Abstract
This study aims to investigate the causal relation between circulating phylloquinone (vitamin K1) concentrations and type 2 diabetes using a Mendelian Randomization (MR) approach. We used data from thee cohorts: the European Prospective Investigation into Cancer and Nutrition (EPIC)-InterAct case-cohort study, Diabetes Genetics Replication and Meta-analysis (DIAGRAM) and the UK Biobank, resulting in 69,647 type 2 diabetes cases. We calculated a weighted genetic risk score including four genetic variants previously found to be associated with circulating phylloquinone concentrations. Inverse-variance weighted analysis was used to obtain a risk ratio (RR) for the causal relation between circulating phylloquinone concentrations and risk of type 2 diabetes. Presence of pleiotropy and the robustness of the results were assessed using MR-Egger and weighted-median analyses. Genetically-predicted concentrations of circulating phylloquinone was associated with lower risk of type 2 diabetes with a RR of 0.93 (95% confidence interval: 0.89;0.97) per every ln-nmol/L unit increase in circulating phylloquinone. The MR-Egger and weighted median analyses showed RRs of 0.94 (0.86;1.02) and 0.93 (0.88;0.98), respectively, indicating no pleiotropy. In conclusion, our study supports that higher circulating phylloquinone may be causally related with lower risk of type 2 diabetes, highlighting the importance of sufficient phylloquinone in the human diet.
Introduction
Vitamin K is a fat-soluble vitamin occurring in two biologically active forms: phylloquinone (vitamin K1) and menaquinones (vitamin K2). Phylloquinone is the most common form in circulation, and is predominantly present in green leafy vegetables (1). Observational studies showed that higher phylloquinone intake was associated with a lower risk of type 2 diabetes (2,3). Intervention studies also showed improved insulin sensitivity following phylloquinone supplementation, although the results were inconsistent (4–6). Moreover, these studies did not use the gold standard (hyperinsulinemic-euglycemic clamp) to measure insulin sensitivity (4–6). The underlying mechanism still is unknown, but vitamin K-dependent carboxylation of osteocalcin and a possible anti-inflammatory effect of vitamin K are suggested (7,8).
Current evidence thus points towards a beneficial role for phylloquinone the prevention of type 2 diabetes, but the study designs used each have certain limitations. Observational studies may be hampered by reverse causation or confounding (9), as phylloquinone co-exists with vitamins and phytochemicals of vegetables, which are likely to affect the risk of non-communicable diseases in diverse pathways (7). Intervention studies are limited by having a short duration and focus on intermediate risk factors for type 2 diabetes, such as insulin sensitivity, instead of risk of type 2 diabetes. Due to these limitations, the causal association between phylloquinone and type 2 diabetes remains debatable.
To date, no study assessed the association between circulating phylloquinone concentrations and type 2 diabetes using a Mendelian Randomization (MR) approach. Therefore, this study aimed to investigate the causal relation between circulating phylloquinone concentrations and type 2 diabetes using a MR approach in which we used a set of genetic variants as an instrumental variable.
Methods
Study populations
For these analyses, we used data from three studies: European Prospective Investigation into Cancer and Nutrition (EPIC)-InterAct case cohort study, DIAGRAM and the UK Biobank. Individual participant data was only available in EPIC-InterAct. EPIC-InterAct is a prospective case-cohort study nested within EPIC, involving 8 European countries (Denmark, France, Germany, Italy, the Netherlands, Spain, Sweden and the United Kingdom) (10). EPIC-InterAct identified 12,403 incident cases of type 2 diabetes between 1991 and 2007 and a randomly selected a subcohort of 16,154 participants (10). After exclusion of individuals with missing genetic data (n=2,332 cases and n=3,115 subcohort members), excluding 1528 participants from EPIC-Norfolk (which contributes to DIAGRAM), 9,400 cases and 12,182 subcohort members were left for analyses (this subcohort also included a set of 598 incident cases reflecting the case-cohort design). The mean age in the subcohort was 52.4 (SD: 9.2) years, 38.4% were men, and mean BMI was 26.0 kg/m2.
Summarized data were included from DIAGRAM and the UK Biobank. The aim of DIAGRAM is to characterize genetic information for type 2 diabetes, mainly in samples of participants of European descent. DIAGRAM included data from 23 studies, which meta-analyzed genetic variants on Metabochip (11). DIAGRAM included both men and women, mainly of European ancestry, aged 43 to 72 years with BMIs varying from 22.3 to 33.4 kg/m2 among the case-control or cohort participants. Data are publicly available at www.diagram-consortium.org.
The UK Biobank is a large, population-based cohort study established to study the interrelationships between environment, lifestyle and genes. The UK Biobank (www.ukbiobank.ac.uk) recruited over 500,000 men and women between 2006 and 2010 (application number 9161, 9905, 12885, 20684) (12). Age varied between 37 to 73 years, and the mean BMI was 27.5 (SD:4.8). The UK Biobank was approved by the North West Multi-Centre Research Ethics Committee and all participants provided written informed consent to participate in the UK Biobank study. EPIC-InterAct, DIAGRAM and the UK Biobank included up to 69,647 type 2 diabetes cases and 551,336 non-diabetic participants.
Genotyping and construction of weighted genetic risk score
For EPIC-InterAct, DNA was extracted from buffy coat from citrated blood samples. Participants were genotyped with the Illumina Human Core Exome chip and the Illumina Human Quad 660 chip. Missing genotypes were imputed by the Haplotype Reference Consortium v1.0, using IMPUTE v2.3.2, and was performed at the Wellcome Trust Centre for Human Genetics.
Genotyping in the UK Biobank was performed using the Affymetrix UK BiLEVE Axiom array and the Affymetrix UK Biobank Axiom Array (13). DIAGRAM participants are genotyped using different assays, as described previously (14).
We included 4 SNPs selected out of 11 SNPs that were likely to predict circulating phylloquinone (plasma or serum) among European descents in a genome-wide meta-analysis study (n=2,138) (rs964184, rs4645543, rs2199565, rs7018214, rs2108622, rs2192574, rs4852146, rs6862909, rs6862071, rs4122275): these SNPs passed the threshold of p<5×10–6, but not p<5×10–8 (15). Among the 11 SNPs, linkage disequilibrium at R2>0.9 was evident among rs2192574 and rs4852146 (chromosome 2); rs6862909, rs6862071 and rs4122275 (chromosome 5); rs4645543, rs2199565 and rs7018214 (chromosome 8); and rs2108622 and rs12609820 (chromosome 19). Therefore, five SNPs with the strongest association with circulating phylloquinone from each chromosome were initially selected (rs2108622, rs2192574, rs4645543, rs6862071 and rs964184).
We further excluded rs964184 with pleiotropic effects on blood lipids and coronary heart disease by searching GWAS catalog and Phenoscanner for the SNPs or its proxies (R2>0.8) and querying associations of the SNPs with variables indicating pleiotropic effects with p<5×10–8 (16,17). After these exclusions, rs2108622, rs2192574, rs4645543 and rs6862071 were retained for the present study.
In all three cohorts, we calculated a weighted genetic risk score (wGRS) by summing the number of effect alleles coded as 0, 1, or 2, at each individual SNP weighted by the respective allelic effect sizes on circulating phylloquinone concentrations from the previous genome-wide meta-analysis study (15).
Diabetes ascertainment
For EPIC-InterAct, a detailed description of ascertainment and verification of incident diabetes cases has been described previously (10). Briefly, occurrence of type 2 diabetes during follow up was identified through self-report, linkage to primary care registers, secondary care registers, medication use, and hospital admissions and mortality data. Type 2 diabetes was determined when at least two sources identified the diagnosis type 2 diabetes. Diagnosis of type 2 diabetes in DIAGRAM differed per study, as described previously (14). Type 2 diabetes diagnosis in the UK Biobank was self-reported at baseline, as previously described (18).
Statistical analysis
Individual-participant data analysis was performed in EPIC-InterAct. Accounting for its case-cohort design, Prentice-weighted Cox regression (19) was used to quantify the association of the individual SNPs and the wGRS with type 2 diabetes risk. Age was used as an underlying timescale. The associations were evaluated in strata of countries and adjusted for sex, study sites, principal components of ancestry and genetic array. For each of the individual SNPs and the wGRS, we combined the hazard ratios obtained from EPIC-InterAct with odds ratios from DIAGRAM and UK Biobank as risk ratios (RRs) using a random effect meta-analysis. Then, inverse-variance weighted (IVW) estimates for the causal association between circulating phylloquinone and type 2 diabetes risk were calculated using the formulae reported by Burgess et al. (20). The ratio estimate of the causal effect of circulating phylloquinone on diabetes risk is calculated as the association between the SNP and diabetes risk divided by the association between the SNP and circulating phylloquinone. The IVW estimate combines the ratio estimates using each variant in a fixed-effect meta-analysis model (21).
We quantified the F-statistic to assess the strength of the genetic instrument, using the proportion of variance explained by the genetic variants, the sample size and number of variants included (22). To assess presence of unbalanced pleiotropy, we performed a MR-Egger and weighted-median analysis in all cohort studies using the ‘Mendelian Randomization’ package in R (23–25). Unbalanced pleiotropy was considered present if the intercept significantly deviated from zero (p <0.05). In the subcohort of EPIC-interact only, we assessed potential pleiotropic effects of the wGRS on demographic, lifestyle, and physiological variables using logistic and linear regression analyses in strata of countries and adjusted for sex, center, principal components and genetic array.
Phylloquinone is carried predominantly in triglyceride-rich lipoproteins and in the previous genome-wide association study, beta coefficients for the gene-phylloquinone associations adjusted for fasting triglycerides were also reported (15). Therefore, we performed sensitivity analyses, in EPIC-InterAct data only, using the beta-coefficients adjusted for triglycerides and corrected the association between the wGRS and diabetes incidence for triglycerides and hours of fasting. Missing values on triglycerides (5.1%) and hours of fasting (32.3%) were imputed with predictive mean matching. We used a maximum of 50 iterations and 10 imputations using the R package ‘mice’ (26). This analysis was also performed in the complete case dataset to assess the influence of the large number of missing values for hours of fasting. Finally, we calculated ORs in EPIC-InterAct and pooled these with the ORs in DIAGRAM and UK Biobank, instead of combining the HR in EPIC-InterAct with OR in DIAGRAM and UK Biobank. All analyses were performed using R Software (version 3.1.1).
Results
For EPIC-InterAct participants only, baseline characteristics are described in supplementary table 1, and the distributions of the alleles of each phylloquinone-related SNP are shown per country in supplementary table 2. We did not detect any association between genetically predicted circulating phylloquinone and possible pleiotropic traits (supplementary table 3).
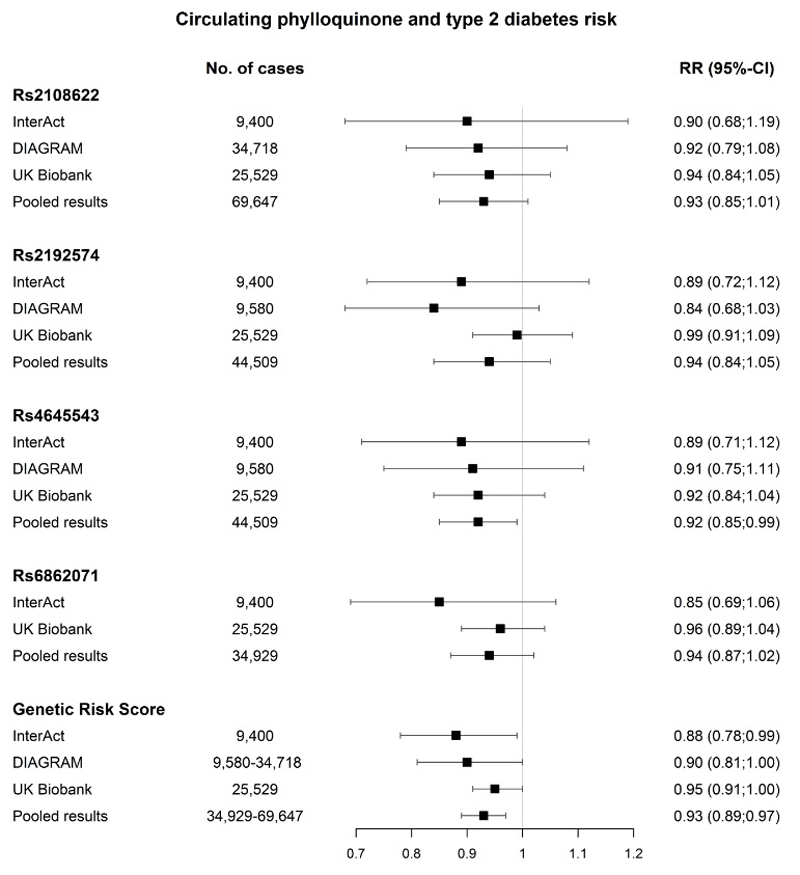
The IVW analyses showed that higher genetically-predicted concentrations of circulating phylloquinone were associated with a lower risk of type 2 diabetes in EPIC-InterAct, with a RR of 0.88 (95%-CI: 0.78;0.99) per ln-nmol/L increase in circulating phylloquinone (Figure 1). Similar results were observed in DIAGRAM (RR 0.90 (95%-CI: 0.81;1.00)) and UK Biobank (RR 0.95 (95%-CI: 0.91;1.00)). Pooling the three datasets (EPIC-InterAct, DIAGRAM and UK Biobank) resulted in a RR for the wGRS of 0.93 (95%-CI: 0.89;0.97) per ln-nmol/L increase in circulating phylloquinone. There was no heterogeneity between the studies (I2=0.3%, p=0.96). No association was observed for the individual SNPs; however, the directions of genetic associations with the outcome were consistent across the four SNPs, and there was some suggestion of a dose-response relationship in the genetic associations with the risk factor and outcome.
The included SNPs explained 4.7% of the variation in circulating phylloquinone in the GWAS, resulting in an F-statistic of 208.9 in our study. The MR-Egger method indicated no pleiotropic effects (intercept=-0.02, SE=0.01, p-value =0.88) and resulted in a RR of 0.94 (95%-CI: 0.86;1.02) per ln-nmol/L in circulating phylloquinone (Supplementary figure 1). The weighted median method resulted in a RR of 0.93 (95%-CI: 0.88;0.98) per ln-nmol/L in circulating phylloquinone.
In EPIC-InterAct data only, additional adjustments for triglycerides and hours of fasting did not alter the results, and resulted in a HR for the wGRS of 0.87 (95%-CI: 0.78;0.98) in the IVW analyses (supplementary table 4). Similar results were observed in a complete cases analysis (HR wGRS 0.82 (95%-CI:0.71;0.95). Finally, calculating OR in EPIC-InterAct resulted in comparable pooled results (OR wGRS 0.93 (95%-CI: 0.89;0.98)).
Discussion
Our study supports that higher circulating phylloquinone may be causally related with lower type 2 diabetes risk.
This is the first MR study linking circulating phylloquinone concentrations to the risk of type 2 diabetes. Our results are in line with the current evidence on this association from observational and intervention studies. Two observational studies found that higher phylloquinone intake was associated with a lower type 2 diabetes incidence (2,3). Current evidence from trials are not conclusive on the effect of phylloquinone supplementation on insulin sensitivity and insulin resistance (4–6). Two studies observed improved insulin sensitivity after phylloquinone supplementation (4,6), after 4 weeks intervention (6) or after 3 years intervention (4). By contrast, another small-scale study did not find improved insulin sensitivity after 1 year phylloquinone supplementation (5). While the inconsistency should be acknowledged, altogether, the available evidence from MR, observational studies, and trials supports a causal relation between higher circulating phylloquinone concentrations and a reduced risk of type 2 diabetes. This evidence highlights the need of sufficient phylloquinone in the diet, and supports the further development of phylloquinone requirements. However, the mechanism is still unknown, and it is possible that the causation is due to a biological pathway retaining high phylloquinone concentrations but not due to phylloquinone itself. Furthermore, since phylloquinone concentrations and phylloquinone intake are only modestly correlated (r=0.51)(27), our results cannot be directly translated to phylloquinone intake. Nonetheless these results highlight the need for further work relating phylloquinone metabolism to the etiology of type 2 diabetes.
MR studies only provide evidence about the causality of an association when three assumptions are met. We will discuss each assumption with its strengths and limitations; the first assumption implies a strong association between the instrumental variable (wGRS) and the risk factor, in our case circulating phylloquinone. None of the SNPs selected for this study reached genome-wide significance in phylloquinone genome-wide meta-analysis study (15). Rs2108622 is a CYP4F2 variant, which functions as a phylloquinone oxidase (28). Previous studies showed that rs2108622 was associated with altered phylloquinone metabolism and warfarin dosage (29). Because of the biologically plausible effect of rs2108622 on circulating concentrations of phylloquinone, we considered this functional SNP causally altering circulating phylloquinone. However, this genetic variant did not have a genome wide significant association with phylloquinone concentrations in the GWAS (15). Presumably, this was due to the relatively small sample size (n=2,138) of the meta-analysis and the low minor allele frequency (15). While the F-statistic showed no indication of weak instrument bias (288.2 in EPIC-InterAct) and the included SNPs explained 4.7% of the variation in circulating phylloquinone in the small-scale GWAS, the F-statistic might be an overestimation of the true effect, since there might be an overestimation of the R2 of the GWAS, due to winners curse resulting in effect size estimates that are upwardly biased (30). Although the F-statistic might be an overestimation, there is still no indication of weak instrument since the F-statistic is much higher than the arbitrary threshold of 10. The wGRS has not been validated in an external dataset, because no large scale cohort studies performed a GWAS and assessed circulating phylloquinone levels apart from those included in the GWAS by Dashti et al. (15). Because of this lack of validation and because none of the SNPs reached genome wide significance, our findings need to be interpreted with caution. Additional GWAS data on circulating phylloquinone or other markers of vitamin K status are warranted to further investigate causal roles of vitamin K and its metabolic pathway in the development of type 2 diabetes.
The second and third assumption comprises no association between the instrumental variable and outcome via confounders or other mechanisms. The MR-Egger and the linear and logistic regression analyses showed no indication of pleiotropy. Still, we note that rs2108622 is a variant in the CYP4F2 gene, may play a role in transport and metabolism of vitamin E (31). Notably, a trial of vitamin E showed no evidence of its effect on type 2 diabetes (32), while vitamin E may antagonize vitamin K (33). Whereas we found no evidence of pleiotropy in the genetically predicted effect of vitamin K on diabetes risk, both genetics and the trial support the causal role of vitamin E in vitamin K metabolism and this nutrient-nutrient interaction is of further interest also in etiology of type 2 diabetes. Although we did not find any indication of pleiotropy, circulating phylloquinone may be a biomarker that reflects healthy lifestyle in general instead of phylloquinone specifically.
In conclusion, this study supports that higher circulating phylloquinone concentrations are causally related with lower type 2 diabetes risk, highlighting the importance of sufficient amounts of phylloquinone in the human diet.
Supplementary Material
Acknowledgements
We thank all EPIC participants and staff for their contribution to the study. We thank Nicola Kerrison (MRC Epidemiology Unit, Cambridge) for managing the data for the EPIC-InterAct Project.
Funding Statement: Funding for the EPIC-InterAct project was provided by the EU FP6 programme (grant number LSHM_CT_2006_037197). This research has been conducted using the UK Biobank Resource (application number 9161, 9905, 12885 and 20684). SRZ and JWJB are supported by the Senior Dr. Dekker grant (2013T120) from The Dutch Heart Foundation. IS is supported by a personal Dr. Dekker Junior Postdoctoral grant (2015T19) from The Dutch Heart Foundation. FI is supported by MRC Epidemiology Unit Core Grant (MC_UU_12015/5). SLB is partially supported by the USDA Agricultural Research Service cooperative agreement 58-1950-7-707. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the USDA.
Guarantor Statement: SZ, SR, JB, YvdS and IS are the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Author contributions: Author contributions were as follows: SZ and SR researched data. SZ, SR, JB, YvdS and IS wrote the manuscript. SLB, SB, HD, FI, EF contributed to discussion and reviewed/edited manuscript.
Conflict of Interest Statement: None of the authors declared a conflict of interest.
References
- 1.Bolton-Smith C, Price RJ, Fenton ST, Harrington DJ, Shearer MJ. Compilation of a provisional UK database for the phylloquinone (vitamin K1) content of foods. Br J Nutr. 2000;83(4):389–99. [PubMed] [Google Scholar]
- 2.Beulens JWJ, Van Der A DL, Grobbee DE, Sluijs I, Spijkerman AMW, Van Der Schouw YT. Dietary phylloquinone and menaquinones intakes and risk of type 2 diabetes. Diabetes Care. 2010;33(8):1699–705. doi: 10.2337/dc09-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ibarrola-Jurado N, Salas-Salvadó J, Martínez-González MA, Bulló M. Dietary phylloquinone intake and risk of type 2 diabetes in elderly subjects at high risk of cardiovascular disease. Am J Clin Nutr. 2012 Nov;96(5):1113–8. doi: 10.3945/ajcn.111.033498. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida M, Jacques PF, Meigs JB, Saltzman E, Shea MK, Gundberg C, et al. Effect of Vitamin K Supplementation on Insulin Resistance in Older Men and Women. Diabetes Care. 2008 Nov;31(11):2092–6. doi: 10.2337/dc08-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar R, Binkley N, Vella A. Effect of phylloquinone supplementation on glucose homeostasis in humans. Am J Clin Nutr. 2010 Dec;92(6):1528–32. doi: 10.3945/ajcn.2010.30108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasekhi H, Karandish M, Jalali MT, Mohammad-shahi M, Zarei M, Saki A, et al. The effect of vitamin K1 supplementation on sensitivity and insulin resistance via osteocalcin in prediabetic women: a double-blind randomized controlled clinical trial. Eur J Clin Nutr. 2015 Aug;69(8):891–5. doi: 10.1038/ejcn.2015.17. [DOI] [PubMed] [Google Scholar]
- 7.Kyla Shea M, Booth SL. Concepts and controversies in evaluating vitamin K status in population-based studies. Nutrients. 2016;8(1) doi: 10.3390/nu8010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci U S A. 2008 Apr;105(13):5266–70. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith GD, Ebrahim S. Mendelian randomization: Prospects, potentials, and limitations. Int J Epidemiol. 2004;33(1):30–42. doi: 10.1093/ije/dyh132. [DOI] [PubMed] [Google Scholar]
- 10.InterAct Consortium TI. Langenberg C, Sharp S, Forouhi NG, Franks PW, Schulze MB, et al. Design and cohort description of the InterAct Project: an examination of the interaction of genetic and lifestyle factors on the incidence of type 2 diabetes in the EPIC Study. Diabetologia. 2011 Sep;54(9):2272–82. doi: 10.1007/s00125-011-2182-9. Europe PMC Funders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris AP, Voight BF, Teslovich TM, Ferreira T, Segrè AV, Steinthorsdottir V, et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012 Aug;44(9):981–90. doi: 10.1038/ng.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins R. What makes UK Biobank special? The Lancet. 2012;379:1173–4. doi: 10.1016/S0140-6736(12)60404-8. [DOI] [PubMed] [Google Scholar]
- 13.UK Biobank. Protocol for a large-scale prospective epidemiological resource. 2006 Available from: www.ukbiobank.ac.uk/resources/
- 14.Gaulton KJ, Ferreira T, Lee Y, Raimondo A, Mägi R, Reschen ME, et al. Genetic fine mapping and genomic annotation defines causal mechanisms at type 2 diabetes susceptibility loci. Nat Genet. 2015;47(12):1415–25. doi: 10.1038/ng.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dashti HS, Shea MK, Smith CE, Tanaka T, Hruby A, Richardson K, et al. Meta-analysis of genome-wide association studies for circulating phylloquinone concentrations. Am J Clin Nutr. 2014 Dec;100(6):1462–9. doi: 10.3945/ajcn.114.093146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacArthur J, Bowler E, Cerezo M, Gil L, Hall P, Hastings E, et al. The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog) Nucleic Acids Res. 2017;45(D1):D896–901. doi: 10.1093/nar/gkw1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staley JR, Blackshaw J, Kamat MA, Ellis S, Surendran P, Sun BB, et al. PhenoScanner: A database of human genotype-phenotype associations. Bioinformatics. 2016;32(20):3207–9. doi: 10.1093/bioinformatics/btw373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eastwood SV, Mathur R, Atkinson M, Brophy S, Sudlow C, Flaig R, et al. Algorithms for the capture and adjudication of prevalent and incident diabetes in UK Biobank. PLoS One. 2016;11(9) doi: 10.1371/journal.pone.0162388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73(1):1–11. [Google Scholar]
- 20.Burgess S, Thompson SG. Mendelian randomization: methods for using genetic variants in causal estimation. 2015:142. [Google Scholar]
- 21.Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658–65. doi: 10.1002/gepi.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burgess S, Thompson SG. Avoiding bias from weak instruments in mendelian randomization studies. Int J Epidemiol. 2011;40(3):755–64. doi: 10.1093/ije/dyr036. [DOI] [PubMed] [Google Scholar]
- 23.Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017 doi: 10.1093/ije/dyx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowden J, Smith GD, Burgess S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–25. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 2016;40(4):304–14. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J Stat Softw. 2011;45(3) [Google Scholar]
- 27.Booth SL, Tucker KL, McKeown NM, Davidson KW, Dallal GE, Sadowski JA. Relationships between dietary intakes and fasting plasma concentrations of fat-soluble vitamins in humans. J Nutr. 1997;127:587–92. doi: 10.1093/jn/127.4.587. [DOI] [PubMed] [Google Scholar]
- 28.Edson KZ, Prasad B, Unadkat JD, Suhara Y, Okano T, Peter Guengerich F, et al. Cytochrome P450-dependent catabolism of vitamin K: ω-hydroxylation catalyzed by human CYP4F2 and CYP4F11. Biochemistry. 2013;52(46):8276–85. doi: 10.1021/bi401208m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDonald MG, Rieder MJ, Nakano M, Hsia CK, Rettie AE. CYP4F2 Is a Vitamin K1 Oxidase: An Explanation for Altered Warfarin Dose in Carriers of the V433M Variant. Mol Pharmacol. 2009;75(6):1337–46. doi: 10.1124/mol.109.054833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao R, Boehnke M. Genet Epidemiol. 5. Vol. 33. Wiley Subscription Services, Inc., A Wiley Company; 2009. Jul, Quantifying and correcting for the winner’s curse in genetic association studies; pp. 453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Major JM, Yu K, Wheeler W, Zhang H, Cornelis MC, Wright ME, et al. Genome-wide association study identifies common variants associated with circulating vitamin E levels. Hum Mol Genet. 2011 Oct;20(19):3876–83. doi: 10.1093/hmg/ddr296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kataja-Tuomola M, Sundell JR, Männistö S, Virtanen MJ, Kontto J, Albanes D, et al. Effect of α-tocopherol and β-carotene supplementation on the incidence of type 2 diabetes. Diabetologia. 2007 Nov;51(1):47–53. doi: 10.1007/s00125-007-0864-0. [DOI] [PubMed] [Google Scholar]
- 33.Booth SL, Golly I, Sacheck JM, Roubenoff R, Dallal GE, Hamada K, et al. Effect of vitamin E supplementation on vitamin K status in adults with normal coagulation status. Am J Clin Nutr. 2004;80(1):143–8. doi: 10.1093/ajcn/80.1.143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.