Abstract
Glycosylation fine-tunes signal transduction of adhesion molecules during neural development and supports synaptic plasticity and repair after injury in the adult nervous system. One abundantly expressed neural glycan is LewisX (LeX). Although it is known that its expression starts at the formation of the neural tube during the second embryonic week in the mouse and peaks during the first postnatal week, its functional relevance is only rudimentarily understood. To gain better insights into the functions of this glycan, we identified small organic compounds that mimic structurally and functionally this glycan glycosidically linked to several neural adhesion molecules. Mimetic compounds were identified by competitive enzyme-linked immunosorbent assay (ELISA) using the LeX-specific monoclonal antibodies L5 and SSEA-1 for screening a library of small organic molecules. In this assay, antibody binding to substrate-coated LeX glycomimetic peptide is measured in the presence of compounds, allowing identification of molecules that inhibit antibody binding and thereby mimic LeX. Gossypol, orlistat, ursolic acid, folic acid, and tosufloxacin inhibited antibody binding in a concentration-dependent manner. With the aim to functionally characterize the molecular consequences of the compounds’ actions, we here present evidence that, at nM concentrations, the mimetic compounds enhance neurite outgrowth and promote neuronal survival of cultured mouse cerebellar granule cells via, notably, distinct signal transduction pathways. These findings raise hopes that these LeX mimetics will be powerful tools for further studying the functions of LeX and its effects in acute and chronic nervous system disease models. It is worth mentioning in this context that the LeX compounds investigated in the present study have been clinically approved for different therapies.
Keywords: LewisX mimetics, Glycomimetic peptide, Small organic compounds, Neurite outgrowth, Neuronal survival, Signal transduction
Introduction
Adhesion molecules at the cell surface and in the extracellular matrix are essential for the development of the nervous system. They are also important in the adult to allow synaptic plasticity and regeneration required for recovery from trauma. Cell surface-localized transmembrane adhesion molecules not only function in recognition between cells, but also act as signal transducers to initiate intracellular signaling cascades, which underlie, for instance, neurite outgrowth and neuronal survival [1]. Glycosylation is a common posttranslational modification of the protein backbone of adhesion molecules, finetuning the signal transduction mechanisms triggered under different functional conditions [2,3]. Elucidating the functional significance of glycosylation in the nervous system is a very challenging area of molecular neurobiology due to the diverse and heterogeneous nature of glycosylation. The discovery and characterization of monoclonal antibodies to classify specific glycan epitopes triggered a break-through in molecular neurobiology [4, 5].
One important glycan identified by monoclonal antibodies is LeX, also known as CD15 or stage-specific embryonic antigen-1 (SSEA-1). The LeX epitope comprises galactose β1–4-linked and fucose α1–3-linked to N-acetylglucosamine (Ga1β1–4(Fucα1–3)GlcNAc). Fucosyltransferase 9 (Fut9) catalyzes the last step in the biosynthesis of LeX by transferring fucose with an α1,3-linkage to N-acetylglucosamine [6]. In a more recent study, a second fucosyltransferase has been identified in the brain, named Fut10, which is essential for the maintenance of neural stem cells [7]. LeX can be part of glycan chains linked via N- or O-glycosylation to proteins or attached to glycolipids. It was reported to be abundantly expressed in the nervous system [8, 9].
LeX expression in mice starts with formation of the neural tube at embryonic day 9 and peaks during the first postnatal week [10]. LeX is expressed by neural stem/progenitor cells in neurogenic brain regions, such as the retina, olfactory epithelium, hippocampus, isthmus, ventricular zone of the cerebellar cortex, and in the developing spinal cord [10–15]. LeX is also prominently expressed in the molecular layer and Purkinje cell layer of the cerebellum and has been found on subpopulations of astrocytes and neurons in the subventricular zone and dentate gyrus of hippocampus, cerebral and cerebellar cortex, septum, striatum, and corpus callosum [16]. Although the LeX expression pattern in the nervous system has been described extensively, the functional significance of this glycan has remained to be explored in more detail. The LeX epitopes were found on many molecules that promote beneficial developmental and regenerative function [13, 17–19]. Considering both, the spatial and temporal distribution of LeX and the developmental and functional relevance of LeX carrying glycoproteins indicate important functions of LeX in the nervous system. Since our study is focused on signal transduction triggered by LeX mimetics, it is worth mentioning that we had identified several small organic compounds that mimic the functions of the cell adhesion molecule L1 or of the glycan polysialic acid (PSA) [20, 21]. Intriguingly, these chemical compounds trigger specifically L1-mediated or PSA-mediated cell signaling in vitro and in vivo. Follow-up studies confirmed that different L1 and PSA mimetics improve functional recovery after spinal cord and femoral nerve injury in mice and zebrafish [20, 22–25].
In our present study, we identified five small organic compounds as structural and functional LeX mimetics, binding to the LeX-specific SSEA-1 antibody These compounds and the linear form of a previously described LeX glycomimetic peptide [26] promote neurite outgrowth and neuronal survival of mouse cerebellar granule cells. However, we expected that the signal transduction mechanisms underlying these different functions are distinct from each other. We now propose that these distinct mechanisms reflect the more complex nature of the LeX glycan in terms of functions.
Materials and Methods
Animals
Six-week-old CB6F1/J mice (Jackson Laboratory, Bar Harbor, ME, USA) were maintained for breeding 6- to 8- day-old (P6-P8) offspring with ad libitum access to food and water at a 12-h light and 12-h dark cycle in the animal facility of the Division of Life Sciences at the Nelson Biology Laboratories of Rutgers University. Offspring of either sex was used for the preparation of brain homogenate and of primary cerebellar granule cell culture. The Institutional Animal Care and Use Committee of Rutgers University approved all animal experiments (protocol no. 09-051).
Antibodies and Reagents
Chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA) if not indicated otherwise. Media and reagents for cell culture were purchased from Gibco (Gaithersburg, MD, USA). The LeX mimetic compounds 7-(3-amino-1-pyrrolidinyl)-1-(2,4-difluorophenyl)-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid tosylate (tosufloxacin tosyl-ate; tosufloxacin; CAS 115964-29-9), 2,2′-bis(8-Formyl-1,6,7-trihydroxy-5-isopropyl-3-methylnaphthalene) (gossypol; CAS 303-45-7), (3β)-3-hydroxyurs-12-en-28-oic acid (ursolic acid; CAS 77-52-1), (2S)-2-[[4-[(2-amino-4-oxo-1H-pteridin-6-yl)methylamino]benzoyl]amino] pentanedioic acid (vitamin B9; folic acid; CAS 59-30-3), and the protein kinase C (PKC) inhibitor 2,2′,3,3′,4,4′-hexahydroxy-1,1′-biphenyl-6,6′-dimethanol-dimethyl ether (HBDDE; CAS 154675–180), casein kinase (CKII) inhibitor (E)-3-(2,3,4,5-tetrabromophenyl)acrylic acid (TBCA; CAS 934358-00-6), Erk inhibitor 1-nitro-2-[(Z)-[5-(3-itrophenyl)furan-2-yl]methylideneamino] guanidine (Erk inhibitor III; CAS 331656-92-9), and phosphatase and tensin homolog (PTEN) and protein tyrosine phosphatase (PTP) inhibitor dipotassium bisperoxo [5-hydroxypyridine-2-carboxyl) oxo-vanadate (bpv(HOpic)] (bpv(HOpic); CAS 722494-26-0) were obtained from Santa Cruz Biotechnology (Dallas, TX, USA). The LeX mimicking compound (–)-tetrahydrolipstatin and N-formyl-L-leucine (1S)-1-[[(2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl ester (orlistat; CAS 96829-58-2) were obtained from Sigma-Aldrich. The PSA mimetic (7S,9S)-9-acetyl-7-(4-amino-5-hydroxy-6-methyloxan-2-yl)oxy-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12- dione hydrochloride (idarubicin hydrochloride; idarubicin; CAS 57852-57-0), Scr and Abl inhibitor 1-cyclopentyl-3-(1H-pyrrolo[2,3-b]pyridin-5-yl)-1H-pyrazolo[3,4-d] pyrimidin-4-amine (PP121; CAS 1092788-83-4), v-Scr and c-Fyn inhibitor 1-(1,1-dimethylethyl)-3-(1-naphthalenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine (1-naphthyl PP1; CAS 221243-82-9) and protein kinase A (PKA) inhibitor (9R, 10S,12S)-2,3,9,10,11,12-hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzo-diazocine-10-carboxylic acid hexyl ester (KT 5720; CAS 108068-98-0) were purchased from Tocris Bioscience (Bristol, UK). The LeX glycomimetic peptide (CSRLNYLHC [26]) was obtained from GenScript (Piscataway Township, NJ, USA). Ortho-phenylenediamine dihydrochloride (OPD, CAS 615-28-1), calcein-AM (CAS 148504-34-1), and propidium iodide (CAS 25535-16-4) were purchased from Thermo Fisher Scientific (Waltham, MA, USA). The NIH Clinical Collection libraries 1 and 2 were from Evotec (Hamburg, Germany) and the Natural Product Library was from Selleckchem (Houston, TX, USA). The LeX-specific rat monoclonal antibody L5 has been described [13, 27]. The LeX-specific mouse monoclonal antibody SSEA-1 was purchased from LifeSpan Biosciences Inc. (Seattle, WA, USA), and secondary anti-mouse IgM antibodies coupled to horseradish peroxidase (HRP) were obtained from Jackson ImmunoResearch (West Grove, PA, USA).
ELISA Screening and Verification of LeX Mimetics
In the attempt to elucidate the functions of this glycan, it should be mentioned that LeX-specific monoclonal antibodies vary in their affinity to the distinct multistructural LeX containing glycans [19]. We therefore specify the monoclonal antibodies used in the present study which are directed against LeX: 487/L5 and MC-480, also referred to as SSEA-1 [4,13]. Monoclonal antibody 487/L5 detects all glycans containing a terminal LeX epitope, and monoclonal antibody SSEA-1 detects only terminal LeX epitopes exposed on long neo-lacto backbones [28]. First, the LeX monoclonal antibody SSEA-1 binding to the linear LeX glycomimetic peptide was verified by a standard ELISA similar as described [26]. Here, the LeX glycomimetic peptide was diluted at different concentrations (5, 10, 20 40, 60, 80, 100, and 120 μM; 100 μl/well) in phosphate-buffered saline, pH 7.3 (PBS), and was immobilized on 96-well plates overnight at 4 °C, which were then blocked by incubation with 1% bovine serum albumin (BSA) for 1 h at 22 °C. Then, the SSEA-1 antibody was added for 1 h at 22 °C to the coated wells which were then washed three times with washing buffer (PBS + 0.05% Tween 20). Subsequently, the wells were incubated with secondary mouse IgM antibody (1:5000 in PBS) coupled to HRP and were washed three times with washing buffer. Peroxidase substrate (1 mg/ml o-phenylenediamine dihydrochloride dissolved in 50 mM citric acid, 50 mM sodium phosphate and 0.03% hydrogen peroxide) was then added to the wells. After 20–30 min, when the signal had developed optimally, 2.5 M sulfuric acid was added to stop the reaction and the optical density (OD) was measured at 450 nm with an ELISA reader (EL800; BioTek Instruments, Winooski, VT, USA). The concentration-dependent OD curve was fitted to a logarithmic regression function (y = 0.0452ln-0.0071; R2 = 0.9761). To identify the compounds that inhibit the binding of LeX-specific antibodies L5 and SSEA-1 to LeX epitopes, the NIH Clinical Collection 1 and 2 Libraries and the Natural Product Library were screened via competitive ELISA. By using this method, our group had previously identified structural and functional mimetic compounds of the glycan PSA and the cell adhesion molecule L1 [20, 21]. In brief, mouse brain homogenate of 7-day-old CB6F1/J mice in PBS (1 mg/ml; 100 μl/well) was incubated in 96-well plates overnight at 4 °C, washed three times with wash buffer and blocked, with 1% BSA for 1 h at 22 °C. Next, the L5 and SSEA-1 LeX-specific antibodies (0.5 μg/ml; 100 μl/well) were preincubated for 1 h at 22 °C with either PBS serving as negative control, PBS containing 1% dimethyl sulfoxide (DMSO) serving as solvent control, brain homogenate (1 mg/ml) serving as positive control or 10 μM of library compounds in DMSO. Subsequently, the antibody-compound mixtures were added to the brain homogenate-coated wells for 1 h at 22 °C, which were washed three times with washing buffer, incubated with secondary mouse IgM antibody coupled to HRP (1:5000 in PBS), washed again, and incubated for 20–30 min at 22 °C with peroxidase substrate. A chemical compound was considered a LeX mimetic, if it inhibited at least 50% of binding of either LeX monoclonal antibody to the brain homogenate. Verification of the structural LeX mimetics was performed by testing their ability to bind to the SSEA-1 antibody in a specific and concentration-dependent manner by competitive ELISA as described [21]. Briefly, LeX glycomimetic peptide was diluted in PBS and immobilized on 96-well plates overnight at 4 °C (80 μM; 100 μl/well). SSEA-1 antibody was preincubated for 1 h at 22 °C with increasing concentrations (1, 5, 10, 20, 40, 60, and 80 nM) of identified LeX mimetic compounds, or the PSA mimetic compound idarubicin [21] that served as negative control and pre-incubated antibodies were added to the coated wells.
Neuronal Survival Assays
Primary cerebellar granule cells were prepared from P6-P8 CB6F1/J mice, and survival assays were conducted as described [21]. Briefly, cells were seeded (250,000 cells/well) on a 0.01% poly-D-lysine (PDL)-coated 48-well Falcon tissue culture plates (Thermo Fisher Scientific). For the toxicity tests, neurons were treated 1 h after seeding with different concentrations (0.001, 0.01, 0.1, 1, 10, and 50 μM) of the LeX mimetic compounds, or with 0.01% DMSO solvent control. Neurons were maintained for 24 h at 37 °C in 5% CO2 and 90% humidity. Living and apoptotic cells were stained for 20 min at 37 °C using calcein-AM and propidium iodide (each 1 μg/ml, 100 μl/well). Live imaging of the cerebellar granule cells was performed using a Zeiss Axiovert 200M inverted transmission-light microscope (Carl Zeiss, Oberkochen, Germany) with a × 20 objective and AxioVision 4.6 software. For the neuronal protection assays, cells were maintained at 37 °C in 5% CO2 and 90% humidity overnight and treated with different concentrations of the LeX mimetic compounds (0.01, 0.1, 1, 10, 100, and 1000 nM), or with 0.01% DMSO solvent control, or the LeX glycomimetic peptide (16,80 μM). Subsequently, oxidative stress was induced by exchanging the medium with Neurobasal-A containing 20 μM hydrogen peroxide. Neurons were maintained for 48 h at 37 °C and in 5% CO2. Images from viable cells were obtained as previously described [21]. Experiments were also conducted as described [21] to study the cell transduction pathways underlying neuronal survival. Briefly, cells were pretreated for 20 min with different signal-transducing molecule inhibitors (120 nM KT5720, PKA inhibitor; 100 μM HBDDE, PKC inhibitor; 220 nM TBCA, CKII inhibitor; 4 μM Erk inhibitor III; 40 nM PP121, proto-oncogene tyrosine-protein kinase Sarcoma (Src) inhibitor; 1.2 μM 1-naphthyl PP1, c-Fyninhibitor; 28 nM bpV (HOpic), PTEN inhibitor) before addition of LeX mimetics. These inhibitors did not influence cell survival as we showed previously [21]. A count of living cells and apoptotic cells was taken from four images for three wells per condition (12 images/condition) using ImageJ software (Version 1.50b; National Institutes of Health, Bethesda, MD, USA), as described [21]. In brief, the contrast/brightness threshold was set on default. Subsequently, the background noise was eliminated using despeckle, erode and dilate functions of ImageJ. Finally, the watershed ImageJ function was implemented to improve accuracy before the cells were counted using the analyze particles function of ImageJ. Cell numbers of living and apoptotic cells were averaged for each image, and the percentage of living cells was calculated.
Neurite Outgrowth Measurements
Neurite outgrowth measurements were performed as described [21]. In brief, primary cerebellar granule cells were prepared from P6-P8 CB6F1/J mice, and cells were seeded (30,000 cells/well) on 0.01% PDL-coated 48-well Falcon tissue culture plates (Thermo Fisher Scientific). Cells were allowed to settle down for 1 h and then incubated with different concentrations of the LeX mimetics (0.001, 0.01, 0.1, 1, 10, and 100 nM), or with 0.01% DMSO solvent control, or with LeX glycomimetic peptide (16, 80 μM). Neurons were maintained for 24 h at 37 °C in 5% CO2 and 90% humidity, fixed with 2.5% glutaraldehyde (Sigma-Aldrich) for 30 min at 22 °C and stained with 1% toluidine blue and 0.1% methylene blue in 1% Na-tetraborate. Neurites were imaged and quantified using an Axio Observer.A1 microscope (Carl Zeiss) with a × 20 objective and AxioVision 4.6 software. The longest neurite lengths were measured from the edge of the cell body to the end of the process, taking into account only neurites with a length equal to or greater than the diameter of the cell soma from which they originated and only from those that showed no contact with other neurites or cell bodies. All measurements were conducted using ImageJ software. The cell signaling experiments were carried out as described for analysis of neuronal survival. For the neurite outgrowth measurements, cells were incubated with the same concentrations of the above mentioned signal-transducing molecule inhibitors.
Statistical Analysis
All experiments were performed and analyzed in a blinded manner as described [21], and each experiment was performed at least three independent times. Statistical comparisons between groups were performed by one-way ANOVA using Fisher’s protected least significant difference (PLSD) test. StatView Version 5.0.1 (SAS Institute Inc., New York, NY, USA) and Microsoft Excel (Redmond, Washington, USA) were used for all calculations. Chemical drawings were prepared with ChemDraw Professional 16.0 Suite (Perkin Elmer, Waltham, MA, USA).
Results
Linear Form of the LeX Glycomimetic Peptide Binds to LeX-Specific Antibody SSEA-1
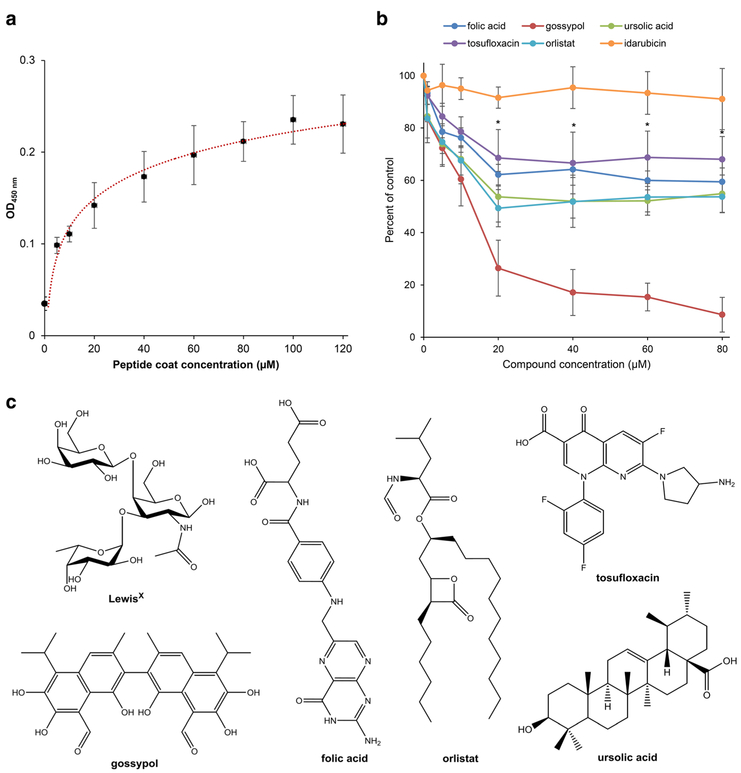
In a previous study, we identified a LeX glycomimetic peptide (CSRLNYLHC) which is recognized in its linear and circular forms by the LeX-specific antibody L5 [26]. To verify the binding of the linear LeX glycomimetic peptide to the LeX-specific antibody SSEA-1, an ELISA with different concentrations of immobilized LeX glycomimetic peptide (5, 10, 20, 40, 60, 80, 100, and 120 μM) was performed. Indeed, the LeX-specific antibody SSEA-1 bound to the linear LeX glycomimetic peptide in a concentration-dependent manner to (Fig. 1a). The differences of the OD values between 80 μM (OD = 0.21, SEM± 0.022) and 120 μM (OD=0.23, SEM± 0.032) immobilized peptide were minimal, so that a peptide coating concentration of 80 μM was chosen for the next experiments (Fig. 1a, b). The background signal detected in the wells containing SSEA-1 antibody without coated LeX glycomimetic peptide had an average OD value of 0.035 (SEM± 0.007) (Fig. 1a).
Fig. 1.
Concentration-dependent binding of the LeX glycomimetic peptide and the LeX mimetic compounds gossypol, folic acid, orlistat, to sufloxacin, and ursolic acid to the LeX-specific monoclonal antibody SSEA-1. a The graph shows a binding curve of the SSEA-1 antibody in different concentrations (5–120 μM) to the substrate-coated linear LeX glycomimetic peptide. The binding curve is fitted to a logarithmic regression function. b LeX-specific antibody SSEA-1 was preincubated with different concentrations (1–80 μM) of gossypol (red), folic acid (blue), orlistat (orange), tosufloxacin tosylate (purple), ursolic acid (green), or the control compound idarubicin (black) and added to wells coated with the LeX glycomimetic peptide. The signal of LeX-specific SSEA-1 antibody alone without any competing chemical compound was set on 100%. Asterisks signify differences in competition between all LeX mimetic compounds and the negative control compound idarubicin as calculated by one-way analysis of variance (ANOVA) followed by Fisher’s PLSD test (F =9.468, p = 0.0001; PLSD *p<0.05). a, b Mean values are shown from eight wells in four independent experiments. c Chemical structure of the LeX epitope and the LeX mimetic compounds gossypol, folic acid, orlistat, tosufloxacin tosylate, and ursolic acid
Gossypol, Orlistat, Ursolic Acid, Folic Acid, and Tosufloxacin Compete with the LeX Glycomimetic Peptide for Binding to LeX-Specific Antibodies L5 and SSEA-1
To identify novel LeX mimetic compounds, the NIH Clinical Collection 1 and 2 Libraries and the Natural Product Library were screened via competitive ELISA for compounds that interfere in the binding of the LeX-specific antibodies SSEA-1 and L5 to LeX epitopes from immobilized mouse brain homogenate of 7-day-old CB6F1/J littermates. Here, we identified the structural LeX mimetics orlistat and tosufloxacin from the NIH Clinical Collection 1 Library, folic acid from the NIH Clinical Collection 2 Library, and gossypol and ursolic acid from the Natural Product Library (Fig. 1b, c). To verify these findings of the initial screen, a second competitive ELISA was performed using different concentrations of orlistat, tosufloxacin, folic acid gossypol, and ursolic acid (1, 5, 10, 20 40, 60, and 80 μM) competing with the antibody SSEA-1 to the immobilized linear LeX glycomimetic peptide. All compounds interfered with the binding of antibody SSEA-1 to the LeX glycomimetic peptide in a concentration-dependent manner with maximal inhibition at approximately 20 μM (Fig. 1b). Gossypol was found to be most efficient at 80 μM with an inhibition of 91.4% (SEM± 6.6%). Orlistat and ursolic acid reached an inhibition of 50.6% (SEM ± 7.1%)at20 μM and 47.8% (SEM± 5.5%) at 60 μM, respectively. Folic acid and tosufloxacin inhibited binding 40.5% (SEM ± 5.3%) at 80 μM and 31.9% (SEM ± 8.7%) at 80 μM. The ability of all identified compounds to inhibit the binding of antibody SSEA-1 to the LeX glycomimetic peptide was significantly higher at all tested concentrations of and above 20 μM compared to the negative control idarubicin, a PSA mimetic compound (Fig. 1b). Idarubicin reached a maximal inhibition of 8.9% (SEM± 11.7%).
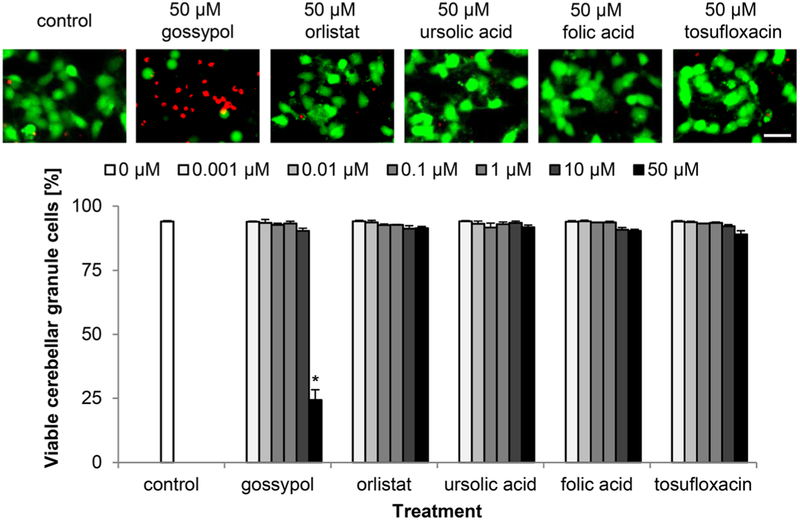
Gossypol Is Neurotoxic for Cerebellar Granule Cells at 50 μM
Since the aim of the study was to investigate functional aspects of the LeX mimetic compounds, it was important to estimate a potential neurotoxicity of these compounds. None of the tested compounds was neurotoxic to cerebellar granule cells at concentrations between 1 nM and 10 μM and only gossypol showed a neurotoxic effect at 50 μM with a decrease in cell viability from 90% (SEM± 1%) at 10 μM to 24% (SEM± 4%) at 50 μM (Fig. 2). These findings indicate that all identified LeX mimetic compounds can be used at 10 μM or lower concentrations to study LeX-mediated functions in neurons.
Fig. 2.
Gossypol, but not orlistat, ursolic acid, folic acid, and tosufloxacin, is toxic to cultured cerebellar granule cells at 50 μM. Representative images of cerebellar granule cells treated only with vehicle DMSO (control), or treated with 50 μM LeX mimetic compounds and stained with calcein-AM (green) and propidium iodide (red). Bar diagram shows percentage of viable cells (mean + SEM, 36 images captured from nine wells carried out in three independent experiments) treated with different concentrations of the mimetics or treated only with vehicle DMSO (control). Asterisk signifies differences between the vehicle control group and the LeX mimetic compound-treated group as determined by one-way ANOVA followed by Fisher’s PLSD test (F = 140.61, p < 0.0001; PLSD *p < 0.05). Scale bar, 20 μm
Gossypol, Orlistat, Ursolic Acid, Folic Acid, and Tosufloxacin Promote Neuronal Survival and Neurite Outgrowth of Cerebellar Granule Cells at Nanomolar Concentrations
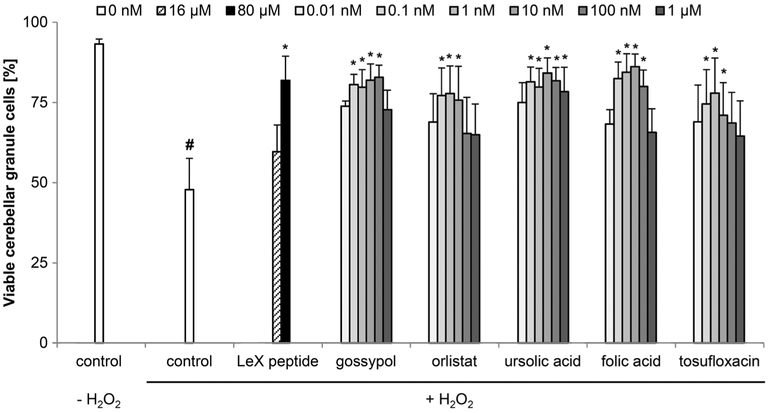
Next, we tested whether the LeX mimetics promote neuronal survival of stressed cerebellar granule cells. Viability of untreated cerebellar granule cells 72 h after seeding was 93.2% (SEM± 1.5%) and decreased thereafter (p < 0.001) to 47.8% (SEM± 9.8%) after hydrogen peroxide treatment (Fig. 3). The LeX glycomimetic peptide enhanced neuronal survival after hydrogen peroxide treatment to 81.9% (SEM ± 7.5%) at 80 μM (Fig. 3). The LeX mimetic compound gossypol was the most effective mimetic with 82.8% (SEM± 3.7%) viable cells at 100 nM. Ursolic acid and folic acid promoted neuronal survival between 0.1 and 100 nM, with the strongest effect being observed at a concentration of 10 nM and a viability of 84.1% (SEM± 4.7%) and 86.1% (SEM± 4.0%), respectively. Orlistat and tosufloxacin increased neuronal survival between 0.1 to 10 nM, with the strongest effect being observed at 1 nM and a viability of 77.8% (SEM± 8.5%) and 77.9% (SEM± 10.9%). The efficiency of neuronal protection decreased again at higher concentrations (Fig. 3).
Fig. 3.
LeX mimetics enhance neuronal survival of stressed cerebellar granule cells. Bar diagram shows the percentage of viable cerebellar granule cells (mean + SEM, 60 images captured from 15 wells carried out in five independent experiments) treated with different concentrations of the linear LeX glycomimetic peptide or with different concentrations of the LeX mimetic compounds and subsequently stressed with 20 μM H2O2. The hash sign indicates significant differences between the unstressed group (control, − H2O2) and stressed group (control, + H2O2). Asterisks signify differences between the stressed group (control, + H2O2) and compound-treated groups as determined by oneway ANOVA with Fisher’s PLSD test (F = 1.61, p <0.0329; PLSD *p <0.05)
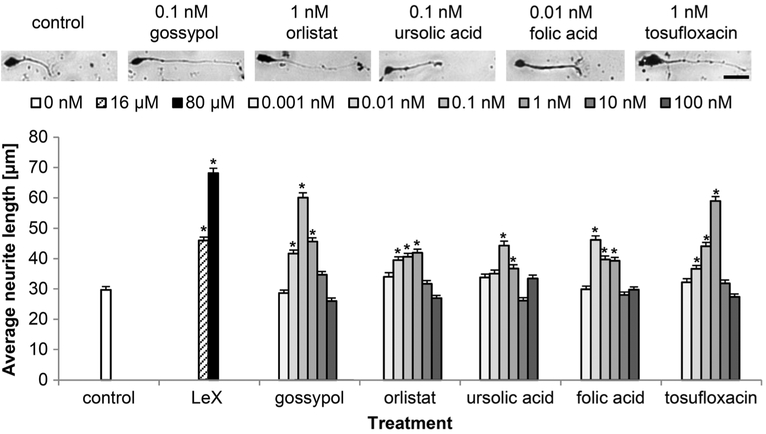
Next, we investigated whether the LeX mimetics affect neurite outgrowth. All LeX mimetics stimulated neurite outgrowth in comparison to the vehicle control (Fig. 4). The LeX glycomimetic peptide was most efficient and promoted neurite outgrowth at 80 μM with an average neurite length of 68 μm (SEM±1.7 μm) compared to 29 μm (SEM± 1.1 μm) average neurite length with the vehicle control. Gossypol and tosufloxacin were the most potent compounds for stimulating neurite outgrowth with average neurite length of 60 μm (SEM± 1.6 μm) and 59 μM (SEM± 1.5 μm) at 1 and 10 nM, respectively. Orlistat, ursolic acid, and folic acid were less efficient in stimulating neurite outgrowth with maximal average neurite lengths of 42 μm (SEM±1.1 μm), 44 μM (SEM ± 1.5 μm), and 46 μm (SEM± 1.3 μM) at 1, 0.1, and 0.01 nM, respectively. Higher concentrations of the LeX mimetic compounds reduced the average neurite length (Fig. 4).
Fig. 4.
LeX mimetics enhance neurite outgrowth of cerebellar granule cells. Representative images of primary cultured cerebellar granule cells treated only with vehicle DMSO (control), or treated with the LeX mimetic compounds. Bar diagram shows average longest neurite lengths (300 neurons from six wells out of three independent experiments, mean + SEM) exposed to different concentrations of the linear LeX glycomimetic peptide (LeX) or the LeX mimetic compounds. Asterisks signify differences versus vehicle control (control) as determined by one-way ANOVA with Fisher’s PLSD test (F =27.744, p < 0.0001; PLSD *p < 0.05). Scale bar, 20 μm
Gossypol, Orlistat, Ursolic Acid, Folic Acid, and Tosufloxacin Enhance Neuronal Survival and Neurite Outgrowth via Similar But Not Identical Signal Transduction Pathways
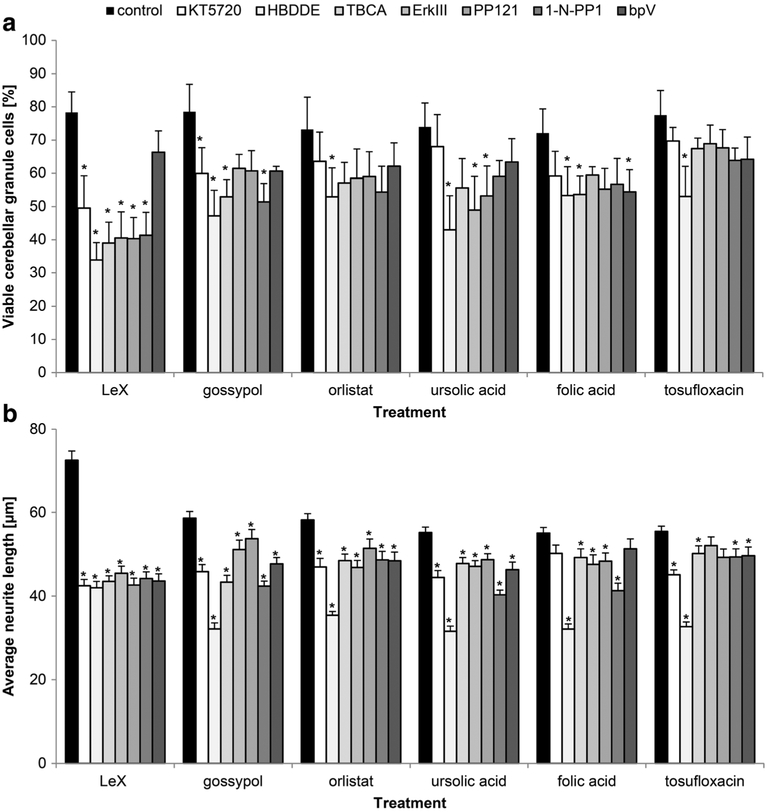
Next, we analyzed the signal transduction mechanisms underlying LeX mimetic-mediated neuronal survival and neurite outgrowth, showing that distinct signal transduction pathways are involved (Fig. 5). For orlistat- and tosufloxacin-mediated protection, only PKC was found to be involved. Inhibitors for PKC, CKII, and PTEN affected folic acid-mediated protection. For the ursolic acid-mediated protection, PKC, the extracellular regulated kinase (Erk) and Src were found to be involved. PKA, PKC, CKII, and Fyn are involved in gossypol-mediated protection. For the LeX glycomimetic peptide-mediated survival, PKA, PKC, CKII, Erk, Src, and Fyn were required (Fig. 5a). In neurite outgrowth, the LeX glycomimetic peptide and the LeX mimetic compounds gossypol, orlistat and ursolic acid showed involvement of PKA, PKC, CKII, Erk, Src, Fyn, and PTEN. Except for PKA and PTEN, all studied signal-transducing molecules contribute to folic acid-mediated neurite outgrowth. For tosufloxacin-mediated neurite outgrowth, all studied signal-transducing molecules are crucial except Erk (Fig. 5b).
Fig. 5.
LeX mimetics trigger cell signaling that is required for neurite outgrowth and neuronal survival. a, b Cerebellar granule cells were pretreated with different inhibitors of signal transducing molecules. Shown are inhibitors (KT 5720 (PKA), HBDDE (PKC), TBCA (CKII), Erk inhibitor III (Erk), PP121 (Src), 1-naphthyl PP1 (Fyn), bpV(HOpic) (PTEN)) versus cells not treated with inhibitor (control, black bars). After exposure to the inhibitors, cells were treated with the linear LeX glycomimetic peptide (LeX) (80 μM) or with LeX mimetic compounds (1 nM). a Bar diagram shows the average percentage ofviable cells (mean + SEM, 48 images captured from 12 wells carried out in four independent experiments) that were stressed with H2O2. b Bar diagram shows average longest neurite lengths (300 neurons from six wells out of three independent experiments, mean + SEM). Asterisks signify differences between cells only treated with the mimetics (control, black bars) and cells pretreated with the inhibitors as determined by oneway ANOVA with Fisher’s PLSD test (a F =3.542, p < 0.0001; b F = 9.505, p <0.0001; PLSD *p <0.05)
Discussion
The functional significance of the LeX glycan in the nervous system is not well understood. Until now, only function-inhibitory LeX antibodies were known to disturb migration, aggregation, adhesion, and neurite outgrowth of astrocytes and neurons [14, 17, 26]. Similarly, the LeX glycomimetic peptide identified by phage display using the LeX-specific monoclonal antibody 487/L5 inhibited in its cyclic form CD24-mediated and LeX-dependent neurite outgrowth, but did not influence functional recovery after femoral nerve or spinal cord injury [26]. Here, we now show that the linear form of the LeX glycomimetic peptide binds in a concentration-dependent manner also to LeX specific monoclonal antibody SSEA-1. The SSEA-1 antibody binds only to terminal LeX epitopes on long neo-lacto backbones, whereas the 487/L5 clone binds with high affinity to short terminal LeX epitopes [19].
It has been shown that the LeX carrying cell adhesion molecule L1 promotes neuronal survival [3, 29]. Interestingly, L1 enhances the expression of Fut9, one key enzyme for LeX biosynthesis, and promotes cell survival of embryonic stem cells via PKC signaling [30]. Thus, L1 is not only a carrier, but also a modulator of LeX synthesis. A dependence in function of LeX was reported for human neuroblastoma cells, where a decreased LeX expression leads to reduced neurite outgrowth [31]. The mucin-type adhesion molecule CD24-regulated neurite outgrowth also depends on LeX [17].
Given these observations, it deemed interesting to screen for and characterize novel LeX small organic compound mimetics. Of the identified compounds, gossypol, orlistat, and ursolic acid, the toxicological and pharmacological profiles are known in other functional systems, rendering the mimetics interesting for applications in neurological disorders [32–34].
Interestingly, very different small compound structures bind to the LeX-specific SSEA-1 antibody. Similarly, very different PSA and L1 mimetic compounds have been identified [20, 21, 25, 35]. Although these structures differ considerably within one group, all PSA and L1 mimetics trigger specifically PSA- or L1-mediated cell signaling pathways, indicating that small compounds with different structures can mimic functions of the same protein or glycan. The smaller size of these mimetic compounds makes them more flexible in binding into binding pockets of specific receptors. A molecular explanation for this phenomenon is provided by our study that describes tegaserod as one PSA mimetic [25]. To elucidate how a small compound can act as mimetic for a more complex protein or glycan structures, we performed molecular modeling of PSA and the PSA mimetic compound tegaserod within the published structure for the PSA binding pocket of the antibody 735 which was used for identification of PSA mimetics. The structure modeling of PSA within the binding pocket of the antibody revealed that an eight-residue PSA fragment forms a half helical turn. PSA binds to a broad groove in the antibody binding region forming multiple hydrogen bonds, whereas tegaserod binds within the groove in only one region of the putative PSA binding site. An explanation why different chemical structures bind into the binding pocket of the same antibody could be that the different chemical compounds bind to either with a different position in the larger binding pocket of the antibody or with different affinities to the same binding site.
We found that the LeX mimetic compounds prevented neuronal cell death and promoted neurite outgrowth at nM concentrations. Yet, higher concentrations than 100 nM of the LeX mimetic compounds, although not being cytotoxic, reduced their beneficial profile, in a manner similar to observations on PSA-mimicking compounds [21]. It is either reported that the here identified LeX mimetic compounds are able to internalize into cells [36–38] or that they pass the plasma membrane due to their hydrophobic properties [39, 40]. The possibility that the intracellular presence of the LeX mimetic compounds leads to a reduction in beneficial effects remains to be investigated.
From the five identified LeX mimetics, only gossypol was neurotoxic at 50 μM. Gossypol is known for its cytotoxic effect on tumor cells in the μM range [32]. Gossypol is a phenolic compound and a male antifertility agent that occurs naturally in certain species of cotton plants of the family Malvaciae. The suggested mechanism behind the cytotoxic effect of gossypol is the inhibition of cellular energy metabolism by being a strong mitochondrial respiratory toxin and may block the microtubule assembly in sciatic nerves at μM concentrations [41]. In the present study, we show that gossypol is neuroprotective at nM concentrations. This result agrees with a report that gossypol protects retinal pigment epithelial cells via the FoxO3/Sestrin2 pathway [42].
Ursolic acid, another identified LeX mimetic, is a ubiquitous triterpenoid in the plant kingdom. Since it was shown that ursolic acid promotes nerve growth factor (NGF)-mediated neurite outgrowth in PCD12C cells [43], it will be interesting to analyze the role of NGF in LeX mimetic-stimulated neurite outgrowth.
Folic acid has also been determined to be an important ingredient in development. Pregnant women who do not receive enough folic acid are more likely to have children with neural tube defects [44]. A low level of folate during development is associated with increased risk of autism, severe language delay, and emotional problems [45, 46]. Moreover, population studies correlate low concentrations of folate in the blood with poor cognitive function, dementia, and Alzheimer’s disease-related neurodegeneration [47, 48]. Although a direct link between LeX and Alzheimer’s disease has not been reported, our results suggest such a link.
Tosufloxacin is an antibacterial agent that inhibits bacterial DNA gyrase and topoisomerase [49]. Our observations show that this compound may have beneficial effects on cerebellar granule cells at nM concentrations.
Based on these findings, the effects of compounds on signal transduction mechanisms were studied. Interestingly, these mechanisms are distinct between protection and neurite outgrowth. Also, the signal transduction mechanisms for neuronal survival and neurite outgrowth are different between the LeX mimetics. In contrast, signaling cascades triggered by the PSA mimetics idarubicin and irinotecan are similar between the PSA mimetics [21]. A possible explanation would be that PSA is predominantly expressed on the cell adhesion molecule NCAM, and thus, PSA mimetics would trigger predominantly NCAM-PSA-mediated cell signaling, while LeX is a functionally more diverse glycan epitope. The variety of triggered signal transduction pathways and the considerable structural diversity of the identified LeX mimetic compounds could reflect the widespread LeX presence is on many glycoproteins and glycolipids [50–52]. We suggest that different LeX mimetics bind to different sets of receptors that lead to similar functional consequences, but via different signal transduction mechanisms. In addition, it is crucial to mention in this context that LeX facilitates cell adhesion by homophilic glycan-glycan interactions [53]. Hence, we will discuss below some prominent LeX-carrying glycoproteins that are important in the function, repair and plasticity of the nervous system as putative receptors for our identified LeX mimetics. Using the 487/L5 and SSEA-1 antibodies, several molecules were identified to carry LeX, such as the neural cell adhesion molecules L1, thymus cell antigen 1 (Thy-1), cluster of differentiation 24 (CD24), the neuron-specific and synaptic vesicle-associated protein synapsin I, and low-density lipoprotein receptor-related protein 1 (LRP1) [13, 17–19].
The immunoglobulin superfamily member L1 cell adhesion molecule is important for the development, plasticity, and repair of the nervous system, with multiple cellular functions, including notably its role in neurite outgrowth, neuronal cell migration, and survival, as well as myelination and synaptic plasticity [1,54]. The signaling pathways underlying L1-mediated neurite outgrowth and neuronal protection are well studied. P13-, Src family-, and mitogen-activated protein kinases are crucial for neuritogenesis and neuroprotection, with the functionally associated fibroblast growth factor (FGF) receptor being involved in PKA and cAMP-mediated signal transduction pathways that are mainly essential in L1-mediated neurite outgrowth, but not for neuronal survival [55]. L1 binds intracellularly to CKII and activates the kinase leading to neuroprotection by inhibiting PTEN and p53 [56]. Inhibitors of Src and PKA attenuate the enhanced neurite outgrowth of gossypol and a CKII inhibitor reduces the neuro-protective effect of gossypol. These results suggest that L1 is one of the receptors for gossypol. Thy-1 is another member of the immunoglobulin superfamily involved in T cell activation, neurite outgrowth, apoptosis, tumor suppression, wound healing, and fibrosis in dorsal root ganglion cells [57]. In the nervous system, Thy-1 is expressed in the striatum, hippocampus, neocortex, cerebellum, spinal cord, and the retina and optic nerve [58]. Thy-1 expression on neuronal cell lines in vitro inhibits neurite outgrowth on top of an astrocyte substrate, but not on a Schwann cell or embryonic glial cell substrate. An anti-Thy-1 monoclonal antibody stimulates neurite outgrowth in a PKA-dependent manner by the c-Src-MEK signaling pathway [57, 59–61]. Thy-1 signals cell death involving apoptotic proteins and cell cycle regulators by Src family kinase-mediated cell signaling. Inhibitors of Src and PKA affect gossypol- and ursolic acid-promoted neurite outgrowth. The same Src inhibitors diminished gossypol’s and ursolic acid’s neuroprotective properties. Thus, Thy-1 may belong to the group of receptors for gossypol and ursolic acid. The highly glycosylated mucin-type glycoprotein, CD24 with a peptide core of only 30 amino acids, expressed by neurons, interacts with L1 to stimulate cell adhesion and to increase intracellular calcium levels. This interaction promotes LeX-dependent neurite outgrowth [17, 62]. CD24 was shown to be a downstream target of Ral and hedgehog signaling pathways, and its expression mediates cell proliferation and survival of tumor cells [63, 64]. Although the cell signal transduction pathways triggered by CD24 are not well understood, CD24 has been suggested to regulate epidermal growth factor receptor (EGFR) signaling by inhibiting EGFR internalization in a RhoA-dependent manner [65]. Src family kinases mediate EGFR signaling in breast cancer cells [66] and are essential for gossypol- and ursolic acid-mediated neurite outgrowth and neuronal survival. Hence, CD24 may also belong to the set of receptors for these two compounds. Synapsin I carries LeX on O-glycans and modulates neurite outgrowth and neuron-glia interaction [67]. It is expressed in the nervous system of vertebrates and is crucial for neuronal development and neurotransmission [68]. Intriguingly, autoantibodies to human synapsin Ia and Ib are detectable in sera from patients with different psychiatric and neurological disorders [69]. Synapsin regulates Fyn-dependent brain-derived neurotrophic factor (BDNF)-mediated synaptic potentiation and neurite outgrowth [70]. Synapsin I contains phosphorylation sites for PKA, calcium/calmodulin-dependent protein kinases (CAMKs) IV and II, mitogen-activated protein kinase (MAPKs), Src- and cyclin-dependent kinase (CDK) 1 and 5. Compounds inhibiting Src, Fyn, and PKA decrease gossypol-, orlistat-, and ursolic acid-enhanced neurite outgrowth, suggesting that synapsin I belongs to the group of receptors important for neurite outgrowth mediated by these molecules. LRP1, a multiligand receptor abundantly expressed in the nervous system, enhances neurite outgrowth and promotes axonal regeneration after spinal cord injury in rat [71]. LRP1 triggers neurogenesis and neuroprotection via TrkC, Akt, and ERK signal transduction pathways [71]. Since Erk is essential for gossypol-, orlistat-, usolic acid-, and folic acid-mediated neurite outgrowth, LRP1 might be part of the receptor group triggered by these small compounds. The cell transduction mechanisms underlying the tosufloxacin-mediated neurite outgrowth and neuronal protection differ from the cell signaling triggered by the discussed LeX carrying glycoproteins. The combined compilation of potential LeX receptors will need verification and need to be accepted as incomplete. Nevertheless, all kinases involved in signal transduction pathways triggered by the cognate LeX carrying glycoproteins, are in agreement with our findings on the signaling activated by the LeX glycomimetic peptide. We thus propose that the LeX glycomimetic peptide binds to all the above mentioned LeX carrying proteins, which lead to a promotion of neurite outgrowth and neuronal survival. To test this view, different sets of receptors to which the different LeX mimetics bind will have to be identified in future studies.
Our combined findings are consistent with the hypothesis that the different LeX epitopes on different long neo-lacto glycan chains mediate neurite outgrowth and neuronal survival via diverse and distinct signal transducing pathways. The diversity of signal transducing pathways indicates that the different, here identified, LeX mimetics represent LeX epitopes on different glycoproteins, leading to different signal transduction mechanisms via different sets of receptors. We propose that the glycomimetics raise hopes for novel and powerful tools to study the heterogeneous nature and functional relevance of the LeX epitope, with the LeX glycomimetic peptide and gossypol being the most potent stimulators of neurite outgrowth and neuronal survival, thus possibly being promising to test in different models of traumatic nerve injury and neurodegenerative diseases.
Acknowledgments
The authors are funded in part by R01 grant R01NS078385-05 (to M.S.) from the National Institute of Neurological Disorders and Stroke. The support of the Li Kashing Foundation (to M.S.) is gratefully acknowledged.
References
- 1.Loers G, Schachner M (2007) Recognition molecules and neural repair. J Neurochem 101:865–882. 10.1111/j.1471-4159.2006.04409.x [DOI] [PubMed] [Google Scholar]
- 2.Jessell TM, Hynes MA, Dodd J (1990) Carbohydrates and carbohydrate-binding proteins in the nervous system. Annu Rev Neurosci 13:227–255. 10.1146/annurev.ne.13.030190.001303 [DOI] [PubMed] [Google Scholar]
- 3.Kleene R, Schachner M (2004) Glycans and neural cell interactions. Nat Rev Neurosci 5:195–208. 10.1038/nrn1349 [DOI] [PubMed] [Google Scholar]
- 4.Solter D, Knowles BB (1978) Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc Natl Acad Sci U S A 75:5565–5569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kruse J, Keilhauer G, Faissner A et al. (1985)The J1 glycoprotein—a novel nervous system cell adhesion molecule of the L2/HNK-1 family. Nature 316:146–148 [DOI] [PubMed] [Google Scholar]
- 6.Nishihara S, Iwasaki H, Nakajima K et al. (2003) Alpha1,3-fucosyltransferase IX (Fut9) determines Lewis X expression in brain. Glycobiology 13:445–455. 10.1093/glycob/cwg048 [DOI] [PubMed] [Google Scholar]
- 7.Kumar A, Torii T, Ishino Y et al. (2013) The Lewis X-related α1,3-fucosyltransferase, Fut10, is required for the maintenance of stem cell populations. J Biol Chem 288:28859–28868. 10.1074/jbc.M113.469403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bleckmann C, Geyer H, Lieberoth A et al. (2009) O-glycosylation pattern of CD24 from mouse brain. Biol Chem 390:627–645. 10.1515/BC.2009.044 [DOI] [PubMed] [Google Scholar]
- 9.Bleckmann C, Geyer H, Reinhold V et al. (2009) Glycomic analysis of N-linked carbohydrate epitopes from CD24 of mouse brain. J Proteome Res 8:567–582. 10.1021/pr800729r [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto M, Boyer AM, Schwarting GA (1985) Fucose-containing glycolipids are stage- and region-specific antigens in developing embryonic brain of rodents. Proc Natl Acad Sci U S A 82:3045–3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capela A, Temple S (2006) LeX is expressed by principle progenitor cells in the embryonic nervous system, is secreted into their environment and binds Wnt-1. Dev Biol 291:300–313. 10.1016/j.ydbio.2005.12.030 [DOI] [PubMed] [Google Scholar]
- 12.Koso H, Ouchi Y, Tabata Y et al. (2006) SSEA-1 marks regionally restricted immature subpopulations of embryonic retinal progenitor cells that are regulated by the Wnt signaling pathway. Dev Biol 292: 265–276. 10.1016/j.ydbio.2005.09.051 [DOI] [PubMed] [Google Scholar]
- 13.Streit A, Faissner A, Gehrig B, Schachner M (1990) Isolation and biochemical characterization of a neural proteoglycan expressing the L5 carbohydrate epitope. J Neurochem 55:1494–1506 [DOI] [PubMed] [Google Scholar]
- 14.Streit A, Nolte C, Rásony T, Schachner M (1993) Interaction of astrochondrin with extracellular matrix components and its involvement in astrocyte process formation and cerebellar granule cell migration. J Cell Biol 120:799–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karus M, Hennen E, Safina D et al. (2013) Differential expression of micro-heterogeneous LewisX-type glycans in the stem cell compartment of the developing mouse spinal cord. Neurochem Res 38:1285–1294. 10.1007/s11064-013-1048-6 [DOI] [PubMed] [Google Scholar]
- 16.Capela A, Temple S (2002) LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron 35: 865–875 [DOI] [PubMed] [Google Scholar]
- 17.Lieberoth A, Splittstoesser F, Katagihallimath N et al. (2009) Lewis(x) and alpha2,3-sialyl glycans and their receptors TAG-1, Contactin, and L1 mediate CD24-dependent neurite outgrowth. J Neurosci 29:6677–6690. 10.1523/JNEUROSCI.4361-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hennen E, Safina D, Haussmann U et al. (2013) A LewisX glycoprotein screen identifies the low density lipoprotein receptor-related protein 1 (LRP1) as a modulator of oligodendrogenesis in mice. J Biol Chem 288:16538–16545. 10.1074/jbc.M112.419812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hennen E, Faissner A (2012) LewisX: a neural stem cell specific glycan? Int J Biochem Cell Biol 44:830–833. 10.1016/j.biocel.2012.02.019 [DOI] [PubMed] [Google Scholar]
- 20.Kataria H, Lutz D, Chaudhary H et al. (2016) Small molecule agonists of cell adhesion molecule L1 mimic L1 functions in vivo. Mol Neurobiol 53:4461–4483. 10.1007/s12035-015-9352-6 [DOI] [PubMed] [Google Scholar]
- 21.Loers G, Astafiev S, Hapiak Y et al. (2017) The polysialic acid mimetics idarubicin and irinotecan stimulate neuronal survival and neurite outgrowth and signal via protein kinase C. J Neurochem. 10.1111/jnc.14076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahu S, Zhang Z, Li R et al. (2017) A small organic compound mimicking the L1 cell adhesion molecule promotes functional recovery after spinal cord injury in zebrafish. Mol Neurobiol. 10.1007/s12035-016-0254-z [DOI] [PubMed] [Google Scholar]
- 23.Xu J, Hu C, Jiang Q et al. (2017) Trimebutine, a small molecule mimetic agonist of adhesion molecule L1, contributes to functional recovery after spinal cord injury in mice. Dis Model Mech 10: 1117–1128. 10.1242/dmm.029801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saini V, Lutz D, Kataria H et al. (2016) The polysialic acid mimetics 5-nonyloxytryptamine and vinorelbine facilitate nervous system repair. Sci Rep 6:26927 10.1038/srep26927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bushman J, Mishra B, Ezra M et al. (2014) Tegaserod mimics the neurostimulatory glycan polysialic acid and promotes nervous system repair. Neuropharmacology 79:456–466. 10.1016/j.neuropharm.2013.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katagihallimath N, Mehanna A, Guseva D et al. (2010) Identification and validation of a Lewisx glycomimetic peptide. Eur J Cell Biol 89:77–86. https://doi.org/10.1016Zj.ejcb.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 27.Streit A, Yuen CT, Loveless RW et al. (1996) The Le(x) carbohydrate sequence is recognized by antibody to L5, a functional antigen in early neural development. J Neurochem 66:834–844 [DOI] [PubMed] [Google Scholar]
- 28.Fukui S, Feizi T, Galustian C et al. (2002) Oligosaccharide micro-arrays for high-throughput detection and specificity assignments of carbohydrate-protein interactions. Nat Biotechnol 20:1011–1017. 10.1038/nbt735 [DOI] [PubMed] [Google Scholar]
- 29.Maness PF, Schachner M (2007) Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci 10:19–26. 10.1038/nn1827 [DOI] [PubMed] [Google Scholar]
- 30.Li Y-L, Wu G-Z, Zeng L et al. (2009) Cell surface sialylation and fucosylation are regulated by the cell recognition molecule L1 via PLCgamma and cooperate to modulate embryonic stem cell survival and proliferation. FEBS Lett 583:703–710. 10.1016/j.febslet.2009.01.013 [DOI] [PubMed] [Google Scholar]
- 31.Gouveia R, Schaffer L, Papp S et al. (2012) Expression of glycogenes in differentiating human NT2N neurons. Downregulation of fucosyltransferase 9 leads to decreased Lewis(x) levels and impaired neurite outgrowth. Biochim Biophys Acta 1820:2007–2019. 10.1016/j.bbagen.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 32.Tuszynski GP, Cossu G (1984) Differential cytotoxic effect of gossypol on human melanoma, colon carcinoma, and other tissue culture cell lines. Cancer Res 44:768–771 [PubMed] [Google Scholar]
- 33.Kridel SJ, Axelrod F, Rozenkrantz N, Smith JW (2004) Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity. Cancer Res 64:2070–2075 [DOI] [PubMed] [Google Scholar]
- 34.Mazumder K, Tanaka K, Fukase K (2013) Cytotoxic activity of ursolic acid derivatives obtained by isolation and oxidative derivatization. Mol Basel Switz 18:8929–8944. 10.3390/molecules18088929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loers G, Saini V, Mishra B et al. (2014) Nonyloxytryptamine mimics polysialic acid and modulates neuronal and glial functions in cell culture. J Neurochem 128:88–100. 10.1111/jnc.12408 [DOI] [PubMed] [Google Scholar]
- 36.Coutinho EM (2002) Gossypol: a contraceptive for men. Contraception 65:259–263 [DOI] [PubMed] [Google Scholar]
- 37.BandaraNA Hansen MJ, Low PS (2014) Effect of receptor occupancy on folate receptor internalization. Mol Pharm 11:1007–1013. 10.1021/mp400659t [DOI] [PubMed] [Google Scholar]
- 38.Noumi T, Nishida N, Minami S et al. (1990) Intracellular activity of tosufloxacin (T-3262) against Salmonella enteritidis and ability to penetrate into tissue culture cells of human origin. Antimicrob Agents Chemother 34:949–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Desai J, Khatri N, Chauhan S, Seth A (2012) Design, development and optimization of self-microemulsifying drug delivery system of an anti-obesity drug. J Pharm Bioallied Sci 4:S21–S22. 10.4103/0975-7406.94124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao R, Li T, Zheng G et al. (2017) Simultaneous inhibition of growth and metastasis of hepatocellular carcinoma by co-delivery of ursolic acid and sorafenib using lactobionic acid modified and pH-sensitive chitosan-conjugated mesoporous silica nanocomplex. Biomaterials 143:1–16. 10.1016/j.biomaterials.2017.07.030 [DOI] [PubMed] [Google Scholar]
- 41.Kanje M, Ekstrom P, Deinum J, Wallin M (1986) The effect of gossypol on fast axonal transport and microtubule assembly. Biochim Biophys Acta 856:437–442 [DOI] [PubMed] [Google Scholar]
- 42.Hanus J, Zhang H, Chen DH et al. (2015) Gossypol acetic acid prevents oxidative stress-induced retinal pigment epithelial necrosis by regulating the FoxO3/Sestrin2 pathway. Mol Cell Biol 35:1952–1963. 10.1128/MCB.00178-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Ohizumi Y (2004) Search for constituents with neurotrophic factor-potentiating activity from the medicinal plants of Paraguay and Thailand. Yakugaku Zasshi 124:417–424 [DOI] [PubMed] [Google Scholar]
- 44.Ahrens K, Yazdy MM, Mitchell AA, Werler MM (2011) Folic acid intake and spina bifida in the era of dietary folic acid fortification. Epidemiol Camb Mass 22:731–737. 10.1097/EDE.0b013e3182227887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steenweg-de Graaff J, Roza SJ, Steegers EA et al. (2012) Maternal folate status in early pregnancy and child emotional and behaviora lproblems: the Generation R Study. Am J Clin Nutr 95:1413–1421. 10.3945/ajcn.111.030791 [DOI] [PubMed] [Google Scholar]
- 46.Suren P, Roth C, Bresnahan M et al. (2013) Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA 309:570–577. 10.1001/jama.2012.155925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snowdon DA, Tully CL, Smith CD et al. (2000) Serum folate and the severity of atrophy of the neocortex in Alzheimer disease: findings from the Nun study. Am J Clin Nutr 71:993–998 [DOI] [PubMed] [Google Scholar]
- 48.Wang HX, Wahlin A, Basun H et al. (2001) Vitamin B(12) and folate in relation to the development of Alzheimer’s disease. Neurology 56:1188–1194 [DOI] [PubMed] [Google Scholar]
- 49.Barry AL, Jones RN (1989) In-vitro activities of temafloxacin, tosufloxacin (A-61827) and five other fluoroquinolone agents. J Antimicrob Chemother 23:527–535 [DOI] [PubMed] [Google Scholar]
- 50.Noro E, Togayachi A, Sato T et al. (2015) Large-scale identification of N-glycan glycoproteins carrying Lewis X and site-specific N-glycan alterations in Fut9 knockout mice. J ProteomeRes 14:3823–3834. 10.1021/acs.jproteome.5b00178 [DOI] [PubMed] [Google Scholar]
- 51.Gourier C, Pincet F, Perez E et al. (2005) The natural LewisX-bearing lipids promote membrane adhesion: Influence of ceramide on carbohydrate-carbohydrate recognition. Angew Chem Int Ed Engl 44:1683–1687. 10.1002/anie.200461224 [DOI] [PubMed] [Google Scholar]
- 52.Allendoerfer KL, Durairaj A, Matthews GA, Patterson PH (1999) Morphological domains of Lewis-X/FORSE-1 immunolabeling in the embryonic neural tube are due to developmental regulation of cell surface carbohydrate expression. Dev Biol 211:208–219. 10.1006/dbio.1999.9308 [DOI] [PubMed] [Google Scholar]
- 53.Hakomori S (1992) Le(X) and related structures as adhesion molecules. Histochem J 24:771–776 [DOI] [PubMed] [Google Scholar]
- 54.Sytnyk V, Leshchyns’ka I, Schachner M (2017) Neural cell adhesion molecules of the immunoglobulin superfamily regulate synapse formation, maintenance, and function. Trends Neurosci 40:295–308. 10.1016/j.tins.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 55.Loers G, Chen S, Grumet M, Schachner M (2005) Signal transduction pathways implicated in neural recognition molecule L1 triggered neuroprotection and neuritogenesis. J Neurochem 92:1463–1476. 10.1111/j.1471-4159.2004.02983.x [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Schachner M (2015) The intracellular domain of L1CAM binds to casein kinase 2α and is neuroprotective via inhibition of the tumor suppressors PTEN and p53. J Neurochem 133:828–843. 10.1111/jnc.13083 [DOI] [PubMed] [Google Scholar]
- 57.Rege TA, Hagood JS (2006) Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J Off Publ Fed Am Soc Exp Biol 20:1045–1054. 10.1096/fj.05-5460rev [DOI] [PubMed] [Google Scholar]
- 58.Seki M, Nawa H, Morioka T et al. (2002) Establishment of a novel enzyme-linked immunosorbent assay for Thy-1; quantitative assessment of neuronal degeneration. Neurosci Lett 329:185–188 [DOI] [PubMed] [Google Scholar]
- 59.Yang S-H, Chen Y-J, Tung P-Y et al. (2008) Anti-Thy-1 antibody-induced neurite outgrowth in cultured dorsal root ganglionic neurons is mediated by the c-Src-MEK signaling pathway. J Cell Biochem 103:67–77. 10.1002/jcb.21387 [DOI] [PubMed] [Google Scholar]
- 60.Chen C-H, Chen Y-J, Jeng C-J et al. (2007) Role of PKA in the anti- Thy-1 antibody-induced neurite outgrowth of dorsal root ganglionic neurons. J Cell Biochem 101:566–575. 10.1002/jcb.21217 [DOI] [PubMed] [Google Scholar]
- 61.Rege TA, Hagood JS (2006) Thy-1, a versatile modulator of signaling affecting cellular adhesion, proliferation, survival, and cytokine/growth factor responses. Biochim Biophys Acta 1763: 991–999. 10.1016/j.bbamcr.2006.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kleene R, Yang H, Kutsche M, Schachner M (2001) The neural recognition molecule L1 is a sialic acid-binding lectin for CD24, which induces promotion and inhibition of neurite outgrowth. J Biol Chem 276:21656–21663. 10.1074/jbc.M101790200 [DOI] [PubMed] [Google Scholar]
- 63.Smith SC, Oxford G, Wu Z et al. (2006) The metastasis-associated gene CD24 is regulated by Ral GTPase and is a mediator of cell proliferation and survival in human cancer. Cancer Res 66:1917–1922. 10.1158/0008-5472.CAN-05-3855 [DOI] [PubMed] [Google Scholar]
- 64.Zeng C, Chen T, Zhang Y, Chen Q (2017) Hedgehog signaling pathway regulates ovarian cancer invasion and migration via adhesion molecule CD24. J Cancer 8:786–792. 10.7150/jca.17712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deng W, Gu L, Li X et al. (2016) CD24 associates with EGFR and supports EGF/EGFR signaling via RhoA in gastric cancer cells. J Transl Med 14:32 10.1186/s12967-016-0787-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Irwin ME, Bohin N, Boerner JL (2011) Src family kinases mediate epidermal growth factor receptor signaling from lipid rafts in breast cancer cells. Cancer Biol Ther 12:718–726. 10.4161/cbt.12.8.16907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang S, Cesca F, Loers G et al. (2011) Synapsin I is an oligomannose-carrying glycoprotein, acts as an oligomannose-binding lectin, and promotes neurite outgrowth and neuronal survival when released via glia-derived exosomes. J Neurosci 31: 7275–7290. 10.1523/JNEUROSCI.6476-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cesca F, Baldelli P, Valtorta F, Benfenati F (2010) The synapsins: key actors of synapse function and plasticity. Prog Neurobiol 91: 313–348. 10.1016/j.pneurobio.2010.04.006 [DOI] [PubMed] [Google Scholar]
- 69.Hültje M, Mertens R, Schou MB et al. (2017) Synapsin-antibodies in psychiatric and neurological disorders: Prevalence and clinical findings. Brain Behav Immun. 10.1016/j.bbi.2017.07.011 [DOI] [PubMed] [Google Scholar]
- 70.Kao H-T, Ryoo K, Lin A et al. (2017) Synapsins regulate brain-derived neurotrophic factor-mediated synaptic potentiation and axon elongation by acting on membrane rafts. Eur J Neurosci 45: 1085–1101. 10.1111/ejn.13552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoon C, Van Niekerk EA, Henry K et al. (2013) Low-density lipoprotein receptor-related protein 1 (LRP1)-dependent cell signaling promotes axonal regeneration. J Biol Chem 288:26557–26568. 10.1074/jbc.M113.478552 [DOI] [PMC free article] [PubMed] [Google Scholar]