Abstract
Objective
Data describing long-term outcomes following intensive care (ICU) for patients with alcohol-related liver disease (ALD) are scarce. We aimed to report long-term mortality and emergency hospital resource use for patients with ALD and compare this with two comparator cohorts.
Design
Retrospective cohort study linking population registry data.
Setting
All adult general Scottish ICUs (2005-2010) serving 5 million population.
Patients
ICU patients with ALD were compared to an unmatched cohort with APACHE-defined diagnoses of severe cardiovascular, respiratory or renal comorbidity and a matched general ICU cohort.
Interventions(none);Measurements and Main Results
Outcomes were five-year mortality, emergency hospital resource use and emergency hospital readmission. Multivariable regression was used to identify risk factors and adjust for confounders. Of 47779 ICU admissions, 2463 patients with ALD and 3590 patients with severe co-morbidities were identified; 2391(97.1%) were matched to a general ICU cohort. The ALD cohort had greater 5-year mortality than comorbid (79.2% vs 75.3%,p<0.001) and matched general (79.8% vs 63.3%,p<0.001) cohorts. High liver SOFA score and three-organ support were associated with 90% 5-year mortality in ALD patients. After confounder adjustment, ALD patients had 31% higher hazard of death (adjusted-HR 1.31,95%CI 1.17,1.47,p<0.001) and used greater resource than the severe comorbid comparator group. Findings were similar compared with the matched cohort.
Conclusions
ICU patients with ALD have higher 5-year mortality and emergency readmission rates than ICU patents with other severe comorbidities and matched general ICU patients. These data can contribute to shared decision-making for ALD patients.
Keywords: Alcoholic liver disease, critical care, patient readmission, epidemiology, outcomes
Introduction
Alcohol-related harm is a significant public health problem. Alcohol-related hospital admissions in England have risen in each of the last 10 years, costing an estimated £3.5 billion/year.(1, 2) Patients with alcohol-related liver disease (ALD) are vulnerable to developing critical illness due to decompensation of chronic liver disease, which is often precipitated by an acute illness such as variceal bleeding, hepatic encephalopathy, or sepsis. ICU admission rates for ALD are increasing,(3) but hospital mortality is high and reported to exceed 85% in patients developing multiorgan failure.(4, 5) Data for long-term survival and other outcomes are limited, but studies in non-ICU patients with cirrhosis report 30-45% one-year mortality(6, 7) and high hospital readmission rates (20-50%).(8–11)
A recent Lancet commission addressing liver disease advocated widened access to critical care.(12) However, a shared decision-making process between clinicians, patients and families should precede admission balancing burdens and benefits.(13) These stakeholders need to understand the consequences of critical care admission based on robust evidence. Few studies have reported longer-term outcomes for critically ill patients with ALD, and none describe both long-term mortality and hospital readmission rates for a complete national cohort.(5) This is particularly important given the increasing evidence of the burden associated with ICU survivorship.(14)
To inform these knowledge gaps, we analysed a national cohort of patients admitted with ALD identified from the Scottish ICU registry in order to (1) report 5-year mortality, emergency readmission risk and subsequent acute hospital resource used in comparison to two cohorts of ICU patients: one with severe chronic diseases known to be independently associated with poor outcomes and a second comprising matched general ICU patients; and (2) identify risk factors for early and late mortality and 5-year emergency readmission risk. We hypothesised that patients with ALD would have greater long-term morbidity and mortality compared with general ICU patients, but not patients with severe chronic diseases.
Methods
Design, setting and data sources
We used a cohort study design using the following linked registries: the Scottish Intensive Care Society Audit Group (SICSAG), Scottish Morbidity Record of acute hospital admissions (SMR01) and Scottish death records. The SICSAG registry captures all adult general intensive care activity within Scotland.(15) Between 2005 and 2010, all 24 adult general ICUs, serving a population of 5.1 million (4.2 million aged ≥16), submitted data. Approvals were obtained from the relevant data-governing body (Privacy Advisory Committee, Information Services Division: ref 55/09). All data were anonymised prior to release to the researchers. The South East Scotland Research Ethics Committee granted a waiver (ref NR/1001AB14).
Population
The ALD cohort comprised Scottish residents ≥16 years with an ALD diagnosis admitted to general Scottish ICUs over a six-year period (01/01/2005-31/12/2010; index admission). We excluded elective ICU admissions and those undergoing liver transplantation. Only first ICU admissions with a valid linkage number were included where there were multiple admissions.
ALD diagnosis was defined by combining SICSAG and SMR01 data sources as following: 1) presence of a SICSAG ICU admission diagnosis code of ALD coded on the index ICU admission; or 2) presence of an SMR01 diagnosis code of ALD (International Classification Disease 10th revision codes (ICD-10) K700-K709 inclusive) on the index hospital SMR01 record or in the one-year preceding ICU admission. Coding in both SICSAG and SMR01 datasets has been validated and demonstrated to be accurate.(16, 17)
We used two comparator cohorts:
-
1)
an unmatched cohort of non-ALD patients admitted to ICU with severe cardiovascular, respiratory or renal disease as defined in the Acute Physiology and Chronic Health Evaluation (APACHE) definition of comorbidity.(18) The APACHE comorbidity definition captures severe chronic disease causing symptoms that significantly interfere with daily life (e.g. severe cardiovascular comorbidity is defined as those with angina/claudication/dyspnoea at rest; see Supplement for full definitions).
-
2)
a cohort of non-ALD patients admitted to ICU matched on age, sex, comorbidity, admission source, socioeconomic status(SES), and severity of illness using Coarsened Exact Matching (Stata command:‘cem’).(19) Instead of traditional 1-to-1 matching, CEM ensures that matched patients have values within a similar range for each variable of interest, though this does not need to be exact. See Supplement for full description.
We chose the first comparator cohort because the presence of severe chronic disease may influence clinician decision-making in relation to ICU admission and ongoing treatment decisions in a similar way that the presence of ALD may influence clinical management.(20) We chose the second comparator cohort to benchmark outcomes against a general ICU population.
Variables
Variables included demographics, pre-admission factors and factors relating to the ICU/hospital stay. SES was measured by the Social Index of Multiple Deprivation (SIMD)(21). Comorbidity was measured using a combination of Charlson and SICSAG-defined comorbidity (see reference (22) for details of comorbidity derivation). ICU admission diagnosis was grouped by organ system for ALD vs comorbid cohort comparisons. Highest bilirubin in the first 24 hours of ICU admission was categorised using Sequential Organ Failure Assessment (SOFA) score thresholds to produce a liver SOFA score.(23) See Supplement for full list of variables and confounders used in multivariable models to adjust outcomes for ALD vs comparator cohorts.
Outcomes and follow-up period
The primary outcomes were 5-year mortality, emergency hospital resource use and cumulative risk of first hospital readmission obtained from linkage to Scottish death records and SMR01 registries. For further information relating to outcomes and cause-specific readmission analyses, see Supplement. Follow-up was censored on 01 January 2011 or after 5-year follow-up and commenced on ICU admission for mortality analyses and index hospital discharge for readmission/resource use analyses.
Statistical analysis
Analyses were undertaken using Stata IC version 13 (StataCorpLP,Texas,USA) and SAS (SAS Institute Inc.,NC,USA). Baseline characteristics were compared between ALD and comorbid comparator cohorts using T-test, Mann-Whitney-U or χ2-tests. A complete case analysis was undertaken. Missing liver SOFA score was included as an independent category. We used Kaplan-Meier estimates to report mortality to 5-years. Cox regression was used to identify independent predictors of mortality for early (0 -30d) and late (31d-5year) periods in the ALD cohort. Z-score and likelihood ratio tests were used to assess significance of individual and all categories of predictors respectively. Cumulative risk of first emergency hospital readmission was estimated using cumulative incidence function (Stata commands:‘stcompet’;‘stccreg’). Fine-Gray regression was used to identify independent predictors of first emergency readmission risk in the ALD cohort allowing for the competing risk of death.(24) Comparisons between ALD and comparator cohorts were undertaken with adjustment for confounders using the multivariable regression models (details in Supplement).
Subgroup and additional analyses
We stratified results within the ALD cohort for patients with and without cirrhosis as outcomes were likely to differ between groups. We presented subgroup analyses for ALD patients and comparator populations for mortality outcomes for the four commonest ICU admission diagnoses: pneumonia, septic shock, acute abdominal pathology and post-cardiac arrest. The rationale for subgroup analyses was to reduce the heterogeneity present in comparisons undertaken at a whole population level in order to better investigate the effect of ALD on outcomes. See Appendix for full definitions of cirrhosis and admission diagnoses. As the primary analysis with the severe comparator comorbid cohort was unmatched, we undertook a matched analysis to identify the presence of residual confounding in the unmatched analysis.
Results
ALD vs severe comorbid cohort
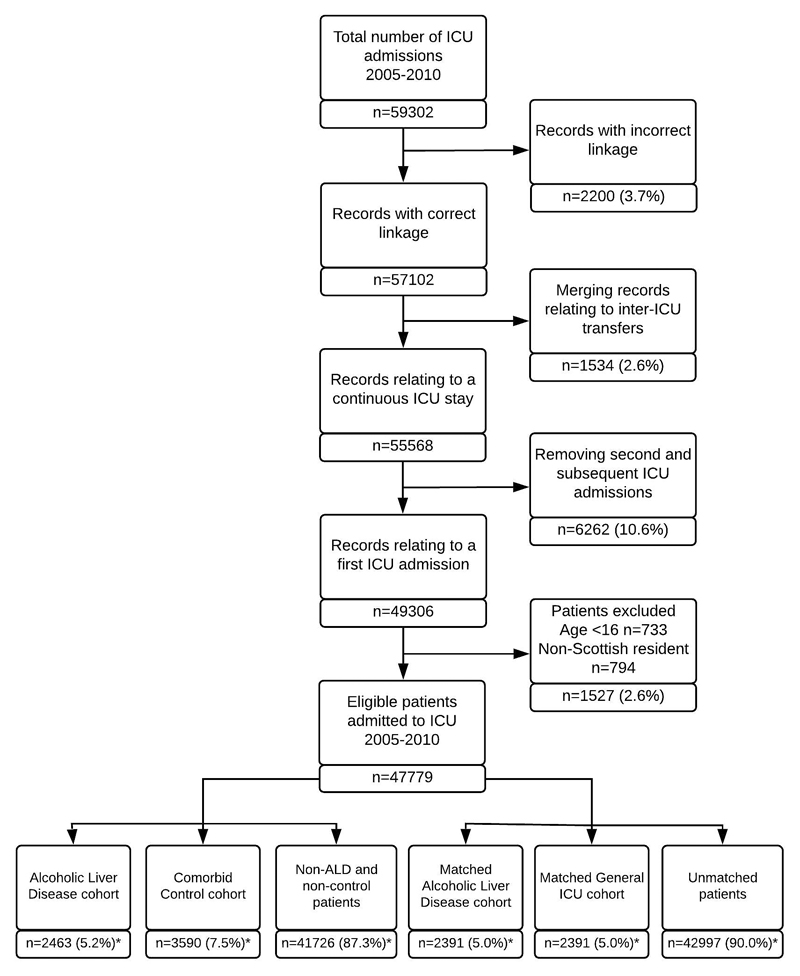
Of 47,779 first ICU admissions, the ALD cohort comprised 2463 patients and comparator cohort with severe comorbidities 3590 patients (cardiovascular 1111,30.9%; respiratory 1981,48.3%; renal 455,12.7%; ≥2 comorbidities 291,8.1%) (Figure 1). Those with ALD were younger at the time of ICU admission (53 vs 68,p<0.001), were more socioeconomically disadvantaged (p<0.001), had fewer comorbidities (p<0.001) and were more severely ill on admission to ICU (mean Acute Physiology Score (APS) 18.3 vs 16.3,p<0.001) (Table 1).
Figure 1. Flow diagram of study population derivation.
Figures marked * are reported as a proportion of the number of eligible patients (n=47,779).
Table 1. Baseline characteristics of ALD cohort and comparator cohort with other severe comorbidities.
| Characteristic | Characteristic | ALD cohort n=2463 |
Comorbid cohort n=3590 |
p-value |
|---|---|---|---|---|
| Age (years) | Median (IQR) | 53 (45, 61) | 68 (59, 76) | <0.001 |
| Sex | Female | 877 (35.6%) | 1681 (46.8%) | <0.001 |
| Socioeconomic status quintile | 1 Least deprived | 225 (9.1%) | 395 (11.0%) | <0.001 |
| Quintiles 2 to 4 | 1197 (48.6%) | 2256 (62.8%) | ||
| 5 Most deprived | 1041 (42.3%) | 939 (26.2%) | ||
| Comorbidity count | 0 | 1958 (79.5%) | 2323 (64.7%) | <0.001 |
| 1 | 385 (15.6%) | 852 (23.7%) | ||
| 2+ | 120 (4.9%) | 415 (11.6%) | ||
| Previous year inpatient hospital admissions | Mean (SD) | 1.6 (2.5) | 1.4 (2.1) | 0.002 |
| Previous year inpatient days in hospital | Mean (SD) | 11.0 (21.8) | 10.2 (23.2) | 0.19 |
| Admission source | Theatre after emergency surgery | 385 (15.7%) | 866 (24.1%) | <0.001 |
| Emergency department | 524 (21.3%) | 736 (20.5%) | ||
| Hospital ward | 771 (31.4%) | 1117 (31.1%) | ||
| Other | 779 (31.7%) | 869 (24.2%) | ||
| ICU admission diagnosis | Cardiovascular | 446 (18.1%) | 1144 (31.9%) | <0.001 |
| Respiratory | 548 (22.2%) | 1268 (35.3%) | ||
| Gastrointestinal | 1028 (41.7%) | 700 (19.5%) | ||
| Renal | 31 (1.3%) | 133 (3.7%) | ||
| Neurological | 281 (11.4%) | 128 (3.6%) | ||
| Trauma | 55 (2.2%) | 91 (2.5%) | ||
| Other | 74 (3.0%) | 126 (3.5%) | ||
| Acute Physiology Score | Median (IQR) | 18.3 (7.9) | 16.3 (6.8) | <0.001 |
| APACHE II score | Median (IQR) | 23.3 (8.1) | 25.5 (7.1) | <0.001 |
| Number of organ systems supported on ICU admission | 0 | 377 (15.3%) | 804 (22.4%) | <0.001 |
| 1 | 1050 (42.6%) | 1383 (38.5%) | ||
| 2 | 860 (34.9%) | 1217 (33.9%) | ||
| 3 | 176 (7.1%) | 186 (5.2%) | ||
| Mechanical ventilation | n (%) | 1908 (77.6%) | 2397 (67.1%) | <0.001 |
| Circulatory support | n (%) | 1145 (46.5%) | 1596 (44.7%) | 0.16 |
| Renal replacement therapy | n (%) | 245 (10.0%) | 382 (10.7%) | 0.35 |
| Liver SOFA score | 0 | 441 (20.8%) | 2294 (77.3%) | <0.001 |
| 1 | 295 (13.9%) | 405 (13.6%) | ||
| 2 | 793 (37.4%) | 229 (7.7%) | ||
| 3 | 354 (16.7%) | 27 (0.9%) | ||
| 4 | 237 (11.2%) | 14 (0.5%) | ||
| Missing | 343 (13.9%) | 621 (17.3%) | ||
| ICU length of stay (days) | Median (IQR) | 2.7 (1.0, 7.4) | 2.2 (1.0, 5.4) | <0.001 |
| Index hospital length of stay (days) | Median (IQR) | 12 (5, 29) | 14 (6, 31) | <0.001 |
| ICU mortality | n (%) | 1087 (44.1%) | 1308 (36.4%) | <0.001 |
| Hospital mortality | n (%) | 1448 (58.8%) | 1791 (49.9%) | <0.001 |
Missing data: admission source n=6, organ support variables n=26, APS n=398, APACHE II score n=38. Abbreviations: IQR interquartile range; APACHE Acute Physiology and Chronic Health Evaluation; SOFA: Sequential Organ Failure Assessment. For comparisons between ALD and comorbid comparator cohort, a count of comorbidities excluded the index comorbidity (e.g. ALD cohort members could not have ‘liver disease’ as a comorbidity).
The most common ICU admission diagnoses in the ALD cohort were variceal hemorrhage (18%), pneumonia (17%) and septic shock (12%). More ALD patients than those with other severe comorbidities required mechanical ventilation on the day of admission (77.6% vs 67.1%,p<0.001). The median ICU length of stay was longer in ALD patients (2.7 vs 2.2 days,p<0.001) but median index hospital length of stay was shorter (12 vs 14 days,p<0.001).
ALD vs matched general ICU cohort
For the matched general ICU population, 2391(97.1%) of ALD patients could be matched to 2391 general ICU patients without ALD (Figure 1). Sex, SES, APS/APACHE score were similar between groups with minor imbalances in age, and admission source (eTable 1). ALD patients required more organ support (p<0.001), had a similar ICU length of stay but shorter hospital stay. Unmatched ALD cohort members were more severely ill, requiring more organ support and with higher ICU mortality (eTable 2).
Early and late mortality
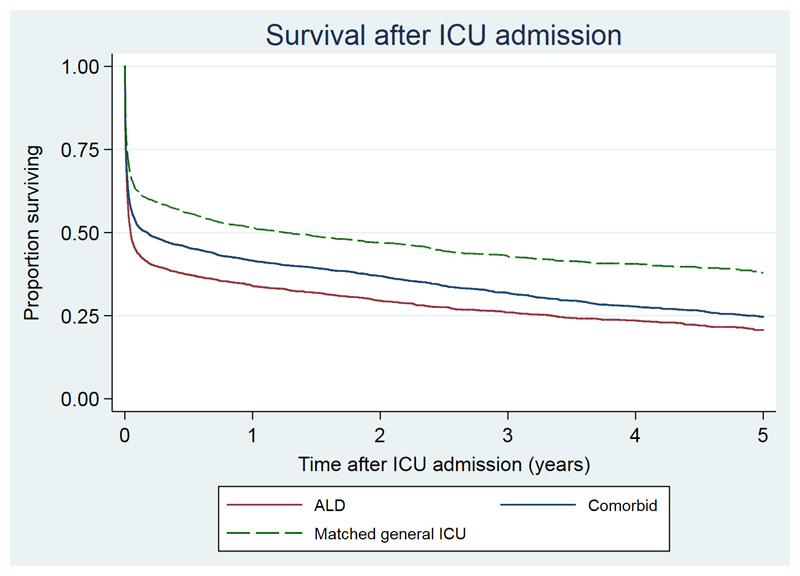
In the ALD cohort, ICU mortality was 44.1% (95%CI 42.2%,46.1%), and a further 361 died in hospital after ICU discharge (hospital mortality 58.8%; 95%CI 56.8%,60.7%). Using fixed time-point outcomes, 30-day mortality was 54.9% (95%CI 53.0%,56.9%) increasing to 66.1% and 79.2% at one and five-years (Figure 2). Median survival was 17 days (IQR 2, 563).
Figure 2. Kaplan-Meier plot of survival in ALD cohort compared with comparator cohorts with other severe comorbidities and matched general ICU patients from time of ICU admission.
| Years | 0 | 1 | 2 | 3 | 4 |
| ALD | 2463 | 730 | 522 | 361 | 231 |
| Comorbid | 3590 | 1337 | 996 | 649 | 410 |
| General ICU | 2391 | 1043 | 766 | 479 | 304 |
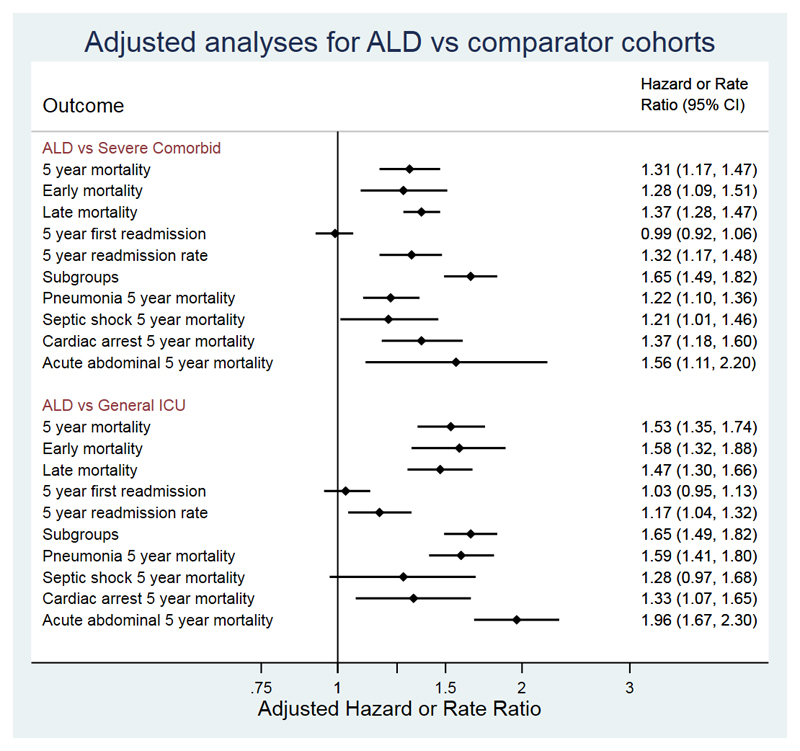
Five-year mortality was greater in the ALD cohort than the comparator cohort with severe comorbidities (79.2% vs 75.3%) and the matched general ICU cohort (79.1% vs 62.1%; Figure 2). After adjustment for confounders, there was a 31% relative excess in 5-year hazard of death in the ALD cohort compared with the severe comorbid cohort (adjHR 1.31,95%CI 1.16,1.47,p<0.001) and 53% excess in comparison with the general ICU cohort (adjHR 1.53,95%CI 1.35,1.74,p<0.001) (Figure 3). Point estimates of excess hazard of death were similar in both early and late periods in both comparator cohorts (Figure 3).
Figure 3. Adjusted analyses for mortality, cumulative incidence of first emergency readmission and emergency readmission rate in ALD cohort compared with comparator cohorts with other severe comorbidities and matched general ICU patients.
Point estimates are hazard ratios for all outcomes other than 5-year readmission rate which is the admission rate ratio.
Predictors of early and late mortality
Patients requiring 3 organ-support (79.0%), admitted after a cardiac arrest (80.9%, eFigure 1) and with a liver SOFA score of 4 (77.6%) had the highest 30-day mortality rates. Age, admission source, diagnosis on ICU admission, number of organs supported and admission liver SOFA score were independent predictors of early (0-30days) and late (31days-5year) mortality (Table 2;eTable 3). Sex, SES and previous number of admissions were predictors of late but not early mortality.
Table 2. Independent factors associated with early and late mortality in the ALD cohort (abbreviated).
See eTable 1 for full table.
| Characteristic | Characteristic | 5-year death (%) | Early 0-30d death (%) | Late 31d-5y death (%) | Early Multivariable regression HR (95%CI) p-value |
Late Multivariable regression HR (95%CI) p-value |
|---|---|---|---|---|---|---|
| n | 2463 | 2463 | 1106 | |||
| Age (years) | per 10 year increase | - | - | - | 1.24 (1.19, 1.29) <0.001 | 1.33 (1.19, 1.50) <0.001 |
| Sex | Male | 83.3% | 54.0% | 57.5% | 1 (Reference) | 1 (Reference) |
| Female | 81.9% | 56.7% | 49.2% | 1.02 (0.92, 1.13) 0.652 | 0.73 (0.58, 0.93) 0.010 | |
| Socioeconomic status quintile | 1 Least deprived | 85.0% | 56.0% | 59.5% | 1 (Reference) | 1 (Reference) |
| 2 | 80.2% | 52.5% | 52.0% | 1.01 (0.64, 1.57) 0.978 | 0.75 (0.61, 0.91) 0.004 | |
| 3 | 84.1% | 49.5% | 63.8% | 1.03 (0.67, 1.58) 0.885 | 1.08 (0.80, 1.46) 0.622 | |
| 4 | 84.5% | 58.2% | 55.3% | 1.19 (0.77, 1.85) 0.438 | 0.89 (0.55, 1.45) 0.642 | |
| 5 Most deprived | 81.7% | 55.7% | 50.2% | 1.11 (0.75, 1.63) 0.601 | 0.78 (0.55, 1.09) 0.140 | |
| Comorbidity count | 0 | 82.4% | 55.0% | 53.9% | 1 (Reference) | 1 (Reference) |
| 1 | 83.6% | 55.3% | 55.3% | 1.13 (0.99, 1.30) 0.063 | 0.78 (0.61, 0.99) 0.043 | |
| 2 | 86.1% | 55.1% | 63.3% | 1.18 (0.78, 1.79) 0.432 | 1.07 (0.69, 1.68) 0.758 | |
| 3+ | 87.3% | 48.4% | 72.0% | 1.12 (0.72, 1.75) 0.621 | 1.04 (0.63, 1.73) 0.871 | |
| Previous year hospital admissions | 0 | 82.4% | 58.9% | 47.7% | 1 (Reference) | 1 (Reference) |
| 1 | 81.5% | 54.4% | 52.0% | 0.96 (0.86, 1.09) 0.550 | 1.25 (0.91, 1.71) 0.172 | |
| 2 | 84.7% | 54.6% | 61.1% | 0.97 (0.80, 1.18) 0.775 | 1.69 (1.03, 2.79) 0.039 | |
| 3 | 84.5% | 48.9% | 65.3% | 0.93 (0.77, 1.12) 0.448 | 2.19 (1.34, 3.55) 0.002 | |
| 4+ | 83.8% | 47.2% | 64.5% | 0.89 (0.72, 1.10) 0.283 | 1.76 (1.12, 2.77) 0.015 | |
| Admission source | See eTable 2 | |||||
| ICU admission diagnosis | Variceal bleed | 78.7% | 42.4% | 57.8% | 1.89 (1.14, 3.15) 0.014 | 1 (Reference) |
| Pneumonia | 83.0% | 54.1% | 56.5% | 1.67 (1.05, 2.66) 0.031 | 1.08 (0.64, 1.84) 0.767 | |
| Septic shock | 88.0% | 67.6% | 54.1% | 1.90 (1.04, 3.49) 0.037 | 1.05 (0.50, 2.17) 0.905 | |
| Liver failure | 89.7% | 73.0% | 53.5% | 2.16 (1.17, 3.99) 0.013 | 1.01 (0.72, 1.41) 0.960 | |
| Post-cardiac arrest | 93.4% | 80.9% | 57.6% | 3.23 (1.93, 5.40) <0.001 | 1.04 (0.65, 1.67) 0.877 | |
| Seizures | 70.9% | 26.0% | 57.4% | 1 (Reference) | 1.80 (1.05, 3.10) 0.034 | |
| Other | 71.4% | 49.2% | 51.8% | 1.91 (1.32, 2.77) 0.001 | 1.04 (0.72, 1.51) 0.819 | |
| Acute Physiology Score | per 10 point increase | - | - | - | 1.83 (1.66, 2.02) <0.001 | 0.89 (0.74, 1.07) 0.205 |
| Number of organs supported at admission | 0 | 71.4% | 33.2% | 52.5% | 1 (Reference) | 1 (Reference) |
| 1 | 81.1% | 48.0% | 58.0% | 1.39 (1.18, 1.64) <0.001 | 1.36 (1.06, 1.74) 0.016 | |
| 2 | 87.9% | 68.0% | 52.6% | 1.94 (1.69, 2.23) <0.001 | 1.23 (1.06, 1.42) 0.007 | |
| 3 | 89.8% | 79.0% | 37.0% | 2.12 (1.50, 3.01) <0.001 | 0.80 (0.55, 1.16) 0.237 | |
| Liver SOFA score | 0 | 70.4% | 36.7% | 46.1% | 1 (Reference) | 1 (Reference) |
| 1 | 78.2% | 46.1% | 53.2% | 1.20 (0.98, 1.46) 0.070 | 1.20 (0.82, 1.75) 0.355 | |
| 2 | 83.4% | 55.6% | 55.6% | 1.79 (1.40, 2.30) <0.001 | 1.42 (1.11, 1.81) 0.006 | |
| 3 | 88.7% | 65.5% | 61.5% | 2.19 (1.76, 2.72) <0.001 | 1.98 (1.53, 2.57) <0.001 | |
| 4 | 93.4% | 77.6% | 63.4% | 2.92 (2.29, 3.73) <0.001 | 2.02 (1.36, 2.99) <0.001 | |
| Missing | 85.9% | 57.7% | 60.7% | 2.09 (1.54, 2.84) <0.001 | 1.66 (1.32, 2.10) <0.001 |
Kaplan-Meier estimator presented for % death. Multivariable Cox models are stratified by health board of residence. HR, hazard ratio; ICU, intensive care unit; SOFA, Sequential Organ Failure Assessment.
Emergency hospital resource use and readmission risk
Hospital resource use
Analyses of resource use measured by multiple emergency readmissions revealed that the ALD cohort had a higher readmission rate compared with both comparator cohorts during 5-year follow-up (ALD vs comorbid vs general ICU:1.64vs 1.28vs 1.43 emergency readmissions/person-year; eFigure 2), a greater number of days in hospital (14.4vs 13.1vs 11.8 days/person-year), and higher costs (£9725($13834)vs £8834($12566)vs £7986($11360)/person-year). After adjustment for potential confounders, 5-year emergency readmission rate was 32% higher in ALD vs severe comorbid cohort and 17% higher compared with a general ICU cohort (p<0.001,Figure 3).
Relative to both comparator cohorts, the ALD cohort had higher emergency readmission rates during the first three years after discharge, but admission rates were comparable in years four/five (eFigure 2). Around half of costs associated with emergency readmissions in the first 4-years after discharge were due to liver disease or alcohol-related diagnoses (eFigure 3).
Cumulative incidence of first emergency readmission
In the ALD cohort, the cumulative risk of first emergency hospital readmission was 21.7% (95%CI 19.2%,24.3%) at 30-days post-index hospital discharsge increasing to 62.7% and 83.4% at one and five-years. This was similar in both comparator cohorts (cumulative risk in severe comorbid: 30d 22.1%; 5y 84.6%; general ICU: 30d 21.8%; 5y 83.9%)) even after adjustment for confounders (Figure 3).
The strongest predictor of first emergency readmission in the ALD cohort was the number of inpatient admissions in the year before ICU admission (χ2=121,4df,p<0.001) (eTable 4). Other independent predictors were number of organs supported, age, SES, diagnosis on admission, APS and liver SOFA score.
Subgroup analyses and additional analyses
Within the ALD cohort, two-thirds of patients had a diagnosis of cirrhosis coded. These patients were younger, were less severely ill (measured by APS) and required less organ support on admission compared with ALD patients without cirrhosis (eTable 5). However, cirrhosis patients experienced higher 5-year mortality, resource use and costs (eTable 6).
For each of the four commonest ICU admission diagnoses (pneumonia, septic shock, post-cardiac arrest and acute abdominal pathology), the ALD cohort had higher adjusted 5-year mortality compared with both severe comorbid and general ICU cohorts (Figure 3). The matched analysis with the severe comorbid cohort confirmed the findings of the primary unmatched analysis (eTable 7).
Discussion
In a complete, national database of ICU admissions, patients with a diagnosis of ALD experienced higher long-term mortality and emergency readmission rates compared with ICU patients with other severe comorbidities and a matched general ICU cohort. For every three patients admitted to ICU with ALD, two had died within one year of ICU admission. In those surviving their index hospitalisation, acute emergency readmission rates were highest in the first year of follow-up with almost half of readmissions related to liver disease or alcohol-related conditions. Morbidity and mortality were higher in those with cirrhosis. These findings highlight the substantial longer term burden experienced by patients with ALD after ICU admission.
The presence of ALD is often viewed as a poor prognostic marker in critically ill patients considered for admission to ICU.(5, 20, 25) Our results, reporting the largest cohort to date, confirm this to be the case, even when compared to a cohort with other severe comorbidities. No previous research has reported hospital readmission rates or healthcare resource use for ICU survivors with ALD. Elevated readmission rates in general ICU survivor cohorts may in part be attributable to morbidity associated with surviving ICU (‘post-intensive care syndrome’ (PICS)).(14, 22, 26) Superimposing a PICS on ALD patients already on a declining chronic disease trajectory may result in a particularly vulnerable patient group. However, in this study almost half of early emergency readmissions in the ALD survivor cohort were caused by liver/alcohol-related conditions suggesting that the underlying disease, rather than other consequences of critical illness, were more important drivers of readmission.
Comparison of our data with studies of non-ICU hospital inpatients with liver disease reveals conflicting results. Hospital 30-day readmission was higher than inpatients with advanced liver disease of any etiology(27) (26.6%vs20%) but lower than inpatients with cirrhosis-related complications (26.6%vs37%).(8) The reasons for this may relate to differences in case-mix and/or health care organisation. In the first cohort, only readmissions to the same institution were included which may have underestimated readmission rates.(27) Furthermore, members of our cohort had been selected by clinicians at the time of ICU admission resulting in likely lower prevalence of frailty, which can influence ICU admission decision-making.(28)
Strengths and limitations
Our study has a number of strengths. We used linked databases with complete coverage in Scotland for ICU admissions ensuring robust, long-term follow-up for mortality and readmission rates. Furthermore, we captured readmissions to any Scottish hospital unlike other studies that were limited to single centres. Our findings were robust when benchmarked against two comparator cohorts, general ICU patients and those with severe comorbidities, as well as in more homogeneous subgroups defined by ICU admission diagnosis. However, the study was conducted in a single country and the results may reflect region-specific societal, cultural and clinical practices. Readers should, therefore, exercise caution when generalising the findings to other countries.
A limitation of our work was that we could only grade severity of ALD using the SOFA liver score. Liver-specific disease scores such as Child-Pugh score were not available. However, in a recent study of critically ill patients with cirrhotic liver disease, ICU-specific scores demonstrated similar ability to predict mortality compared with liver-specific scores.(29) Furthermore, a systematic review of scores showed Child-Pugh score is likely the weakest discriminator of mortality.(4, 30)
We used retrospectively collected registry data to define the study cohort by combining diagnostic coding in ICU and hospital datasets, which could have misclassified some patients. However, in Scottish datasets case-note validation of ICU diagnosis coding has been shown to be of ‘very high quality’(17) and coding accuracy in hospital datasets approaches 90% for the primary diagnosis code.(16)
Unlike severe cardiovascular and respiratory comorbid illnesses, patients with ALD who remain abstinent through treatment for alcohol dependency have reduced long-term mortality.(31) We were unable to identify such patients in our cohort.
Implications for clinical practice and future work
Our findings have implications for clinical practice and health policy. Our data can inform discussions between clinicians, patients and families relating to the consequences of admission to critical care in the context of ALD. There is a risk that population-level data exacerbate the stigma which may be associated with ALD or may promote a self-fulfilling prophecy of high mortality if used to apply a blanket denial of critical care admission. Nuanced decision-making is needed, in conjunction with hepatologists, patients and families to fully consider an individual patient’s circumstances and their perspective on balancing burdens of treatment with benefits. Our data provide a valuable resource in such discussions. High post-discharge healthcare resource use for ICU survivors with ALD may highlight improvements that are needed in community strategies to reduce alcohol-dependence and optimising chronic disease management for the subgroup with alcohol-related cirrhosis.
There have been recent calls for targeted discussions with patients with end-stage chronic liver disease to ensure their treatment wishes are fully understood after an admission to hospital.(32) These discussions may involve shared decision-making relating to treatment escalation, anticipatory care planning and referral to palliative care services. National guidelines recommend this process for patients with severe respiratory and heart disease, who comprised a high proportion of our comparator cohort.(33, 34) Given outcomes were significantly worse among the ALD cohort than those with severe chronic comorbidities, our study highlights the need for clinicians to better recognise patients with end-stage liver disease, and consider similar anticipatory planning where appropriate.
Conclusion
In summary, patients admitted to ICU with ALD experience higher mortality and hospital readmission rates than patients with other severe chronic diseases and similar general ICU patients. Our data provide important information that can be used to inform shared decision-making for clinicians and patients with ALD.
Supplementary Material
Online supplement
This article has an online data supplement.
Acknowledgements
We are grateful to clinicians in ICUs in Scotland who contribute data to the Scottish Intensive Care Society Audit Group registry and Information Services Division (ISD Scotland) for providing data and undertaking linkage.
Role of the funding source
NL was supported by a fellowship from the Chief Scientist Office (CAF/08/12). The funder had no role in the study design; the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Copyright form disclosure: Dr. Lone received support for article research from the Research Councils UK. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Declaration of interests
All authors declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval
Approvals were obtained from the relevant data-governing body (Privacy Advisory Committee, Information Services Division: ref 55/09). All data were anonymised prior to release to the researchers. The South East Scotland Research Ethics Committee granted a waiver (ref NR/1001AB14).
References
- 1.NHS Digital. Statistics on Alcohol England,2017. [cited 16/08/2017];2017 Available from: https://www.gov.uk/government/statistics/statistics-on-alcohol-england-2017.
- 2.Information Services Division. Alcohol-Related Hospital Statistics Scotland 2015/16. [cited 08/08/2017];2016 Available from: https://www.isdscotland.org/Health-Topics/Drugs-and-Alcohol-Misuse/Publications/2016-10-25/2016-10-25-ARHS-Report.pdf?12266176940.
- 3.Welch C, Harrison D, Short A, et al. The increasing burden of alcoholic liver disease on United Kingdom critical care units: secondary analysis of a high quality clinical database. Journal of health services research & policy. 2008;13(Suppl 2):40–44. doi: 10.1258/jhsrp.2007.007101. [DOI] [PubMed] [Google Scholar]
- 4.Flood S, Bodenham A, Jackson P. Mortality of patients with alcoholic liver disease admitted to critical care: A systematic review. Journal of the Intensive Care Society. 2012;13(2):130–135. [Google Scholar]
- 5.Weil D, Levesque E, McPhail M, et al. Prognosis of cirrhotic patients admitted to intensive care unit: a meta-analysis. Annals of intensive care. 2017;7(1):33. doi: 10.1186/s13613-017-0249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ratib S, Fleming KM, Crooks CJ, et al. 1 and 5 year survival estimates for people with cirrhosis of the liver in England, 1998-2009: a large population study. Journal of hepatology. 2014;60(2):282–289. doi: 10.1016/j.jhep.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 7.Kanwal F, Tansel A, Kramer JR, et al. Trends in 30-Day and 1-Year Mortality Among Patients Hospitalized With Cirrhosis From 2004 to 2013. The American journal of gastroenterology. 2017;112(8):1287–1297. doi: 10.1038/ajg.2017.175. [DOI] [PubMed] [Google Scholar]
- 8.Volk ML, Tocco RS, Bazick J, et al. Hospital readmissions among patients with decompensated cirrhosis. American Journal of Gastroenterology. 2012;107(2):247–252. doi: 10.1038/ajg.2011.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganesh S, Rogal SS, Yadav D, et al. Risk factors for frequent readmissions and barriers to transplantation in patients with cirrhosis. PloS one. 2013;8(1):e55140. doi: 10.1371/journal.pone.0055140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bajaj JS, Reddy KR, Tandon P, et al. The 3-month readmission rate remains unacceptably high in a large North American cohort of patients with cirrhosis. Hepatology (Baltimore, Md) 2016;64(1):200–208. doi: 10.1002/hep.28414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scaglione SJ, Metcalfe L, Kliethermes S, et al. Early Hospital Readmissions and Mortality in Patients With Decompensated Cirrhosis Enrolled in a Large National Health Insurance Administrative Database. Journal of clinical gastroenterology. 2017 doi: 10.1097/MCG.0000000000000826. [DOI] [PubMed] [Google Scholar]
- 12.Williams R, Aspinall R, Bellis M, et al. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet (London, England) 2014;384(9958):1953–1997. doi: 10.1016/S0140-6736(14)61838-9. [DOI] [PubMed] [Google Scholar]
- 13.Nates JL, Nunnally M, Kleinpell R, et al. ICU Admission, Discharge, and Triage Guidelines: A Framework to Enhance Clinical Operations, Development of Institutional Policies, and Further Research. Critical care medicine. 2016;44(8):1553–1602. doi: 10.1097/CCM.0000000000001856. [DOI] [PubMed] [Google Scholar]
- 14.Desai SV, Law TJ, Needham DM. Long-term complications of critical care. Critical care medicine. 2011;39(2):371–379. doi: 10.1097/CCM.0b013e3181fd66e5. [DOI] [PubMed] [Google Scholar]
- 15.SICSAG. Scottish Intensive Care Society Audit Group. Annual Report: Audit of Intensive Care Units in Scotland 2017 Reporting on 2016. [cited 08/08/2017];2017 Available from: http://www.sicsag.scot.nhs.uk/docs/2017/2017-08-08-SICSAG-Report.pdf?33.
- 16.NSS. Assessment of SMR01 Data Scotland 2014-2015. [cited 06/04/2017];2015 [Online] Available from: http://www.isdscotland.org/Health-Topics/Hospital-Care/Publications/2012-05-08/Assessment-of-SMR01Data-2010-2011-ScotlandReport.pdf.
- 17.SICSAG. Scottish Intensive Care Society Audit Group. Annual Report: Data Quality. [cited 06/04/2017];2017 Available from: http://www.sicsag.scot.nhs.uk/quality/data.html.
- 18.SICSAG. Scottish Intensive Care Society Audit Group. WardWatcher (2014 version). Help pages: Definitions for all mandatory pages/fields. [cited 08/08/2017];2014 Available from: http://www.sicsag.scot.nhs.uk/data/Help_042014.pdf.
- 19.Blackwell M, Iacus S, King G, et al. Cem: Coarsened exact matching in Stata. Stata J. 2009;9(4):524–546. [Google Scholar]
- 20.McPhail MJW, Parrott F, Wendon JA, et al. Incidence and Outcomes for Patients With Cirrhosis Admitted to the United Kingdom Critical Care Units. Critical care medicine. 2018;46(5):705–712. doi: 10.1097/CCM.0000000000002961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scottish Government. SIMD Scottish Index of Multiple Deprivation 2009 General Report. [cited 04/05/2017];2009 Available from: http://www.gov.scot/resource/doc/289599/0088642.pdf.
- 22.Lone NI, Gillies MA, Haddow C, et al. Five-Year Mortality and Hospital Costs Associated with Surviving Intensive Care. Am J Respir Crit Care Med. 2016;194(2):198–208. doi: 10.1164/rccm.201511-2234OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vincent JL, de Mendonca A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on "sepsis-related problems" of the European Society of Intensive Care Medicine. Critical care medicine. 1998;26(11):1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation. 2016;133(6):601–609. doi: 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackle IJ, Swann DG, Cook B. One year outcome of intensive care patients with decompensated alcoholic liver disease. British Journal of Anaesthesia. 2006;97(4):496–498. doi: 10.1093/bja/ael177. [DOI] [PubMed] [Google Scholar]
- 26.Elliott D, Davidson JE, Harvey MA, et al. Exploring the scope of post-intensive care syndrome therapy and care: engagement of non-critical care providers and survivors in a second stakeholders meeting. Critical care medicine. 2014;42(12):2518–2526. doi: 10.1097/CCM.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 27.Berman K, Tandra S, Forssell K, et al. Incidence and predictors of 30-day readmission among patients hospitalized for advanced liver disease. Clinical Gastroenterology & Hepatology. 2011;9(3):254–259. doi: 10.1016/j.cgh.2010.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sprung CL, Baras M, Iapichino G, et al. The Eldicus prospective, observational study of triage decision making in European intensive care units: part I--European Intensive Care Admission Triage Scores. Critical care medicine. 2012;40(1):125–131. doi: 10.1097/CCM.0b013e31822e5692. [DOI] [PubMed] [Google Scholar]
- 29.Theocharidou E, Pieri G, Mohammad AO, et al. The Royal Free Hospital score: a calibrated prognostic model for patients with cirrhosis admitted to intensive care unit. Comparison with current models and CLIF-SOFA score. The American journal of gastroenterology. 2014;109(4):554–562. doi: 10.1038/ajg.2013.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karvellas CJ, Bagshaw SM. Advances in management and prognostication in critically ill cirrhotic patients. Current opinion in critical care. 2014;20(2):210–217. doi: 10.1097/MCC.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 31.Pessione F, Ramond MJ, Peters L, et al. Five-year survival predictive factors in patients with excessive alcohol intake and cirrhosis. Effect of alcoholic hepatitis, smoking and abstinence. Liver international : official journal of the International Association for the Study of the Liver. 2003;23(1):45–53. doi: 10.1034/j.1600-0676.2003.01804.x. [DOI] [PubMed] [Google Scholar]
- 32.Kimbell B, Boyd K, MacGilchrist A, et al. Liver disease in the UK. Lancet (London, England) 2015;385(9967):503. doi: 10.1016/S0140-6736(15)60191-X. [DOI] [PubMed] [Google Scholar]
- 33.National Institute for Health and Clinical Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management (CG101) [cited 08/08/2017];2010 Available from: http://www.nice.org.uk/CG101. [PubMed]
- 34.National Institute for Health and Clinical Excellence. Chronic heart failure in adults: management (CG108) [cited 08/08/2017];2010 Available from: https://www.nice.org.uk/guidance/CG108.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.