Abstract
Aims:
The purpose of this study was to objectively measure, with wrist-worn actigraphy, free-living sleeping patterns in Icelandic adolescents, and to compare sleep duration, sleep quality and clock times between school days (SchD) and non-school days (NSchD) and the association between sleep and body mass index (BMI).
Methods:
A cross-sectional study on 15.9-year-old (±0.3) adolescents from six schools in Reykjavík, Iceland, took place in the spring of 2015. Free-living sleep was measured on 301 subjects (122 boys and 179 girls) over seven days using wrist-worn actigraphy accelerometers. Total rest time (TRT), total sleep time (TST), sleep quality markers, and clock times for sleep were quantified and compared between SchD and NSchD and between the sexes, using paired and group t-tests as appropriate. Linear regression was used to assess the association between sleep parameters and BMI.
Results:
On SchD, TST was 6.2 ± 0.7 h, with sleep efficiency (SLE) of 87.9 ± 4.4% for the group. On NSchD, TST increased to 7.3 ± 1.1 h (p < 0.001), although SLE decreased to 87.4 ± 4.7% (p < 0.05). On SchD and NSchD, 67% and 93% had bed times after midnight, respectively, and on SchD 10.7% met sleep recommendations (8 h/night). There was no association between BMI and average sleep parameters.
Conclusion:
The majority of Icelandic adolescents did not get the recommended number of hours of sleep, especially on SchD. While TST increased on NSchD, many participants still did not achieve the recommendations. These findings provide information on the sleep patterns of adolescents and may serve as reference for development of policies and interventions to promote better sleep practices.
Keywords: Sleep, Adolescents, Accelerometry, Actigraphy, Sleep duration, Body mass index (BMI)
1. Introduction
Sleep plays an important role in adolescents’ health and well-being. Adequate sleep is an essential element for proper function of body and mind, which influences quality of life. The National Sleep Foundation recommends that teenagers, aged 14–17 years old, should sleep at least 8–10 h a night [1]. Inadequate sleep duration in adolescents is associated with higher body mass index (BMI) [2,3], greater body fat [4], increased insulin resistance [5], and reduced academic performance [6].
A previous study using questionnaires and sleep diaries found that Icelandic youth had shorter sleep duration than their European peers [7]. Although subjective, self-report methods were commonly used in the past to assess adolescent sleep patterns [8], they tend to overestimate actual sleep length, suggesting that adolescents may sleep even less than previously reported [9]. Wrist actigraphy is an objective method of studying free-living sleep patterns via a watch-like accelerometer and well-validated sleep detection algorithms [10–14]. Although an actigraphy based sensor using the chosen algorithm has been validated against polysomnography (PSG), this specific device is newer to the market and has only been used in conjunction with PSG in other populations. However, it is designed and marketed specifically to researchers and not for the commercial market. It is believed that no study, to date, has objectively measured free-living sleeping patterns during school and non-school days in Icelandic children or adolescents.
Whilst sleep duration, or the minutes of sleep per night, is the most widely reported and studied sleep measure, emerging research has also shown that sleep timing is another important component of sleep that may influence overall metabolic health [5]. The biological circadian clock is an important regulator of sleep and other individual behaviors. However, in modern society, the sleep–wake cycle is heavily influenced by factors like school, work, or other social schedules [15,16]. The term “social jetlag” has been coined to describe the misalignment between social schedules and the circadian clock [16,17]. Consequently, along with investigating sleep duration, it is also important to study the influence of other sleep dimensions, such as sleep quality, and the consistency in bed time (BT) and wake-up time [3] on health outcomes such as BMI.
1.1. Aims of the study
The primary purpose of this study was to objectively investigate, with wrist actigraphy, free-living sleeping patterns in Icelandic adolescents. Secondary aims included: (a) assessing differences in sleep duration, sleep quality and clock times between the sexes; (b) examining differences in sleep patterns between school days (SchD) and non-school days (NSchD); and (c) investigating the association between sleep and BMI.
2. Methods
2.1. Participation
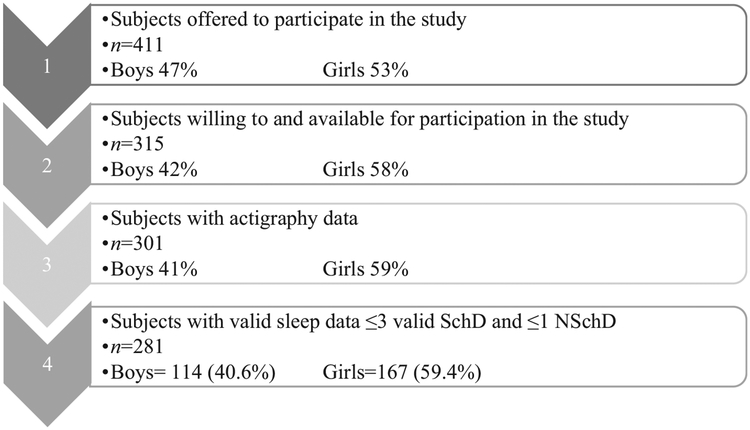
A total of 411 tenth-grade students (15–16 years old) – 47% boys and 53% girls – from six schools in Reykjavík, Iceland, received an invitation letter to participate in this study. The data collection took place in the spring of 2015 (April–June). A total of 315 pupils participated in the study (Fig. 1). Non-participation (n = 104) was mainly due to absence from school during measurement days and lack of interest in the study. Study participation is shown in Fig. 1.
Fig. 1.
Participation in the study. SchD, school days; NSchD, non-school days.
Written informed consent was obtained from all participants and their guardians. Strict procedures were followed to ensure confidentiality. The study was approved by the National Bioethics Committee and the Icelandic Data Protection Authority (Study number: VSN b200605002&03).
2.2. Sleep parameters
Free-living sleep was measured with wrist-worn raw signal accelerometers: ActiGraph GT3X+ (ActiSleep by Actigraph Inc., Pensacola, Florida, USA). The small (3.8 cm × 3.7 cm × 1.8 cm) and light (27 g) Actigraphy watches were placed on the non-dominant wrist of each subject, at school, and each was asked to continuously wear the monitor for seven days. Raw triaxial data were sampled at 80 samples/second (Hz). Sleep parameters were derived from the Actilife software from Actigraph (Pensacola, FL, USA) (version 6.13.0.) using a sleep detection algorithm specifically validated for adolescents [13]. Wrist actigraphy has high sensitivity and moderate specificity, and overall high accuracy when compared to PSG [12], which is the gold standard in sleep research. Wrist actigraphy has been recommended for characterization of sleep parameters in population-based studies of young adults [10].
Rest and sleep durations, timing, and other sleep quality parameters (such as sleep efficiency (SLE), and wakening after sleep onset (WASO), as shown in Table 1) were first determined by auto-detection of the Actilife sleep analysis. Self-reported sleep logs of BT and rise time were then used to confirm the rest intervals determined by the software detection, and two expert scorers adjusted them, when necessary (inter-scorer variability was evaluated, data not shown). Total rest time (TRT) was compared to the recommended 8 h of sleep for adolescents in this age range [1], and the proportion of participants going to bed after midnight was also calculated. Measurements of daily sleep data for at least three valid SchD and one valid NSchD with wear time >14 h were considered valid. Naptime was not included in the analyses, due to low incidents of naps detected in the data (22 total naps taken by 18 different subjects).
Table 1.
Sleep parameters for analysis.
| Abbreviation | Sleep parameter | Definition |
|---|---|---|
| TRT | Total rest time | Resting period: the time spent in bed (minutes) |
| TST | Total sleep time | Actual sleep time during rest period (minutes) |
| SLE | Sleep efficiency | Minutes of total sleep divided by minutes available for sleep (TST/TRT) multiplied by 100 (%) |
| SOL | Sleep onset latency | Time it takes from wakefulness to sleep |
| NOA | Number of awakenings | Wake-ups during rest period (counts) |
| WASO | Wakening after sleep onset | Minutes of non-sleep from the resting period (TRT) after sleep onset due to awakening/s |
| SFI | Total sleep fragmentation index | Summation of the movement index (percentage of epochs >0), and the fragmentation index (percentage of 1-min periods of sleep vs all periods of sleep during the sleep period) (%) |
| BT | Bed time | Clock time of bed time |
| MS | Midpoint of sleep | The clock time at the midpoint of the sleeping period (between sleep onset and rise time) |
| RT | Rise time | Clock time of wake up |
2.3. Anthropometric measures
Standing height was measured with a stadiometer (Seca model 217, Seca Ltd. Birmingham, UK) to the nearest 0.1 cm. Body weight was measured on a balance scale (Seca model 813, Seca Ttd., Birmingham, UK) to the nearest 0.1 kg, with participants wearing light clothes. BMI was calculated by dividing weight by height squared (kg/m2). All measurements were performed at individual schools.
2.4. School hours
School time for this age group in Iceland is 37 school hours/week. The timing and duration of the school day varied little between schools and individual participants, with the school day starting for most participants between 08:10–08:20 in the morning and finished around 14:00, and the latest school day finishing at 16:00.
2.5. Statistical analysis
All finalized and exported Actilife reports were compiled into daily and weekly averages using customized programs written with Matlab software (The Mathworks, Natick, MA, USA) (version R2013a). Mean ± standard deviation was reported as summary statistics. Paired t-tests were used for comparisons between SchD and NSchD; unpaired t-tests were used for between-sex comparisons. The TRT was compared with recommended sleep hours for adolescents (8–10 h). Linear regression was used to assess the association between sleep parameters (independent variables) and BMI (dependent variable). Separate regressions were run for boys and girls and SchD and NSchD. Statistical analyses were carried out using RStudio statistical software (Rstudio headquarters, Boston, MA, USA) (version 0.99.482).
3. Results
Participants’ characteristics are shown in Table 2. The final sample included 114 boys and 167 girls who had valid data on the sleep parameters (measurements for ≥3 SchD and ≥1 NSchD were considered valid).
Table 2.
Characteristics of the study subjects.
| Characteristics | Boys (n = 114) | Girls (n = 167) |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| Age (years) | 15.8 ± 0.3 | 15.9 ± 0.3 |
| Height (cm) | 178.2 ± 5.9 | 167.0 ± 5.7 |
| Weight (kg) | 68.9 ± 11.6 | 62.0 ± 10.1 |
| BMI (kg/m2) | 21.7 ± 3.2 | 22.2 ± 3.2 |
n, number of participants; BMI, body mass index.
The average age of participants was 15.9 ± 0.3 years, with a BMI of 22.2 ± 3.7 kg/m2. The prevalence of being overweight (BMI between 25 and 30 kg/m2) was 10.4% (7.1% of boys and 12.7% of girls), and 2.9% were obese (BMI ≥30 kg/m2, 2.7% of boys and 3.0% of girls).
The average day length was 17.6 h of daylight, with the shortest day being 15.1 h and the longest day 20.4 h. There was no relationship between day length and TST on SchD or NSchD.
Sleep parameters for SchD and NSchD are summarized in Table 3. Markers of sleep quality or duration did not differ between the sexes on SchD or NSchD. All participants had shorter TRT(7.05 ± 0.83 vs 8.40 ± 1.23 h) and TST (6.19 ± 0.74 vs 7.33 ± 1.10 h) on SchD vs NSchD (both p < 0.001). Over the entire week, 22.1% of participants met the sleep recommendation of 8 h per night (based on TRT). Similarly, when TRT (time in bed) was compared to recommendations, 10.7% got the recommended 8 h of sleep on SchD, while 66.9% achieved the recommended sleep duration on NSchD. Despite a longer TST on NSchD, objective sleep parameters (SLE, WASO, number of awakenings (NOA) and Sleep Fragmentation Index (SFI), see Table 3) indicated lower sleep quality on NSchD than SchD (p < 0.005).
Table 3.
Sleep parameter mean values for both sexes on school days and non-school days.
| Sleep parameters | Boys (n = 114) | Girls (n = 167) | All (n = 281) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SchD | NSchD | p | SchD | NchD | p | SchD | NSchD | p | |
| Sleep duration | |||||||||
| TRT (hours) | 7.01 ± 0.90 | 8.36 ± 1.45 | <0.001 | 7.07 ± 0.78 | 8.42 ± 1.06 | <0.001 | 7.05 ± 0.83 | 8.40 ± 1.23 | <0.001 |
| TST (hours) | 6.16 ± 0.82 | 7.25 ± 1.29 | <0.001 | 6.21 ± 0.69 | 8.42 ± 1.06 | <0.001 | 6.20 ± 0.74 | 7.33 ± 1.10 | <0.001 |
| Sleep quality | |||||||||
| SLE (%) | 88.0 ± 4.4 | 86.8 ± 4.2 | 0.01 | 87.9 ± 4.4 | 87.9 ± 5.1 | 0.837 | 87.9 ± 4.4 | 87.4 ± 4.7 | 0.038 |
| SOL (minutes) | 1.6 ± 1.0 | 1.6 ± 1.1 | 0.790 | 1.5 ± 1.0 | 1.6 ± 1.2 | 0.767 | 1.6 ± 1.0 | 1.6 ± 1.2 | <0.001 |
| WASO (minutes) | 50.0 ± 21.1 | 66.3 ± 24.8 | <0.001 | 50.2 ± 21.4 | 60.3 ± 28.4 | <0.001 | 50.1 ± 21.2 | 62.7 ± 27.1 | <0.001 |
| NOA (count) | 18.3 ± 6.3 | 23.4 ± 9.4 | <0.001 | 18.5 ± 4.8 | 22.7 ± 7.8 | <0.001 | 18.4 ± 5.5 | 23.0 ± 7.8 | <0.001 |
| SFI (%) | 19.7 ± 5.8 | 22.3 ± 6.7 | <0.001 | 20.1 ± 4.2 | 20.7 ± 7.5 | 0.175 | 19.9 ± 6.1 | 21.3 ± 7.2 | 0.846 |
SchD, school days; NSchD, non-school days; TRT, total rest time; TST, total sleep time; SLE, sleep efficiency; SOL, sleep onset latency; WASO, wake time after sleep onset; NOA, number of awakenings during rest period; SFI, Sleep Fragmentation Index.
Clock times for sleep are summarized in Table 4. There was a shift in the sleep schedule of both sexes from SchD to NSchD. Boys went to bed 91 ± 78 min later (00:28 ± 56 min SchD vs 01:58 ± 83 min NSchD, p < 0.001) and rose 178 ± 81 min later on NSchD (07:33 ± 48 min SchD vs 10:32 ± 86 min NSchD, p < 0.001), leading to a 135 ± 66 min shift in midpoint of sleep (MS). The SchD to NSchD shifts for girls were all less than the shifts for boys (all p < 0.02), as girls went to bed 73 ± 50 min later (00:18 ± 51 min SchD vs 01:31 ± 64 min NSchD, p < 0.001) and rose 154 ± 71 min later on NSchD (07:23 ± 33 min SchD vs 09:57 ± 74 min NSchD, p < 0.001), with a MS shift of 113 ± 50 min. Similarly, the average bedt ime (BT) and rise time (RT) for boys was 33 min later and 35 min later than that for girls on NSchD (p < 0.005 and p < 0.001, respectively), and both were about 10 min later than that for girls on SchD (p = 0.121 and p = 0.05, respectively).
Table 4.
Mean sleep schedule times for both sexes on school days and non-school days.
| Time of day | Boys (n = 114) | Girls (n = 167) | All (n = 281) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SchD | NSchD | p | SchD | NchD | p | SchD | NSchD | p | |
| Bed time (o’clock ± minutes) | 00:28 ± 56 | 01:58 ± 83* | <0.001 | 00:18 ± 51 | 01:31 ± 64 | <0.001 | 00:22 ± 54 | 01:42 ± 74 | 0.05 |
| Mid-sleep time (o’clock ± minutes) | 04:00 ± 45* | 06:14 ± 77** | <0.001 | 03:50 ± 36 | 05:43 ± 61 | <0.001 | 03:54 ± 40 | 05:56 ± 69 | 0.158 |
| Rise time (o’clock ± minutes) | 07:33 ± 48* | 10:32 ± 86** | <0.001 | 07:23 ± 33 | 09:57 ± 73 | <0.001 | 07:27 ± 40 | 10:11 ± 80 | 0.735 |
SchD, school days; NSchD, non-school days.
Difference between the sexes is marked *p < 0.05 and **p < 0.001.
There was no association between BMI and average sleep times, SchD to NSchD sleep timing shifts, or average sleep duration in either sex (p-values 0.115–0.376). Results of linear regression between sleep parameters and BMI are shown in Table 5.
Table 5.
Results of linear regression between sleep parameters and body mass index (BMI).
| Linear relationship to BMI | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sleep parameters (predictor variables) | Boys (n = 114) | Girls (n = 167) | All (n = 281) | |||||||||
| β | SE | r | p | β | SE | r | p | β | SE | r | p | |
| TRT SchD (hours) | −0.410 | 0.327 | −0.12 | 0.21 | −0.415 | 0.318 | −0.10 | 0.19 | −0.403 | 0.230 | −0.10 | 0.08 |
| TRT NSchD (hours) | −0.275 | 0.197 | −0.13 | 0.16 | 0.011 | 0.236 | 0.00 | 0.96 | −0.142 | 0.154 | −0.06 | 0.35 |
| TST SchD (hours) | −0.366 | 0.357 | −0.10 | 0.31 | −0.464 | 0.362 | −0.10 | 0.20 | −0.407 | 0.256 | −0.09 | 0.11 |
| TST NSchD (hours) | −0.346 | 0.221 | −0.15 | 0.12 | −0.059 | 0.261 | −0.02 | 0.82 | −0.197 | 0.171 | −0.07 | 0.25 |
| SLE SchD (%) | 0.029 | 0.065 | 0.04 | 0.66 | −0.015 | 0.057 | −0.02 | 0.80 | 0.003 | 0.043 | 0.00 | 0.95 |
| SLE NSchD (%) | −0.036 | 0.069 | −0.05 | 0.60 | −0.024 | 0.049 | −0.04 | 0.63 | −0.020 | 0.040 | −0.03 | 0.62 |
| SOL SchD (minutes) | −0.031 | 0.280 | −0.01 | 0.91 | −0.162 | 0.257 | −0.05 | 0.53 | −0.114 | 0.190 | −0.04 | 0.55 |
| SOL NSchD (minutes) | 0.110 | 0.230 | 0.05 | 0.63 | 0.162 | 0.207 | 0.06 | 0.43 | 0.135 | 0.155 | 0.05 | 0.38 |
| WASO SchD (minutes) | −0.012 | 0.014 | −0.08 | 0.37 | −0.004 | 0.012 | −0.03 | 0.72 | −0.007 | 0.009 | −0.05 | 0.41 |
| WASO NSchD (minutes) | −0.001 | 0.012 | −0.01 | 0.93 | 0.005 | 0.009 | 0.04 | 0.59 | 0.001 | 0.007 | 0.01 | 0.84 |
| NOA SchD (n) | −0.052 | 0.050 | −0.10 | 0.30 | −0.054 | 0.048 | −0.09 | 0.26 | −0.052 | 0.035 | −0.09 | 0.13 |
| NOA NSchD (n) | 0.016 | 0.035 | 0.04 | 0.64 | 0.018 | 0.033 | 0.04 | 0.59 | 0.013 | 0.024 | 0.03 | 0.58 |
| SFI SchD | −0.008 | 0.050 | −0.02 | 0.87 | −0.008 | 0.039 | −0.02 | 0.85 | −0.007 | 0.031 | −0.01 | 0.83 |
| SFI NSchD | 0.040 | 0.043 | 0.09 | 0.35 | −0.018 | 0.033 | −0.04 | 0.59 | −0.003 | 0.026 | −0.01 | 0.92 |
| BT SchD (hours) | 0.347 | 0.308 | 0.11 | 0.26 | 0.250 | 0.292 | 0.07 | 0.39 | 0.258 | 0.213 | 0.07 | 0.23 |
| BT NSchD (hours) | −0.024 | 0.207 | −0.01 | 0.91 | 0.050 | 0.234 | 0.02 | 0.83 | −0.040 | 0.154 | −0.02 | 0.79 |
| MS SchD (hours) | 0.142 | 0.387 | 0.03 | 0.71 | 0.153 | 0.412 | 0.03 | 0.71 | 0.083 | 0.283 | 0.02 | 0.77 |
| MS NSchD (hours) | −0.204 | 0.225 | −0.09 | 0.37 | 0.061 | 0.245 | 0.02 | 0.80 | −0.136 | 0.163 | −0.05 | 0.41 |
| RT SchD (hours) | −0.218 | 0.356 | −0.06 | 0.54 | −0.226 | 0.449 | −0.04 | 0.61 | −0.283 | 0.281 | −0.06 | 0.31 |
| RT NSchD (hours) | −0.302 | 0.199 | −0.14 | 0.13 | 0.047 | 0.204 | 0.02 | 0.82 | −0.169 | 0.141 | −0.07 | 0.23 |
| BT NSchD – BT SchD (hours) | −0.338 | 0.259 | −0.12 | 0.19 | 0.011 | 0.305 | 0.00 | 0.97 | −0.239 | 0.197 | −0.07 | 0.23 |
| MS NSchD – MS SchD (hours) | −0.208 | 0.223 | −0.09 | 0.35 | −0.181 | 0.299 | −0.05 | 0.55 | −0.239 | 0.180 | −0.08 | 0.18 |
| RT SchD – RT SchD (hours) | −0.263 | 0.212 | −0.12 | 0.22 | 0.100 | 0.211 | 0.04 | 0.64 | −0.109 | 0.149 | −0.04 | 0.46 |
SchD, school days; NSchD, non-school days; TRT, total rest time; TST, total sleep time; SLE, sleep efficiency; SOL, sleep onset latency; WASO, wake time after sleep onset; NOA, number of awakenings during rest period; SFI, Sleep Fragmentation Index; BT, bed time; MS, midpoint of sleep; RT, rise time.
4. Discussion
The main findings in this study revealed that the majority of Icelandic adolescents aged 15–16 years do not get the recommended 8–10 h of sleep each night [1], especially on school nights. Although individual need for sleep may vary, sleep duration far outside the normal range should raise concerns, since sleep recommendations are established to improve overall health and well-being [18]. The main influence on insufficient sleep (averaging about 7 h in bed, with 6.2 h of actual sleep time per night) was that Icelandic adolescents appeared to have a very late BT routine (00:22). When combined with early school schedules, time for sleep was substantially suppressed on school days. The insufficient sleep on school days was only partially “recovered” by sleeping longer (about 80 min) on non-school days, although the quality of sleep was reduced. The “social jetlag” phenomenon was also present, with a shift in mid-sleep time of about 2.3 and 1.9 h later on non-school days for boys and girls, respectively.
The wrist-worn actigraphy derived sleep data confirmed previous observations of short sleep duration in Icelandic adolescents on SchD using subjective measures [7]. Furthermore, the Icelandic adolescents measured in this study went to bed rather late compared with previous self-reported data from various other countries [19], which ultimately leads to shorter sleep duration. The habit of later BT is most likely cultural for Icelanders, as previous studies, both within the same age group and amongst other age groups, have also reported late BTs [7,20,21]. As early as the 1980s, Kristbjarnarson et al. surveyed Icelanders (n = 668) and found an average BT for 15-year-olds of 00:18 and an average nocturnal sleep of 8.1 h [20]. A follow-up study by Thorleifsdottir et al. confirmed that Icelandic adolescents (11, 13 and 15 year-olds) had later BTs and shorter nocturnal sleep than their peers in neighboring countries [7,20]. The even later BT and RT on NSchD in the present study further suggests that Icelandic adolescents may “prefer” a later circadian profile compared to what is now required by current school schedules, which may be set to meet the adults’ (teachers and parents) schedules.
The present study suggests that over the course of the measured school week, 21% of Icelandic adolescents (18.6% of boys and 22.8% of girls) incurred a substantial sleep debt, defined as ≥2 h difference in TST between SchD and NSchD [22]. This is in line with the findings of an analogous actigraphy based study of adolescents aged 15–16 years in the USA, who had a similarly short TST (6.4 h overall and 6.0 h on weekdays), with a high percentage of sleep debt (35%) [22]. This differential between SchD and NSchD TST is not unusual, as short-term sleep deficiency during SchD is typically followed by longer sleep durations of “recovery” or “catch-up” sleep on NSchD [3,15]. On average, the present data seem to support the recovery sleep phenomenon, as the adolescents slept about 1.2 h longer on NSchDs than SchDs. However, it did not find an inverse correlation between TST during SchD and NSchD, and most measures indicated that sleep quality was reduced on NSchD for both sexes, despite a longer average TST. One plausible explanation is that the shift in their MS of roughly 2 h may have created inconsistency in their sleep routine, hindering their ability to simultaneously increase their sleep quantity and quality. Sleep guidelines for adolescents typically recommend that they maintain consistent BTs and wakeup times during the week for overall health benefits [23].
During puberty, biological rhythms normally change so that adolescents develop sleepiness later at night and need to sleep longer in the morning [24]. Delayed BTs and early school start result in inadequate sleep for a large portion of the adolescent population [25]. During the school week, the main determinant of RT is the start time of school [2]. This is a challenge for adolescents, where the social/cultural/biological pressures for staying up late combined with early school times reduce sleep time and are inconsistent with healthy sleep patterns [17]. The American Academy of Pediatrics advised US middle and high schools to modify start time to no earlier than 08:30, as an approach to enable students to get adequate sleep and improve their health, safety, academic achievement and quality of life [25].
A wide range of evidence, from cross-sectional associations [4,5,26] to mechanistic-based laboratory studies [27], has demonstrated links between short sleep duration and an increased risk of obesity in both children and adults. Similarly, sleep timing is thought to independently influence BMI in adolescents [28]. However, the present study did not find an association between BMI and TST, sleep timing, or shifts in sleep schedule. The lack of an association may have been due, in part, to the small and homogeneous participant population, which had a relatively low prevalence of obesity (<3%) combined with a high prevalence of short sleep (~88%). Studies that focus on the association between sleep duration and BMI in adolescents show inconsistent results. One cross-sectional analysis of a similarly aged (13–15 years) subset (n = 1290) of a larger Australian cohort also failed to identify a relationship between obesity prevalence and TST with subjectively assessed sleep duration [29]. However, another study showed a negative association between BMI and sleep duration for boys, but not for girls [30]. Similarly, a third study on BMI and sleep duration in young adults (mean age 27.7 ± 3.8 years, n = 430) demonstrated a negative association between objectively measured sleep duration and BMI [31]. Future studies should further investigate the link between sleep patterns and adiposity in this population, using more precise measurements such as the dual-energy X-ray absorptiometry, since BMI does not differentiate between lean mass and fat mass of the individual, even though it correlates well with body fat percent [32].
4.1. Strengths and limitations
It is believed that this is the first study to objectively measure free-living sleep patterns in a large sample of Icelandic youth. Actigraphy has been shown to provide more accurate sleep assessments than subjective methods, particularly during longer, free-living, population-based studies, where daily sleep logs are prone to increased non-compliance [33] and self-report tends to over-estimate sleep time [9]. However, wrist-worn actigraphy tends to underestimate waking, when subjects lay perfectly still while awake, compared to the gold standard in sleep research – PSG [12]. Although wrist actigraphy has high sensitivity and moderate specificity, and overall high accuracy when compared to PSG[12], it has only been used in conjunction with PSG in other populations than adolescents.
It should be noted that the present study compared objective assessments of total rest duration (in bed intended to sleep) to sleep duration guidelines, which have been established using self-reported data [3]. The actual sleep times were shorter than the total rest time.
There were other limitations of the present study. The school hours between schools and individuals varied slightly (<15 min), which could have possibly resulted in shorter or longer sleep on SchD for the individual at times. It is also suspected that day length and the demands of the school season might have affected the sleep length and quality in the study participants. Due to the study design, the study was conducted during spring (average day length17.6 h) and participants were in the last year of primary school in Iceland (equivalent to American high school). The authors plan to repeat the study during a different season (e.g., winter and another school year) to assess how these factors may impact sleep in Icelandic adolescents.
5. Conclusion
The actigraphy measured sleep pattern in a group of Icelandic boys and girls aged 15–16 years revealed that the majority of them did not get sufficient sleep on school days. Icelandic boys had later BTs than girls on school days and non-school days. Collectively, these findings provide information on the sleep patterns of adolescents and may serve as reference for development of policies and interventions to promote better sleep practices.
Acknowledgments
Funding
The study was primarily funded by the Icelandic Centre for Research (RANNIS) (grant number: 152509–051), but also supported by the Ministry of Education, Science and Culture and The Icelandic Primary Health Care Research Fund.
Footnotes
Conflict of interest
The ICMJE Uniform Disclosure Form for Potential Conflicts of Interest associated with this article can be viewed by clicking on the following link: http://dx.doi.org/10.1016/j.sleep.2016.12.028.
References
- [1].Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health 2015;1(4):233–43. 10.1016/j.sleh.2015.10.004. [DOI] [PubMed] [Google Scholar]
- [2].Knutson KL, Lauderdale DS. Sociodemographic and behavioral predictors of bed time and wake time among US adolescents aged 15 to 17 years. J Pediatr 2009;154(3):426–30. 10.1016/j.jpeds.2008.08.035.30.e1. Epub 2008/10/14. PubMed PMID: ; PubMed Central PMCID: PMCPmc2783185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chaput JP, Janssen I. Sleep duration estimates of Canadian children and adolescents. J Sleep Res 2016. October;25(5):541–8. [DOI] [PubMed] [Google Scholar]
- [4].Garaulet M, Ortega FB, Ruiz JR, et al. Short sleep duration is associated with increased obesity markers in European adolescents: effect of physical activity and dietary habits. The HELENA study. Int J Obes (Lond) 2011;35(10): 1308–17. 10.1038/ijo.2011.149. Epub 2011/07/28. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- [5].Matthews KA, Dahl RE, Owens JF, et al. Sleep duration and insulin resistance in healthy black and white adolescents. Sleep 2012;35(10):1353–8. 10.5665/sleep.2112. Epub 2012/10/02. PubMed PMID: ; PubMed Central PMCID: PMCPMC3443761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Owens J Insufficient sleep in adolescents and young adults: an update on causes and consequences. Pediatrics 2014;134(3):e921–32. 10.1542/peds.2014-1696. Epub 2014/08/27. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Thorleifsdottir B, Bjornsson JK, Benediktsdottir B, et al. Sleep and sleep habits from childhood to young adulthood over a 10-year period. J Psychosom Res 2002;53(1):529–37. Epub 2002/07/20. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- [8].Lang C, Kalak N, Brand S, et al. The relationship between physical activity and sleep from mid adolescence to early adulthood. A systematic review of methodological approaches and meta-analysis. Sleep Med Rev 2015;28: 28–41. 10.1016/j.smrv.2015.07.004. Epub 2015/10/09. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- [9].Arora T, Broglia E, Pushpakumar D, et al. An investigation into the strength of the association and agreement levels between subjective and objective sleep duration in adolescents. Plos One 2013;8(8):e72406 10.1371/journal.pone.0072406. Epub 2013/08/21. PubMed PMID: ; PubMed Central PMCID: PMCPmc3739794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zinkhan M, Berger K, Hense S, et al. Agreement of different methods for assessing sleep characteristics: a comparison of two actigraphs, wrist and hip placement, and self-report with polysomnography. Sleep Med 2014;15(9): 1107–14. 10.1016/j.sleep.2014.04.015. Epub 2014/07/16 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- [11].Weiss AR, Johnson NL, Berger NA, et al. Validity of activity-based devices to estimate sleep. J Clin Sleep Med 2010;6(4):336–42. Epub 2010/08/24. PubMed PMID: ; PubMed Central PMCID: PMCPmc2919663. [PMC free article] [PubMed] [Google Scholar]
- [12].Slater JA, Botsis T, Walsh J, et al. Assessing sleep using hip and wrist actigraphy. Sleep Biol Rhythms 2015;13(2):172–80. 10.1111/sbr.12103. [DOI] [Google Scholar]
- [13].Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep 1994;17(3):201–7. Epub 1994/04/01. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- [14].de Souza L, Benedito-Silva AA, Pires ML, et al. Further validation of actigraphy for sleep studies. Sleep 2003;26(1):81–5. Epub 2003/03/12. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- [15].Roenneberg T, Allebrandt KV, Merrow M, et al. Social jetlag and obesity. Curr Biol 2012;22(10):939–43. 10.1016/j.cub.2012.03.038. Epub 2012/05/15. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- [16].Wittmann M, Dinich J, Merrow M, et al. Social jetlag: misalignment of biological and social time. Chronobiol Int 2006;23(1–2):497–509. 10.1080/07420520500545979. Epub 2006/05/12. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- [17].Barnes CM, Drake CL. Prioritizing sleep health: public health policy recommendations. Perspect Psychol Sci 2015;10(6):733–7. 10.1177/1745691615598509. Epub 2015/11/20. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- [18].Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health 2015;1(1):40–3. 10.1016/j.sleh.2014.12.010. [DOI] [PubMed] [Google Scholar]
- [19].Ortega FB, Chillon P, Ruiz JR, et al. Sleep patterns in Spanish adolescents: associations with TV watching and leisure-time physical activity. Eur J Appl Physiol 2010;110(3):563–73. 10.1007/s00421-010-1536-1. PubMed PMID: ISI:000282214600012. [DOI] [PubMed] [Google Scholar]
- [20].Kristbjarnarson Helgi MH, Sverrisson GI, Arnarsson EӦ, et al. Survey on sleep habits in Iceland. Læknablaðið 1985;71(6):193. [Google Scholar]
- [21].Brychta RJ, Arnardottir NY, Johannsson E, et al. Influence of day length and physical activity on sleep patterns in older Icelandic men and women. J Clin Sleep Med 2016;12(2):203–13. 10.5664/jcsm.5486. Epub 2015/09/29 PubMed PMID: ; PubMed Central PMCID: PMCPmc4751419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Matthews KA, Hall M, Dahl RE. Sleep in healthy black and white adolescents. Pediatrics 2014;133(5):e1189–96. 10.1542/peds.2013-2399. Epub 2014/04/23. PubMed PMID: ; PubMed Central PMCID: PMCPMC4006433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gruber R, Carrey N, Weiss SK, et al. Position statement on pediatric sleep for psychiatrists. J Can Acad Child Adolesc Psychiatry 2014;23(3):174–95. Epub 2014/10/17. PubMed PMID: ; PubMed Central PMCID: PMCPMC4197518. [PMC free article] [PubMed] [Google Scholar]
- [24].Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med 2007;8(6):602–12. 10.1016/j.sleep.2006.12.002. Epub 2007/03/27. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- [25].Wheaton AG, Ferro GA, Croft JB. School start times for middle school and high school students – United States, 2011–12 school year. MMWR Morb Mortal Wkly Rep 2015;64(30):809–13. Epub 2015/08/08. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Megdal SP, Schernhammer ES. Correlates for poor sleepers in a Los Angeles high school. Sleep Med 2007;9(1):60–3. 10.1016/j.sleep.2007.01.012. Epub 2007/09/18. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- [27].Depner CM, Stothard ER, Wright KP Jr. Metabolic consequences of sleep and circadian disorders. Curr Diab Rep 2014;14(7):507 10.1007/s11892-014-0507-z. Epub 2014/05/13 PubMed PMID: ; PubMed Central PMCID: PMCPMC4308960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Golley RK, Maher CA, Matricciani L, et al. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int J Obes (Lond) 2013;37(4):546–51. 10.1038/ijo.2012.212. Epub 2013/01/09 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- [29].Shi Z, Taylor AW, Gill TK, et al. Short sleep duration and obesity among Australian children. BMC Public Health 2010;10(1):609 10.1186/1471-2458-10-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Storfer-Isser A, Patel SR, Babineau DC, et al. Relation between sleep duration and BMI varies by age and sex in youth age 8e19. Pediatr Obes 2012;7(1): 53–64. 10.1111/j.2047-6310.2011.00008.x. Epub 2012/03/22. PubMed PMID: ; PubMed Central PMCID: PMCPMC3313079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wirth MD, Hebert JR, Hand GA, et al. Association between actigraphic sleep metrics and body composition. Ann Epidemiol 2015;25(10):773–8. 10.1016/j.annepidem.2015.05.001. Epub 2015/06/14. PubMed PMID: ; PubMed Central PMCID: PMCPMC4567903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wellens RI, Roche AF, Khamis HJ, et al. Relationships between the body mass index and body composition. Obes Res 1996;4(1):35–44. Epub 1996/01/01. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- [33].Sadeh ACA. The role of actigraphy in sleep medicine. Sleep Med Rev 2002;6(2):113–24. [DOI] [PubMed] [Google Scholar]