Abstract
Gastrointestinal symptoms are among the most common complaints in patients with postural tachycardia syndrome (POTS). In some cases, they dominate the clinical presentation and cause substantial disabilities including significant weight loss and malnutrition that require the use of invasive treatment to support caloric intake. Multiple cross-sectional studies have reported a high prevalence of gastrointestinal symptoms in POTS patients with connective tissue diseases such as Ehlers Danlos, hypermobile type, and in patients with evidence of autonomic neuropathy. Previous studies that evaluated gastric motility in these patients reported a wide range of abnormalities, particularly delayed gastric emptying. The pathophysiology of gastrointestinal symptoms in POTS is likely multifactorial and probably depends on the co-morbid conditions. In patients with POTS and Ehlers Danlos syndrome, structural and functional abnormalities in the GI connective tissue may play a significant role; whereas in neuropathic POTS, the gastrointestinal tract motility, and gut hormonal secretion maybe directly impaired due to localized autonomic denervation. In patients with normal gastrointestinal motility but persistent gastrointestinal symptoms, gastrointestinal functional disorders should be considered. We performed a systematic review of the literature related to POTS and gastrointestinal symptoms, proposed possible mechanisms and discussed diagnosis and treatment approaches for delayed gastric emptying, the most common GI abnormality reported in POTS.
Keywords: Postural Tachycardia Syndrome, Gastroparesis, Nausea, Vomiting
Introduction
Postural Tachycardia Syndrome (POTS) is a heterogeneous condition, which mostly affects young women in their reproductive years. Clinically, POTS is characterized by a rapid increase in heart rate (≤30 bpm) that occurs within 10 minutes upon standing, in the absence of orthostatic hypotension, and chronic (>6 months) symptoms of cerebral hypoperfusion such as lightheadedness, blurred vision, and mental clouding [21]. These symptoms are worse while standing and improve after resting supine. In severe cases, POTS could induce substantial disability; data from a cross-sectional survey suggest that around 25% of patients with POTS file for disability and more than 50% of these patients disrupt their education [55].
POTS is a syndrome rather than a disease; multiple overlapping pathophysiologies have been proposed for POTS, including a partial peripheral neuropathy [9, 31], a deficit in blood volume, abnormalities in the renin-angiotensin-aldosterone system (RAAS) [32, 46, 53, 54], physical deconditioning [34] autoantibodies targeting peripheral adrenergic receptors [37], and a hyperadrenergic state resulting from central activation of the sympathetic nervous system [9].
Despite the heterogeneity of this syndrome, cardiovascular symptoms such as orthostatic tachycardia, chronic pre-syncopal symptoms and syncope often dominate its clinical presentation [21]. Orthostatic tachycardia could be a compensatory phenomenon to hypovolemia, impaired sympathetic-mediated vasoconstriction or increased vascular compliance. The latter could induce an exaggerated fluid shift upon standing from the thorax to the lower body. Depending on the mechanisms involved, different POTS phenotypes have been described: hypovolemic POTS, neuropathic POTS, primary hyperadrenergic POTS, and POTS associated with Ehlers-Danlos, hypermobile type (h-EDS) and mast cell activation syndrome (Figure 1). In clinical practice, there is overlap in the pathophysiology of POTS with patients having more than one etiology. Furthermore, POTS is commonly associated with other co-morbid conditions such as somatic hypervigilance, behavioral amplification, anxiety, depression, and high rates of suicidal ideation [9, 50].
Figure 1. Pathophysiology of Postural Tachycardia Syndrome and major sub-types.
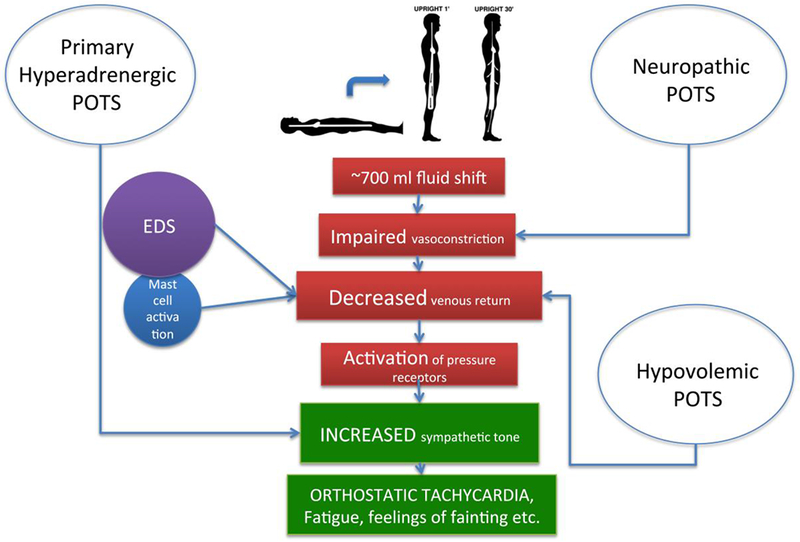
EDS, Ehlers Danlos syndrome
POTS patients also experience a wide array of noncardiovascular complaints, particularly gastrointestinal (GI) symptoms such as chronic nausea, vomiting, bloating, diarrhea or severe constipation. These symptoms are debilitating and have negative consequences as they limit oral intake of fluids and electrolytes, which is the core of POTS treatment. In severe cases, these patients may require intravenous parenteral nutrition or placement of a gastrostomy tube for feeding to treat severe weight loss and malnutrition.
Cross-sectional studies have shown an association between POTS and GI symptoms. The methods and techniques used to assess GI function in POTS varied significantly. Hence, it is difficult to understand the impact of these symptoms in POTS, and the possible mechanism(s) linked to these abnormalities. The purpose of this review article is to systematically examine the available literature on this particular topic by reviewing peer-reviewed articles, and publicly available literature.
Methods
Following the PRISMA guidelines for systematic reviews [38], we performed a comprehensive search in MedLine/PubMed, and Google Scholar for peer-reviewed articles, case series, cross-sectional studies and review manuscripts on gastrointestinal complications in POTS. We only included articles published in the English Language, until November 2017, see flowchart, figure 2. We used the key words “postural tachycardia syndrome, gastrointestinal symptoms,” “postural tachycardia syndrome gastric emptying,” “POTS gastrointestinal symptoms,” “POTS gastrointestinal complications.” We also searched the bibliographies listed in the two review manuscripts that result from our initial search, for additional references to ensure a thorough and complete review of the literature. For specific co-morbid conditions such as h-EDS and gluten sensitivity, we only included the studies that used these terms in addition to the key words “postural tachycardia syndrome, POTS, and orthostatic intolerance.” Single case reports, studies in pregnant women and in pediatric POTS population were excluded in this review article because these cases by definition are unique and may not represent the general POTS population.
Figure 2.
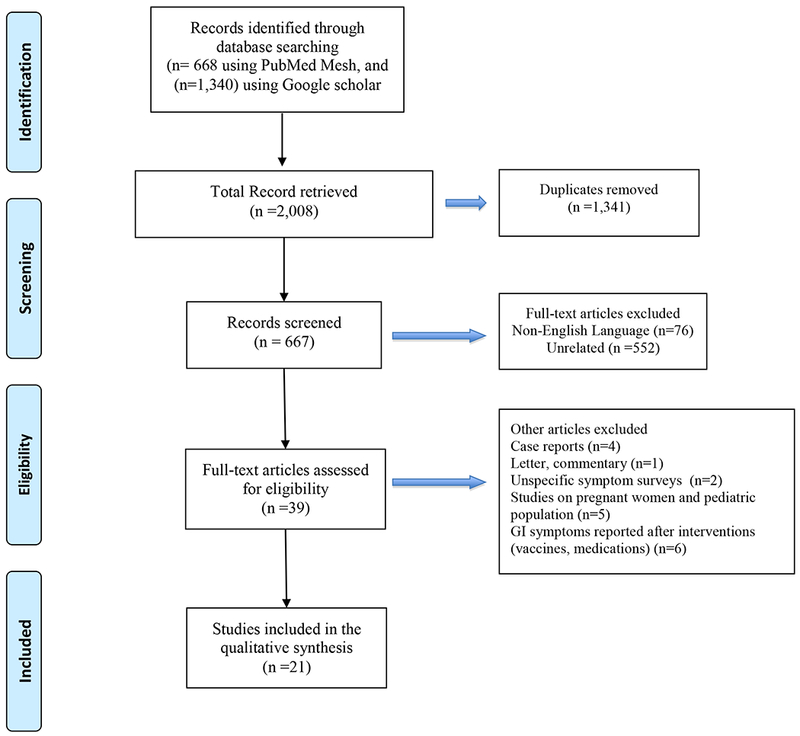
PRISMA flow diagram for systematic review of the literature. On POTS and gastrointestinal symptoms.
Results
We identified a total of 21 manuscripts, six case-control studies, two case series, four cross-sectional studies, six retrospective case series, two review articles, and one editorial. The list of manuscripts, authors, and publication information included in this review are presented in Table 1.
Table 1.
Listing of Peer-Reviewed Literature
| Author (s) | Title | Publication Information |
|---|---|---|
| Retrospective case series | ||
| Al-Shekhlee et al. [5] | The value of autonomic testing in postural tachycardia syndrome | Clinical Autonomic Research. 2005,15:219-222. |
| Antiel et al. [6] | Orthostatic intolerance and gastrointestinal motility in adolescents with nausea and abdominal pain | Journal of Pediatric Gastroenterology and Nutrition. 2008, 46:285-288. |
| Loavenbruck et al. [37] | Disturbances of gastrointestinal transit and autonomic functions in postural orthostatic tachycardia syndrome. | Neurogastroenterology and Motility. 2015, 27:92-98. |
| Nelson et al. [45] | Ehlers Danlos syndrome and gastrointestinal manifestations: a 20-year experience at Mayo Clinic. | Neurogastroenterology and Motility. 2015, 27:1657-1666. |
| Park et al. [46] | Gastric emptying in postural tachycardia syndrome: a preliminary report. | Clinical Autonomic Research. 2013, 23:163-167. |
| Review | ||
| Benarroch [10] | Postural tachycardia syndrome: a heterogeneous and multifactorial disorder. | Mayo Clinic Proceedings. 2012, 87:1214-1225. |
| Fikree et al. [21] | Gastrointestinal involvement in the Ehlers-Danlos syndromes. | American Journal of Medical Genetics. Part C, Seminars in Medical Genetics. 2017, 175:181-187. |
| Cross-sectional | ||
| De Wandele et al. [20] | Clinical heterogeneity in patients with the hypermobility type of Ehlers-Danlos syndrome. | Research in Developmental Diabetes. 2013, 34:873. |
| Lawal et al. [35] | Rapid gastric emptying is more common than gastroparesis in patients with autonomic dysfunction. | The American Journal of Gastroenterology. 2007 102:618-623. |
| Seligman et al. [56] | Abnormal gastric myoelectrical activity in postural tachycardia syndrome. | Clinical Autonomic Research. 2013, 23:73-80. |
| Wang et al. [64] | Gastrointestinal dysfunction in postural tachycardia syndrome. | J Neurol Sci. 2015, 359:193-196. |
| Case control | ||
| Chaudhuri et al. [16] | Abnormality of superior mesenteric artery blood flow responses in human sympathetic failure. | The Journal of Physiology. 1992, 457:477-489 |
| Gazit et al. [24] | Dysautonomia in the joint hypermobility syndrome. | The American Journal of Medicine. 2003, 115:33-40. |
| Gibbons et al. [25] | Structural and functional small fiber abnormalities in the neuropathic postural tachycardia syndrome. | PloS One. 2013, 8:e84716. |
| Penny et al. [48] | Is there a relationship between gluten sensitivity and postural tachycardia syndrome? | European Journal of Gastroenterology & Hepatology. 2016, 28:1383-1387. |
| Tani et al. [61] | Splanchnic-mesenteric capacitance bed in the postural tachycardia syndrome (POTS). | Autonomic Neuroscience:Basic & Clinical. 2000,86:107-113. |
| Editorial | ||
| Hadjivassiliou et al. | Gluten sensitivity as a neurological illness. | Journal of Neurology, Neurosurgery, and Psychiatry. 2002, 72:560-563. |
Postural Tachycardia Syndrome and Gastrointestinal Symptoms
In POTS patients, nausea and abdominal pain are the most frequent noncardiovascular symptoms; pooled data from six studies (N=352 patients) show a prevalence of ≈69%, Table 2. Of note, abnormalities in GI motility provoke symptoms such as early satiety, nausea, bloating, and abdominal pain [30]. Four of these studies that describe the GI symptoms in POTS also reported evidence of abnormal gastric motility- either rapid gastric emptying (43%) or delayed gastric emptying (gastroparesis, 20%) [5, 36, 48, 67].
Table 2.
Prevalence of gastrointestinal symptoms in POTS patients
| Author (s) | Title | Population(n) | Gastrointestinal symptoms | GI motility studies |
|---|---|---|---|---|
| Lawal et al. (2007)[35] | Rapid Gastric Emptying Is More Common than Gastroparesis in Patients With Autonomic Dysfunction | 61 POTS (all with mild to moderate CASS*) | 49(80%) Nausea, 28(46%) Vomiting, 54 (67%) Abdominal pain | 27 (44.2%) Rapid, 17 (27.9) Delayed, 17 (27.9) Normal |
| Antiel et al. (2008)[6] | Orthostatic Intolerance and Gastrointestinal Motility in Adolescents With Nausea and Abdominal Pain | 21 POTS (24% with abnormal sudomotor function) | 15 (71%) Nausea, 10 (47%) Vomiting, 14 (67%) Abdominal pain | 5 (24%) Rapid, 4 (19%) Delayed, 12 (57%) Normal |
| Wang et al. (2015)[64] | Gastrointestinal dysfunction in postural tachycardia syndrome | 28 POTS | 24 (86%) Nausea, 11 (40%) Vomiting, 20 (70%) Abdominal pain | NA |
| Loavenbruck et al. (2015)[37] | Ehlers Danlos syndrome and gastrointestinal manifestations: a 20-year experience at Mayo Clinic. | 163 POTS (36% with abnormal sudomotor function) | 34 (21%) Nausea, 16 (10%) Vomiting | 78 (48%) Rapid, 30 (18%) Delayed, 5 (34%) Normal |
| Park et al. (2013)[46] | Gastric emptying in postural tachycardia syndrome: a preliminary report. | 22 POTS (32% with abnormal sudomotor function) | 18 (82%) Nausea or vomiting, 8 (59%) Abdominal pain | 6 (27%) Rapid, 2 (9%) Delayed, 14 (63%) Normal |
| Al-Shekhlee et al. (2005)[5] | The value of autonomic testing in postural tachycardia syndrome | 19 neuropathic-POTS/38 non-neuropathic POTS | 13 (68%)/10(26%) Combined symptoms (nausea and vomiting) | NA |
CASS, composite autonomic scoring scale
Only one study evaluated the pathophysiology of the abnormal gastric motility in POTS. Seligman et al. [58] reported the gastric myoelectrical activity in 15 POTS patients versus 11 healthy individuals before and after a standard liquid meal ingestion. The author showed greater pre-and post-prandial variability in the gastric pacemaker rhythm in POTS patients compared to controls.
Three different studies reported that the majority of POTS patients with GI symptoms have evidence of autonomic neuropathy. For instance, Loavenbruck et al. [39] and Park et al. [48] showed that nearly 30% of POTS patients with GI symptoms have an abnormal quantitative sudomotor axon reflex test (QSART), which is a surrogate measure of post-ganglionic autonomic innervation. Al-Shekhlee et al. [4] stratified the occurrence of GI symptoms based on the presence or absence of autonomic neuropathy using QSART. In this study, POTS patients with autonomic neuropathy had 68% prevalence of GI symptoms compared with 26% in patients without evidence of autonomic neuropathy.
One of the major challenges when comparing studies is the lack of consensus on the definition and diagnosis of autonomic neuropathy in POTS. Our group previously defined neuropathic POTS as a subgroup of POTS with a partial peripheral autonomic neuropathy primarily affecting lower extremities. Neuro-hormonal characterization of these patients showed a decrease in norepinephrine spillover in response to sympathetic activation in the legs but not in the arms. Furthermore, these patients had an abnormal sweat volume and prolonged latency using QSART [31].
Gibbons and Freeman [25] defined neuropathic POTS as a reduced intra-epidermal nerve fiber density through skin biopsies with impaired sensory or sudomotor function. It is noteworthy that there is not concordance between the different markers of autonomic neuropathy (abnormal skin biopsies, sudomotor testing or quantitative sensory analyses) in the same patient. The definition of neuropathic POTS should use a combination of different biomarkers to define this condition.
In summary, the review of the available literature on GI symptoms in POTS showed that these symptoms particularly early satiety, nausea, bloating, and abdominal pain are very common in POTS; these symptoms are associated with abnormalities in GI motility. Abnormal gastric pacemaker rhythm has been proposed as the underlying mechanism. However, there is a paucity of data with only one study reporting differences between POTS versus controls. Moreover, the prevalence of GI symptoms is greater in POTS patients with autonomic neuropathy. These findings suggest a possible mechanistic link between GI symptoms, abnormal GI motility and autonomic neuropathy in POTS.
Postural Tachycardia Syndrome, Gastrointestinal Symptoms, and related co-morbidities GI symptoms in Postural Tachycardia Syndrome and Ehlers-Danlos
Ehlers-Danlos is a group of heritable disorders of connective tissue [8], and according to the recent classification of the International EDS Consortium, there are 13 major types of EDS [41]. Type V (formerly known as EDS-III) or h-EDS (hypermobile-EDS) is one of the most common varieties, affecting 1 in 5000 individuals, and this type is frequently associated with POTS [24]. h-EDS is a chronic, disabling condition, characterized by large and small joint and spine hypermobility, frequent joint dislocation, chronic pain and chronic GI symptoms [13]. GI co-morbid conditions in EDS include reflux, dysphagia, hiatal hernias, visceroptosis, rectocele, and rectal prolapse, as well as GI functional disorders related to abnormal gut motility in fore- and hind-gut [20, 68]. In patients with h-EDS, the GI symptoms are very prominent and often present as the primary complaint.
In a retrospective observational study, around 60% of patients with colonic transit abnormalities had h-EDS, and 22% had abnormal gastric emptying [47]. In a cohort of patients with h-EDS (N=78), De Wandele et al. using self-reported questionnaires described that GI symptoms such as motility dysfunction, bloating and swallowing problems clustered with cardiovascular symptoms such as POTS and other forms of orthostatic intolerance [18] .
More recently, Fikree et al. studied a similar patient population (N=30 h-EDS; 12 without POTS and 18 with POTS) and performed GI physiological studies (multichannel intraluminal impedance-pH testing and high-resolution manometry testing). In this cohort, 53% of patients had pathological acid reflux, 21% had hypersensitive reflux, and 25% had functional reflux. Furthermore, 60% had functional dysphagia with normal esophageal motility. The POTS group was more likely to have esophageal hypomobility and pathological gastro-esophageal reflux [19].
It remains unknown why patients with POTS and h-EDS have a higher prevalence of GI symptoms and dysmotility. Structural and functional abnormalities in the GI connective tissue may play a significant role; however, further studies are needed.
Postural Tachycardia Syndrome and Gluten sensitivity
Celiac disease (CD) and non-celiac gluten sensitivity (NCGS) are immune-mediated small bowel enteropathies [14]. GI symptoms such as abdominal pain, bloating and diarrhea is frequent in both conditions. Previous studies also reported cardiovascular symptoms such as palpitations, dizziness, and presyncope in CD and NCGS suggesting autonomic dysfunction [27, 33]. However, the association between POTS and CD is still debatable.
Only one study met our eligibility criteria. Penny et al., [51] reported that the prevalence of CD was 4% in a large POTS cohort; this prevalence was higher than the one reported in a control group (1%) matched for age and gender (OR: 4.1, 95% CI:1.3-13.0; p=0.03). The prevalence of self-reported gluten sensitivity and IBS were also higher in the POTS cohort than in controls. Approximately 6% of POTS patients complained of gluten sensitivity despite having negative celiac serology and normal duodenal biopsy. The mechanism by which gluten sensitivity may result in neurological complications including autonomic dysfunction has not been elucidated. Among the possible culprits are: 1) malabsorption of nutrients such as thiamine, folic acid, vitamin B12, and vitamin E that are important for neuronal protection and plasticity [17, 42, 66]; 2) the presence of antigliadin and transglutaminase antibodies which can target neural tissue particularly in the central nervous system [12, 26, 59]; 3) the co-existence with other autoimmune disorders commonly observed in POTS. Even though patients are likely to reduce gluten in their diet, there are no well-controlled studies in POTS patients with either CD or NCGS that evaluate the efficacy of gluten-free diet on GI and cardiovascular symptoms in this population.
Proposed Pathophysiology for Gastrointestinal Symptoms in Postural Tachycardia Syndrome
In normal circumstances, an autonomous integrated neural mechanism controls GI tract motility, secretion, and blood flow to enable digestion, absorption of fluid and nutrients and elimination of waste. Different neuronal cell types and glia formed the enteric nervous system (ENS) that dwell in the myenteric and submucosal plexus of the gastrointestinal tract (GIT). GI motility is primarily under the control of the myenteric plexus between the longitudinal and circular muscles. The submucosal plexus, located between circular muscles and the GI mucosa, regulates fluid absorption, secretion, and blood flow, and responds to exogenous stimuli to maintain GI function [7].
While the ENS provides a significant degree of autonomy for different functions of the GIT, the central nervous system sends extrinsic neural inputs that modulate and control these functions as well. In contrast to the stomach and the esophagus, the small and large intestine show a remarkable amount of independence from CNS control [11].
Denervation of the stomach causes abnormal gastric electrical activity and produces GI symptoms such as nausea and vomiting, abdominal discomfort pain, and diarrhea. However, the gastric activity partly recovers after a period of time [11]. Extrinsic innervation of the GIT is mainly through sympathetic and parasympathetic afferent and efferent nerves.
The sympathetic nervous system (SNS) provides innervation to the enteric ganglia, the circular muscles of sphincters, and the mucosa of the stomach and intestines [40]. The SNS also negatively regulates its motor and secretory functions. The absence of sympathetic inhibitory innervation, as seen in POTS patients with partial autonomic neuropathy, may cause excessive and uncoordinated activity in the GI tract [11]. This could be, in theory, a possible mechanism to explore when evaluated the pathophysiology of GI symptoms in POTS, particularly the neuropathic type. It is noteworthy that patients with a higher occurrence of GI complications also complaint of increased post-prandial orthostatic symptoms. Therefore, it is possible that an uncoupling effect between the neuromuscular and neurovascular regulation of the splanchnic regulation induce an increase in the blood pooling in this vascular bed, which could worsen the orthostatic symptoms. This hypothesis, however, requires further studies.
Postural Tachycardia Syndrome, Gastrointestinal Symptoms and Splanchnic Circulation
In a healthy person, more than one-third of the total blood volume is compartmentalized in the splanchnic vascular bed which receives up to 25% of the resting cardiac output [62]. The splanchnic circulation is a larger reservoir than the limbs, therefore is particularly crucial during postural changes and after meals. A standard meal results in about a ~300% increase in mesenteric blood volume [23]. Previous studies in humans showed that the splanchnic blood flow is modulated through sympathoneural stimulation [10, 62].
In the Vanderbilt Autonomic Dysfunction Center, POTS patients usually complain of worsening pre-syncopal symptoms and tachycardia after meals, particularly meals rich in carbohydrates. Eating small meals throughout the day and limiting carbohydrate content in their diet curbs the pre-syncopal symptoms. We observed a very similar pattern in our patients with severe autonomic failure and neurogenic orthostatic hypotension; these patients have severe post-prandial hypotension. The hemodynamic changes after a meal are related to the release of vasoactive gut hormones that increase splanchnic blood sequestration. Chaudhuri et al., [15] showed that in patients with autonomic failure, active vasoconstriction of the superior mesenteric artery (SMA) after a sympathetic stimulus does not occur which could contribute to the post-prandial hypotension. In these patients, the increased blood sequestration in the splanchnic circulation is related to the release of vasoactive gut hormones. It is plausible, therefore, that similar hemodynamic changes occur after meal ingestion in POTS patients but to a lesser extent than in autonomic failure.
In this context, Tani et al. [64] reported an increase in resting mesenteric blood flow and reduced splanchnic vascular resistance in spite of normal systemic vascular resistance. After a meal, POTS patients had a larger increase in mesenteric blood flow than in controls, and this suggests that they have excessive splanchnic capacitance during resting conditions and possibly after a meal. The increase splanchnic capacitance in POTS, and the augmented requirements of the SNS to counteract these changes, could explain in part, the worsening orthostatic tolerance in POTS patients after meals.
Diagnosis and Treatment of Impaired Gastric Motility in POTS
As previously discussed, POTS patients, particularly those with h-EDS and autonomic neuropathy have a high prevalence of abnormal gastric motility. On the other hand, POTS patients also have a high prevalence of functional GI disorders. Treatment differs from these conditions, and therefore a careful evaluation is needed to provide an adequate diagnosis. In addition to a careful history and physical examination that include the removal of medications that could affect GI motility; some patients may also require gastric motility studies.
In table 3, we summarized all the methods available to evaluate gastric motility and discussed the advantages and disadvantages of each technique. The majority of the studies that have evaluated gastric motility in POTS used gamma camera scintigraphy because this is a non-invasive test that is available in most radiological centers. However, these studies differ in the diet used and duration of the measurement. The American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine published a consensus standard for gastric emptying studies that include standardization of diet and appropriate duration of the study (4 hours). Future studies should use these recommendations and compare results with the current normative data [2].
Table 3.
Comparison of different methods to assess gastric motility in humans
| Diagnostic Test | Advantages | Disadvantages |
|---|---|---|
| Gamma camera scintigraphy | Available for clinical use, allows direct measurement of gastric motility, non-invasive. | Radiation exposure, prolonged study |
| Wireless pH and motility capsule | Non-invasive, direct measurement of gastric and intestinal motility | Limits patient’s activity, risk of capsule retention |
| Stable isotope breath tests | Non-invasive measurement, applicable for children and pregnant women | Lack of standardized meal |
| Magnetic resonance image | Measures multiple parameters, assesses emptying fat and water separately | Can be used in supine position, not commonly available, elevated cost, subject must hold breath during testing |
| Balloon measurement | Gold standard for measurement of volume | Needs intubation |
| SPECT | Non-invasive, rate and volume measurement simultaneously | Not widely available, only used in supine position |
| Manometry | Records pressure of distal stomach, pylorus, and duodenum | Invasive and painful |
| Acetaminophen intestinal absorption test | Non-invasive, non-expensive | Just for liquid phase of gastric emptying, need several blood draws |
One of the most common conditions reported in POTS is delayed gastric emptying or gastroparesis. This is a difficult and still ill-defined syndrome to manage, with few effective treatments and a paucity of well-controlled trials. The symptoms are non-specific, and the gold standard diagnostic test remains the 4-hour gastric emptying scintigraphy with a solid meal.
Medical Treatment
In the literature, there are no clinical trials that test the effect of specific interventions in patients with POTS and gastrointestinal symptoms. Furthermore, there are no studies that evaluated differences in the pathophysiology of the most common gastrointestinal conditions described in POTS versus patients without POTS with similar symptoms.
In the following section, we briefly summarized the current medical, and surgical treatment approaches for the most common gastrointestinal symptoms reported in POTS based on evidence from the literature derived from studies that enrolled non-POTS patients with these conditions.
Dietary modifications
Dietary modifications are among the first measures to be taken in the treatment of gastroparesis. In addition to addressing nutritional deficiencies that are often present, such changes can impact the severity of the symptoms experienced by patients. Many gastroparetic patients have intakes deficient in calories, vitamins, and minerals and often tend to reduce meals to one or two foods or food categories. Dietary recommendations include modifications to meal content and frequency. More liquid-based or blenderized meals are emphasized, as the gastric emptying of liquids appears more conserved in gastroparesis. Further, reducing the size of single meals and consuming 6-8 small feedings/day also appear helpful[44, 45]. Reduction in fat and fiber intake has also been recommended, as these tend to prolong gastric emptying. Smoking cessation should also be emphasized as smoking may worsen gastric function. Compliance with these measures is poor.
Pharmacological Therapy
The pharmacotherapy of gastroparesis is aimed at increasing gastric emptying, reducing nausea and promoting a sense of well-being. The most commonly used medications are discussed in the next section.
Prokinetic agents
This group of drugs increases antral contractility, reduces gastric dysrhythmias, and improves coordination between the antrum and duodenum. The effect is modest, and responses are assessed clinically [63]. There is a poor correlation between the improvement in gastric emptying secondary to use of pro-kinetic agents and the improvement in symptoms.
The macrolide, erythromycin is a potent prokinetic through activating the motilin receptors, but the rapid onset of tachyphylaxis and many adverse effects precludes long-term efficacy and use. Metoclopramide is currently the only US Food and Drug Administration (FDA)-approved medication for the treatment of gastroparesis. It is mainly a dopamine type 2 receptor (D2) antagonist, aiding in the release of acetylcholine which increases antral contractility, antroduodenal tone, and gastric emptying [52]. Long-term data on the efficacy of metoclopramide is not available. Its most serious side effect is the occurrence of extrapyramidal movement disorders, tardive dyskinesia, and akathisia in a significant number of patients (30-40%). Drowsiness, fatigue, and depression also occur frequently. The Food and Drug Administration has a black box warning against the chronic use of metoclopramide [56]. Domperidone, another D2-antagonist, has much less penetration through the blood-brain barrier when compared to metoclopramide. In use throughout the world, it has not been approved by the FDA, because of unconvincing efficacy data, although it possesses a good safety profile [61].
Anti-emetic agents
Nausea is the most common gastroparesis symptom, reported by up to 90% to 95% of patients, although only 55%-80% report vomiting [16]. It is, therefore, no surprise that antiemetic agents without prokinetic activity are often used in addressing symptoms of gastroparesis. Antiemetic therapy comprises 5-HT3-antagonists (ondansetron), cannabinoids (Marinol), benzodiazepines (lorazepam) and H1-antagonists (promethazine); all can be of benefit in relieving nausea [28]. Symptom improvement at 48 weeks did not differ between patients using and not using antiemetics, suggesting that anti-emetics provide at best a modest and short-lived benefit. Histamine H1 antagonists (i.e., promethazine, meclizine, dimenhydrinate), although used in gastroparetic patients, have been more useful in conditions with activation of the vestibular system and in postoperative settings. However, promethazine as a phenothiazine analog exerts its central antiemetic effects partly through D2 receptors antagonism [60]. Psychotropic agents, such as tricyclic antidepressants at doses lower than those used to treat depression, are also frequently used for refractory symptoms of nausea, vomiting, and abdominal pain [49]. Evidence of efficacy for this use is lacking.
Surgical Treatment
Failure of nutritional and dietary and intensive medical treatments and the presence of severe nutritional deficits are often reasons for seeking surgical treatment. The consensus is lacking for surgical and endoscopic treatment options, reflective of the highly variable responses of patients. Endoscopic treatment of gastroparesis involves the injection of Botox into the pyloric sphincter. Although a large percentage of patients respond initially, the results are temporary and second injections do not result in any added benefit [6, 22, 35]. Currently, Botox injections are used sparingly and do not reliably predict responses to surgical pyloroplasty.
In patients with severe gastroparesis refractory to medical management, particularly in those with malnutrition, surgical placement of a gastro-jejunostomy or jejunostomy tube can be performed. This provides an effective means of infusing fluids, nutrition, and medications if oral intake is not tolerated. The procedures can be performed via endoscopy or laparoscopy; the combined use of a gastro-jejunal tube allows for simultaneous gastric decompression.
Gastric electrical stimulation (GSE) is a surgical option that was granted humanitarian approval from the FDA in 2000 for the treatment of chronic, refractory nausea and vomiting for diabetic or idiopathic gastroparesis. Approval was based on a study showing improvement in gastroparesis symptoms and gastric emptying [3]. Long-term studies have confirmed that about 70% of patients treated with GSE have significantly decreased gastrointestinal symptoms, improved quality of life and recovery of nutritional status that can last for several years [1, 43]. Even though GI symptoms improve, there is little data showing improvement in gastric emptying.
Since the GES does not improve gastric emptying, several groups have considered that a surgical pyloroplasty may improve gastric emptying by creating a larger outlet. Surgical pyloroplasty can be safely performed either alone or in conjunction with GES implantation. Although injection of Botox can result in a transient decrease in symptoms, unfortunately, this does not predict a positive response to pyloroplasty [29, 57, 65]. Moreover, many of the patients with an initial response to pyloroplasty alone, require subsequent placement of GES [29]. Controlled, randomized studies are needed to confirm these preliminary positive results.
As a last resort, sub-total or total gastrectomy has been undertaken in patients refractory to all other treatment modalities. Although some positive results have been reported, long-term nutritional deficiencies persist.
Conclusions
In addition to the cardiovascular symptoms, POTS patients have ≈69 % prevalence of GI symptoms and impaired GI motility such as delayed gastric emptying. Furthermore, meals not only trigger GI symptoms but also worsen orthostatic intolerance in POTS. Cross-sectional studies suggest that GI symptoms and GI abnormal motility are more frequent in patients with h-EDS and neuropathic POTS than in other groups. The field is in need of further studies that address the pathophysiology of these symptoms. These studies should aim to assess differences in autonomic regulation of GI-motility, GI-hormonal secretion and GI-splanchnic circulation between patients with POTS sub-groups and normal controls.
Acknowledgement
Source of Funding
S.E.M. is supported by grant NIH 5T32GM007569, C.A.S. was supported by Doris Duke Foundation Career Development Award. The study was supported by Vanderbilt CTSA, NACTS/NIH grant 1UL1 RR000445, and NINDS/NIH U54NS065736
Footnotes
Conflict of Interest
C.S. is consultant for Lundbeck Pharmaceuticals.
References
- 1.Abell T, McCallum R, Hocking M, Koch K, Abrahamsson H, Leblanc I, Lindberg G, Konturek J, Nowak T, Quigley EMM, Tougas G, Starkebaum W (2003) Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology 125:421–428 [DOI] [PubMed] [Google Scholar]
- 2.Abell TL, Camilleri M, Donohoe K, Hasler WL, Lin HC, Maurer AH, McCallum RW, Nowak T, Nusynowitz ML, Parkman HP, Shreve P, Szarka LA, Snape WJ, Ziessman HA, Medicine ANaMSatSoN (2008) Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. The American Journal of Gastroenterology 103:753–763 [DOI] [PubMed] [Google Scholar]
- 3.Abell TL, Van Cutsem E, Abrahamsson H, Huizinga JD, Konturek JW, Galmiche JP, VoelIer G, Filez L, Everts B, Waterfall WE, Domschke W, Bruley des Varannes S, Familoni BO, Bourgeois IM, Janssens J, Tougas G (2002) Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion 66:204–212 [DOI] [PubMed] [Google Scholar]
- 4.Al-Shekhlee A, Lindenberg JR, Hachwi RN, Chelimsky TC (2005) The value of autonomic testing in postural tachycardia syndrome. Clinical Autonomic Research: Official Journal of the Clinical Autonomic Research Society 15:219–222 [DOI] [PubMed] [Google Scholar]
- 5.Antiel RM, Risma JM, Grothe RM, Brands CK, Fischer PR (2008) Orthostatic intolerance and gastrointestinal motility in adolescents with nausea and abdominal pain. Journal of Pediatric Gastroenterology and Nutrition 46:285–288 [DOI] [PubMed] [Google Scholar]
- 6.Arts J, Holvoet L, Caenepeel P, Bisschops R, Sifrim D, Verbeke K, Janssens J, Tack J (2007) Clinical trial: a randomized-controlled crossover study of intrapyloric injection of botulinum toxin in gastroparesis. Alimentary Pharmacology & Therapeutics 26:1251–1258 [DOI] [PubMed] [Google Scholar]
- 7.Avetisyan M, Schill EM, Heuckeroth RO (2015) Building a second brain in the bowel. The Journal of Clinical Investigation 125:899–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ (1998) Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK). American Journal of Medical Genetics 77:31–37 [DOI] [PubMed] [Google Scholar]
- 9.Benarroch EE (2012) Postural tachycardia syndrome: a heterogeneous and multifactorial disorder. Mayo Clinic Proceedings 87:1214–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradley SE, Childs AW, Combes B, Cournand A, Wade OL, Wheeler HO (1956) The effect of exercise on the splanchnic blood flow and splanchnic blood volume in normal man. Clinical Science 15:457–463 [PubMed] [Google Scholar]
- 11.Browning KN, Travagli RA (2014) Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Comprehensive Physiology 4:1339–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caio G, De Giorgio R, Venturi A, Giancola F, Latorre R, Boschetti E, Serra M, Ruggeri E, Volta U (2015) Clinical and immunological relevance of anti-neuronal antibodies in celiac disease with neurological manifestations. Gastroenterology and Hepatology from Bed to Bench 8:146–152 [PMC free article] [PubMed] [Google Scholar]
- 13.Castori M, Morlino S, Celletti C, Celli M, Morrone A, Colombi M, Camerota F, Grammatico P (2012) Management of pain and fatigue in the joint hypermobility syndrome (a.k.a. Ehlers-Danlos syndrome, hypermobility type): principles and proposal for a multidisciplinary approach. American Journal of Medical Genetics. Part A 158A:2055–2070 [DOI] [PubMed] [Google Scholar]
- 14.Catassi C, Rätsch IM, Fabiani E, Rossini M, Bordicchia F, Candela F, Coppa GV, Giorgi PL (1994) Coeliac disease in the year 2000: exploring the iceberg. Lancet (London, England) 343:200–203 [DOI] [PubMed] [Google Scholar]
- 15.Chaudhuri KR, Thomaides T, Mathias CJ (1992) Abnormality of superior mesenteric artery blood flow responses in human sympathetic failure. The Journal of Physiology 457:477–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cherian D, Parkman HP (2012) Nausea and vomiting in diabetic and idiopathic gastroparesis. Neurogastroenterology and Motility: The Official Journal of the European Gastrointestinal Motility Society 24:217–222, e103 [DOI] [PubMed] [Google Scholar]
- 17.Chin RL, Sander HW, Brannagan TH, Green PHR, Hays AP, Alaedini A, Latov N (2003) Celiac neuropathy. Neurology 60:1581–1585 [DOI] [PubMed] [Google Scholar]
- 18.De Wandele I, Rombaut L, Malfait F, De Backer T, De Paepe A, Calders P (2013) Clinical heterogeneity in patients with the hypermobility type of Ehlers-Danlos syndrome. Research in Developmental Disabilities 34:873–881 [DOI] [PubMed] [Google Scholar]
- 19.Fikree A, Aziz Q, Sifrim D (2017) Mechanisms underlying reflux symptoms and dysphagia in patients with joint hypermobility syndrome, with and without postural tachycardia syndrome. Neurogastroenterol Motil 29(6) [DOI] [PubMed] [Google Scholar]
- 20.Fikree A, Chelimsky G, Collins H, Kovacic K, Aziz Q (2017) Gastrointestinal involvement in the Ehlers-Danlos syndromes. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics 175:181–187 [DOI] [PubMed] [Google Scholar]
- 21.Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz I, Schondorff R, Stewart JM, van Dijk JG (2011) Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 21:69–72 [DOI] [PubMed] [Google Scholar]
- 22.Friedenberg FK, Palit A, Parkman HP, Hanlon A, Nelson DB (2008) Botulinum toxin A for the treatment of delayed gastric emptying. The American Journal of Gastroenterology 103:416–423 [DOI] [PubMed] [Google Scholar]
- 23.Fujimura J, Camilleri M, Low PA, Novak V, Novak P, Opfer-Gehrking TL (1997) Effect of perturbations and a meal on superior mesenteric artery flow in patients with orthostatic hypotension. J Auton Nerv Syst 67:15–23 [DOI] [PubMed] [Google Scholar]
- 24.Gazit Y, Nahir AM, Grahame R, Jacob G (2003) Dysautonomia in the joint hypermobility syndrome. The American Journal of Medicine 115:33–40 [DOI] [PubMed] [Google Scholar]
- 25.Gibbons CH, Bonyhay I, Benson A, Wang N, Freeman R (2013) Structural and functional small fiber abnormalities in the neuropathic postural tachycardia syndrome. PloS One 8:e84716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibbons CH, Freeman R (2005) Autonomic neuropathy and coeliac disease. Journal of Neurology, Neurosurgery, and Psychiatry 76:579–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hadjivassiliou M, Grünewald RA, Davies-Jones GaB (2002) Gluten sensitivity as a neurological illness. Journal of Neurology, Neurosurgery, and Psychiatry 72:560–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasler WL, Wilson LA, Parkman HP, Koch KL, Abell TL, Nguyen L, Pasricha PJ, Snape WJ, McCallum RW, Sarosiek I, Farrugia G, Calles J, Lee L, Tonascia J, Unalp-Arida A, Hamilton F (2013) Factors related to abdominal pain in gastroparesis: contrast to patients with predominant nausea and vomiting. Neurogastroenterology and Motility: The Official Journal of the European Gastrointestinal Motility Society 25:427–438, e300–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hibbard ML, Dunst CM, Swanström LL (2011) Laparoscopic and endoscopic pyloroplasty for gastroparesis results in sustained symptom improvement. Journal of Gastrointestinal Surgery: Official Journal of the Society for Surgery of the Alimentary Tract 15:1513–1519 [DOI] [PubMed] [Google Scholar]
- 30.Holmes GM (2012) Upper gastrointestinal dysmotility after spinal cord injury: is diminished vagal sensory processing one culprit? Frontiers in Physiology 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacob G, Costa F, Shannon JR, Robertson RM, Wathen M, Stein M, Biaggioni I, Ertl A, Black B, Robertson D (2000) The neuropathic postural tachycardia syndrome. The New England Journal of Medicine 343:1008–1014 [DOI] [PubMed] [Google Scholar]
- 32.Jacob G, Robertson D, Mosqueda-Garcia R, Ertl AC, Robertson RM, Biaggioni I (1997) Hypovolemia in syncope and orthostatic intolerance role of the renin-angiotensin system. The American Journal of Medicine 103:128–133 [DOI] [PubMed] [Google Scholar]
- 33.Johnston SD, Watson RG, McMillan SA, Sloan J, Love AH (1997) Prevalence of coeliac disease in Northern Ireland. Lancet (London, England) 350:1370. [DOI] [PubMed] [Google Scholar]
- 34.Joyner MJ, Masuki S (2008) POTS versus deconditioning: the same or different? Clin Auton Res 18:300–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lacy BE, Crowell MD, Schettler-Duncan A, Mathis C, Pasricha PJ (2004) The treatment of diabetic gastroparesis with botulinum toxin injection of the pylorus. Diabetes Care 27:2341–2347 [DOI] [PubMed] [Google Scholar]
- 36.Lawal A, Barboi A, Krasnow A, Hellman R, Jaradeh S, Massey BT (2007) Rapid gastric emptying is more common than gastroparesis in patients with autonomic dysfunction. The American Journal of Gastroenterology 102:618–623 [DOI] [PubMed] [Google Scholar]
- 37.Li H, Yu X, Liles C, Khan M, Vanderlinde-Wood M, Galloway A, Zillner C, Benbrook A, Reim S, Collier D, Hill MA, Raj SR, Okamoto LE, Cunningham MW, Aston CE, Kem DC (2014) Autoimmune basis for postural tachycardia syndrome. Journal of the American Heart Association 3:e000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loavenbruck A, Iturrino J, Singer W, Sletten DM, Low PA, Zinsmeister AR, Bharucha AE (2015) Disturbances of gastrointestinal transit and autonomic functions in postural orthostatic tachycardia syndrome. Neurogastroenterology and Motility: The Official Journal of the European Gastrointestinal Motility Society 27:92–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lomax AE, Sharkey KA, Furness JB (2010) The participation of the sympathetic innervation of the gastrointestinal tract in disease states. Neurogastroenterology and Motility: The Official Journal of the European Gastrointestinal Motility Society 22:7–18 [DOI] [PubMed] [Google Scholar]
- 41.Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, Bloom L, Bowen JM, Brady AF, Burrows NP, Castori M, Cohen H, Colombi M, Demirdas S, De Backer J, De Paepe A, Fournel-Gigleux S, Frank M, Ghali N, Giunta C, Grahame R, Hakim A, Jeunemaitre X, Johnson D, Juul-Kristensen B, Kapferer-Seebacher I, Kazkaz H, Kosho T, Lavallee ME, Levy H, Mendoza-Londono R, Pepin M, Pope FM, Reinstein E, Robert L, Rohrbach M, Sanders L, Sobey GJ, Van Damme T, Vandersteen A, van Mourik C, Voermans N, Wheeldon N, Zschocke J, Tinkle B (2017) The 2017 international classification of the Ehlers-Danlos syndromes. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics 175:8–26 [DOI] [PubMed] [Google Scholar]
- 42.Mauro A, Orsi L, Mortara P, Costa P, Schiffer D (1991) Cerebellar syndrome in adult celiac disease with vitamin E deficiency. Acta Neurologica Scandinavica 84:167–170 [DOI] [PubMed] [Google Scholar]
- 43.McCallum RW, Snape W, Brody F, Wo J, Parkman HP, Nowak T (2010) Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association 8:947–954; quiz e116 [DOI] [PubMed] [Google Scholar]
- 44.Moore JG, Christian PE, Brown JA, Brophy C, Datz F, Taylor A, Alazraki N (1984) Influence of meal weight and caloric content on gastric emptying of meals in man. Digestive Diseases and Sciences 29:513–519 [DOI] [PubMed] [Google Scholar]
- 45.Moore JG, Christian PE, Coleman RE (1981) Gastric emptying of varying meal weight and composition in man. Evaluation by dual liquid- and solid-phase isotopic method. Digestive Diseases and Sciences 26:16–22 [DOI] [PubMed] [Google Scholar]
- 46.Mustafa HI, Garland EM, Biaggioni I, Black BK, Dupont WD, Robertson D, Raj SR (2011) Abnormalities of angiotensin regulation in postural tachycardia syndrome. Heart Rhythm 8:422–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nelson AD, Mouchli MA, Valentin N, Deyle D, Pichurin P, Acosta A, Camilleri M (2015) Ehlers Danlos syndrome and gastrointestinal manifestations: a 20-year experience at Mayo Clinic. Neurogastroenterology and Motility: The Official Journal of the European Gastrointestinal Motility Society 27:1657–1666 [DOI] [PubMed] [Google Scholar]
- 48.Park K-J, Singer W, Sletten DM, Low PA, Bharucha AE (2013) Gastric emptying in postural tachycardia syndrome: a preliminary report. Clinical Autonomic Research: Official Journal of the Clinical Autonomic Research Society 23:163–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parkman HP, Van Natta ML, Abell TL, McCallum RW, Sarosiek I, Nguyen L, Snape WJ, Koch KL, Hasler WL, Farrugia G, Lee L, Unalp-Arida A, Tonascia J, Hamilton F, Pasricha PJ (2013) Effect of nortriptyline on symptoms of idiopathic gastroparesis: the NORIG randomized clinical trial. JAMA 310:2640–2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pederson CL, Brook JB (2017) Health-related quality of life and suicide risk in postural tachycardia syndrome. Clin Auton Res 27:75–81 [DOI] [PubMed] [Google Scholar]
- 51.Penny HA, Aziz I, Ferrar M, Atkinson J, Hoggard N, Hadjivassiliou M, West JN, Sanders DS (2016) Is there a relationship between gluten sensitivity and postural tachycardia syndrome? European Journal of Gastroenterology & Hepatology 28:1383–1387 [DOI] [PubMed] [Google Scholar]
- 52.Perkel MS, Moore C, Hersh T, Davidson ED (1979) Metoclopramide therapy in patients with delayed gastric emptying: a randomized, double-blind study. Digestive Diseases and Sciences 24:662–666 [DOI] [PubMed] [Google Scholar]
- 53.Raj SR (2006) The Postural Tachycardia Syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing and Electrophysiology Journal 6:84–99 [PMC free article] [PubMed] [Google Scholar]
- 54.Raj SR, Robertson D (2007) Blood volume perturbations in the postural tachycardia syndrome. The American Journal of the Medical Sciences 334:57–60 [DOI] [PubMed] [Google Scholar]
- 55.Raj SR, Stiles L, Shaw B, Green EA, Dorminy CA, Shibao CA, Okamoto LE, Garland E, Diedrich A, Biaggioni I, Robertson D (2016) The diagnostic experience in postural tachycardia syndrome: insights from a cross-sectional community-based survey. In, 27TH INTERNATIONAL SYMPOSIUM 27TH INTERNATIONAL SYMPOSIUM ON THEON THE AUTONOMIC NERVOUS SYSTEM [Google Scholar]
- 56.Rao AS, Camilleri M (2010) Review article: metoclopramide and tardive dyskinesia. Alimentary Pharmacology & Therapeutics 31:11–19 [DOI] [PubMed] [Google Scholar]
- 57.Sarosiek I, Forster J, Lin Z, Cherry S, Sarosiek J, McCallum R (2013) The addition of pyloroplasty as a new surgical approach to enhance effectiveness of gastric electrical stimulation therapy in patients with gastroparesis. Neurogastroenterology and Motility: The Official Journal of the European Gastrointestinal Motility Society 25:134–e180 [DOI] [PubMed] [Google Scholar]
- 58.Seligman WH, Low DA, Asahina M, Mathias CJ (2013) Abnormal gastric myoelectrical activity in postural tachycardia syndrome. Clinical Autonomic Research: Official Journal of the Clinical Autonomic Research Society 23:73–80 [DOI] [PubMed] [Google Scholar]
- 59.Shill HA, Alaedini A, Bushara KO, Latov N, Hallett M (2003) Anti-ganglioside antibodies in idiopathic and hereditary cerebellar degeneration. Neurology 60:1672–1673 [DOI] [PubMed] [Google Scholar]
- 60.Smith HS, Cox LR, Smith BR (2012) Dopamine receptor antagonists. Ann Palliat Med 1:137–142 [DOI] [PubMed] [Google Scholar]
- 61.Sugumar A, Singh A, Pasricha PJ (2008) A systematic review of the efficacy of domperidone for the treatment of diabetic gastroparesis. Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association 6:726–733 [DOI] [PubMed] [Google Scholar]
- 62.Takala J (1996) Determinants of splanchnic blood flow. British Journal of Anaesthesia 77:50–58 [DOI] [PubMed] [Google Scholar]
- 63.Talley NJ (2003) Diabetic gastropathy and prokinetics. The American Journal of Gastroenterology 98:264–271 [DOI] [PubMed] [Google Scholar]
- 64.Tani H, Singer W, McPhee BR, Opfer-Gehrking TL, Haruma K, Kajiyama G, Low PA (2000) Splanchnic-mesenteric capacitance bed in the postural tachycardia syndrome (POTS). Autonomic Neuroscience: Basic & Clinical 86:107–113 [DOI] [PubMed] [Google Scholar]
- 65.Toro JP, Lytle NW, Patel AD, Davis SS, Christie JA, Waring JP, Sweeney JF, Lin E (2014) Efficacy of laparoscopic pyloroplasty for the treatment of gastroparesis. Journal of the American College of Surgeons 218:652–660 [DOI] [PubMed] [Google Scholar]
- 66.Tursi A, Giorgetti GM, Iani C, Arciprete F, Brandimarte G, Capria A, Fontana L (2006) Peripheral neurological disturbances, autonomic dysfunction, and antineuronal antibodies in adult celiac disease before and after a gluten-free diet. Digestive Diseases and Sciences 51:1869–1874 [DOI] [PubMed] [Google Scholar]
- 67.Wang LB, Culbertson CJ, Deb A, Morgenshtern K, Huang H, Hohler AD (2015) Gastrointestinal dysfunction in postural tachycardia syndrome. J Neurol Sci 359:193–196 [DOI] [PubMed] [Google Scholar]
- 68.Zarate N, Farmer AD, Grahame R, Mohammed SD, Knowles CH, Scott SM, Aziz Q (2010) Unexplained gastrointestinal symptoms and joint hypermobility: is connective tissue the missing link? Neurogastroenterol Motil 22:252–e278 [DOI] [PubMed] [Google Scholar]


