Abstract
Centrally administered glucagon-like peptide 1 (GLP-1) supresses food intake. Here we demonstrate that GLP-1–producing (PPG) neurons in the nucleus tractus solitarii (NTS) are the predominant source of endogenous GLP-1 within the brain. Selective ablation of NTS PPG neurons by viral expression of diphtheria toxin subunit A substantially reduced active GLP-1 concentrations in brain and spinal cord. Contrary to expectations, this loss of central GLP-1 had no significant effect on the ad libitum feeding of mice, affecting neither daily chow intake nor body weight or glucose tolerance. Only after bigger challenges to homeostasis were PPG neurons necessary for food intake control. PPG-ablated mice increased food intake after a prolonged fast and after a liquid diet preload. Consistent with our ablation data, acute inhibition of hM4Di-expressing PPG neurons did not affect ad libitum feeding; however, it increased refeeding intake after fast and blocked stress-induced hypophagia. Additionally, chemogenetic PPG neuron activation through hM3Dq caused a strong acute anorectic effect. We conclude that PPG neurons are not involved in primary intake regulation but form part of a secondary satiation/satiety circuit, which is activated by both psychogenic stress and large meals. Given their hypophagic capacity, PPG neurons might be an attractive drug target in obesity treatment.
Introduction
Glucagon-like peptide 1 (GLP-1) is found in the central nervous system (CNS), with the highest levels reported in the hypothalamus, a major projection target of preproglucagon (PPG) neurons. PPG neurons are the presumptive main source of endogenous central GLP-1 and are expected to exert effects similar to those of exogenously delivered GLP-1. The most notable of these are reduced food intake and body weight loss. PPG neurons are in a prime position to fulfill this role since they innervate all areas identified as mediating GLP-1 effects in the CNS (1–4) and are sensitive to peripheral satiety signals, including gastric distension, leptin, and cholecystokinin (CCK) (5–8). However, doubts have been raised whether PPG neurons are involved in the homeostatic regulation of food intake or whether they signal interoceptive stress and only regulate food intake under pathophysiological conditions (9–13). Three recent studies (14–16) demonstrated that the activation of PPG neurons in vivo using Gq-coupled DREADD (designer receptors exclusively activated by designer drugs) or optogenetic stimulation reduces food intake and maintains glucose homeostasis. Although these studies confirmed that PPG neurons have the capacity to modulate food intake and glucose tolerance, they did not address the question of whether PPG neurons play a role in the regulation of appetite or blood glucose under physiological conditions.
Here we use a mouse line expressing Cre-recombinase (Cre) under the control of the glucagon promoter (17) to selectively target nucleus tractus solitarii (NTS) PPG neurons. Pharmacogenetic activation, confirmed using in vitro Ca2+ imaging, acutely reduced food intake, replicating previous studies, but failed to have a lasting impact on body weight. Selective ablation of these neurons significantly reduced active GLP-1 concentrations in hypothalamus, brainstem, and spinal cord but did not affect body weight or daily food intake. However, PPG-ablated mice ate more chow postfast than control littermates and were less sensitive to the satiating effects of a liquid diet preload. Similarly, pharmacogenetic inhibition of NTS PPG neurons, confirmed in vitro using patch-clamp electrophysiology, had no effect in ad libitum–fed mice, but significantly increased food intake after a long fast and prevented the hypophagic effect of acute restraint stress.
Research Design and Methods
Animal Models
Adult male and female Glu-Cre/tdRFP (tandem red fluorescent protein) (17), Glu-Cre/GCaMP3 (18,19), and Glu-YFP (yellow fluorescent protein) (20) mice were group housed, whenever possible, on a 12-h light/dark cycle with chow and water available ad libitum unless otherwise stated. All experiments were performed in accordance with the U.K. Animals (Scientific Procedures) Act, 1986, with appropriate ethical approval.
Mice were anesthetized with intramuscular ketamine (50 mg/kg) and medetomidine (1 mg/kg) or 1.5–2.5% isoflurane and were injected with virus (200–250 nL, bilaterally) (Table 1) using a pressurized glass needle at the following coordinates from the calamus scriptorius: 500 μm lateral, 100 μm rostral, and 350 μm ventral to transduce PPG neurons. Mice were left for at least 2 weeks before experiments started.
Table 1.
Sources of virus and antibody preparations used
| Virus/antibody; titer/dilution | Application | Source | References |
|---|---|---|---|
| AAV2-FLEX-hM3Dq:mCherry; 6.1 × 1012 | Activation of Cre-expressing PPG neurons | UNC Vector Core, Chapel Hill, NC | pAAV-hSyn-DIO-hM3D(Gq)-mCherry was a gift from Bryan Roth (49) |
| AAV8-FLEX-hM3Dq:mCherry; 4 × 1012 | Activation of Cre-expressing PPG neurons | VVF, ZNZ, Zurich, Switzerland | pAAV-hSyn-DIO-hM3D(Gq)-mCherry was a gift from Bryan Roth (49) |
| AAV8-mCherry-FLEX-DTA; 3.3 × 1012 | Ablation of Cre-expressing PPG neurons | UNC Vector Core, Chapel Hill, NC | pAAV-mCherry-flex-dtA was a gift from Naoshige Uchida |
| AAV2-FLEX-hM4Di:mCherry; 6.4 × 1012 | Inhibition of Cre-expressing PPG neurons | VVF, ZNZ, Zurich, Switzerland | pAAV-hSyn-DIO-hM4D(Gi)-mCherry was a gift from Bryan Roth (49) |
| AAV1/2-FLEX-Perceval; titer not determined | Control for viral transduction | Made in-house | pAAV-FLEX-empty was a gift from Bill Wisden (50) pShuttleCMV-Perceval was a gift from Guy Rutter |
| AAV8-FLEX-EGFP; 8 × 1012 | Control for viral transduction | VVF, ZNZ, Zurich, Switzerland | pAAV-hSyn-DIO-EGFP was a gift from Bryan Roth |
| PRSx8-AlstR-EGFP-LV; 1 × 1010 | Identification of Phox2b neurons | Sergei Kasparov | (26) |
| Chicken anti-GFP, Alexa Fluor 488 goat anti-chicken; 1:1,000 | GCaMP3, EGFP, YFP | AB13970; Abcam/#A-11039; Invitrogen | (18) |
| Rabbit anti-dsRed, Cy3 sheep anti-rabbit; 1:1,000 | mCherry, tdRFP | #632496; TaKaRa Bio Inc./#C2306; Sigma | |
| Rabbit anti-cFOS; 1:500 Alexa Fluor 488 goat anti-rabbit; 1:1,000 | cFOS | #ABE457; Merck/#A-11008; Invitrogen |
UNC, University of North Carolina; VVF, Viral Vector Facility; ZNZ, Neuroscience Center Zurich.
Ca2+ Imaging and Electrophysiology
Coronal brainstem sections (200 μm) were prepared as previously described (18).
Ca2+ imaging was performed in wide-field configuration using a 40× water immersion lens (18). Excitation and emission light were filtered at 470 ± 20 and 515 ± 17 nm, respectively (Chroma 59004), and images captured on a charge-coupled device camera (Q-click; QImaging). Camera and light-emitting diode light source were controlled using Micro-Manager (21). Electrical activity of PPG neurons was recorded in cell-attached configuration, as described previously (22). Currents were filtered at 1.0 kHz and digitized at 3 kHz. Recordings were analyzed with WinEDR Software (University of Strathclyde, Glasgow, U.K.).
Active GLP-1 Assay
Brains were rapidly extracted from the skull, and lower brainstem, hypothalamus, cerebellum, olfactory bulbs, and spinal cord were isolated, snap frozen, and homogenized by pestle and mortar then trituration in 500 μL of artificial cerebrospinal fluid supplemented with dipeptidyl peptidase 4 inhibitor (Millipore) through a 29-gauge insulin syringe, before storage for 24 h at −80°C. To generate a crude protein lysate, samples were thawed on ice and clarified twice by centrifugation at 500g for 10 min and the supernatant collected. The active GLP-1 concentration was determined using an MSD Kit (K150JWC-1; Meso Scale Diagnostics, Rockville, MD). A Bradford protein assay was performed (Bio-Rad). Absorbance was determined using a LabSystems Multiskan MS Microplate Reader.
Immunohistochemistry
Mice were transcardially perfused-fixed with 4% paraformaldehyde, and brains were sectioned at 30 μm and immunostained for GCaMP3, EGFP, YFP, tdRFP, mCherry, or cFOS (for details, see Table 1), as previously described (18). Sections were incubated overnight at 4°C with primary antibodies in blocking solution followed by fluorophore-conjugated secondary antibody in blocking solution for 2 h. Immunofluorescence was visualized on an upright microscope (Leica). Images were captured using a Retiga 3000 Camera (QImaging). Brightness and contrast were adjusted using Fiji software (23).
cFOS Expression in PPG Neurons
Mice expressing hM3Dq in NTS PPG neurons were food restricted for 3 h prior to the injection of vehicle or clozapine-N-oxide (CNO), which was administered 2 mg/kg i.p. in 5 mL/kg saline, 30 min before dark onset for all in vivo chemogenetic experiments. Mice were transcardially perfused 90 min after injection and tissue processed and immunostained for cFOS as described above.
Glu-YFP mice were trained to consume Vanilla Ensure. After 3 days of stable Ensure intake, mice were randomly allocated into control (n = 5) and Ensure-fed groups (n = 5) and fasted for 3 h. Control mice had no access to chow or Ensure, whereas Ensure-fed mice had access to Vanilla Ensure for 120 min. At that point, mice were transcardially perfused, and tissues were immunostained for cFOS and YFP.
Glucose Tolerance Test
Mice were placed into new cages, given ad libitum access to water and fasted for 18 h. Animals were injected with glucose (1 g/kg i.p., 5 mL/kg) (Sigma-Aldrich). Blood glucose measurements were repeatedly taken from the tail over 2 h and analyzed using a Roche Accu-Chek Glucose Meter.
Feeding Paradigms
Intake was measured using standard chow or Vanilla Ensure liquid diet. Mice were weighed daily and habituated to 5 mL/kg i.p. saline injection and food intake measurements. On experimental days, food was removed 3 h prior to dark onset (except for the overnight fast/refeed paradigm), thus standardizing the time all mice had their first meal.
Repeated Injections of CNO
Using a between-subjects, repeated-measures design, the body weight and food intake of control and hM3Dq-expressing mice were measured daily for 5 days. On the 6th day, twice-daily injections of CNO were started.
Normal Chow Intake
The PPG ablation study was a between-subjects design, whereas the activation study (hM3Dq) was a counterbalanced, within-subject design and the acute inhibition study (hM4Di) was a mixed-model design. Mice were transferred to individual cages, and food was removed 3 or 18 h prior to dark onset. Chow was returned at dark onset, and intake was measured manually in the following hours.
Ensure Preload
This experiment was a between-subjects design. PPG-ablated and control mice were transferred to individual cages, and food was removed 3 h prior to dark onset. Vanilla Ensure was provided for 15 min at dark onset, and intake was measured manually, with this protocol repeated for several days until intake was stable (Fig. 6C). On test day, chow intake was measured for 1 h immediately after Ensure access.
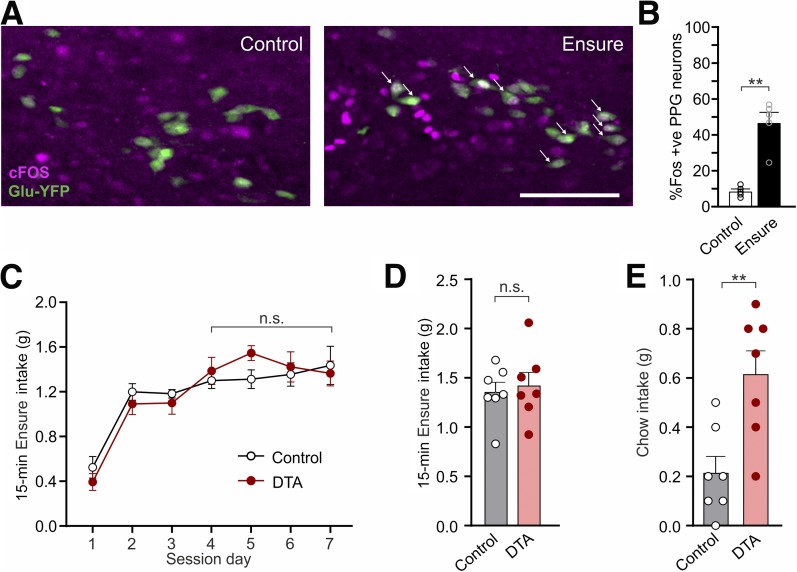
Figure 6.
Intake of large volumes of highly palatable diet activates PPG neurons. A: Expression of the immediate early gene cFOS (green) in PPG neurons after 30 min access to Vanilla Ensure or no access to food (control). White arrows: representative cFOS-positive PPG neurons. Scale bar, 100 μm. B: Percentage of PPG neurons expressing cFOS 90 min after 30-min access to Vanilla Ensure or no access to food. Data are given as the mean ± SEM; n = 3 (control), n = 3 (Ensure). P = 0.0079 (Mann-Whitney U test). C: Ensure intake during 15-min access at dark onset over several days of habituation in control and PPG-ablated (DTA) mice. Data are given as the mean ± SEM; n = 7 (control), n = 7 (DTA). No interaction of virus × time (F(3,36) = 0.592, P = 0.62) and no significant main effect of virus (F(1,12) = 1.135, P = 0.31) or time (F(3,36) = 0.19, P = 0.90).). D: Ensure intake during the 15-min access period on the test day in control and PPG-ablated (DTA) mice. Data are given as the mean ± SEM; n = 7 (control), n = 7 (DTA). P = 0.70 (unpaired t test). E: Chow intake of control and PPG-ablated (DTA) mice for 1 h after 15-min access to Vanilla Ensure. Data given as the mean ± SEM; n = 7 (control), n = 7 (DTA). P = 0.0052 (unpaired t test). **P < 0.01. n.s., not significant.
Stress-Induced Hypophagia
Stress-induced hypophagia was assessed using a mixed-model design. Control (EGFP-expressing) and hM4Di-expressing mice were transferred to individual cages without food 3 h prior to dark onset. All mice received CNO 1 h before dark onset. Thirty minutes before dark onset, mice were restrained in plastic bags with a breathing hole for 30 min. At dark onset, they were returned to their cages, and chow intake was measured in the following hours.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 7.0. Summary data are presented as the mean ± SEM. Statistical significance was tested using nonparametric tests, t tests, three-way mixed-model ANOVAs, and two-way mixed-model or repeated-measures ANOVAs; simple main effects were tested as appropriate and as indicated in figure legends.
Results
Pharmacogenetic Activation of PPG Neurons Robustly Reduces Food Intake
To assess the role of PPG neurons in feeding, we first confirmed and extended previous findings that PPG neurons have the capacity to reduce food intake (14,15). We used transgenic mice expressing Cre under glucagon promotor control (Glu-Cre) and a Cre-dependent reporter (RFP or the Ca2+ indicator GCaMP3) (18,19). GCaMP3-positive cells in this mouse are GLP-1 immunoreactive (19), and, as an additional control, we crossed this mouse with the Glu-YFP mouse that expresses YFP under glucagon promoter control. YFP-expressing neurons in the NTS of Glu-YFP mice have been shown to express PPG mRNA by single-cell RT-PCR (6). The Glu-YFP/Glu-Cre cross revealed an almost complete overlap of these cell populations; >85% of YFP-expressing cells expressed RFP, and <5% of RFP cells did not express YFP (Supplementary Fig. 1A). Previous reports (24,25) used a Phox2b transgenic mouse to target PPG neurons, assuming that Phox2b is expressed and active in PPG neurons. However, targeting the lower brainstem with a lentivirus expressing GFP under the promoter responsive to Phox2b (PRSx8-AlstR-IRES-EGFP-LV) (26) yielded no colocalization between GFP and RFP (Supplementary Fig. 1B).
To selectively activate NTS PPG neurons in vivo, the excitatory DREADD hM3Dq was expressed by stereotaxic injection of AAV2-FLEX-hM3Dq:mcherry into Glu-Cre mice (Fig. 1A). Activation of hM3Dq with CNO substantially increased [Ca2+]i in brainstem slices (Fig. 1B). Similarly, in awake mice, CNO injection elicited cFOS immunoreactivity in PPG neurons (Fig. 1C).
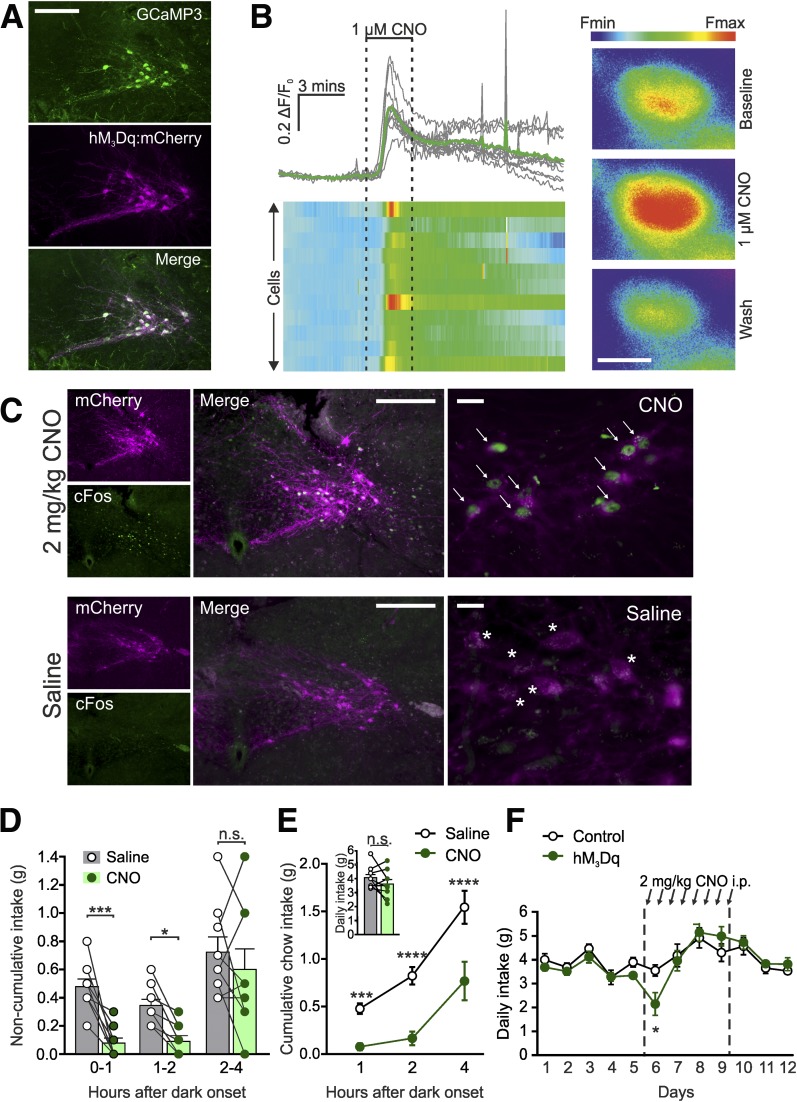
Figure 1.
Pharmacogenetic activation of PPG neurons robustly reduces food intake. A: Expression of hM3Dq:mCherry (magenta) in PPG neurons (detected with an anti-GFP antibody; green) 3 weeks after stereotaxic injection of AAV2-hM3Dq:mCherry into the NTS of Glu-Cre/GCaMP3 mice. Scale bar, 100 µm. B: Increase in [Ca2+]i in GCaMP3-expressing PPG neurons in response to superfusion of ex vivo brainstem slices with 1 μmol/L CNO. Data are displayed as traces (top left panel) and a heat map (bottom left panel) representing the fractional change in fluorescence from baseline. Right panel: Representative pseudocolored cell responding to 1 μmol/L CNO with an increase in [Ca2+]i. Scale bar, 10 μm; n = 11 cells. C: Expression of the immediate early gene cFOS (green) in PPG neurons expressing hM3Dq:mCherry (magenta) after i.p. injection of 2 mg/kg CNO (top panels) or saline (bottom panels). White arrows, representative cFOS-positive hM3Dq-expressing cells; white stars, representative cFOS-negative hM3Dq-positive cells. Scale bars: middle panel, 100 μm; right panel, 20 μm. Noncumulative (D) and cumulative (E) food intake in the first 4 h of dark phase after injection of CNO (2 mg/kg i.p.) or saline. CNO was delivered 30 min prior to dark onset. Data are given as the mean ± SEM; n = 9 mice. D: No significant time × drug interaction (F(2,16) = 2.897, P = 0.0844), but a significant main effect of drug treatment (F(1,8) = 17.31, P = 0.0032). E: Significant time × drug interaction (F(2,16) = 5.626, P = 0.0141). One-hour P = 0.0005; 2-h P < 0.0001; 4-h P < 0.0001 (Sidak multiple-comparisons test). Inset: P = 0.20 (paired t test). F: Daily chow intake in hM3Dq-expressing (n = 7) and control (n = 6) Glu-Cre mice in response to twice-daily i.p. injection of 2 mg/kg CNO (indicated with black arrows). Significant time × virus interaction (F(11,121) = 2.06, P = 0.0283); day 6 P = 0.0119 (Sidak multiple-comparisons test). Data are given as the mean ± SEM. *P < 0.05, ***P < 0.001, ****P < 0.0001.
Having confirmed that CNO activates PPG neurons both in vitro and in vivo, we explored whether in vivo activation suppresses feeding. First, we confirmed that viral overexpression of hM3Dq in PPG neurons had no intrinsic effect on food intake and that the administration of CNO in mice transduced with a control virus did not affect food intake (Supplementary Fig. 2). Subsequently, ad libitum dark-onset food intake was measured in mice expressing hM3Dq in NTS PPG neurons in a counterbalanced crossover design. CNO-injected mice consumed substantially less chow than when injected with saline in both the first and second hour (Fig. 1D), and cumulative intake was suppressed over the first 4 h after dark onset (Fig. 1E). The effect of CNO disappeared over 21 h with cumulative daily intake unaffected by PPG activation (Fig. 1E).
To determine whether this was simply due to the washout of CNO, we injected another cohort of hM3Dq-expressing and control mice with CNO every 12 h (at dark and light onset) for 4 days. Twice-daily PPG activation initially suppressed feeding, with significantly lower 24-h intake after the first injections; however, intake suppression was not sustained (Fig. 1F).
NTS PPG Neurons Are the Main Source of GLP-1 in Brain
Although the above results demonstrate the capacity of PPG neurons to reduce feeding, they do not prove that brain GLP-1 is derived from these neurons. To address this, we used an adeno-associated virus (AAV) Cre-dependently encoding diphtheria toxin subunit A (DTA). Unilateral targeting of the NTS in Glu-Cre mice with AAV8-mCherry-FLEX-DTA selectively ablated PPG neurons, with complete disappearance of cell bodies within 14 days, while contralateral PPG neurons remained intact (Fig. 2A). Bilateral ablation of NTS PPG neurons dramatically reduced active GLP-1 levels in brainstem, hypothalamus, and spinal cord (Fig. 2B), demonstrating that NTS PPG neurons are the main source of GLP-1 in these areas.
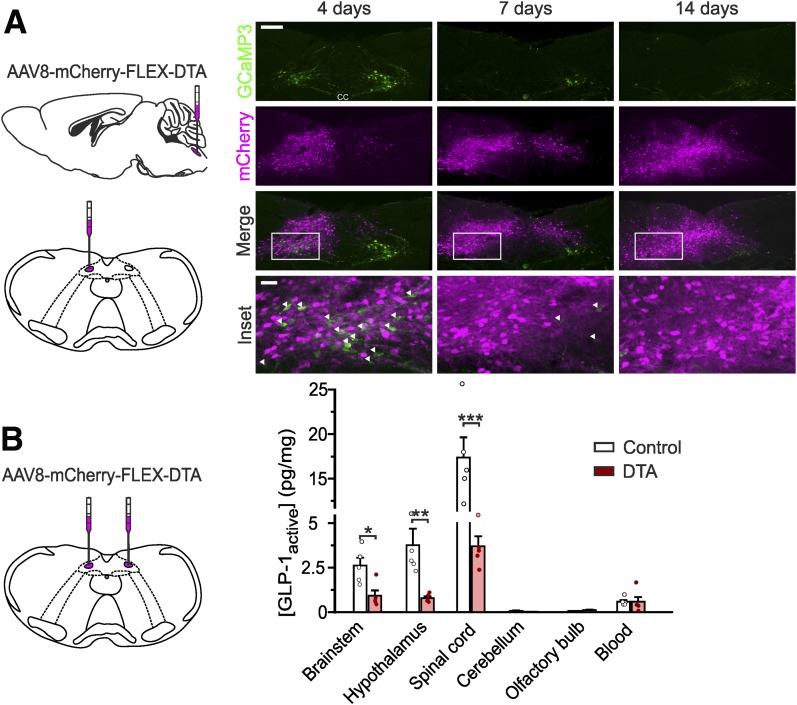
Figure 2.
NTS PPG neurons are the main source of brain GLP-1. A: Expression of mCherry and GCaMP3 (as a marker for PPG neurons) 4, 7, and 14 days after unilateral stereotaxic injection of AAV8-mCherry-FLEX-DTA into the NTS of a Glu-Cre/GCaMP3 mouse (schematic on left). White arrows indicate the remaining GCaMP3-positive PPG neurons. Scale bars: top panels, 100 μm; inset, 20 μm. B: Protein levels of active GLP-1 (normalized to total protein) detected in several brain regions after bilateral stereotaxic injection of AAV8-mCherry-FLEX-DTA or a control virus (AAV1/2-FLEX-Perceval). Brainstem P = 0.0317; hypothalamus P = 0.0079 (Mann-Whitney U test); spinal cord P = 0.0004 (unpaired t test). Data are given as the mean ± SEM; n = 5 in each group. *P < 0.05, **P < 0.01, ***P < 0.001.
Active GLP-1 concentrations in brainstem, hypothalamus, and spinal cord were 4-fold, 6-fold, and 29-fold larger, respectively, than those from concurrent systemic blood samples, indicating that blood contained within the brain samples was not the source of the GLP-1. Consistent with this, amounts of active GLP-1 in cerebellum, which does not receive projections from PPG neurons (3), were negligible, and neither blood nor cerebellar concentrations were affected by PPG neuron ablation. Similarly, the GLP-1 concentration in the olfactory bulb, a confirmed location of additional PPG neurons (27,28), was very low and not affected by DTA ablation in the brainstem.
Ablation of NTS PPG Neurons Does Not Impact Body Weight, Daily Food Intake, or Glucose Tolerance
Although the activation of PPG neurons robustly suppressed short-term feeding, sufficiency does not prove necessity. We therefore explored physiological conditions under which PPG neurons might regulate food intake. Body weight and daily food intake were recorded over 2 months in Glu-Cre mice injected bilaterally with AAV8-mCherry-FLEX-DTA or AAV1/2-FLEX-Perceval as a control. Ablation of NTS PPG neurons did not affect body weight (Fig. 3A) or daily food intake when fed ad libitum (Fig. 3B), suggesting that there is no significant impact on long-term energy balance under these conditions.
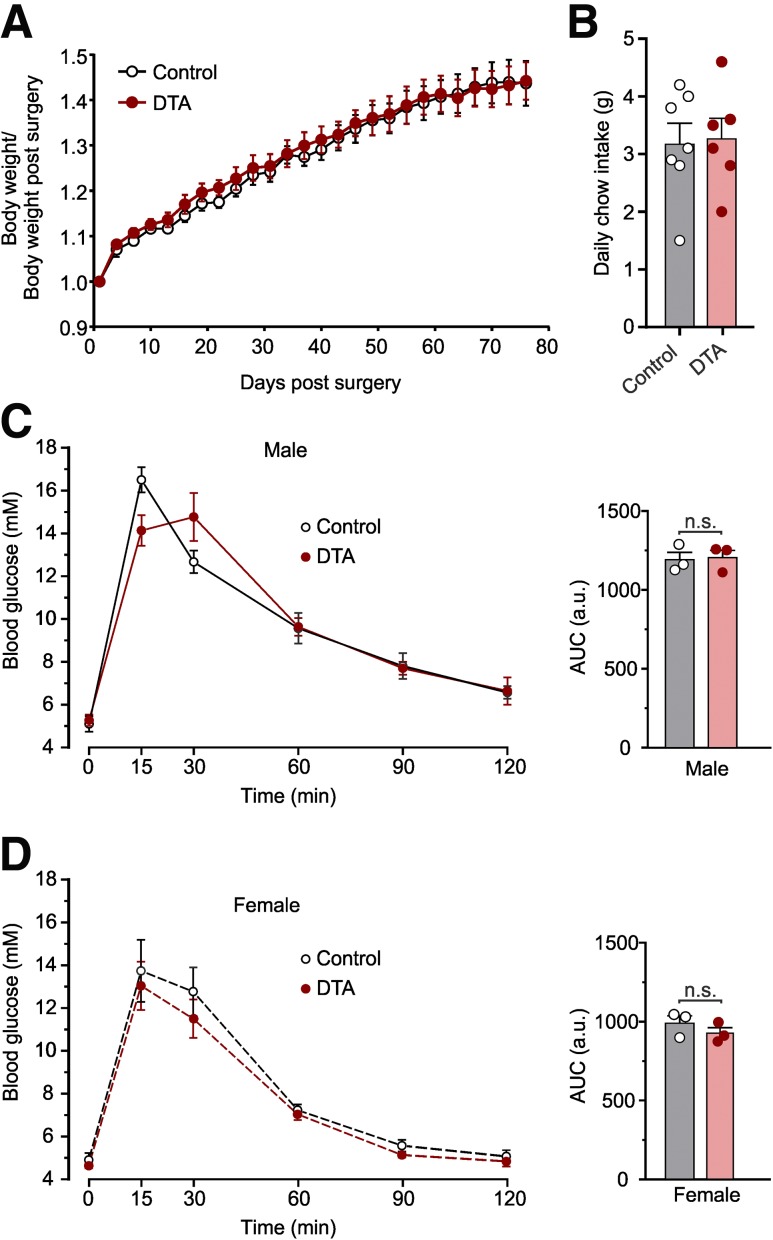
Figure 3.
Ablation of NTS PPG neurons has no impact on body weight, food intake, or glucose tolerance. Body weight change (A) and daily chow intake (B) after stereotaxic injection of AAV8-mCherry-FLEX-DTA or control virus. Body weight was measured every 2–3 days over 2 months. Data are given as the mean ± SEM; n = 7 (control), n = 6 (DTA). A: No significant time × virus interaction (F(1,12) = 0.08578, P = 0.7746) and no significant main effect of virus (F(1,12) = 0.08578, P = 0.7746). B: P = 0.45 (unpaired t test). Blood glucose in response to an i.p. injection of glucose (1 g/kg) at t = 0 7 weeks after stereotaxic injection of DTA or control virus in six male (C) and six female (D) Glu-Cre mice. Area under the curve (AUC) of the i.p. glucose tolerance test for each group is given on the right of each graph. Data are given as the mean ± SEM. Glucose concentrations: there was a significant time × virus interaction for males (F(5,20) = 3.83, P = 0.014), but no significant difference between DTA and control mice at any timepoint. There was no significant time × virus interaction for females (F(5,20) = 0.19, P = 0.96) and no significant main effect of virus (F(1,4) = 1.08, P = 0.36). AUC: no effect of virus for males (P > 0.99, Mann-Whitney U test) or females (P = 0.34, unpaired t test). a.u., arbitrary units; n.s., not significant.
PPG neurons were recently shown to be sufficient to improve glucose tolerance (16). We therefore investigated whether NTS PPG ablation affects the response to an i.p. glucose load (Fig. 3C and D). Before ablation, there was no difference in glucose tolerance, with males having poorer glucose tolerance than females (Supplementary Fig. 3). Seven weeks after PPG ablation, sex differences were still evident, but loss of NTS PPG neurons did not affect glucose tolerance (Fig. 3C and D).
Ad Libitum Food Intake Is Unaffected by Ablation or Acute Inhibition of NTS PPG Neurons
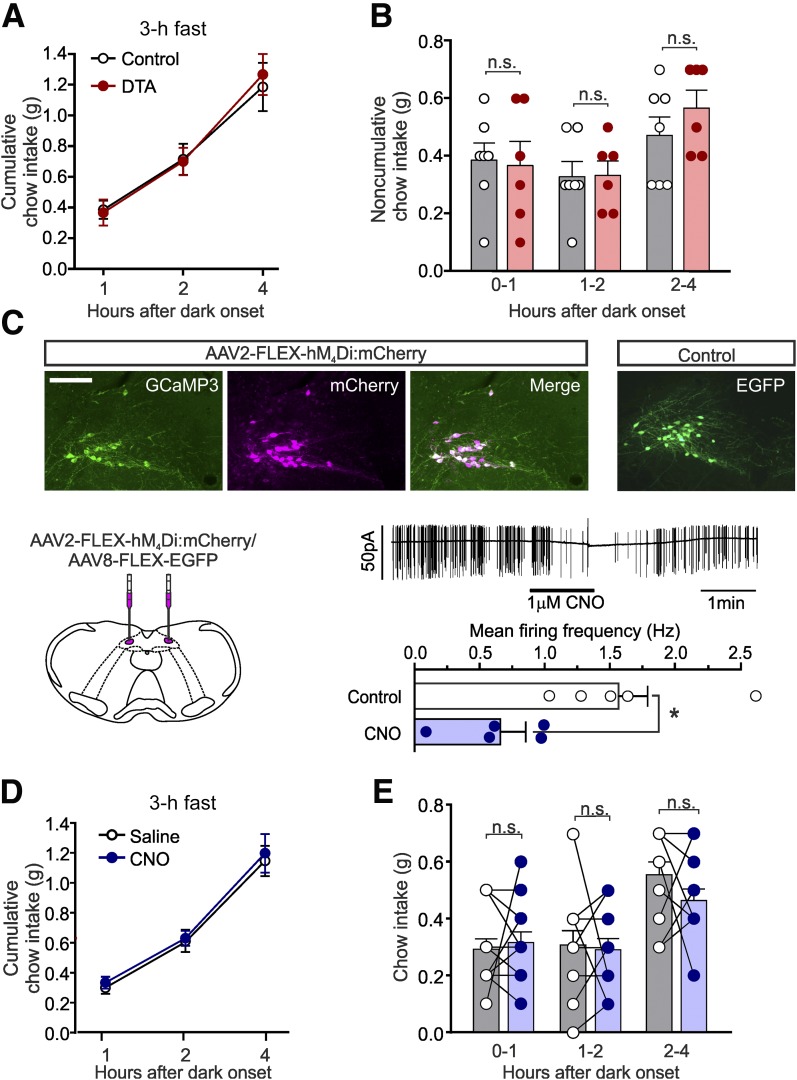
Having found no evidence that NTS PPG neurons regulate long-term energy balance, we next asked whether PPG neurons are necessary for short-term regulation of feeding. PPG ablation had no effect on cumulative (Fig. 4A) or noncumulative chow intake (Fig. 4B) over 4 h after dark onset, suggesting that although activation of PPG neurons is sufficient to reduce food intake, it is not necessary to regulate ad libitum feeding.
Figure 4.
Ad libitum food intake is unaffected by ablation or acute inhibition of NTS PPG neurons. Cumulative (A) and noncumulative (B) food intake of control and PPG-ablated (DTA) mice in the first 4 h of the dark phase. Data are given as the mean ± SEM; n = 7 (control), n = 6 (DTA). n.s., not significant. A: No significant time × virus interaction (F(2,22) = 0.5406, P = 0.5900) and no significant main effect of virus (F(1,11) = 0.012, P = 0.91). B: No significant time × virus interaction (F(2,22) = 0.834, P = 0.4476) and no significant main effect of virus (F(1,11) = 0.1472, P = 0.7085). C: Cre-dependent expression of hM4Di and EGFP as control in the NTS of Glu-Cre/GCaMP3 mice. Scale bar, 100 μm. Bottom left: Schematic of bilateral injections. Bottom right: Representative voltage-clamp recording (top) and summary data (bottom) of hM4Di-expressing PPG neurons in ex vivo slice preparation superfused with CNO (1 μmol/L). Data are given as the mean ± SEM; n = 5. *P < 0.05 (paired t test). Cumulative (D) and noncumulative (E) food intake of hM4Di-expressing mice injected with saline or CNO (2 mg/kg i.p.; 30 min prior to dark onset) in the first 4 h of the dark phase. Data are given as the mean ± SEM; n = 12. D: No significant main effect of virus (F(1,21) = 1.19, P = 0.29) or drug (F(1,21) = 0.002, P = 0.96). E: No significant main effect of virus (F(1,20) = 0.11, P = 0.75) or drug (F(1,20) = 0.045, P = 0.83). Data from mice expressing control virus (Supplementary Fig. 4) are included in the analysis. n.s., not significant.
Although ablations were inflicted in adult mice, compensatory responses could account for the lack of effect on long-term energy balance. We therefore assessed the effect of acute inhibition of PPG neurons using the inhibitory DREADD hM4Di.
To assess whether hM4Di stimulation inhibits PPG neurons in vitro, virally transduced PPG neurons were identified by mCherry fluorescence in brainstem slices, and electrical activity was recorded in the cell-attached configuration. PPG neurons were spontaneously firing at 1.57 ± 0.22 Hz, consistent with previous observations (6,18). CNO superfusion reduced firing frequency to 0.66 ± 0.20 Hz (Fig. 4C).
hM4Di expression did not affect body weight or food intake in the absence of CNO (Supplementary Fig. 4), and daily food intake was unaffected after a single dose of CNO at dark onset (Supplementary Fig. 4C). As with ablation, acute inhibition of PPG neurons did not affect dark-onset feeding (Fig. 4D and E). The data from both ablation and acute inhibition therefore suggest that PPG neurons are not necessary for the regulation of long-term or short-term food intake in ad libitum–fed mice.
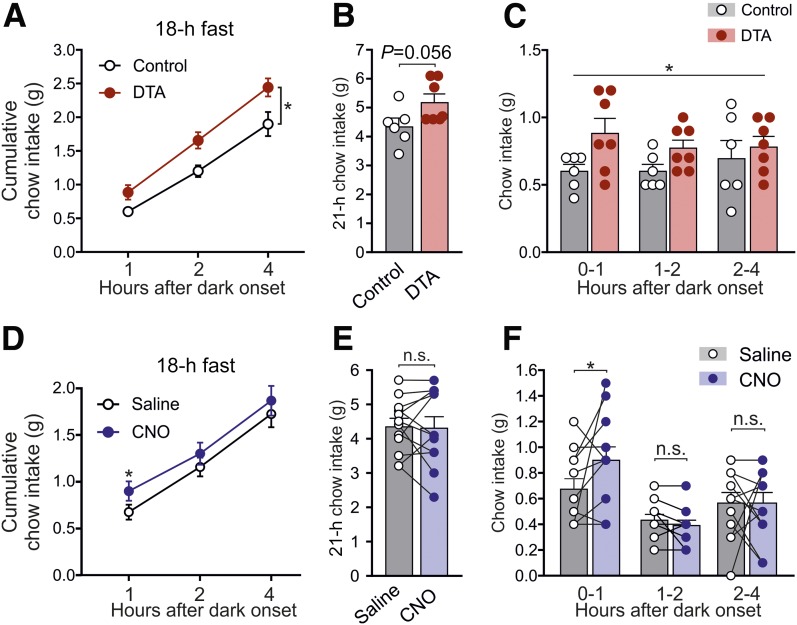
PPG Neurons Limit Fasting-Induced Refeeding
Having found no major role of NTS PPG neurons in ad libitum feeding, we next investigated whether PPG neurons need abnormally large meals to become engaged, as suggested for rats (9,10). To encourage significant refeeding, we fasted mice for 18 h prior to intake measurements from dark onset. Control animals ate 0.6 ± 0.05 g chow in the first hour compared with 0.4 ± 0.06 g when food access was restricted only briefly (Figs. 5C and 4A, respectively), whereas PPG-ablated animals ate 0.9 ± 0.11 g (Fig. 5C). This elevated postfast chow intake was evident over 4 h (Fig. 5A) but did not last overnight, although there was a trend toward increased intake (Fig. 5B).
Figure 5.
Ablation or acute inhibition of PPG neurons increases food intake only after a large meal. Cumulative (A and B) and noncumulative (C) food intake of control and PPG-ablated mice in the first 4 h (A and C) and 21 h (B) after the onset of the dark phase after 18 h of food deprivation prior to the onset of dark. Data are given as the mean ± SEM; n = 7 (control), n = 6 (DTA). A: No significant virus × time interaction (F(2,22) = 1.81, P = 0.19), but a significant main effect of virus (F(1,11) = 8.0, P = 0.016). B: P = 0.056 (unpaired t test). C: No significant virus × time interaction (F(2,22) = 0.75, P = 0.49), but a significant main effect of virus (F(1,11) = 6.1, P = 0.031). Cumulative (D and E) and noncumulative (F) food intake of hM4Di-expressing mice injected with saline or CNO (2 mg/kg i.p.; 30 min prior to dark onset) in the first 4 h of the dark phase after 18 h of food deprivation prior to the onset of dark. Data are given as the mean ± SEM; n = 12. D: There was a significant virus × drug interaction at hour 1 (F(1,21) = 4.733, P = 0.0411), P = 0.038 (CNO vs. saline, Sidak multiple comparisons test). E: No significant virus × drug interaction (F(1,21) = 1.245, P = 0.28) and no significant main effect of drug (F(1,21) = 1.67, P = 0.21) or virus (F(1,21) = 0.084, P = 0.77). F: There was a significant virus × drug interaction at hour 1 (F(1,21) = 4.733, P = 0.0411), P = 0.038 (CNO vs. saline; Sidak multiple-comparisons test). Data from mice expressing control virus are included in analysis. *P < 0.05. n.s., not significant.
Similarly, CNO-injected hM4Di-expressing mice ate 0.23 ± 0.1 g more chow in the first hour of refeeding compared with when injected with saline (Fig. 5F). This hyperphagic effect was not sustained beyond 1 h (Fig. 5D), and there was no impact on 21 h of chow intake (Fig. 5E).
PPG Neurons Limit Chow Intake After a Liquid Diet Preload
Results from these two independent, complementary experiments suggest that PPG neurons may be recruited after unusually large intakes to limit subsequent feeding. To further investigate this hypothesis, we encouraged high intakes by provision of a highly palatable liquid diet (Vanilla Ensure). Mice were habituated to dark-onset Ensure access for 8 days, during which 30-min intake stabilized at 1.7 ± 0.1 g from day 4. On day 9, mice were randomly allocated to Ensure or fasted groups, and subsequent analysis of cFOS immunoreactivity revealed that 45% of PPG neurons were activated after Ensure intake, compared with 10% activation in fasted controls (Fig. 6A and B).
To determine whether the ablation of PPG neurons affects feeding under these conditions, mice were habituated to consume a 15-min Ensure preload at dark onset, which stabilized at 1.4 ± 0.04 g after four sessions (Fig. 6C). Ensure intake did not differ between PPG-ablated and control mice at the beginning (Fig. 6C) or end (Fig. 6D) of the habituation. After the final Ensure session, chow intake was lower in the control group than usually seen 1 h into the dark phase, with control mice eating approximately half their normal intake (Fig. 6E) (0.21 ± 0.06 vs. 0.4 ± 0.06 g in Fig. 4B). In contrast, PPG-ablated mice ate significantly more than control mice, with 0.61 ± 0.1 g of chow eaten 1 h after the Ensure preload (Fig. 6E). These data support the hypothesis that unusually large intakes recruit NTS PPG neurons to limit subsequent feeding.
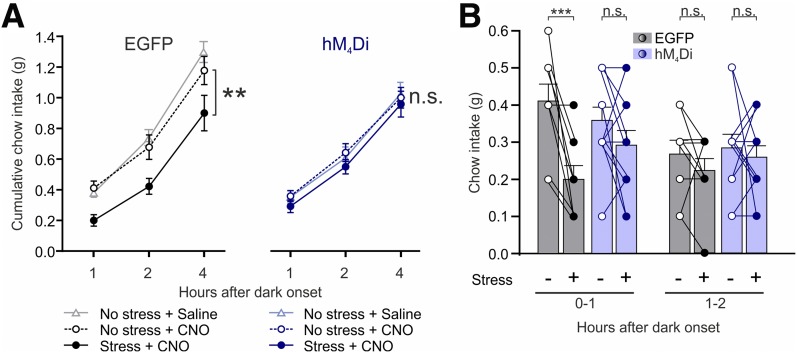
Stress-Induced Hypophagia Requires PPG Neurons
We next explored whether there are other conditions under which PPG neurons are necessary for anorectic effects. Previous work (29) suggests that central GLP-1 contributes to the intake suppression after acute stress in rats. To investigate whether PPG neurons contribute to the hypophagic response to restraint stress, we acutely inhibited PPG neurons in vivo by activating the hM4Di receptor. Control and PPG-inhibited mice were exposed to 30-min restraint stress, and subsequent food intake was measured. Restraint stress significantly suppressed cumulative feeding over 4 h in control mice, while PPG-inhibited mice displayed no stress-induced hypophagia (Fig. 7A). In the absence of stress, CNO had no effect on food intake in both control and PPG-inhibited mice compared with vehicle (Fig. 7A). Stress-induced hypophagia was seen in the first hour (Fig. 7B), during which intake in control mice was reduced by 50 ± 8%, whereas intake was unaffected by stress in the PPG-inhibited group. There was no clear suppression of chow intake in hour 2. These data demonstrate that PPG neurons are required for the hypophagic response to acute restraint stress.
Figure 7.
Stress-induced hypophagia requires PPG neurons. Cumulative (A) and noncumulative (B) chow intake of mice expressing EGFP or hM4Di injected with 2 mg/kg CNO i.p. 60 min prior to dark onset and left undisturbed or exposed to 30 min of restraint stress 30 min prior to dark onset. Also included in A are data from the same EGFP- and hM4Di-expressing mice injected with saline and left undisturbed. Data are given as the mean ± SEM; n = 9 (control), n = 12 (hM4Di). A: Significant main effect of stress for control (F(1,8) = 14.14, P = 0.0055), but not hM4Di (F(1,11) = 0.8684, P = 0.37). No effect of CNO on food intake in both control (P = 0.5) and PPG-inhibited mice (P = 0.98) compared with saline vehicle in the absence of stress. B: Hour 0–1: Significant effect of stress in the EGFP group (P = 0.0008), but not in the hM4Di group (P = 0.25) (Sidak multiple-comparisons test). Hour 1–2: No significant virus × stress interaction (F(1,19) = 0.091, P = 0.77) and no main effect of virus (F(1,19) = 0.49, P = 0.49) or stress (F(1,19) = 1.16, P = 0.29). **P < 0.01; ***P < 0.001. n.s., not significant.
Discussion
We demonstrate here that NTS PPG neurons are the main source of brain GLP-1. Active GLP-1 was reduced by >60% in brainstem and almost 80% in hypothalamus and spinal cord after ablation of NTS PPG neurons. In contrast, circulating GLP-1 levels, which were substantially lower than in the brain, were unaffected by ablation and levels in cerebellum, which receives no projections from PPG neurons (3), were negligible and likely reflect only the vascular supply throughout the brain. The remaining GLP-1 levels in the sampled CNS regions after ablation likely reflect the intermediate reticular nucleus and ventral midline PPG cells, which were left intact in our study. These neurons make up 44% (29) and 32% (1) of all PPG neurons in the brainstem in rat and mouse, respectively. Outside the brainstem, small populations of PPG neurons have been described in olfactory bulb (27,28), piriform cortex (3), and lumbar-sacral spinal cord (1). Of these, the olfactory bulb neurons project only locally (28), and the spinal neurons do not project to the brain (1), excluding these populations as the source of remaining GLP-1 after ablation of NTS PPG neurons. Approximately 50% of PPG neurons have axons projecting to the autonomic control areas of the spinal cord (1). The current study adds to the significance of these projections by demonstrating a high GLP-1 content in spinal cord, the majority of which is supplied by NTS PPG neurons. This suggests a significant physiological role for spinal GLP-1 release, which is an intriguing path for future research considering the role of GLP-1 in sympathetic outflow (30–33).
In addition to providing unequivocal evidence that PPG neurons are the main source of brain GLP-1, these results validate that our genetic approach targets the cells that provide GLP-1 in the CNS. In contrast, the injection of a lentivirus that expresses GFP in Phox2b-expressing cells (PRSx8-AlstR-EGFP-LV) (26) failed to show any colocalization with Cre-expressing PPG neurons, indicating that the transcription factor Phox2b is not expressed in adult PPG neurons. Transgenic mice expressing Cre under the control of Phox2b have been used previously to target PPG neurons (24,25). Although our results do not exclude the possibility that PPG neurons express Phox2b during development, they clearly show that using adult Phox2b-Cre mice combined with Cre-dependent viruses is not a valid approach to target PPG neurons. This substantiates previous concerns about that approach (34,35).
Recent studies (14–16) have pharmacogenetically and optogenetically activated PPG neurons, using a mouse model produced independently from ours but using an equivalent strategy. Based on those results and our observations, NTS PPG neurons are sufficient to suppress feeding in mice. Here we also demonstrate that repeated, twice-daily chemogenetic activation of NTS PPG neurons leads to a transient decrease in food intake reminiscent of the results reported for both CCK- and dopamine β-hydroxylase–expressing NTS neurons (36). In both cases, there was a strong short-term reduction in intake, but either no effect (CCK cells) or only a small effect (dopamine β-hydroxylase cells) on 24-h food intake. A similar lack of long-term effects has been observed with intracerebroventricular GLP-1 infusions in rat (37), and it seems likely that compensatory mechanisms are activated to maintain energy balance and avoid excessive weight loss, although we cannot exclude that continued activation downregulates DREADD receptors.
Gaykema et al. (14) argued that chemogenetic activation using hM3Dq is comparable to activation by physiological stimuli, such as leptin and CCK. However, with <50% of PPG neurons activated, our cFOS expression study demonstrated that even a large volume of Ensure did not activate PPG neurons to the same degree as hM3Dq activation (98%) (14). Similarly, acute stress in rats activated ∼74% of GLP-1–producing neurons (29), suggesting that chemogenetic activation of PPG neurons is a supraphysiological stimulus.
Prompted by these limitations, we attempted to determine the physiological role of PPG neurons in food intake. Although Liu et al. (15) did address the role of endogenous GLP-1 in food intake by optogenetically inhibiting NTSPPG → paraventricular hypothalamus projections, the specific physiological conditions under which PPG neurons influence food intake were not investigated. Although chemogenetic activation strongly suppressed feeding, the ablation of NTS PPG neurons had no effect on 24-h intake or body weight in mice with ad libitum access to food. Not only was long-term energy balance unaltered by the loss of NTS PPG neurons, neither ablation nor acute inhibition affected dark-onset food intake when mice were fed ad libitum. Similarly, Liu et al. (15) failed to observe persistent increases in food intake over seven daily injections of 0.3 mg/kg CNO, although this dose schedule may not provide lasting inhibition of the PPG neurons (38). This suggests that PPG neurons do not produce an obligatory meal termination signal that is required for normal satiation, a conclusion supported by both experimental approaches used here. Loss of NTS PPG neurons also failed to affect glucose tolerance, which is consistent with previous reports that central GLP-1 receptors (GLP-1Rs) are not necessary for glucose control in mouse (39). However, both ablation and acute inactivation left a proportion of PPG neurons, in the intermediate reticular nucleus, intact. It is conceivable that only a small fraction of PPG neurons is needed to maintain satiation and that a complete loss of PPG neurons could reveal a role in ad libitum feeding. This reliance on a few neurons only was demonstrated for orexin neurons, where >90% loss was necessary to reveal the cataplexy phenotype (40). Although this is difficult to categorically exclude, our results suggest that PPG neurons are not normally recruited in response to ad libitum feeding, but point to a role for PPG neurons in suppressing food intake in response to stronger physiological stimuli, such as a large meal or acute stress. In support, global and central knockdown of GLP-1R has little impact on food intake and body weight in mice (39,41).
In contrast, studies in rats have provided some evidence for a role of endogenous GLP-1 in the control of normal feeding and glucose control (35,42,43). The infusion of exendin-9, a GLP-1R antagonist, into the ventricular system or the knockdown of GLP-1Rs in discrete nuclei increase food intake, and shRNA-mediated knockdown of PPG in the brainstem led to increased body weight gain and hyperphagia (35,44). It is unclear whether these different observations reflect species differences and, in that case, which rodent species is the best model of human physiology (35,45).
Several studies in the rat support the idea that GLP-1–producing neurons are mainly recruited after the intake of a large meal. An unusually large meal is required to activate GLP-1–producing neurons in the NTS (10), gastric distension activates GLP-1 neurons (7), and the ensuing decrease in food intake is mediated by central GLP-1 signaling (46). In support of these findings, we demonstrate here that NTS PPG neurons are necessary for satiation/satiety after the intake of a large meal, either encouraged by depriving mice of food for 18 h or by providing them with access to a highly palatable diet. Seemingly contradictory findings by previous studies in the rat (29,47) suggest that GLP-1 neurons are inhibited by a negative energy balance after an overnight fast, rendering them less sensitive to stimulation with CCK or acute stress. However, we demonstrate here that a large meal after food deprivation is sufficient to recruit NTS PPG neurons to limit overeating, as was previously suggested in the rat (10).
In addition to playing a role in satiation/satiety after a large meal, we found that hypophagia induced by acute restraint stress was dependent on PPG neuron activity. In support of a role for central GLP-1 in stress regulation, central infusion of GLP-1 increases plasma ACTH and corticosterone levels and elicits anxiety-like behavior in rats (48). Conversely, third ventricular exendin-9 attenuated the rise in corticosterone after psychogenic stress, demonstrating hypothalamic-pituitary-adrenal axis activation by exogenous as well as endogenous GLP-1. However, chemogenetic activation of PPG neurons in the mouse did not affect stress hormone levels or anxiety-related behavior (14), suggesting the restraint stress–induced hypophagia investigated in our study may not involve the activation of the hypothalamic-pituitary-adrenal axis, but uses different pathways. In ad libitum–fed rats, restraint stress induces cFOS expression in GLP-1–immunoreactive neurons, and restraint stress–induced hypophagia can be reversed by lateral ventricular exendin-9 (29). Those findings are in line with the effects of the direct inhibition of PPG neurons that we report here and suggest that these neurons play a central role in restraint stress–induced hypophagia in both mouse and rat. This places PPG neurons at the center of behavioral decisions to maintain energy intake versus avoiding danger and stress.
Conclusions
We report that NTS PPG neurons of the lower brainstem are the main source of the GLP-1 found in the CNS and that these neurons have the capacity to significantly suppress food intake, and we describe the conditions under which they are necessary for the control of feeding. Our results suggest that PPG neurons may not control ad libitum food intake but are essential for short-term limitation of feeding after unusually large intakes and in mediating stress-induced hypophagia. We thus conclude that PPG neurons likely form part of a secondary satiation/satiety circuit, activated by both psychogenic stress and presumptive gastric distension from unusually large intake. PPG neurons thus constitute a regulator with scope for substantial hypophagia, without being involved in day-to-day energy balance, and, as such, they may be an attractive target for pharmaceutical intervention to reduce body weight.
Supplementary Material
Article Information
Acknowledgments. The authors thank Bill Wisden (Imperial College London), Guy Rutter (Imperial College London), Sergey Kasparov (University of Bristol), Naoshige Uchida (Harvard University), and Bryan Roth (University of North Carolina) for plasmids and viruses, as listed in Table 1. The authors also thank Alexander Gourine, University College London (UCL), for advice regarding the stereotaxic brainstem injections; Ian Edwards, UCL, for assistance with the extraction of spinal cords; and Linda Rinaman, Florida State University, for critical comments on the manuscript.
Funding. This study was supported by a UCL Graduate Research Studentship and a Bogue Fellowship to M.K.H. and by Medical Research Council grants MR/J013293/2 and MR/N02589X/1 and British Heart Foundation grant FS/14/43/30960 (PhD Studentship D.R.C.) to S.T.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.K.H. contributed to the methodology, investigation, and conceptualization of the study; funding acquisition; writing of the original draft; and review and editing of the manuscript. J.E.R. contributed to the conceptualization, methodology, investigation, and supervision of the study; writing of the original draft; and review and editing of the manuscript. D.R.C. contributed to investigation of the study and the review and editing of the manuscript. D.I.B. contributed to the methodology and investigation of the study and the review and editing of the manuscript. D.L.W. contributed to the methodology and supervision of the study and the review and editing of the manuscript. F.R. and F.M.G. contributed to study resources and the review and editing of the manuscript. S.T. contributed to the conceptualization, methodology, investigation, and supervision of the study; funding acquisition; writing of the original draft; and review and editing of the manuscript. S.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db18-0729/-/DC1.
See accompanying article, p. 15.
References
- 1.Llewellyn-Smith IJ, Marina N, Manton RN, Reimann F, Gribble FM, Trapp S. Spinally projecting preproglucagon axons preferentially innervate sympathetic preganglionic neurons. Neuroscience 2015;284:872–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Llewellyn-Smith IJ, Gnanamanickam GJ, Reimann F, Gribble FM, Trapp S. Preproglucagon (PPG) neurons innervate neurochemically identified autonomic neurons in the mouse brainstem. Neuroscience 2013;229:130–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S. Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience 2011;180:111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 1997;77:257–270 [DOI] [PubMed] [Google Scholar]
- 5.Hisadome K, Reimann F, Gribble FM, Trapp S. CCK stimulation of GLP-1 neurons involves α1-adrenoceptor-mediated increase in glutamatergic synaptic inputs. Diabetes 2011;60:2701–2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hisadome K, Reimann F, Gribble FM, Trapp S. Leptin directly depolarizes preproglucagon neurons in the nucleus tractus solitarius: electrical properties of glucagon-like peptide 1 neurons. Diabetes 2010;59:1890–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vrang N, Phifer CB, Corkern MM, Berthoud HR. Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am J Physiol Regul Integr Comp Physiol 2003;285:R470–R478 [DOI] [PubMed] [Google Scholar]
- 8.Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am J Physiol 1999;277:R582–R590 [DOI] [PubMed] [Google Scholar]
- 9.Kreisler AD, Rinaman L. Hindbrain glucagon-like peptide-1 neurons track intake volume and contribute to injection stress-induced hypophagia in meal-entrained rats. Am J Physiol Regul Integr Comp Physiol 2016;310:R906–R916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreisler AD, Davis EA, Rinaman L. Differential activation of chemically identified neurons in the caudal nucleus of the solitary tract in non-entrained rats after intake of satiating vs. non-satiating meals. Physiol Behav 2014;136:47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maniscalco JW, Kreisler AD, Rinaman L. Satiation and stress-induced hypophagia: examining the role of hindbrain neurons expressing prolactin-releasing Peptide or glucagon-like Peptide 1. Front Neurosci 2013;6:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holt MK, Trapp S. The physiological role of the brain GLP-1 system in stress. Cogent Biol 2016;2:1229086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maniscalco JW, Rinaman L. Interoceptive modulation of neuroendocrine, emotional, and hypophagic responses to stress. Physiol Behav 2017;176:195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaykema RP, Newmyer BA, Ottolini M, et al. . Activation of murine pre-proglucagon-producing neurons reduces food intake and body weight. J Clin Invest 2017;127:1031–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Conde K, Zhang P, et al. . Enhanced AMPA receptor trafficking mediates the anorexigenic effect of endogenous glucagon-like Peptide-1 in the paraventricular hypothalamus. Neuron 2017;96:897–909.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi X, Chacko S, Li F, et al. . Acute activation of GLP-1-expressing neurons promotes glucose homeostasis and insulin sensitivity. Mol Metab 2017;6:1350–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker HE, Adriaenssens A, Rogers G, et al. . Predominant role of active versus facilitative glucose transport for glucagon-like peptide-1 secretion. Diabetologia 2012;55:2445–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holt MK, Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S. Serotonergic modulation of the activity of GLP-1 producing neurons in the nucleus of the solitary tract in mouse. Mol Metab 2017;6:909–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anesten F, Holt MK, Schéle E, et al. . Preproglucagon neurons in the hindbrain have IL-6 receptor-α and show Ca2+ influx in response to IL-6. Am J Physiol Regul Integr Comp Physiol 2016;311:R115–R123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab 2008;8:532–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edelstein AD, Tsuchida MA, Amodaj N, Pinkard H, Vale RD, Stuurman N. Advanced methods of microscope control using μManager software. J Biol Methods 2014;1:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machhada A, Trapp S, Marina N, et al. . Vagal determinants of exercise capacity. Nat Commun 2017;8:15097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schindelin J, Arganda-Carreras I, Frise E, et al. . Fiji: an open-source platform for biological-image analysis. Nat Methods 2012;9:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott MM, Williams KW, Rossi J, Lee CE, Elmquist JK. Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. J Clin Invest 2011;121:2413–2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang XF, Liu JJ, Xia J, Liu J, Mirabella V, Pang ZP. Endogenous glucagon-like peptide-1 suppresses high-fat food intake by reducing synaptic drive onto mesolimbic dopamine neurons. Cell Reports 2015;12:726–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marina N, Abdala AP, Trapp S, et al. . Essential role of Phox2b-expressing ventrolateral brainstem neurons in the chemosensory control of inspiration and expiration. J Neurosci 2010;30:12466–12473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 1999;403:261–280 [DOI] [PubMed] [Google Scholar]
- 28.Thiebaud N, Llewellyn-Smith IJ, Gribble F, Reimann F, Trapp S, Fadool DA. The incretin hormone glucagon-like peptide 1 increases mitral cell excitability by decreasing conductance of a voltage-dependent potassium channel. J Physiol 2016;594:2607–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maniscalco JW, Zheng H, Gordon PJ, Rinaman L. Negative energy balance blocks neural and behavioral responses to acute stress by “silencing” central glucagon-like peptide 1 signaling in rats. J Neurosci 2015;35:10701–10714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto H, Kishi T, Lee CE, et al. . Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci 2003;23:2939–2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto H, Lee CE, Marcus JN, et al. . Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest 2002;110:43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosal S, Packard AEB, Mahbod P, et al. . Disruption of glucagon-like peptide 1 signaling in Sim1 neurons reduces physiological and behavioral reactivity to acute and chronic stress. J Neurosci 2017;37:184–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SJ, Sanchez-Watts G, Krieger JP, et al. . Loss of dorsomedial hypothalamic GLP-1 signaling reduces BAT thermogenesis and increases adiposity. Mol Metab 2018;11:33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuesta LM, Chen Z, Duncan A, et al. . GLP-1 acts on habenular avoidance circuits to control nicotine intake. Nat Neurosci 2017;20:708–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanoski SE, Hayes MR, Skibicka KP. GLP-1 and weight loss: unraveling the diverse neural circuitry. Am J Physiol Regul Integr Comp Physiol 2016;310:R885–R895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roman CW, Derkach VA, Palmiter RD. Genetically and functionally defined NTS to PBN brain circuits mediating anorexia. Nat Commun 2016;7:11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donahey JC, van Dijk G, Woods SC, Seeley RJ. Intraventricular GLP-1 reduces short- but not long-term food intake or body weight in lean and obese rats. Brain Res 1998;779:75–83 [DOI] [PubMed] [Google Scholar]
- 38.Roth BL. DREADDs for neuroscientists. Neuron 2016;89:683–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sisley S, Gutierrez-Aguilar R, Scott M, D’Alessio DA, Sandoval DA, Seeley RJ. Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. J Clin Invest 2014;124:2456–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tabuchi S, Tsunematsu T, Black SW, et al. . Conditional ablation of orexin/hypocretin neurons: a new mouse model for the study of narcolepsy and orexin system function. J Neurosci 2014;34:6495–6509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scrocchi LA, Hill ME, Saleh J, Perkins B, Drucker DJ. Elimination of glucagon-like peptide 1R signaling does not modify weight gain and islet adaptation in mice with combined disruption of leptin and GLP-1 action. Diabetes 2000;49:1552–1560 [DOI] [PubMed] [Google Scholar]
- 42.Sandoval DA, D’Alessio DA. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev 2015;95:513–548 [DOI] [PubMed] [Google Scholar]
- 43.Jessen L, Smith EP, Ulrich-Lai Y, et al. . Central nervous system GLP-1 receptors regulate islet hormone secretion and glucose homeostasis in male rats. Endocrinology 2017;158:2124–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barrera JG, Jones KR, Herman JP, D’Alessio DA, Woods SC, Seeley RJ. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci 2011;31:3904–3913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mietlicki-Baase EG, Liberini CG, Workinger JL, et al. . A vitamin B12 conjugate of exendin-4 improves glucose tolerance without associated nausea or hypophagia in rodents. Diabetes Obes Metab 2018;20:1223–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 2009;150:2654–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maniscalco JW, Rinaman L. Overnight food deprivation markedly attenuates hindbrain noradrenergic, glucagon-like peptide-1, and hypothalamic neural responses to exogenous cholecystokinin in male rats. Physiol Behav 2013;121:35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kinzig KP, D’Alessio DA, Herman JP, et al. . CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors [published correction appears in J Neurosci 2003;23:following 8158]. J Neurosci 2003;23:6163–6170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krashes MJ, Koda S, Ye C, et al. . Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest 2011;121:1424–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murray AJ, Sauer JF, Riedel G, et al. . Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat Neurosci 2011;14:297–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.