normally distributed and therefore the Wilcoxon signed rank test was applied. Comparing Rest to Anticipation, mean HR increased from 67.1 bpm (range 62-76) to 69.8 bpm (range 65-80), t=-12.6, df=5, p<0.0005; mean sBP increased from 120.6mmHg (range 80-158) to 123.5mmHg (range 86-160), Z=-2.003, p=0.045; and mean dBP increased from 89.5mmHg (range 55-137) to 91.6mmHg (range 59-138), Z=-2.003, p=0.045. Comparing Rest to Exercise, mean HR increased from 67.1bpm (range 65-80) to 72.8bpm (range 66-86), t=-7.6, df=5, p=0.001; mean sBP increased from 120.6mmHg (range 80-158) to 125.7mmHg (range 84-166), Z=-2.214, p=0.027; and mean dBP increased from 89.5mmHg (range 55-137) to 93.9mmHg (range 59-143), Z=-2.214, p=0.027.
Figure 2.
Graph to show change in mean heart rate during Rest, Anticipation and Exercise (confidence intervals represent standard error).
Neurophysiological Variables
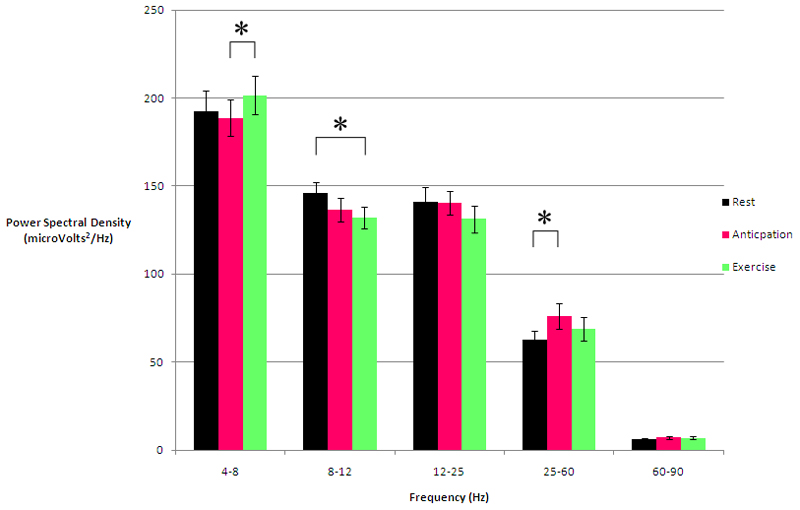
LFP power spectral densities (PSD) during Rest versus Anticipation are shown in Table 1 and Figures 3 & 5. The significant change occurred in the 25-60Hz band: mean PSD increased by 21% from 62.66μV2/Hz (+/-SE 4.94) at Rest to 76.0μV2/Hz (+/-SE 7.24) during Anticipation which was statistically significant (t=-3.145, df=33, p=0.004). There was a 7% mean PSD fall in the 8-12Hz band from 146.21μV2/Hz (+/-SE 6.17) at Rest to 136.41μV2/Hz (+/-SE 6.87) during Anticipation. However, this did not reach statistical significance (t=2.029, df=33, p=0.051).
Table 1. Differences in power spectral density (PSD) within each frequency band during Rest and Anticipation.
| Frequency Band (Hz) | Rest PSD (µV2/Hz) | Anticipation PSD (µV2/Hz) | p | ||
|---|---|---|---|---|---|
| Mean | SE | Mean | SE | ||
| 4-8 | 192.42 | 11.52 | 188.59 | 10.26 | 0.473 |
| 8-12 | 146.21 | 6.17 | 136.41 | 6.87 | 0.051 |
| 12-25 | 141.05 | 8.26 | 140.32 | 6.82 | 0.850 |
| 25-60 | 62.66 | 4.94 | 76.00 | 7.24 | 0.004* |
| 60-90 | 6.31 | 0.71 | 7.11 | 0.81 | 0.051 |
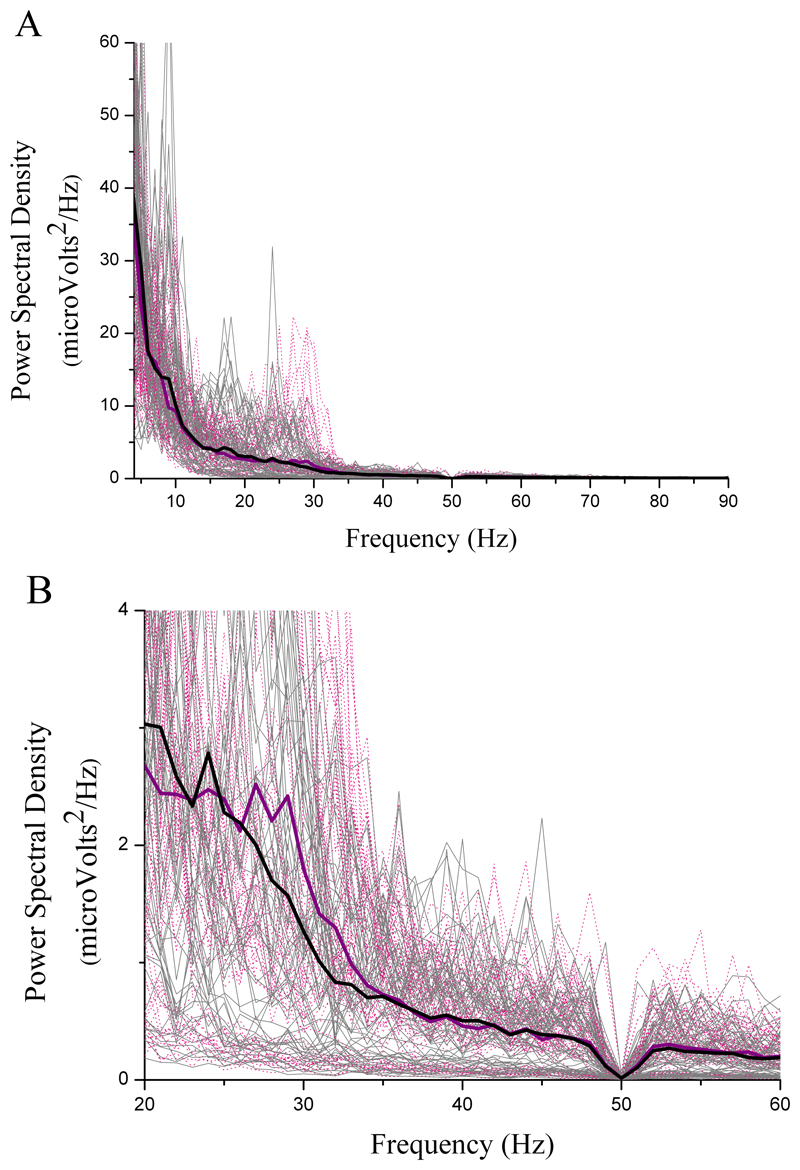
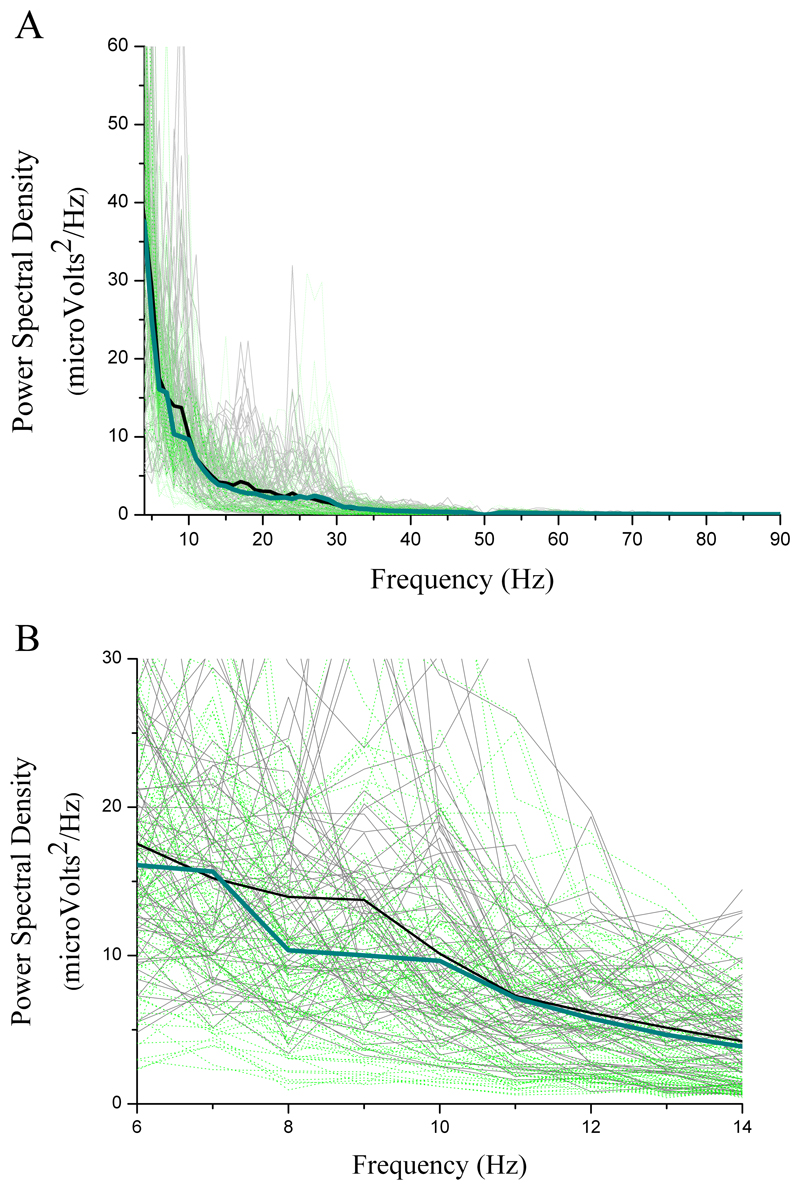
Figure 3.
Local field potential power spectral density during Anticipation of exercise (pink lines) versus Rest (black lines) from 102 electrode channel recordings: A) across all frequencies; B) within the 25-60Hz band only, where increased synchronisation is seen during Anticipation.
Figure 5.
Graph to show local field potential power spectral density changes between Rest, Anticipation and Exercise divided into frequency bands (* p<0.01).
LFP PSDs during Rest versus Exercise are shown in Table 2 and Figures 4 & 5. The significant change occurred in the 8-12Hz band: mean PSD decreased by 10% from 146.21μV2/Hz (+/-SE 6.17) at Rest to 132.20μV2/Hz (+/-SE 6.24) during Exercise which was statistically significant (t=2.916, df=33, p=0.006). There was a 10% mean PSD increase in the 25-60Hz band from 62.66μV2/Hz (+/-SE 4.94) at Rest to 68.88μV2/Hz (+/-SE 6.52) during Exercise. However this did not reach statistical significance (t=-1.962, df=33, p=0.058). Comparing Anticipation to Exercise, the significant change occurred in the 4-8Hz theta band, shown in Figure 5. Mean PSD increased by 7% from 188.59μV2/Hz (+/-SE 10.26) to 201.45μV2/Hz (+/-SE 10.90), t=-2.774, df=33, p=0.009.
Table 2. Differences in power spectral density within each frequency band during Rest and Exercise.
| Frequency Band (Hz) | Rest PSD (µV2/Hz) | Exercise PSD (µV2/Hz) | p | ||
|---|---|---|---|---|---|
| Mean | SE | Mean | SE | ||
| 4-8 | 192.42 | 11.52 | 201.45 | 10.90 | 0.105 |
| 8-12 | 146.21 | 6.17 | 132.20 | 6.24 | 0.006* |
| 12-25 | 141.05 | 8.26 | 131.35 | 7.55 | 0.019 |
| 25-60 | 62.66 | 4.94 | 68.88 | 6.52 | 0.058 |
| 60-90 | 6.31 | 0.71 | 6.88 | 0.86 | 0.276 |
Figure 4.
Local field potential power spectral density during Exercise (green lines) versus Rest (black lines) from 102 electrode channel recordings: A) across all frequencies; B) within 6-14Hz range only. A desynchronisation during Exercise is seen in the 8-12Hz band compared to Rest.
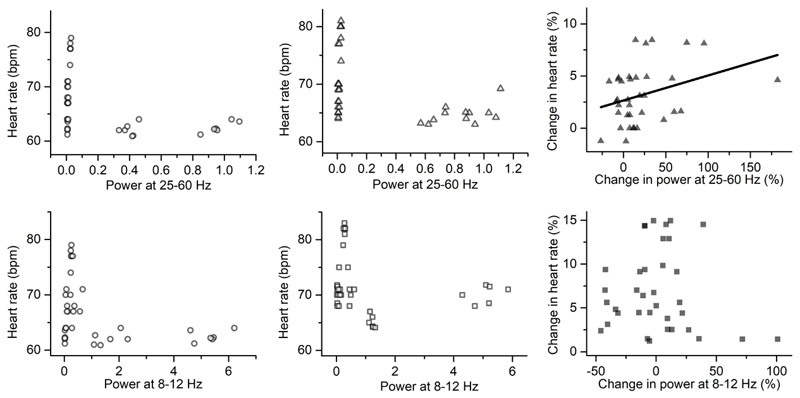
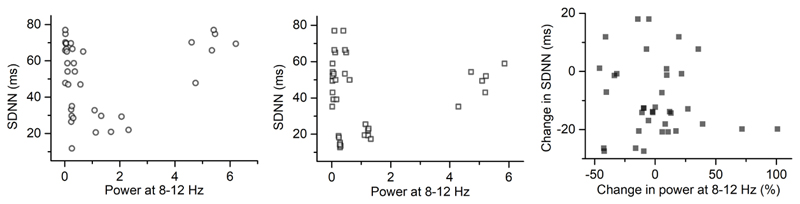
Pearson’s correlation was applied to link the power changes in ACC LFPs with the heart rate changes during conditions. There was no significant correlation between the power in 25-60 Hz or 8-12 Hz and heart rate in each condition (Figure 6A, B, D, E). However, there was a significant positive correlation (Pearson’s r=0.417, p=0.016, n=33) between the power change in 25-60 Hz and the change in HR during Anticipation relative to Rest (Figure 6C). Furthermore, the relationship between the power changes in LFPs and the HRV, i.e. SDNN, was analysed. There was no significant correlation between the power in 8-12 Hz and SDNN or between the power change and the SDNN change during Exercise relative to Rest (Figure 7).
Figure 6.
Scatter plot to show the correlation: A) between heart rate and local field potential power in 25-60 Hz during Rest; B) between heart rate and local field potential power in 25-60 Hz during Anticipation; C) between the changes in heart rate and changes in local field potential power in 25-60 Hz during Anticipation relative to Rest (R=0.417, p=0.016); D) between heart rate and local field potential power in 8-12 Hz during Rest; E) between heart rate and local field potential power in 8-12 Hz during Exercise; F) between the changes in heart rate and changes in local field potential power in 8-12 Hz during Exercise relative to Rest.
Figure 7.
Scatter plot to show the correlation: A) between SDNN and local field potential power in 8-12 Hz during Rest; B) between SDNN and local field potential power in 8-12 Hz during Exercise; C) between the changes in SDNN and changes in local field potential power in 8-12 Hz during Exercise relative to Rest.
Discussion
In this study we invasively examined the electrophysiology of the human dorsal ACC whilst simultaneously measuring heart rate with respect to a task to dissociate central command from proprioceptive influences in order to investigate the role of the dorsal ACC in the autonomic/enteroceptive nervous system in mediating the body’s response to its evaluation of the outside world. In awake humans, we recorded dorsal ACC neural activity during conditions in which participants rested and during conditions in which upcoming exercise was anticipated. By comparing activity in these two periods prior to the commencement of exercise we were able to examine the changes in neural activity associated with central command that are separable from activity associated with cardiovascular and muscular activity during exercise itself. It is significant that we recorded activity changes in the low gamma (25-30 Hz) range in the anticipation phase as beta and gamma frequency activity is associated with intrinsic ACC activity in rodent models of ACC activity albeit at a faster frequency in rats (31). Changes in this spectral band correlated with changes in heart rate at a phase in the task not associated with somatosensory input that would influence heart rate, arguing that heart rate changes in this phase of the task were driven by top down central command. Our experimental design does not permit the unambiguous conclusion that the dACC drives heart rate changes during the anticipation of exercise. We can postulate that periods of increased dACC intrinsic activity are associated selectively with periods of anticipatory tachycardia prior to exercise, but to unambiguously show a direct relationship would require neural stimulation experimental designs. Given these caveats, our data is unique as it provides awake human dACC recordings whilst subjects perform an experimental task that dissociates central from peripheral influences on heart rate, thereby providing evidence the dACC is active selectively during periods associated with central command of cardiovascular activity. This serves the additional purpose of allowing comparison with human fMRI data, which lacks the temporal resolution and power spectral information of our technique, and allows cross species comparisons allowing experimental data from for example non-human primates to be compared from other experimental designs not feasible in humans. A major limitation of our study is that the mechanism by which the dACC might influence heart rate during exercise anticipation is not discernible. ACC has been found to be associated autonomic arousal (23), and baroceptor sensitivity suppression (32) Whilst heart rate variability could be measured for rest and exercise periods, the anticipatory period was too short (10 secs) to allow HRV to be calculated in this period. Additionally we did not measure blood pressure in a manner that would allow calculation of baroceptor sensitivity. We cannot say whether heart rate changes observed in anticipatory periods were a result of alterations to the parasympathetic or sympathetic tone, baroceptor sensitivity or indirect influences of the ACC on the pyramidal tract (33) or a combination of these. There is nonetheless some evidence in humans that the ACC forms part of a central neural network that drives sympathetic autonomic processing, both during anticipation and experience of pain (34) suggesting ACC may be involved in the upregulation of sympathetic tone in an anticipatory fashion. Anticipatory responses associated with ACC (inc subgenual ACC) have been associated with sympathetic features (for example anxiety in anticipation of pain, (35), reward anticipation (36), but anticipatory responses in general are not exclusively associated with sympathetic activation (forewarned reaction time (37), threat (38)). The evidence suggests ACC and autonomic nervous system have a demonstrable relationship (18, 39–41). Our study found dorsal ACC activity that can be described as being anticipatory for exercise. The ACC is known to participate in a diverse range of emotional, cognitive, affective and motor pathways in addition to autonomic pathway. Using neuro-imaging, the ACC has been indirectly implicated during the anticipatory phase of several cognitively-demanding tasks. For example, Murtha et al. studied ten healthy human males undergoing PET scanning after presentation of the task instructions during a 90 second anticipatory period before the experimental stimuli were presented and then scanned again during the task itself (42). The ACC CBF signal was increased during the anticipation phase compared to baseline prior to any experimental stimulus or motor activity. Critchley et al. demonstrated that the ACC generated cardiovascular arousal which was dissociated from cognitive and motor activity (17). We have shown increased ACC neural activity at the time of anticipation of exercise, coincident with escalation of cardiovascular activity, specifically heart rate. Therefore we have linked ACC activation to the phase when cardiovascular performance is augmenting in preparation for exercise. This suggests,in combination with fMRI and anatomical studies, the dorsal ACC is not just involved in task preparation, but forms part of the central command network. Significantly we found low gamma (25-30Hz) activity to be increased in the anticipatory phase. This frequency of neural activity has been associated with ‘predictive coding theory’ such that cortical beta signals are associated with top down predictive signals preceding action, gamma band activity conversely being associated with prediction error signalling (43). Our low gamma activity (25-30Hz) is germane to cortical beta activity reported in this paper (15-31Hz) suggesting dACC activity reported here is anticipatory or feed forward rather than the result of peripheral feedback. The task rule was known to the participant therefore anticipatory and predictive could be argued to be synonymous in this context.
The ACC is known to be active in situations where the rapid activation of motor and cardiorespiratory systems is crucial, specifically during ‘circa strike’, the reaction to an imminent threat. This ecologically-important state results in active escape and avoidance with activation of critical midbrain regions such as the dorsal PAG which facilitates the ‘flight or fight’ response, whilst forebrain circuits are simultaneously inhibited (44). Mobbs et al. demonstrated increased dorsal ACC (dACC) and midbrain BOLD signal on fMRI in humans during circa strike threat in the performance of a maze task incorporating a predator. Conversely, when the threat was remote, the subgenual ACC, amygdala and hypothalamus were active instead. Therefore it appears that the dACC is the cortical structure responsible when activity must be performed imminently and is the direct cortical outflow to the brainstem to facilitate this. This complements our results as, although there was no element of threat in our protocol, there was the need to perform exercise imminently and during this preparation period dACC activity increased in the 25-60Hz band. Further, increased skin conductance level, a presumed autonomic sympathetic arousal state surrogate marker was identified by Mobbs et al. during circa strike rather than simply the presence of a remote threat which also parallels the autonomic augmentation of heart rate seen in our study during anticipation of exercise. It makes biological sense that the brain centre which detects there is a problem requiring imminent exercise based on cognitive and emotional information could orchestrate the ensuing activity for the purposes of gain or self-preservation. As roles of the ACC are recognised to include cognitive and affective regulation including conditional emotional learning, pain perception, maternal behaviour and motor control it is therefore the ideal candidate to coordinate the parallel modulation of the cardiovascular and motor systems to facilitate this survival activity (42).
This study is also the first to directly record human ACC neural activity during exercise. We found three different neural signatures within the ACC for the states of Rest, Anticipation and Exercise. During anticipation, there was an increase in synchronised activity in the 25-60Hz band compared to rest, in contrast there was a reduction in synchrony within the 8-12Hz band which was not statistically-significant during anticipation but was significant during exercise. Although during the anticipatory/preparatory period the dorsal ACC is active, it is then subsequently less active in the 8-12Hz band during the particular upper limb motor task used in our protocol. Activity in this frequency band may therefore be a marker of awareness/attention/arousal. Electroencephalogram studies have shown cortical activity in this band occurs during the transition between wakefulness and sleep (45–47). Further, an observational study in humans attempting to characterise the neural signature of pain implicated activity in this alpha band within the pain matrix, specifically in the sensory thalamus and PAG (48). However, that study was not controlled for attention and a criticism of the finding was that the signal may have been a result of attention/arousal during pain. The ACC is a recognised component of the pain neural circuitry (49). It would be congruous that during the performance of a routine, repetitive motor task there was a decrease in attention. Alternatively, the preparation and engagement in exercise may cause an electrical uncoupling of the dorsal ACC from the pain matrix. In fact the act of exercise may be distracting and the electrophysiological findings in this study may explain how distraction reduces the perception of pain (50, 51). Future studies of pain neurophysiology and arousal state are needed to further support these speculations.
An increase in theta oscillations in our study was evident when comparing the period of Anticipation to Exercise. Electroencephalogaphy has identified neural activity in the theta frequency band during attentional tasks recorded from frontal midline scalp electrodes which have been attributed to the prefrontal and anterior cingulate cortices (43,44). From our own work, we have identified an increase in theta activity in response to task error messages in an intra- extra-dimensional set-shift task from a comparable area to electrophysiology detailed here (Weiss, unpublished). Similarly, when Womelsdorf et al. recorded from area 24c of ACC, comparable to the location of our electrodes, in macaques performing a task involving learning stimulus-response (SR) mapping rules, they found that theta-activity predicted which of the two SR rules will be established in the period before visual target information was presented (52). Thus it is apparent that different types of oscillatory activity may be task dependent. It is also the case that oscillatory activity may vary according to sub-regions of the ACC.
There was no significant PSD increase in the 25-60Hz band during exercise compared to rest. Therefore it appears that the ACC activity peaked during the anticipation phase before subsiding during the motor task itself. Laberge proposed that when a task was routine or had become automatic with practice it required less attention and therefore less ACC cerebral blood flowto perform (53). Notably, no increase in ACC activity was seen in tasks which were routine, practiced or required attention to only a single factor (54, 55). The nature of our motor task was a repetitive flexion/extension of the elbow against a constant weight and was, therefore, not only highly repetitive and predictable, but also a very simple motor algorithm. This may explain the lack of significant 25-60Hz ACC neural activity in our study during exercise. Further studies should employ different motor tasks to test whether the nature of the exercise has an effect on ACC neural activity.
We have recorded the activity of the human dorsal ACC from intraparenchymal electrodes within an experimental paradigm whereby anticipation of exercise was uncoupled from skeletal activity itself. This was a novel approach to the study of central command and the ACC. During exercise anticipation, we demonstrated an increase in dorsal ACC activity within the 25-60Hz band which also correlated with a simultaneous escalation in cardiovascular performance prior to the onset of physical exertion. This is therefore the first invasive electrophysiological evidence to support the role of the ACC in both motor preparation and the top-down control of cardiovascular function in exercise and implicates the ACC as the site linking cognition, arousal and emotion to cardiovascular drive.
Acknowledgements & Financial Disclosure
The authors wish to thank DJ Patterson and M Buckley (Department of Physiology, University of Oxford) for their help in designing the exercise element of the experimental Protocol. JAH wishes to thank JS Brittain (Nuffield Department of Clinical Neurosciences, University of Oxford) for assistance with electrophysiological analysis. Oxford Functional Neurosurgery is supported by educational grants from the Oxford Biomedical Research Centre of the UK National Institute for Health & Research, the Charles Wolfson Charitable Trust, Medtronic Inc. and the Norman Collisson Foundation. MJG is a Clinical Lecturer funded by the NIHR TCC (National Institute for Health Research Trainee Coordinating Centre) ref CL-2013-13-001 and the Academy of Medical Sciences (London, UK). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
References
- 1.Shenhav A, Cohen JD, Botvinick MM. Dorsal anterior cingulate cortex and the value of control. Nat Neurosci. 2016;19(10):1286–91. doi: 10.1038/nn.4384. [DOI] [PubMed] [Google Scholar]
- 2.Boccard SGJ, Prangnell SJ, Pycroft L, Cheeran B, Moir L, Pereira EAC, et al. Long-Term Results of Deep Brain Stimulation of the Anterior Cingulate Cortex for Neuropathic Pain. World Neurosurg. 2017;106:625–37. doi: 10.1016/j.wneu.2017.06.173. [DOI] [PubMed] [Google Scholar]
- 3.Critchley HD, Tang J, Glaser D, Butterworth B, Dolan RJ. Anterior cingulate activity during error and autonomic response. Neuroimage. 2005;27(4):885–95. doi: 10.1016/j.neuroimage.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 4.Krogh A, Lindhard J. The regulation of respiration and circulation during the initial stages of muscular work. J Physiol. 1913;47(1–2):112–36. doi: 10.1113/jphysiol.1913.sp001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williamson JW, Fadel PJ, Mitchell JH. New insights into central cardiovascular control during exercise in humans: a central command update. Exp Physiol. 2006;91(1):51–8. doi: 10.1113/expphysiol.2005.032037. [DOI] [PubMed] [Google Scholar]
- 6.Dampney RA, Coleman MJ, Fontes MA, Hirooka Y, Horiuchi J, Li YW, et al. Central mechanisms underlying short- and long-term regulation of the cardiovascular system. Clin Exp Pharmacol Physiol. 2002;29(4):261–8. doi: 10.1046/j.1440-1681.2002.03640.x. [DOI] [PubMed] [Google Scholar]
- 7.Thornton JM, Guz A, Murphy K, Griffith AR, Pedersen DL, Kardos A, et al. Identification of higher brain centres that may encode the cardiorespiratory response to exercise in humans. J Physiol. 2001;533(Pt 3):823–36. doi: 10.1111/j.1469-7793.2001.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King AB, Menon RS, Hachinski V, Cechetto DF. Human forebrain activation by visceral stimuli. J Comp Neurol. 1999;413(4):572–82. [PubMed] [Google Scholar]
- 9.Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. J Physiol. 2000;523(Pt 1):259–70. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williamson JW, McColl R, Mathews D, Mitchell JH, Raven PB, Morgan WP. Hypnotic manipulation of effort sense during dynamic exercise: cardiovascular responses and brain activation. J Appl Physiol (1985) 2001;90(4):1392–9. doi: 10.1152/jappl.2001.90.4.1392. [DOI] [PubMed] [Google Scholar]
- 11.Williamson JW, McColl R, Mathews D, Mitchell JH, Raven PB, Morgan WP. Brain activation by central command during actual and imagined handgrip under hypnosis. J Appl Physiol (1985) 2002;92(3):1317–24. doi: 10.1152/japplphysiol.00939.2001. [DOI] [PubMed] [Google Scholar]
- 12.Williamson JW, McColl R, Mathews D. Evidence for central command activation of the human insular cortex during exercise. J Appl Physiol (1985) 2003;94(5):1726–34. doi: 10.1152/japplphysiol.01152.2002. [DOI] [PubMed] [Google Scholar]
- 13.Green AL, Wang S, Purvis S, Owen SL, Bain PG, Stein JF, et al. Identifying cardiorespiratory neurocircuitry involved in central command during exercise in humans. J Physiol. 2007;578(Pt 2):605–12. doi: 10.1113/jphysiol.2006.122549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lempka SF, McIntyre CC. Theoretical analysis of the local field potential in deep brain stimulation applications. PLoS One. 2013;8(3):e59839. doi: 10.1371/journal.pone.0059839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ongur D, An X, Price JL. Prefrontal cortical projections to the hypothalamus in macaque monkeys. J Comp Neurol. 1998;401(4):480–505. [PubMed] [Google Scholar]
- 16.An X, Bandler R, Ongur D, Price JL. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J Comp Neurol. 1998;401(4):455–79. [PubMed] [Google Scholar]
- 17.Critchley HD, Mathias CJ, Josephs O, O'Doherty J, Zanini S, Dewar BK, et al. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126(Pt 10):2139–52. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- 18.Matthews SC, Paulus MP, Simmons AN, Nelesen RA, Dimsdale JE. Functional subdivisions within anterior cingulate cortex and their relationship to autonomic nervous system function. Neuroimage. 2004;22(3):1151–6. doi: 10.1016/j.neuroimage.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Burns SM, Wyss JM. The involvement of the anterior cingulate cortex in blood pressure control. Brain Res. 1985;340(1):71–7. doi: 10.1016/0006-8993(85)90774-7. [DOI] [PubMed] [Google Scholar]
- 20.Chefer SI, Talan MI, Engel BT. Central neural correlates of learned heart rate control during exercise: central command demystified. J Appl Physiol (1985) 1997;83(5):1448–53. doi: 10.1152/jappl.1997.83.5.1448. [DOI] [PubMed] [Google Scholar]
- 21.Kaada BR. Somato-motor, autonomic and electrocorticographic responses to electrical stimulation of rhinencephalic and other structures in primates, cat, and dog; a study of responses from the limbic, subcallosal, orbito-insular, piriform and temporal cortex, hippocampus-fornix and amygdala. Acta Physiol Scand Suppl. 1951;24(83):1–262. [PubMed] [Google Scholar]
- 22.Kaada BR, Pribram KH, Epstein JA. Respiratory and vascular responses in monkeys from temporal pole, insula, orbital surface and cingulate gyrus; a preliminary report. J Neurophysiol. 1949;12(5):347–56. doi: 10.1152/jn.1949.12.5.347. [DOI] [PubMed] [Google Scholar]
- 23.Gentil AF, Eskandar EN, Marci CD, Evans KC, Dougherty DD. Physiological responses to brain stimulation during limbic surgery: further evidence of anterior cingulate modulation of autonomic arousal. Biol Psychiatry. 2009;66(7):695–701. doi: 10.1016/j.biopsych.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Pool JL, Ransohoff J. Autonomic effects on stimulating rostral portion of cingulate gyri in man. J Neurophysiol. 1949;12(6):385–92. doi: 10.1152/jn.1949.12.6.385. [DOI] [PubMed] [Google Scholar]
- 25.Zahn TP, Grafman J, Tranel D. Frontal lobe lesions and electrodermal activity: effects of significance. Neuropsychologia. 1999;37(11):1227–41. doi: 10.1016/s0028-3932(99)00020-2. [DOI] [PubMed] [Google Scholar]
- 26.Seeber M, Scherer R, Wagner J, Solis-Escalante T, Muller-Putz GR. High and low gamma EEG oscillations in central sensorimotor areas are conversely modulated during the human gait cycle. Neuroimage. 2015;112:318–26. doi: 10.1016/j.neuroimage.2015.03.045. [DOI] [PubMed] [Google Scholar]
- 27.Drijvers L, Ozyurek A, Jensen O. Hearing and seeing meaning in noise: Alpha, beta, and gamma oscillations predict gestural enhancement of degraded speech comprehension. Hum Brain Mapp. 2018;39(5):2075–87. doi: 10.1002/hbm.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan J, Tompkins WJ. A real-time QRS detection algorithm. IEEE Trans Biomed Eng. 1985;32(3):230–6. doi: 10.1109/TBME.1985.325532. [DOI] [PubMed] [Google Scholar]
- 29.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 30.Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, et al. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45(1 Suppl):S173–86. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- 31.Adams NE, Sherfey JS, Kopell NJ, Whittington MA, LeBeau FE. Hetereogeneity in Neuronal Intrinsic Properties: A Possible Mechanism for Hub-Like Properties of the Rat Anterior Cingulate Cortex during Network Activity. eNeuro. 2017;4(1) doi: 10.1523/ENEURO.0313-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gianaros PJ, Onyewuenyi IC, Sheu LK, Christie IC, Critchley HD. Brain systems for baroreflex suppression during stress in humans. Hum Brain Mapp. 2012;33(7):1700–16. doi: 10.1002/hbm.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sequeira H, Ba-M'hamed S. Pyramidal control of heart rate and arterial pressure in cats. Arch Ital Biol. 1999;137(1):47–62. [PubMed] [Google Scholar]
- 34.Seifert F, Schuberth N, De Col R, Peltz E, Nickel FT, Maihofner C. Brain activity during sympathetic response in anticipation and experience of pain. Hum Brain Mapp. 2013;34(8):1768–82. doi: 10.1002/hbm.22035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brinkmann L, Poller H, Herrmann MJ, Miltner W, Straube T. Initial and sustained brain responses to threat anticipation in blood-injection-injury phobia. Neuroimage Clin. 2017;13:320–9. doi: 10.1016/j.nicl.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudebeck PH, Putnam PT, Daniels TE, Yang T, Mitz AR, Rhodes SE, et al. A role for primate subgenual cingulate cortex in sustaining autonomic arousal. Proc Natl Acad Sci U S A. 2014;111(14):5391–6. doi: 10.1073/pnas.1317695111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frith CD, Allen HA. The skin conductance orienting response as an index of attention. Biol Psychol. 1983;17(1):27–39. doi: 10.1016/0301-0511(83)90064-9. [DOI] [PubMed] [Google Scholar]
- 38.Gladwin TE, Hashemi MM, van Ast V, Roelofs K. Ready and waiting: Freezing as active action preparation under threat. Neurosci Lett. 2016;619:182–8. doi: 10.1016/j.neulet.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 39.Touj S, Houle S, Ramla D, Jeffrey-Gauthier R, Hotta H, Bronchti G, et al. Sympathetic regulation and anterior cingulate cortex volume are altered in a rat model of chronic back pain. Neuroscience. 2017;352:9–18. doi: 10.1016/j.neuroscience.2017.03.047. [DOI] [PubMed] [Google Scholar]
- 40.Maihofner C, Seifert F, Decol R. Activation of central sympathetic networks during innocuous and noxious somatosensory stimulation. Neuroimage. 2011;55(1):216–24. doi: 10.1016/j.neuroimage.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 41.Jennings JR, Sheu LK, Kuan DC, Manuck SB, Gianaros PJ. Resting state connectivity of the medial prefrontal cortex covaries with individual differences in high-frequency heart rate variability. Psychophysiology. 2016;53(4):444–54. doi: 10.1111/psyp.12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murtha S, Chertkow H, Beauregard M, Dixon R, Evans A. Anticipation causes increased blood flow to the anterior cingulate cortex. Hum Brain Mapp. 1996;4(2):103–12. doi: 10.1002/(SICI)1097-0193(1996)4:2<103::AID-HBM2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 43.van Pelt S, Heil L, Kwisthout J, Ondobaka S, van Rooij I, Bekkering H. Beta- and gamma-band activity reflect predictive coding in the processing of causal events. Soc Cogn Affect Neurosci. 2016;11(6):973–80. doi: 10.1093/scan/nsw017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mobbs D, Marchant JL, Hassabis D, Seymour B, Tan G, Gray M, et al. From threat to fear: the neural organization of defensive fear systems in humans. J Neurosci. 2009;29(39):12236–43. doi: 10.1523/JNEUROSCI.2378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9(5):370–86. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 46.McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- 47.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262(5134):679–85. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 48.Green AL, Wang S, Stein JF, Pereira EA, Kringelbach ML, Liu X, et al. Neural signatures in patients with neuropathic pain. Neurology. 2009;72(6):569–71. doi: 10.1212/01.wnl.0000342122.25498.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2000;30(5):263–88. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 50.Inanaga K. Frontal midline theta rhythm and mental activity. Psychiatry Clin Neurosci. 1998;52(6):555–66. doi: 10.1046/j.1440-1819.1998.00452.x. [DOI] [PubMed] [Google Scholar]
- 51.Ishii R, Shinosaki K, Ukai S, Inouye T, Ishihara T, Yoshimine T, et al. Medial prefrontal cortex generates frontal midline theta rhythm. Neuroreport. 1999;10(4):675–9. doi: 10.1097/00001756-199903170-00003. [DOI] [PubMed] [Google Scholar]
- 52.Womelsdorf T, Johnston K, Vinck M, Everling S. Theta-activity in anterior cingulate cortex predicts task rules and their adjustments following errors. Proc Natl Acad Sci U S A. 2010;107(11):5248–53. doi: 10.1073/pnas.0906194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laberge D. Thalamic and cortical mechanisms of attention suggested by recent positron emission tomographic experiments. J Cogn Neurosci. 1990;2(4):358–72. doi: 10.1162/jocn.1990.2.4.358. [DOI] [PubMed] [Google Scholar]
- 54.Posner MI. Seeing the mind. Science. 1993;262(5134):673–4. doi: 10.1126/science.8235585. [DOI] [PubMed] [Google Scholar]
- 55.Posner MI, Petersen SE, Fox PT, Raichle ME. Localization of cognitive operations in the human brain. Science. 1988;240(4859):1627–31. doi: 10.1126/science.3289116. [DOI] [PubMed] [Google Scholar]