Abstract
Antigenic peptide-loaded MHC class II molecules (peptide–MHC class II) are constitutively expressed on the surface of professional antigen-presenting cells (APCs), including dendritic cells, B cells, macrophages and thymic epithelial cells, and are presented to antigen-specific CD4+ T cells. The mechanisms of antigen uptake, the nature of the antigen processing compartments and the lifetime of cell surface peptide–MHC class II complexes can vary depending on the type of APC. It is likely that these differences are important for the function of each distinct APC subset in the generation of effective adaptive immune responses. In this Review, we describe our current knowledge of the mechanisms of uptake and processing of antigens, the intracellular formation of peptide–MHC class II complexes, the intracellular trafficking of peptide–MHC class II complexes to the APC plasma membrane and their ultimate degradation.
MHC class II-restricted antigen presentation is essential for CD4+ T cell-dependent immune responses. Different professional antigen-presenting cells (APCs) participate in a wide range of processes that are necessary for the generation of an effective and specific immune response. Dendritic cells (DCs) sample their environment and capture foreign antigens, such as those derived from bacteria or viruses, and initiate adaptive immune responses against these antigens by activating CD4+ and CD8+ T cells1. DCs and epithelial cells in the thymus express (and also capture) numerous self proteins and contribute to central tolerance and peripheral tolerance. By contrast, each B cell generally captures a single antigen after binding to its antigen-specific surface B cell receptor (BCR)2 and then presents peptides from this antigen to specific T cells. Regardless of APC type, all APCs specifically interact with distinct subsets of T cells that express antigen-specific T cell receptors (TCRs) on their surface. The specificity of this interaction depends on the ability of APCs to display antigenic peptides, immobilized by MHC class I and class II molecules, on their surface. MHC class II binds antigenic peptides that are generated by proteolysis of self and non-self proteins in endosomes and lysosomes, and ‘presents’ them to antigen-specific CD4+ T cells3. Recognition of peptide–MHC class II by CD4+ T cells stimulates their activation and differentiation into T helper cell subsets and also mediates interactions between antigen-specific B cells and T helper cells. Recognition of peptide–MHC class I complexes on DCs by CD8+ T cells stimulates the differentiation of CD8+ T cells into cytotoxic T cells. Understanding the mechanisms that are involved in the effective generation (and maintenance) of peptide–MHC class II complexes in APCs provides much needed insight into T cell function in normal and pathological states. In this Review, we discuss the existing literature regarding MHC class II biosynthesis, maintenance and degradation, with a particular emphasis on the processes that are important for APC function.
Basics of peptide loading of MHC class II
The basic steps that are required for MHC class II molecules to bind antigenic peptides are similar in all APCs and have been recently reviewed4, and for this reason we briefly summarize these steps before discussing cell type-specific differences in antigen processing and presentation in APCs. The antigenic peptides presented by MHC class II molecules on splenic DCs and B cells are derived from almost all intracellular locations5 and use a variety of mechanisms to access endosomal and/or lysosomal antigen-processing compartments (described below). MHC class II molecules must also access these compartments for efficient peptide binding (FIG. 1). Newly synthesized MHC class II molecules bind to a non-polymorphic protein termed invariant chain (Ii), which contains targeting motifs that direct the Ii–MHC class II complex to endosomal–lysosomal antigen-processing compartments6. After trafficking from the trans-Golgi network to the plasma membrane, recognition of the targeting motifs in Ii enables cell surface Ii–MHC class II complexes to undergo clathrin-mediated endocytosis7,8. Following endocytosis9, Ii–MHC class II complexes traffic through the endocytic pathway from early endosomes to late endosomal–lysosomal antigen-processing compartments, which contain antigenic proteins and peptides.
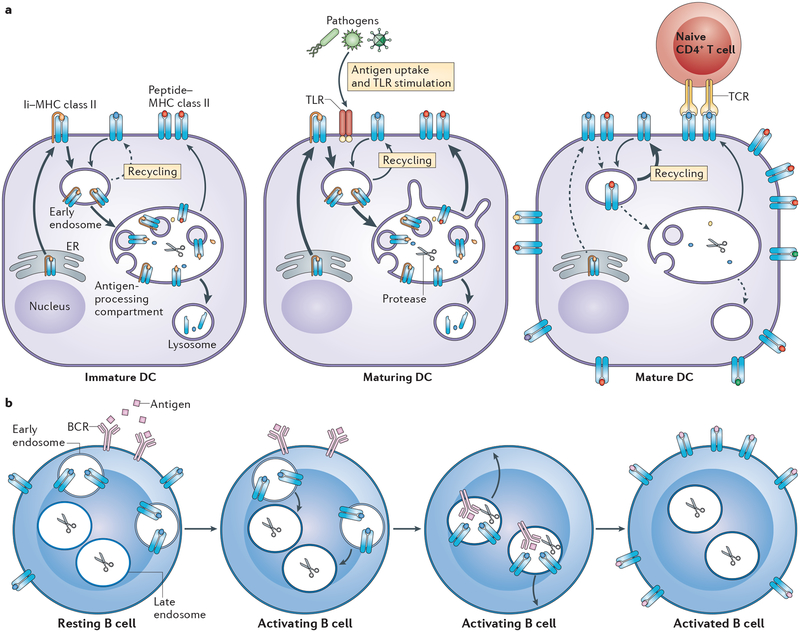
Figure 1 |. Generation of peptide–MHC class II complexes in antigen-presenting cells.
MHC class II αβ dimers associate with the invariant chain (Ii) in the endoplasmic reticulum (ER), traffic through the Golgi apparatus and are delivered to the plasma membrane. Ii–MHC class II complexes are internalized by clathrin-mediated endocytosis and traffic to multivesicular antigen-processing compartments. Some of these complexes are sorted into the intraluminal vesicles (ILVs) of multivesicular bodies (MVBs), in which sequential Ii proteolysis leads to the generation of a fragment of Ii, termed class II-associated invariant chain peptide (CLIP), which remains in the peptide-binding groove of MHC class II. CLIP is removed from CLIP–MHC class II complexes by the enzyme HLA-DM, which is present in the MVB internal and limiting membranes, thereby facilitating peptide binding onto nascent MHC class II. The activity of HLA-DM is regulated by HLA-DO, but the mechanism of regulation remains unknown. ILV-associated peptide–MHC class II somehow associates with the MVB limiting membrane, and endosomal–lysosomal tubules directed towards the plasma membrane either directly fuse or give rise to transport vesicles that fuse with the plasma membrane. MVB membranes are rich in the lipids that constitute lipid raft membrane microdomains, and fusion of peptide–MHC class II from MVB-derived membranes with the plasma membrane leads to the deposition of lipid raft-associated peptide–MHC class II directly into the plasma membrane. In circumstances in which an entire MVB fuses with the plasma membrane, the ILVs of MVBs are released from the APC in the form of exosomes. Surface expressed peptide–MHC class II can be internalized through a clathrin-independent endocytosis pathway and can be targeted for lysosomal degradation or can recycle back to the plasma membrane.
MHC class II is not able to bind antigenic peptides until Ii is proteolytically degraded and dissociates from the Ii–MHC class II complex10. Although peptide–MHC class II complexes can be generated throughout the endocytic pathway11, the typical antigen-processing compartment is a late endosomal or lysosomal structure that is enriched in proteolytic enzymes and disulphide reductases and has a sufficiently low pH to activate proteases for antigen proteolysis3,4,12. In B cells, DCs and macrophages, these compartments exist in various forms, have complex internal membrane structures and are all forms of multivesicular bodies (MVBs). Ii is degraded by stepwise proteolysis in these compartments and a small fragment of Ii, termed the class II-associated invariant chain peptide (CLIP), remains bound to the MHC class II peptide-binding groove. This fragment of Ii must be removed from MHC class II to allow binding of antigenic peptides. The release of CLIP is facilitated by the enzyme HLA-DM (known as H2-M in mice), which is an MHC class II-like protein encoded in the MHC locus13. HLA-DO (known as H2-O in mice) is another MHC class II-like protein that suppresses HLA-DM activity in mildly acidic antigen-processing compartments14. After CLIP removal, the nascent MHC class II molecule then binds antigenic peptides, leaves the MVB in tubulovesicular endosomes and is inserted into the plasma membrane for recognition by CD4+ T cells.
Mechanisms of antigen acquisition and processing
Macropinocytosis.
APCs use various different cellular processes to capture and to deliver diverse antigens to antigen-processing compartments (FIG. 2). DCs are very efficient APCs as a result of their ability to internalize extracellular material through a nonspecific process termed macropinocytosis15. Immature DCs have a very robust process of constitutive macropinocytosis and this is thought to be a major mechanism of antigen acquisition by DCs and the main mode of entry of vaccinia virus, adenovirus type 3 and herpes simplex virus into cells16. By contrast, B cells have little macropinocytic activity; these cells rely almost exclusively on receptor-mediated endocytosis for antigen acquisition (described below). It has also been suggested that the self proteins and peptides present in lymph fluid are important for peripheral tolerance17, although the extent to which macropinocytosis mediates the uptake of these antigens by APCs remains to be determined.
Figure 2 |. Pathways of antigen endocytosis in antigen-presenting cells.
Antigens can enter the endocytic pathway of antigen-presenting cells (APCs) by several distinct mechanisms. Receptor-mediated endocytosis via clathrin-coated vesicles requires antigen binding to one of several receptors on APCs — such as lectin receptors — which results in their internalization into early endosomes. Macropinocytosis, which is a form of cell ‘drinking’, is an actin-dependent process that leads to the uptake of soluble material into the cell in a macropinosome. In each of these processes, the internalized early endosomes eventually fuse with multivesicular late endosomal–lysosomal antigen-processing compartments. It is in these compartments that internalized antigen proteolysis and peptide–MHC class II complex formation takes place. Phagocytosis is an endocytic process in which opsonized particles bind to various receptors and, following actin-dependent membrane reorganization around the particle, enter cells in membrane-derived phagosomes. Phagosomes are not particularly rich in proteases or MHC class II and, after Toll-like receptor (TLR)-induced fusion with lysosomes or potentially with MHC class II-containing late endosomal–lysosomal compartments, the resulting phagolysosome generates peptide–MHC class II complexes. Autophagy is a process by which membranes (often derived from the endoplasmic reticulum (ER)) envelop cytosolic antigens to form an autophagosome. Upon autophagosome fusion with lysosomal compartments, the resulting autophagolysosome generates peptide–MHC class II complexes. It is important to remember that this is a schematic and that each APC does not contain only one early endosome and one antigen-processing compartment; there is a gradient of endosomes containing varying amounts of the essential components that are necessary to generate peptide–MHC class II complexes in every cell and it is for this reason that the nature of the antigen-processing compartment used by different antigens will differ.
Activation of DCs in vitro leads to a transient burst in macropinocytosis that is eventually (within hours) terminated18,19. Macropinocytosis involves the formation of plasma membrane ruffles and is regulated by the RHO GTPase cell division cycle 42 (CDC42)20 and by RAC-dependent remodelling of the actin cytoskeleton19. Studies using bone marrow-derived DCs revealed that DC maturation inhibits macropinocytosis by reducing the amount of GTP-bound CDC42 and that macropinocytosis can be restored in mature DCs by overexpressing a constitutively active form of CDC42 (REF. 20). Similarly, DC activation is associated with the suppression of uptake of larger particles (such as 1 μm latex beads) by nonspecific phagocytosis21,22. Given this suppression of nonspecific antigen uptake in mature DCs, the general tenet was that immature DCs are the main samplers of antigen in peripheral tissues and that maturation converts DCs from antigen-sampling cells to antigen-presenting cells. Recent studies have challenged this dogma. Activation of DCs in vivo, by injection of the Toll-like receptor (TLR) ligands CpG-containing oligodeoxynucleotides, lipopolysaccharide (LPS) or Escherichia coli, did not markedly suppress soluble antigen uptake23,24. Accordingly, in vivo-matured DCs could effectively process and present soluble (pinocytosed) antigens to antigen-specific CD4+ T cells ex vivo22,24. Moreover, in vivo maturation of DCs followed by immunization with soluble antigen in the presence of adjuvant has been reported to promote antigen-specific CD4+ T cell proliferation in mice24, but this has not been observed in other studies22,23.
Receptor-mediated endocytosis.
In addition to nonspecific antigen uptake, APCs have several cell surface receptors that facilitate antigen entry into antigen-processing compartments through clathrin-mediated endocytosis. B cells with antigen-specific BCRs are 1,000-times more efficient in processing and presenting antigens to cognate T cells than B cells with BCRs specific for an irrelevant antigen25. B cells also have cell surface complement receptors and Fc receptors for IgG (FcγRs) that are involved in both clathrin-dependent and clathrin-independent antigen internalization, processing and presentation26,27. Unlike macropinocytosis, clathrin-coated pits and vesicles persist in activated DCs20. In fact, FcγRs that are present on both immature and mature DCs target immune complexes to antigen-processing compartments for efficient processing and presentation to antigen-specific CD4+ T cells22. DCs and B cells also express lectin receptors, such as the mannose receptor and DEC205 (also known as LY75), which recognize carbohydrate residues on self proteins and on some pathogens and target them for internalization either by clathrin-dependent endocytosis or by phagocytosis. Conjugation of antigens to ligands for these endocytic receptors has been used to deliver them to endosomes and/or lysosomes for efficient antigen processing and presentation22. Targeting of antigens to endocytic receptors on DCs such as DEC205 can enhance the efficiency of priming of naive CD4+ T cells by 1,000-fold compared with antigen internalized by macropinocytosis28. Similarly, it has recently been shown that binding of DEC205-targeted antigen by B cells amplifies antigen-specific CD4+ T cell responses in vivo29.
Phagocytosis.
Phagocytosis is probably the most important mechanism of foreign antigen uptake by DCs and macrophages in vivo30. Not all DC subtypes efficiently phagocytose foreign antigens; plasmacytoid DCs have severely compromised phagocytosis compared with CD11chi conventional DCs31. Phagocytosis of apoptotic cells by APCs generates a wide range of self peptides that, when presented on the surface of a peripheral DC, can induce peripheral tolerance against self antigens32. Similarly to macropinocytosis, nonspecific phagocytosis is also suppressed during DC activation20–22, a finding that is attributed to decreased expression of active CDC42 by mature DCs20. However, phagocytosis can also be mediated by receptors such as C-type lectins, Fc receptors, complement receptors, scavenger receptor A and CD36, which recognize ligands on pathogens and apoptotic cells. Although many of these receptors also internalize ligands by clathrin-mediated endocytosis, their ability to facilitate the internalization of large (>3 μm) particles (such as opsonized pathogens) is clathrin independent and does not rely on other components of the clathrin-mediated endocytosis machinery33. Activation of DCs in vivo does not markedly alter their ability to capture antigens by receptor-dependent phagocytosis and even after in vivo activation, DCs are capable of internalizing and processing phagocytosed cargo, and presenting it to CD4+ T cells22.
Phagocytosis requires large amounts of membrane for the capture of cargo, and proteomic analysis of purified phagosomes has implicated both early endosomes and the endoplasmic reticulum (ER) as sources of phagosome membrane. The recruitment of ER membrane to phagosomes is especially interesting given that DCs can activate MHC class I-restricted CD8+ T cells through cross-presentation of phagocytosed antigen34–36. Individual phagosomes are fairly non-proteolytic compartments; however, phagosomes undergo a series of membrane fusion events with lysosomes to generate hybrid organelles termed phagolysosomes30. The phagolysosome is a highly destructive organelle that contains MHC class II molecules and the complete MHC class II peptide-loading machinery. It has been reported that TLR signalling enhances phagosome ‘maturation’ in macrophages and DCs37,38; however, another study showed that TLR signalling suppresses phagosome maturation in macrophages39. Recruitment of TLRs to the phagosome is mainly mediated by the delivery of the endosome-associated adaptor protein AP3 to the phagosome membrane40, probably during the process of phagosome–endosome fusion.
Most pathogens that are internalized by phagocytosis will ultimately be destroyed in phagolysosomes. However, some pathogens, such as Mycobacterium tuberculosis, have evolved mechanisms to avoid destruction in a phagosome (BOX 1). Proteolysis of pathogen-derived proteins that are present in phagolysosomes provides a plentiful source of antigenic peptides for loading onto MHC class II molecules, although it should be noted that the proteolytic activity of phagolysosomes in DCs is regulated to prevent the complete destruction of immunogenic peptides (as described below). Curiously, different phagosomes within a single DC can behave autonomously, such that one phagosome can mediate the presentation of antigens containing a TLR ligand, whereas a different phagosome is unable to present antigen (that does not contain a TLR ligand)37,41. A recent study found that phagosome content could mix between individual phagosomes as long as each phagosome contained a TLR-activating signal42. One advantage of such a mechanism would be to allow APCs to distinguish between phagosomes with self (non-stimulating) antigens and non-self (stimulating) antigens; however, definitive testing of this model has not been carried out.
Box 1 |. Post-translational regulation of MHC class II expression by pathogens.
Downregulation of MHC class II transcription (usually by suppressing the activity of the MHC class II transactivator (CIITA)) is a common strategy used by pathogens to circumvent immune recognition. This topic has been extensively reviewed137 and is not addressed in this Review. Instead, we summarize the data showing that pathogens can also suppress MHC class II expression post-translationally — that is, by downregulating the expression of pre-existing MHC class II. Infection with either Salmonella enterica subsp. enterica serovar Typhimurium138 or Francisella tularensis139 promotes ubiquitylation and degradation of MHC class II in antigen-presenting cells (APCs). F. tularensis-induced MHC class II downregulation is mediated by infection-stimulated prostaglandin E2 release and the subsequent interleukin-10 (IL-10)-stimulated expression of MARCH1 by macrophages140. The finding that S. Typhimurium does not reduce the expression of both MHC class II and the MARCH1 substrate CD86 (REF. 138) suggests that MHC class II downregulation by S. Typhimurium is MARCH1 independent.
Mycobacterium tuberculosis is another organism that attempts to subvert immune recognition by altering the MHC class II antigen-processing pathways. The M. tuberculosis protein TACO (also known as coronin 1A) suppresses phagosome fusion with lysosomes, thereby inhibiting antigen presentation of mycobacterial antigens141. Other studies have supported the idea that phagosome maturation is important for immune recognition of mycobacterial antigens by showing a role for live, but not heat-killed, M. tuberculosis in suppressing peptide–MHC class II formation in phagosomes of infected macrophages142. However, induction of autophagy promotes the fusion of M. tuberculosis-containing phagosomes with lysosomes143, thereby leading to destruction of the pathogen.
Human cytomegalovirus (CMV) has adopted various strategies to escape immune evasion. In addition to the well-documented role of the CMV genes US2 and US11 in promoting retrotranslocation and proteosomal degradation of MHC class I molecules144, US2 promotes retrotranslocation and degradation of MHC class II and HLA-DM, thereby suppressing recognition of viral antigens by CD4+ T cells145. CMV US3 inhibits MHC class II expression by a different mechanism; US3 binds to MHC class II αβ heterodimers in the endoplasmic reticulum and prevents their association with the invariant chain (Ii), thereby inhibiting Ii-dependent MHC class II sorting and peptide loading onto MHC class II146. The CMV protein pp65 also reduces MHC class II expression147, although the mechanism remains to be determined. It should be pointed out that each of these studies relied on infection of non-professional APCs, and the limited experiments done with dendritic cells (DCs) did not confirm marked effects on MHC class II expression by these CMV gene products144.
Diversion of the normal process of intracellular sorting of MHC class II molecules to antigen-processing compartments is another mechanism used by pathogens in an attempt to evade immune recognition. Herpes simplex virus 1 glycoprotein B reduces cell surface expression of MHC class II by diverting MHC class II to multivesicular bodies (MVBs), thereby increasing the secretion of MHC class II in exosomes148. The HIV protein Nef directly interferes with clathrin-dependent internalization of Ii–MHC class II complexes from the cell surface149 and impairs Ii proteolysis in MVBs150, both of which limit the availability of nascent MHC class II, which is required for efficient binding of antigenic peptides for recognition by CD4+ T cells.
Epstein–Barr virus (EBV) encodes several genes, including BZLF1 and BZLF2, that have a role in immune evasion by infected B cells. BZLF1 reduces expression of Ii but has no effect on MHC class II cell surface expression, which suggests a complicated mechanism of action151. BZLF2 encodes a protein that, when processed and secreted from infected cells, can bind to MHC class II and block antigen-specific recognition by CD4+ T cells152. Although EBV is not known to infect DCs directly, it remains to be seen whether EBV gene products secreted from infected cells can alter DC function and suppress naive CD4+ T cell activation.
Autophagy.
Approximately 20–30% of the peptides that are eluted from MHC class II in B cells and human DCs are derived from cytosolic or nuclear proteins43, which suggests a role for macroautophagy in transporting cytosolic antigens to antigen-processing compartments44. Autophagosomes engulf cytosolic macromolecules and organelles and can fuse with endosomal–lysosomal antigen-processing compartments to form auto phagolysosomes45. The presentation of autophagosome-encapsulated antigens requires intact lysosomal function, which highlights the importance of lysosomal proteolysis in T cell epitope formation46.
Autophagy also leads to the generation of citrullinated proteins and peptides by peptidylarginine deiminase47, and antibody responses to citrullinated proteins have been linked to autoimmune rheumatoid arthritis48. Mutation of autophagy gene 5 (ATG5) significantly disrupts both positive and negative selection of CD4+ T cells by thymic epithelial cells49,50 and leads to a quantitative alteration of the MHC class II peptidome in cortical thymic epithelial cells49. ATG5-deficient DCs are also unable to activate herpes simplex virus-specific CD4+ T cells51, which suggests an important role for macroautophagy in peripheral DC function.
Regulation of endosomal and lysosomal proteolysis in APCs.
Regulation of proteolysis in endosomes and lysosomes is essential for many aspects of MHC-restricted antigen processing and presentation. ‘Lysosomal’ pro-teases, such as asparaginyl endopeptidase52 and cathepsin S53,54, are essential not only for Ii degradation and dissociation from MHC class II but also for the generation of the antigenic peptides that ultimately bind to MHC class II. Cathepsin S, B, H and L redistribute from true lysosomes to MHC class II-containing late endosomal antigen-processing compartments upon DC activation55 — a process that promotes peptide-MHC class II complex assembly. Activation of DCs also increases the activity of the ATP-dependent vacuolar proton pump and acidifies antigen-processing compartments56, thereby acutely enhancing antigen proteolysis and peptide–MHC class II complex formation in maturing DCs56–58.
Although enhanced protease activity leads to increased antigen proteolysis, this does not necessarily lead to enhanced epitope generation and antigen presentation by DCs. Indeed, there are many T cell epitopes that are generated in early endosomes that are actually destroyed by lysosomal proteolysis59,60. A recent study has shown that the delivery of a model antigen to early endosomes by conjugation to a CD40-specific monoclonal antibody resulted in efficient antigen presentation to CD4+ T cells, whereas delivery of antigen to late endosomes or early lysosomes by conjugation to a DEC205-specific antibody resulted in fairly poor presentation61. Therefore, professional APCs have developed mechanisms to limit antigen proteolysis, thereby preserving antigenic peptide integrity. Although DCs are better than macrophages at stimulating naive T cells, lysosomal extracts of DCs are 50-times less active than those of macrophages60. Unlike macrophages (which rapidly and completely degrade internalized proteins), DCs can retain intact internalized antigens for many hours in vivo57,58,60.
Phagosomal compartments in DCs are less acidic than their counterparts in macrophages and this difference is mediated by the RAC2-dependent association of NADPH oxidase 2 (NOX2; also known as cytochrome b245 heavy chain) to the membrane of the developing phagosome62. It has recently been shown that during phagocytosis (but not macropinocytosis or receptor-mediated endocytosis), TLR2 signalling recruits the classic autophagy markers ATG8 and LC3 to the developing phagosome63. ATG8-and LC3-tagged phagosomes also recruit NOX2, which results in increased phagosome pH, prolonged antigen persistence in developing phagosomes and prolonged antigen presentation by MHC class II to CD4+ T cells63. These studies highlight the importance of maintaining a balance between antigenic protein proteolysis and complete proteolytic destruction of antigenic MHC class II-binding peptides in antigen-processing compartments in APCs.
Regulation of MHC class II biosynthesis
The expression of MHC class II is tightly regulated in all APCs both transcriptionally and post-transcriptionally. MHC class II transactivator (CIITA) is the master regulator of MHC class II gene expression64. CIITA is constitutively expressed only by professional APCs, such as B cells, DCs, macrophages and thymic epithelial cells. However, CIITA activity and ultimately MHC class II expression can be regulated in both professional and non-professional APCs65; for example, interferon-γ (IFNγ) stimulates CIITA expression and converts monocytes from MHC class II-negative cells to MHC class II-expressing functional APCs. Expression of mRNA encoding MHC class II also depends on the maturation status of APCs. Resting B cells express MHC class II mRNA and protein, and activation of B cells in vitro and in vivo upregulates this expression. Activation of CD11c+ conventional DCs in the spleen also leads to an increase in MHC class II protein synthesis; however, within 24 hours of stimulation, MHC class II synthesis in DCs is abrogated66. This termination of MHC class II biosynthesis does not occur following activation of plasmacytoid DCs67 owing to their sustained expression of CIITA68, and this suggests that there are fundamental differences in the role of newly synthesized MHC class II molecules in conventional DCs and plasmacytoid DCs69. Enhanced MHC class II synthesis following TLR signalling is thought to enable maturing CD11c+ DCs to respond to pathogens through the formation of new pathogen-specific peptide–MHC class II complexes in antigen-processing compartments, whereas the subsequent termination of MHC class II biosynthesis in mature DCs is thought to limit the generation of peptide–MHC class II complexes that are irrelevant to the invading pathogen70. As discussed below, the ability of DCs to effectively generate antigen-specific peptide–MHC class II complexes even when protein synthesis is terminated22 suggests that previously synthesized MHC class II molecules can enter antigen-processing compartments. Thus, the termination of MHC class II synthesis upon DC activation might limit, but not completely prevent, the formation of new antigenic peptide–MHC class II complexes.
MHC class II trafficking
Transport to antigen-processing compartments.
In immature DCs, Langerhans cells and macrophages, large amounts of MHC class II are present in endosomal–lysosomal antigen-processing compartments before cell activation71–73. Activation of DCs by various TLR ligands leads to a marked change in MHC class II expression and cellular localization; in immature DCs, considerable amounts of MHC class II reside in both intracellular endosomes and lysosomes and on the plasma membrane, whereas in mature DCs, the vast majority of MHC class II resides on the plasma membrane (FIG. 3a). The increased expression of MHC class II on the plasma membrane following DC activation can probably be accounted for by considering several factors: the retention of MHC class II in antigen-processing compartments and the lysosomal degradation of intra cellular MHC class II in immature DCs66,74,75; the increased biosynthesis of MHC class II upon DC activation67,76; the movement of intracellular MHC class II to the plasma membrane upon DC activation74,77–79; and the increased stability of MHC class II complexes in mature DCs74,76.
Figure 3 |. Activation of antigen-presenting cells redistributes intracellular MHC class II molecules.
a | In immature dendritic cells (DCs), up to 75% of all MHC class II molecules reside in lysosome-like antigen-processing compartments. It is in these compartments that peptide–MHC class II complexes are generated (albeit slowly in immature DCs). The predominant intracellular localization of MHC class II in immature DCs is due to robust synthesis of invariant chain (Ii)–MHC class II complexes and the movement of these complexes to antigen-processing compartments. Although some peptide–MHC class II complexes formed in these compartments do traffic to the plasma membrane, much of the MHC class II that is synthesized in immature DCs is degraded in lysosomes. Maturation of DCs by pathogen uptake and/or Toll-like receptor (TLR) stimulation leads a transient burst in Ii–MHC class II biosynthesis, efficient generation of peptide–MHC class II complexes that traffic to the plasma membrane in tubular endosomes and degradation of residual MHC class II present in lysosomes. Fully mature conventional DCs do not synthesize Ii–MHC class II and therefore express very little MHC class II in antigen-processing compartments. Arrow thickness indicates prominence of a particular pathway and dashed arrows indicate minor pathways. In mature DCs, any internalized MHC class II is simply recycled to the plasma membrane where peptide–MHC class II complexes can interact with naive antigen-specific CD4+ T cells. Note that after DC activation, structures such as antigen-processing compartments still exist in the mature DC, but peptide–MHC class II complexes do not accumulate in these compartments. b | In resting B cells, large amounts of MHC class II are present on the plasma membrane and in peripheral early endosomal vesicles. Binding of antigen to the B cell receptor (BCR) triggers BCR endocytosis and recruits the peripheral MHC class II-containing vesicles towards the antigen–BCR-containing lysosome-like antigen-processing compartments. Internalized antigen is processed into peptides in these compartments and the resulting peptide–MHC class II complexes traffic to the plasma membrane for recognition by primed antigen-specific CD4+ T cells. TCR, T cell receptor.
In contrast to DCs and macrophages, very little MHC class II resides in late endosomal compartments in resting B cells80 (FIG. 3b). MHC class II trafficking to the conventional antigen-processing compartment is induced by BCR signalling, which targets both MHC class II and BCR-associated antigen to the same late endosomal HLA-DM+ antigen-processing compartment for efficient peptide–MHC class II complex formation80,81. MHC class II redistribution is regulated by the actin-based motor protein myosin II and the spleen tyrosine kinase (SYK)-dependent reorganization of the actin cytoskeleton82,83. BCR-stimulated recruitment of MHC class II to antigen-processing compartments is not only an interesting cell biological process but also has important implications for immune function. Delivery of the model antigen hen egg lysozyme (HEL) to B cells by the HEL-specific BCR or by the macropinocytosis pathway generates HEL46–61 peptide–MHC class II complexes with different conformations that can be recognized by different HEL-specific CD4+ T cell clones84. This is presumably due to the initial formation of HEL46–61 peptide–MHC class II complexes in either MHC class II+HLA-DM+ late endosomes (following BCR-mediated HEL processing) or endosomal compartments that contain MHC class II but are devoid of HLA-DM (following soluble HEL processing). Macropinocytosis of soluble HEL together with nonspecific BCR crosslinking leads to generation of the macropinocytosis-associated peptide–MHC class II conformation84. This suggests that, similarly to phagosome autonomy in DCs38, dichotomy of antigen loading onto MHC class II in distinct intracellular compartments occurs in B cells. Similarly, autoreactive pathogenic CD4+ T cells in non-obese diabetic mice recognize an insulin peptide that binds to MHC class II in a different orientation to that of insulin-peptide–MHC class II complexes that are generated in the thymus and are therefore not negatively selected85. These peptide–MHC class II complexes form when preprocessed peptide (but not intact insulin protein) is incubated with APCs ex vivo85. Although the mechanism responsible for the generation of these distinct insulin-peptide–MHC class II complexes is not completely understood, it is likely that they form in distinct intracellular compartments that may have differences in pH, protease activity and levels of HLA-DM.
Transport to the plasma membrane.
Following peptide binding, the peptide–MHC class II complexes leave the antigen-processing compartments and traffic to the plasma membrane. Activation of DCs results in the formation of elongated tubules that emanate from antigen-processing compartments77–79 and these tubules (or vesicles derived from these tubules) constitutively deliver peptide–MHC class II complexes to the DC plasma membrane. Interaction of antigen-loaded DCs with antigen-specific CD4+ T cells results in the transport of these peptide–MHC class II-containing tubules directly towards the immunological synapse that is formed between a DC and a T cell78. During DC activation, the late endosomal pool of MHC class II redistributes to peripheral vesicles that no longer contain late endosomal markers and finally the MHC class II is inserted into the plasma membrane74. It has recently been shown that complexes of MHC class II with an Eα fusion protein that is internalized by phagocytosis accumulate in punctate cytoplasmic structures that are devoid of markers of either endosomes or lysosomes40. It remains to be deter-mined whether these structures are equivalent to the class II-containing transport vesicles (CIIVs) observed in B cells86; however, it is likely that these structures are MVB-derived transport vesicles that are destined for the plasma membrane.
Although B cells can clearly bind soluble antigens in vitro, most B cells in vivo acquire antigen that is immobilized on the surface of other cells2. The process by which this occurs has recently been identified. Similar to the polarized secretion of lysosome-derived peptide–MHC class II complexes at the immunological synapse between DCs and T cells78, B cells were shown to polarize their lysosomes towards the synapse formed at the site of contact with antigen immobilized on latex beads87. This process depends on reorganization of the B cell microtubule-organizing centre and leads to the secretion of lysosomal enzymes into the synapse, the proteolysis-dependent extraction of antigen from the surface of latex beads and the generation of specific peptide–MHC class II complexes for subsequent presentation to antigen-specific CD4+ T cells.
The precise molecular mechanisms that regulate the movement and the fusion of peptide–MHC class II-containing vesicles and tubules with the plasma membrane are poorly understood. Peptide–MHC class II-containing vesicles move in a ‘stop-and-go’ manner along microtubules towards the plasma membrane and various molecular motors, actin-binding proteins and GTPases control this process88. Disruption of the actin cytoskeleton inhibits LPS-induced peptide-MHC class II movement to the DC plasma membrane58. It is likely that peptide–MHC class II complex redistribution from intracellular antigen-processing compartments to the plasma membrane in activated DCs is regulated by the TRIF (also known as TICAM1)–RHOB pathway89,90. Genome-wide short interfering RNA screens for genes that regulate the movement of peptide–MHC class II molecules revealed that the GTPase ADP ribosylation factor-like protein 14 (ARL14; also known as ARF7) and myosin 1E are important for the actin-based movement of antigen-processing compartments to the DC surface91. However, exactly how these proteins regulate vesicle movement remains to be elucidated.
MHC class II secretion from APCs.
When an MVB fuses directly with the plasma membrane, the entire contents of the MVB (including the intraluminal vesicles (ILVs)) are released into the extracellular space and the secreted ILVs are termed exosomes. Exosomes are readily released from all APC subtypes examined; these small (<100 nm) vesicles contain various T cell ligands (such as peptide–MHC class II, co-stimulatory molecules and adhesion molecules) that can both directly and indirectly stimulate antigen-specific CD4+ T cells92,93. Exosome release is constitutive in APCs, but it can also be stimulated following encounter of antigen-loaded APCs with antigen-specific CD4+ T cells94,95, which can then trap the antigen-bearing exosomes on their surface95. In addition to functioning as ‘mini-APCs’ to stimulate primed CD4+ T cells, exosomes can be internalized by APCs and can function as a source of antigen. Plasmacytoid DCs are fairly poor stimulators of naive CD4+ T cells, partly as a result of their poor phagocytic capacity, but these cells can effectively internalize exosomes and apoptotic bodies, which results in efficient T cell proliferation96. Peptide–MHC class II-bearing exosomes can also become attached to the surface of cells — such as follicular DCs — that do not themselves synthesize MHC class II proteins, enabling them to present antigen97. MHC class II molecules are also known to be present on mouse CD4+ T cells and this acquisition is likely to be a consequence of exosome binding to the T cell surface.
Given their ability to stimulate immune responses in vivo, DC-derived exosomes are promising candidates for use in cancer immunotherapy98. Tumour peptide-pulsed exosomes bearing MHC class II, adhesion and co-stimulatory molecules have been used as cell-free vaccines to eliminate tumours99, and tumour-derived exosomes have also been used as a source of antigen in DC-based therapy for malignant melanoma100. The many clinical protocols using a range of cell-derived exosomes highlight the promise of using these MVB-derived vesicles in a clinical setting.
Clustering in membrane microdomains.
A considerable proportion of cell surface peptide–MHC class II is localized in discrete membrane microdomains known as lipid rafts. These cholesterol-rich microdomains are found in all mammalian cells and the association of MHC class II with lipid rafts has been observed in human and mouse B cells, DCs, monocytes, macrophages and thymic epithelial cells (reviewed in REF. 101). MHC class II has also been reported to associate with tetraspanin proteins in structures termed tetraspan webs102. However, given the close relationship between membrane cholesterol, lipid rafts and tetraspan webs, it is unclear how these microdomains differ in vivo101. The presence of peptide–MHC class II in membrane microdomains is particularly important for T cell activation by APCs that have low levels of specific peptide–MHC class II on their surface103–105, a finding that has been attributed to the local concentration of small amounts of relevant peptide–MHC class II in discrete domains. Pulse–chase biosynthetic radiolabelling studies and the use of protein transport inhibitors have shown that MHC class II localizes to lipid rafts in antigen-processing compartments before peptide binding106,107. MVBs are enriched in the lipids that are constituents of lipid rafts, and we have proposed that coordinated peptide loading onto MHC class II in these compartments and transport of the complexes to the plasma membrane mediates the delivery of a portion of relevant peptide–MHC class II complexes that is present in a pre-formed lipid raft108. We have recently confirmed that peptide–MHC class II complexes generated in antigen-processing compartments first appear at the plasma membrane of DCs in small clusters and that the integrity of these clusters is altered by the disruption of lipid rafts105. These studies show that DCs have a mechanism that allows temporal coordination of intracellular peptide–MHC class II formation and the subsequent insertion of these same peptide–MHC class II complexes into the plasma membrane in membrane microdomains. Whether this is true in APCs other than DCs remains to be determined.
The fate of surface MHC class II in APCs
Recycling from the plasma membrane.
Once expressed on the APC surface, peptide–MHC class II complexes do not simply persist there permanently. The plasma membrane of eukaryotic cells is constantly being internalized; macrophages internalize the equivalent of their entire plasma membrane in only 30 minutes109. Although many internalized plasma membrane proteins are transported to lysosomes for eventual degradation, many proteins efficiently recycle from the plasma membrane to early endosomes and back to the plasma membrane. However, little is known about the molecular machinery that specifically regulates MHC class II recycling in APCs.
Although some antigenic epitopes are only generated in the acidic antigen-processing compartments, others are only generated in early endosomes by limited proteolysis110. For example, influenza virus has an antigenic epitope that simply requires unfolding and mild proteolysis in the early endosomes of DCs111. Presentation of these ‘early endosome’ epitopes to T cells does not require Ii-dependent transport of MHC class II or HLA-DM-mediated peptide editing of CLIP–MHC class II, or even neosynthesis of MHC class II molecules112,113. Together, these findings suggest that such epitopes are presented on pre-existing MHC class II molecules that have been recycled from the plasma membrane into the early endocytic pathway. This alternative pathway of processing and presentation is likely to be important not only for epitopes that are easily destroyed in very acidic antigen-processing compartments but also for conditions in which MHC biosynthesis is terminated (such as following DC activation in vivo).
However, not all antigens that bind to recycling MHC class II molecules do so in early endosomes. Mature DCs that internalize antigenic Eα protein via receptor-mediated endocytosis generate Eα peptide–MHC class II complexes in conventional late endosomal–lysosomal antigen-processing compartments that are independent of new MHC class II biosynthesis22. Taken together, these data are consistent with a model in which ‘old’ (perhaps recycling) MHC class II complexes in mature DCs can generate ‘new’ peptide–MHC class II complexes following antigen internalization; this model challenges the long-held belief that mature DCs are unable to acquire, process and present antigens to T cells.
Regulation by ubiquitylation.
Perhaps the greatest effect of DC activation on MHC class II cell biology is the regulation of peptide–MHC class II stability. Immature DCs synthesize large amounts of MHC class II, express peptide–MHC class II complexes on their surface and, under steady-state conditions, have a high rate of constitutive MHC class II degradation76. Activation of DCs dramatically reduces the kinetics of peptide–MHC class II turnover74,76 and in fully activated DCs, the half-life of surface peptide–MHC class II complexes is more than 100 hours76. The rapid turnover of peptide-MHC class II in immature DCs is primarily due to the ubiquitylation of a conserved lysine residue that is present in the MHC class II β-chain by the E3 ubiquitin ligase MARCH1 (REFS 114–117). Analysis of APCs isolated from MARCH1-deficient mice, or from mice in which this conserved MHC class II lysine residue has been mutated, reveals a direct role for ubiquitylation by MARCH1 in regulating intracellular accumulation and stability of peptide–MHC class II complexes117–119.
Ubiquitylation is not the only mechanism that regulates the turnover of peptide–MHC class II from the surface of DCs. The encounter of antigen-loaded APCs with antigen-specific CD4+ T cells results in the release of exosomes loaded with peptide–MHC class II complexes from APCs94,95, thereby reducing the cell-associated level of peptide–MHC class II. Whereas the targeting of MHC class II molecules for lysosomal degradation is ubiquitin dependent115–117,119, the sorting of MHC class II into the exosome pathway is ubiquitin independent95,120, which shows the complexity of protein sorting in the late endosomal and MVB system in DCs. We have recently shown that encounter of antigen-specific primed CD4+ T cells with antigen-pulsed DCs or crosslinking of surface peptide–MHC class II with an MHC class II-specific monoclonal antibody leads to rapid peptide–MHC class II endocytosis and lysosomal degradation121. This pheno menon is also independent of MHC class II ubiquitylation, as it is observed in DCs isolated from MARCH1-deficient mice121. Surprisingly, engagement of antigen-pulsed DCs with antigen-specific T cells also results in the degradation of immunologically irrelevant peptide–MHC class II complexes, and this is due to the presence of both relevant and irrelevant peptide–MHC class II complexes in lipid raft microdomains121. So, although ubiquitylation seems to regulate the steady-state turn-over of MHC class II in DCs, it does not seem to control ligation-induced changes in peptide–MHC class II expression on the surface of DCs.
MARCH1 is expressed by resting B cells, immature DCs, monocytes and macrophages122, but not by epithelial cells in the thymus123. The mechanisms that control this selective expression of MARCH1 remain to be determined. MARCH1 targets not only MHC class II but also CD86 for degradation124,125, and therefore regulates the basal expression of at least two important T cell ligands in APCs. The anti-inflammatory cytokine interleukin-10 (IL-10) stimulates the synthesis of mRNA encoding MARCH1 in monocytes126, which explains the observation that IL-10 downregulates MHC class II expression and promotes lysosomal MHC class II accumulation in human monocytes127.
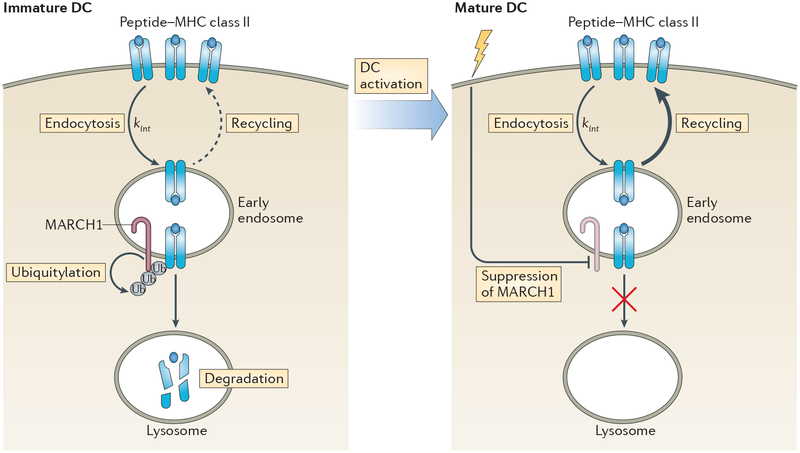
Expression of MARCH1 mRNA is rapidly downregulated following activation of conventional DCs118,128, thereby limiting the expression of MARCH1 to immature DCs. MARCH1 is also expressed by plasmacytoid DCs, but its levels are not altered by activation of these cells67, which might reflect important differences in the role of MHC class II turnover in conventional DCs and plasmacytoid DCs. Although MARCH1 is also expressed by resting B cells and macrophages, it remains to be determined whether activation of these cells also suppresses its expression. MARCH1 has a very short half-life (less than 30 minutes) and its expression is partly regulated by auto-ubiquitylation129,130. Therefore, DC activation rapidly leads to the termination of MARCH1 mRNA synthesis118,128, the downregulation of MARCH1 protein118 and the termination of MARCH1-mediated MHC class II ubiquitylation115,116. Interestingly, MARCH1 targets the internalizing peptide–MHC class II complexes in early endosomes of immature DCs131 in a process that could promote the degradation of ‘older’ surface-expressed peptide–MHC class II complexes and spare the ‘new’ peptide–MHC class II complexes that are still present in the antigen-processing compartment (FIG. 4).
Figure 4 |. Degradation of peptide–MHC class II complexes in antigen-presenting cells.
After arrival at the cell surface, peptide–MHC class II complexes are internalized into early endosomes by clathrin-independent endocytosis in both immature and mature dendritic cells (DCs) at the same rate (kint); however, whether this is true in all antigen-presenting cell (APC) subsets remains to be determined. The E3 ubiquitin ligase MARCH1 is constitutively expressed by resting B cells and DCs, and its expression can be enhanced by exposure of monocytes or macrophages to interleukin-10 (IL-10; not shown). In immature DCs, ubiquitylation of internalized peptide–MHC class II complexes by MARCH1 at the plasma membrane and in early endosomes targets these complexes for lysosomal degradation. By contrast, inefficient ubiquitylation of peptide–MHC class II complexes by MARCH1 in B cells prevents their delivery to lysosomes and instead redirects them back to the plasma membrane (indicated by the dashed arrow). Activation of immature DCs rapidly terminates MARCH1 expression and peptide–MHC class II ubiquitylation. As internalized peptide–MHC class II complexes are not ubiquitylated in mature DCs, these complexes are spared from lysosomal degradation and enter a re-iterative recycling pathway back to the plasma membrane (indicated by the thicker arrow).
In addition to transcriptional and translational control of MARCH1 expression, MARCH1 activity and MHC class II ubiquitylation are regulated by protein–protein interactions. The DC activation marker CD83 enhances MHC class II expression by suppressing the interaction of MHC class II with MARCH1 (REF. 132). Both CD83 and MARCH1 can be found in early endosomes and recycling endosomes118,133, which are the intracellular compartments that are thought to be the sites of MHC class II ubiquitylation131. Recent data showing that Toll-interacting protein (TOLLIP) reduces the expression of MARCH1 and enhances the expression of MHC class II134 confirm the hypothesis that modulation of MHC class II ubiquitylation is a major factor in the regulation of MHC class II expression in APCs.
Mechanism of MARCH1-mediated MHC class II down-regulation.
In B cells, MHC class II is also ubiquitylated, but it does not seem to accumulate in lysosomes. Recent data suggest that differences in ubiquitin chain length in B cells and DCs may affect MHC class II localization. In DCs, MHC class II molecules contain longer ubiquitin chains that preferentially target MHC class II molecules to late endosomal–lysosomal compartments, whereas in B cells MHC class II molecules contain short ubiquitin chains that target MHC class II molecules to earlier endosomes135. The relationship between ubiquitin chain length and the steady-state intracellular distribution of MHC class II was confirmed by expressing genetically engineered MHC class II containing different ubiquitin conjugates in each cell type135. Similar to many other ubiquitylated integral membrane proteins, it is probable that ubiquitylated MHC class II molecules interact with the multiprotein endosomal sorting complex required for transport (ESCRT) that directs ubiquitylated proteins to MVBs for eventual lysosomal degradation136. It is probable that MHC class II molecules with longer ubiquitin chains bind with higher affinity to ESCRT components and traffic to MVBs more effectively than those with short ubiquitin chains (or none at all). We have recently found that internalized peptide–MHC class II recycles very poorly in immature DCs and very efficiently in mature DCs (P.A.R. and K.-J. Cho, unpublished observations). The failure of peptide–MHC class II complexes to recycle in immature DCs is MARCH1 dependent, which is consistent with the idea that ubiquitylation targets internalizing peptide–MHC class II for ESCRT-dependent lysosomal degradation. Such a process could be important for generating a rapidly changing peptide–MHC class II repertoire in immature DCs that can be ‘fixed’ upon DC activation.
Concluding remarks
To maximize the chance of antigen presentation to antigen-specific T cells, professional APCs have adapted various processes to deliver both exogenous and endogenous antigens, whether self or foreign, into antigen-processing compartments. Depending on its form and on the role of the APC that is recognizing the antigen, different endocytic processes are involved in antigen processing; for example, phagocytosis of pathogens for stimulation of naive CD4+ T cells by DCs, or BCR-mediated endocytosis of pathogens or pathogen proteins by B cells for stimulation of primed CD4+ T cells. In each of these diverse situations, peptide–MHC class II complexes form in intracellular compartments and traffic to the APC plasma membrane, where they are either very stable (as in mature DCs) or undergo rapid turnover (as in immature DCs). This Review highlights the steps involved in peptide–MHC class II biosynthesis, transport and turn over in different types of APC and provides an insight into the complexity of these processes. Understanding the molecular mechanisms underlying these processes can potentially initiate new approaches to manipulate distinct steps in the antigen processing and presentation pathway in APCs in an attempt to combat human disease.
Acknowledgements
The authors acknowledge the many investigators in the field whose primary data could not be cited in this Review owing to space limitations. The authors also thank the anonymous referees who provided excellent advice in the preparation of this manuscript. This work was supported by the Japan Society for the Promotion of Science (to K.F.) and by the Intramural Research Program of the US National Institutes of Health (to P.A.R.).
Glossary
- Central tolerance
Self-tolerance that is created at the level of the central lymphoid organs. Developing T cells in the thymus and B cells in the bone marrow that strongly recognize self antigen undergo deletion or marked suppression
- Peripheral tolerance
Refers to mechanisms that control the reactivity of self antigen-specific lymphocytes that have escaped central tolerance. These mechanisms include ‘active’ suppression by cells that have immunomodulatory functions (such as regulatory T cells), as well as the induction of anergy or deletion, for example, through antigen presentation to T cells in the absence of co-stimulation
- Invariant chain
(Ii). A protein that binds to newly synthesized MHC class II molecules and promotes their egress from the endoplasmic reticulum. It blocks the peptide-binding site on nascent MHC class II molecules and targets Ii–MHC class II complexes to late endosomal and lysosomal antigen-processing compartments
- Multivesicular bodies
(MVBs). Forms of late endosomes that contain numerous intraluminal vesicles. MVBs can either fuse with lysosomes (degrading the intraluminal vesicles and associated cargo proteins) or with the plasma membrane (releasing the intracellular vesicles from the cell in the form of exosomes)
- Macropinocytosis
A nonspecific endocytosis pathway that facilitates the uptake of extracellular material that can vary in size from small molecules to intact cells. Plasma membrane ruffles entrap extracellular material and the resulting macropinosomes internalize and deliver their cargo to the endosomal–lysosomal pathway
- Clathrin-mediated endocytosis
The specific uptake of extracellular material that binds to membrane receptors and enters the cell through clathrin-coated vesicles. Integral membrane proteins directly bind to clathrin-associated adaptor molecules to facilitate their uptake
- Plasmacytoid DCs
Immature dendritic cells (DCs) with a morphology that resembles that of plasma cells. Plasmacytoid DCs produce large amounts of type I interferons in response to viral infection
- Cross-presentation
The process by which antigen-presenting cells (APCs) load peptides that are derived from extracellular antigens onto MHC class I molecules. Cross-presentation is essential for the initiation of immune responses to pathogens that do not infect APCs
- Macroautophagy
A process by which intracellular proteins, organelles and invading microorganisms are encapsulated in cytosolic vacuoles. These vacuoles (known as autophagosomes) fuse with lysosomes and degrade encapsulated cargo for antigen presentation. Bulk cytoplasmic autophagy occurs as a starvation response
- Immunological synapse
A junctional structure that is formed at the interface between T cells and target cells (including antigen-presenting cells). The molecular organization within this structure concentrates signalling molecules and directs the release of cytokines and lytic granules towards the target cell
- Follicular DCs
Specialized non-haematopoietic stromal cells that reside in the lymphoid follicles and germinal centres. These dendritic cells (DCs) have long dendrites and carry intact antigens on their surface. They are crucial for the optimal selection of B cells that produce antigen-binding antibodies
- Lipid rafts
Structures that arise from phase separation of different plasma membrane lipids as a result of their physical properties. This results in the formation of distinct and stable lipid domains in membranes, which might provide a platform for membrane-associated protein organization
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Banchereau J et al. Immunobiology of dendritic cells. Annu. Rev. Immunol 18, 767–811 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Harwood NE & Batista FD Early events in B cell activation. Annu. Rev. Immunol 28, 185–210 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Trombetta ES & Mellman I Cell biology of antigen processing in vitro and in vivo. Annu. Rev. Immunol 23, 975–1028 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Blum JS, Wearsch PA & Cresswell P Pathways of antigen processing. Annu. Rev. Immunol 31, 443–473 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozzacco L et al. Mass spectrometry analysis and quantitation of peptides presented on the MHC II molecules of mouse spleen dendritic cells. J. Proteome Res 10, 5016–5030 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cresswell P Invariant chain structure and MHC class II function. Cell 84, 505–507 (1996). [DOI] [PubMed] [Google Scholar]
- 7.Dugast M, Toussaint H, Dousset C & Benaroch P AP2 clathrin adaptor complex, but not AP1, controls the access of the major histocompatibility complex (MHC) class II to endosomes. J. Biol. Chem 280, 19656–19664 (2005). [DOI] [PubMed] [Google Scholar]
- 8.McCormick PJ, Martina JA & Bonifacino JS Involvement of clathrin and AP-2 in the trafficking of MHC class II molecules to antigen-processing compartments. Proc. Natl Acad. Sci. USA 102, 7910–7915 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roche PA, Teletski CL, Stang E, Bakke O & Long EO Cell surface HLA-DR-invariant chain complexes are targeted to endosomes by rapid internalization. Proc. Natl Acad. Sci. USA 90, 8581–8585 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roche PA & Cresswell P Invariant chain association with HLA-DR molecules inhibits immunogenic peptide binding. Nature 345, 615–618 (1990). [DOI] [PubMed] [Google Scholar]
- 11.Castellino F & Germain RN Extensive trafficking of MHC class II-invariant chain complexes in the endocytic pathway and appearance of peptide-loaded class II in multiple compartments. Immunity. 2, 73–88 (1995). [DOI] [PubMed] [Google Scholar]
- 12.Neefjes J CIIV, MIIC and other compartments for MHC class II loading. Eur. J. Immunol 29, 1421–1425 (1999). [DOI] [PubMed] [Google Scholar]
- 13.Busch R et al. Achieving stability through editing and chaperoning: regulation of MHC class II peptide binding and expression. Immunol. Rev 207, 242–260 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Denzin LK, Fallas JL, Prendes M & Yi W Right place, right time, right peptide: DO keeps DM focused. Immunol. Rev 207, 279–292 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Lim JP & Gleeson PA Macropinocytosis: an endocytic pathway for internalising large gulps. Immunol. Cell Biol 89, 836–843 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Mercer J & Helenius A Virus entry by macropinocytosis. Nature Cell Biol. 11, 510–520 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Clement CC, Rotzschke O & Santambrogio L The lymph as a pool of self-antigens. Trends Immunol. 32, 6–11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sallusto F, Cella M, Danieli C & Lanzavecchia A Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med 182, 389–400 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West MA et al. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science 305, 1153–1157 (2004).This study shows that acute activation of DCs leads to a transient burst of macropinocytosis, which enables DCs to internalize a large ‘gulp’ of exogenous antigens.
- 20.Garrett WS et al. Developmental control of endocytosis in dendritic cells by Cdc42. Cell 102, 325–334 (2000). [DOI] [PubMed] [Google Scholar]
- 21.Reis e Sousa C, Stahl PD & Austyn JM Phagocytosis of antigens by Langerhans cells in vitro. J. Exp. Med 178, 509–519 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Platt CD et al. Mature dendritic cells use endocytic receptors to capture and present antigens. Proc. Natl Acad. Sci. USA 107, 4287–4292 (2010).In this study, the authors show that mature DCs maintain the ability to capture exogenous antigens if the antigen binds to endocytic receptors that are present on the mature DC surface.
- 23.Young LJ et al. Dendritic cell preactivation impairs MHC class II presentation of vaccines and endogenous viral antigens. Proc. Natl Acad. Sci. USA 104, 17753–17758 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drutman SB & Trombetta ES Dendritic cells continue to capture and present antigens after maturation in vivo. J. Immunol 185, 2140–2146 (2010).This intriguing study challenges the dogma that mature DCs are incapable of nonspecific endocytosis by showing that in vivo-matured DCs are capable of internalizing, processing and presenting various ‘soluble’ antigens to CD4+ T cells.
- 25.Rock KL, Benacerraf B & Abbas AK Antigen presentation by hapten-specific B lymphocytes. I. Role of surface immunoglobulin receptors. J. Exp. Med 160, 1102–1113 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fearon DT & Carroll MC Regulation of B lymphocyte responses to foreign and self-antigens by the CD19/CD21 complex. Annu. Rev. Immunol 18, 393–422 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Antoniou AN & Watts C Antibody modulation of antigen presentation: positive and negative effects on presentation of the tetanus toxin antigen via the murine B cell isoform of FcγRII. Eur. J. Immunol 32, 530–540 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Bonifaz LC et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J. Exp. Med 199, 815–824 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leung CS et al. Robust T-cell stimulation by Epstein–Barr virus-transformed B cells after antigen targeting to DEC-205. Blood 121, 1584–1594 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stuart LM & Ezekowitz RA Phagocytosis: elegant complexity. Immunity 22, 539–550 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Bonaccorsi I et al. Membrane transfer from tumor cells overcomes deficient phagocytic ability of plasmacytoid dendritic cells for the acquisition and presentation of tumor antigens. J. Immunol 192, 824–832 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Steinman RM, Turley S, Mellman I & Inaba K The induction of tolerance by dendritic cells that have captured apoptotic cells. J. Exp. Med 191, 411–416 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tse SM et al. Differential role of actin, clathrin, and dynamin in Fcγ receptor-mediated endocytosis and phagocytosis. J. Biol. Chem 278, 3331–3338 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Guermonprez P et al. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature 425, 397–402 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Houde M et al. Phagosomes are competent organelles for antigen cross-presentation. Nature 425, 402–406 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Joffre OP, Segura E, Savina A & Amigorena S Cross-presentation by dendritic cells. Nature Rev. Immunol 12, 557–569 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Blander JM & Medzhitov R Regulation of phagosome maturation by signals from toll-like receptors. Science 304, 1014–1018 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Blander JM & Medzhitov R Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 440, 808–812 (2006).This study shows that individual phagosomes in a single DC behave autonomously, and that only phagosomes containing TLR ligands process and present their antigens to CD4+ T cells.
- 39.Russell DG & Yates RM TLR signalling and phagosome maturation: an alternative viewpoint. Cell. Microbiol 9, 849–850 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Mantegazza AR et al. Adaptor protein-3 in dendritic cells facilitates phagosomal toll-like receptor signaling and antigen presentation to CD4+ T cells. Immunity 36, 782–794 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann E et al. Autonomous phagosomal degradation and antigen presentation in dendritic cells. Proc. Natl Acad. Sci. USA 109, 14556–14561 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mantegazza AR et al. TLR-dependent phagosome tubulation in dendritic cells promotes phagosome cross-talk to optimize MHC-II antigen presentation. Proc. Natl Acad. Sci. USA 111, 15508–15513 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adamopoulou E et al. Exploring the MHC-peptide matrix of central tolerance in the human thymus. Nature Commun. 4, 2039 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Crotzer VL & Blum JS Autophagy and adaptive immunity. Immunology 131, 9–17 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmid D, Pypaert M & Munz C Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity 26, 79–92 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paludan C et al. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science 307, 593–596 (2005). [DOI] [PubMed] [Google Scholar]
- 47.Ireland JM & Unanue ER Autophagy in antigen-presenting cells results in presentation of citrullinated peptides to CD4 T cells. J. Exp. Med 208, 2625–2632 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wegner N et al. Autoimmunity to specific citrullinated proteins gives the first clues to the etiology of rheumatoid arthritis. Immunol. Rev 233, 34–54 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Nedjic J, Aichinger M, Emmerich J, Mizushima N & Klein L Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nature 455, 396–400 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Aichinger M, Wu C, Nedjic J & Klein L Macroautophagy substrates are loaded onto MHC class II of medullary thymic epithelial cells for central tolerance. J. Exp. Med 210, 287–300 (2013).References 49 and 50 reveal an important role for macroautophagy in the generation of peptides required for both the positive and the negative selection of the CD4+ T cell repertoire.
- 51.Lee HK et al. In vivo requirement for Atg5 in antigen presentation by dendritic cells. Immunity 32, 227–239 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manoury B et al. Asparagine endopeptidase can initiate the removal of the MHC class II invariant chain chaperone. Immunity 18, 489–498 (2003). [DOI] [PubMed] [Google Scholar]
- 53.Shi GP et al. Cathepsin S required for normal MHC class II peptide loading and germinal center development. Immunity 10, 197–206 (1999). [DOI] [PubMed] [Google Scholar]
- 54.Nakagawa TY et al. Impaired invariant chain degradation and antigen presentation and diminished collagen-induced arthritis in cathepsin S null mice. Immunity 10, 207–217 (1999). [DOI] [PubMed] [Google Scholar]
- 55.Lautwein A et al. Inflammatory stimuli recruit cathepsin activity to late endosomal compartments in human dendritic cells. Eur. J. Immunol 32, 3348–3357 (2002). [DOI] [PubMed] [Google Scholar]
- 56.Trombetta ES, Ebersold M, Garrett W, Pypaert M & Mellman I Activation of lysosomal function during dendritic cell maturation. Science 299, 1400–1403 (2003). [DOI] [PubMed] [Google Scholar]
- 57.Inaba K et al. The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli. J. Exp. Med 191, 927–936 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Turley SJ et al. Transport of peptide-MHC class II complexes in developing dendritic cells. Science 288, 522–527 (2000). [DOI] [PubMed] [Google Scholar]
- 59.Manoury B et al. Destructive processing by asparagine endopeptidase limits presentation of a dominant T cell epitope in MBP. Nature Immunol. 3, 169–174 (2002). [DOI] [PubMed] [Google Scholar]
- 60.Delamarre L, Pack M, Chang H, Mellman I & Trombetta ES Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science 307, 1630–1634 (2005).This important study shows that DCs are better APCs than macrophages, partly because their late endosomal–lysosomal compartments are less proteolytic, thereby limiting the complete destruction of internalized antigens and promoting antigenic peptide generation.
- 61.Chatterjee B et al. Internalization and endosomal degradation of receptor-bound antigens regulate the efficiency of cross presentation by human dendritic cells. Blood 120, 2011–2020 (2012). [DOI] [PubMed] [Google Scholar]
- 62.Savina A et al. The small GTPase Rac2 controls phagosomal alkalinization and antigen crosspresentation selectively in CD8+ dendritic cells. Immunity 30, 544–555 (2009). [DOI] [PubMed] [Google Scholar]
- 63.Romao S et al. Autophagy proteins stabilize pathogen-containing phagosomes for prolonged MHC II antigen processing. J. Cell Biol 203, 757–766 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reith W, LeibundGut-Landmann S & Waldburger JM Regulation of MHC class II gene expression by the class II transactivator. Nature Rev. Immunol 5, 793–806 (2005). [DOI] [PubMed] [Google Scholar]
- 65.Steimle V, Siegrist CA, Mottet A, Lisowska-Grospierre B & Mach B Regulation of MHC class II expression by interferon-γ mediated by the transactivator gene CIITA. Science 265, 106–109 (1994). [DOI] [PubMed] [Google Scholar]
- 66.Wilson NS, El-Sukkari D & Villadangos JA Dendritic cells constitutively present self antigens in their immature state in vivo and regulate antigen presentation by controlling the rates of MHC class II synthesis and endocytosis. Blood 103, 2187–2195 (2004). [DOI] [PubMed] [Google Scholar]
- 67.Young LJ et al. Differential MHC class II synthesis and ubiquitination confers distinct antigen-presenting properties on conventional and plasmacytoid dendritic cells. Nature Immunol. 9, 1244–1252 (2008). [DOI] [PubMed] [Google Scholar]
- 68.LeibundGut-Landmann S, Waldburger JM, Reis e Sousa C, Acha-Orbea H & Reith W MHC class II expression is differentially regulated in plasmacytoid and conventional dendritic cells. Nature Immunol. 5, 899–908 (2004). [DOI] [PubMed] [Google Scholar]
- 69.Villadangos JA & Young L Antigen-presentation properties of plasmacytoid dendritic cells. Immunity 29, 352–361 (2008). [DOI] [PubMed] [Google Scholar]
- 70.Villadangos JA, Schnorrer P & Wilson NS Control of MHC class II antigen presentation in dendritic cells: a balance between creative and destructive forces. Immunol. Rev 207, 191–205 (2005). [DOI] [PubMed] [Google Scholar]
- 71.Kleijmeer MJ et al. MHC class II compartments and the kinetics of antigen presentation in activated mouse spleen dendritic cells. J. Immunol 154, 5715–5724 (1995). [PubMed] [Google Scholar]
- 72.Harding CV & Geuze HJ Class II MHC molecules are present in macrophage lysosomes and phagolysosomes that function in the phagocytic processing of Listeria monocytogenes for presentation to T cells. J. Cell Biol 119, 531–542 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kleijmeer MJ, Oorschot VM & Geuze HJ Human resident langerhans cells display a lysosomal compartment enriched in MHC class II. J. Invest. Dermatol 103, 516–523 (1994). [DOI] [PubMed] [Google Scholar]
- 74.Pierre P et al. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature 388, 787–792 (1997). [DOI] [PubMed] [Google Scholar]
- 75.ten Broeke T, van Niel G, Wauben MH, Wubbolts R & Stoorvogel W Endosomally stored MHC class II does not contribute to antigen presentation by dendritic cells at inflammatory conditions. Traffic 12, 1025–1036 (2011). [DOI] [PubMed] [Google Scholar]
- 76.Cella M, Engering A, Pinet V, Pieters J & Lanzavecchia A Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature 388, 782–787 (1997).This seminal study shows that activation of DCs with LPS or tumour necrosis factor transiently increases MHC class II biosynthesis, enhances cell surface MHC class II stability and enables DCs to maintain functional memory of acquired antigens.
- 77.Kleijmeer M et al. Reorganization of multivesicular bodies regulates MHC class II antigen presentation by dendritic cells. J. Cell Biol 155, 53–63 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boes M et al. T-cell engagement of dendritic cells rapidly rearranges MHC class II transport. Nature 418, 983–988 (2002). [DOI] [PubMed] [Google Scholar]
- 79.Chow A, Toomre D, Garrett W & Mellman I Dendritic cell maturation triggers retrograde MHC class II transport from lysosomes to the plasma membrane. Nature 418, 988–994 (2002).References 77–79 show that maturation of DCs promotes tubulation of antigen-processing compartments, which results in the delivery of peptide–MHC class II to the plasma membrane.
- 80.Lankar D et al. Dynamics of major histocompatibility complex class II compartments during B cell receptor-mediated cell activation. J. Exp. Med 195, 461–472 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Siemasko K, Eisfelder BJ, Williamson E, Kabak S & Clark MR Signals from the B lymphocyte antigen receptor regulate MHC class II containing late endosomes. J. Immunol 160, 5203–5208 (1998). [PubMed] [Google Scholar]
- 82.Vascotto F et al. The actin-based motor protein myosin II regulates MHC class II trafficking and BCR-driven antigen presentation. J. Cell Biol 176, 1007–1019 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Le Roux D et al. Syk-dependent actin dynamics regulate endocytic trafficking and processing of antigens internalized through the B-cell receptor. Mol. Biol. Cell 18, 3451–3462 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nashar TO & Drake JR The pathway of antigen uptake and processing dictates MHC class II-mediated B cell survival and activation. J. Immunol 174, 1306–1316 (2005). [DOI] [PubMed] [Google Scholar]
- 85.Mohan JF, Calderon B, Anderson MS & Unanue ER Pathogenic CD4+ T cells recognizing an unstable peptide of insulin are directly recruited into islets bypassing local lymph nodes. J. Exp. Med 210, 2403–2414 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Amigorena S, Drake JR, Webster P & Mellman I Transient accumulation of new class II MHC molecules in a novel endocytic compartment in B lymphocytes. Nature 369, 113–120 (1994). [DOI] [PubMed] [Google Scholar]
- 87.Yuseff MI et al. Polarized secretion of lysosomes at the B cell synapse couples antigen extraction to processing and presentation. Immunity 35, 361–374 (2011).This study shows that the engagement of B cells with an immobilized antigen leads to polarized lysosome exocytosis, delivery of lysosomal enzymes into the B cell–antigen interface and allows antigen extraction from the immobilized surface into the B cell.
- 88.Rocha N & Neefjes J MHC class II molecules on the move for successful antigen presentation. EMBO J. 27, 1–5 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kamon H et al. TRIF–GEFH1–RhoB pathway is involved in MHCII expression on dendritic cells that is critical for CD4 T-cell activation. EMBO J. 25, 4108–4119 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ocana-Morgner C, Wahren C & Jessberger R SWAP-70 regulates RhoA/RhoB-dependent MHCII surface localization in dendritic cells. Blood 113, 1474–1482 (2009). [DOI] [PubMed] [Google Scholar]
- 91.Paul P et al. A genome-wide multidimensional RNAi screen reveals pathways controlling MHC class II antigen presentation. Cell 145, 268–283 (2011). [DOI] [PubMed] [Google Scholar]
- 92.Thery C et al. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nature Immunol. 3, 1156–1162 (2002). [DOI] [PubMed] [Google Scholar]
- 93.Segura E et al. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood 106, 216–223 (2005). [DOI] [PubMed] [Google Scholar]
- 94.Muntasell A, Berger AC & Roche PA T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes. EMBO J. 26, 4263–4272 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Buschow SI et al. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic 10, 1528–1542 (2009). [DOI] [PubMed] [Google Scholar]
- 96.Bastos-Amador P et al. Capture of cell-derived microvesicles (exosomes and apoptotic bodies) by human plasmacytoid dendritic cells. J. Leukocyte Biol 91, 751–758 (2012). [DOI] [PubMed] [Google Scholar]
- 97.Denzer K et al. Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J. Immunol 165, 1259–1265 (2000). [DOI] [PubMed] [Google Scholar]
- 98.Viaud S et al. Dendritic cell-derived exosomes for cancer immunotherapy: what’s next? Cancer Res. 70, 1281–1285 (2010). [DOI] [PubMed] [Google Scholar]
- 99.Zitvogel L et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nature Med. 4, 594–600 (1998). [DOI] [PubMed] [Google Scholar]
- 100.Mahaweni NM, Kaijen-Lambers ME, Dekkers J, Aerts JG & Hegmans JP Tumour-derived exosomes as antigen delivery carriers in dendritic cell-based immunotherapy for malignant mesothelioma. J. Extracell. Vesicles 2, 22492 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Anderson HA & Roche PA MHC class II association with lipid rafts on the antigen presenting cell surface. Biochim. Biophys. Acta 10.1016/j.bbamcr.2014.09.019 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kropshofer H et al. Tetraspan microdomains distinct from lipid rafts enrich select peptide-MHC class II complexes. Nature Immunol. 3, 61–68 (2002). [DOI] [PubMed] [Google Scholar]
- 103.Anderson HA, Hiltbold EM & Roche PA Concentration of MHC class II molecules in lipid rafts facilitates antigen presentation. Nature Immunol. 1, 156–162 (2000).In this study, we show that MHC class II molecules are constitutive residents of lipid raft membrane microdomains and that raft association is important for CD4+ T cell activation under conditions of limiting antigen dose.
- 104.Eren E et al. Location of major histocompatibility complex class II molecules in rafts on dendritic cells enhances the efficiency of T-cell activation and proliferation. Scand. J. Immunol 63, 7–16 (2006). [DOI] [PubMed] [Google Scholar]
- 105.Bosch B, Heipertz EL, Drake JR & Roche PA Major histocompatibility complex (MHC) class II-peptide complexes arrive at the plasma membrane in cholesterol-rich microclusters. J. Biol. Chem 288, 13236–13242 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Poloso NJ, Muntasell A & Roche PA MHC class II molecules traffic into lipid rafts during intracellular transport. J. Immunol 173, 4539–4546 (2004). [DOI] [PubMed] [Google Scholar]
- 107.Karacsonyi C, Knorr R, Fulbier A & Lindner R Association of major histocompatibility complex II with cholesterol- and sphingolipid-rich membranes precedes peptide loading. J. Biol. Chem 279, 34818–34826 (2004). [DOI] [PubMed] [Google Scholar]
- 108.Poloso NJ & Roche PA Association of MHC class II-peptide complexes with plasma membrane lipid microdomains. Curr. Opin. Immunol 16, 103–107 (2004). [DOI] [PubMed] [Google Scholar]
- 109.Steinman RM, Mellman IS, Muller WA & Cohn ZA Endocytosis and the recycling of plasma membrane. J. Cell Biol 96, 1–27 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Momburg F et al. Epitope-specific enhancement of antigen presentation by invariant chain. J. Exp. Med 178, 1453–1458 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tewari MK, Sinnathamby G, Rajagopal D & Eisenlohr LC A cytosolic pathway for MHC class II-restricted antigen processing that is proteasome and TAP dependent. Nature Immunol. 6, 287–294 (2005). [DOI] [PubMed] [Google Scholar]
- 112.Pinet V, Malnati MS & Long EO Two processing pathways for the MHC class II-restricted presentation of exogenous influenza virus antigen. J. Immunol 152, 4852–4860 (1994). [PubMed] [Google Scholar]
- 113.Griffin JP, Chu R & Harding CV Early endosomes and a late endocytic compartment generate different peptide-class II MHC complexes via distinct processing mechanisms. J. Immunol 158, 1523–1532 (1997). [PubMed] [Google Scholar]
- 114.Ohmura-Hoshino M et al. Inhibition of MHC class II expression and immune responses by c-MIR. J. Immunol 177, 341–354 (2006). [DOI] [PubMed] [Google Scholar]
- 115.Shin JS et al. Surface expression of MHC class II in dendritic cells is controlled by regulated ubiquitination. Nature 444, 115–118 (2006). [DOI] [PubMed] [Google Scholar]
- 116.van Niel G et al. Dendritic cells regulate exposure of MHC class II at their plasma membrane by oligoubiquitination. Immunity 25, 885–894 (2006).References 115 and 116 show that selective ubiquitylation of MHC class II in immature DCs regulates intracellular MHC class II localization.
- 117.Matsuki Y et al. Novel regulation of MHC class II function in B cells. EMBO J. 26, 846–854 (2007).This study confirms that MARCH1 is the main E3 ubiquitin ligase that is responsible for MHC class II ubiquitylation and that it regulates MHC class II expression in B cells.
- 118.De Gassart A et al. MHC class II stabilization at the surface of human dendritic cells is the result of maturation-dependent MARCH I down-regulation. Proc. Natl Acad. Sci. USA 105, 3491–3496 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Walseng E et al. Ubiquitination regulates MHC class II-peptide complex retention and degradation in dendritic cells. Proc. Natl Acad. Sci. USA 107, 20465–20470 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gauvreau ME et al. Sorting of MHC class II molecules into exosomes through a ubiquitin-independent pathway. Traffic 10, 1518–1527 (2009). [DOI] [PubMed] [Google Scholar]
- 121.Furuta K, Ishido S & Roche PA Encounter with antigen-specific primed CD4 T cells promotes MHC class II degradation in dendritic cells. Proc. Natl Acad. Sci. USA 109, 19380–19385 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bartee E, Mansouri M, Hovey Nerenberg BT, Gouveia K & Fruh K Downregulation of major histocompatibility complex class I by human ubiquitin ligases related to viral immune evasion proteins. J. Virol 78, 1109–1120 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Oh J et al. MARCH1-mediated MHCII ubiquitination promotes dendritic cell selection of natural regulatory T cells. J. Exp. Med 210, 1069–1077 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Baravalle G et al. Ubiquitination of CD86 is a key mechanism in regulating antigen presentation by dendritic cells. J. Immunol 187, 2966–2973 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Corcoran K et al. Ubiquitin-mediated regulation of CD86 protein expression by the ubiquitin ligase membrane-associated RING-CH-1 (MARCH1). J. Biol. Chem 286, 37168–37180 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thibodeau J et al. Interleukin-10-induced MARCH1 mediates intracellular sequestration of MHC class II in monocytes. Eur. J. Immunol 38, 1225–1230 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Koppelman B, Neefjes JJ, de Vries JE & de Waal Malefyt R Interleukin-10 down-regulates MHC class II αβ-peptide complexes at the plasma membrane of monocytes by affecting arrival and recycling. Immunity. 7, 861–871 (1997). [DOI] [PubMed] [Google Scholar]
- 128.Walseng E et al. Dendritic cell activation prevents MHC class II ubiquitination and promotes MHC class II survival regardless of the activation stimulus. J. Biol. Chem 285, 41749–41754 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jabbour M, Campbell EM, Fares H & Lybarger L Discrete domains of MARCH1 mediate its localization, functional interactions, and posttranscriptional control of expression. J. Immunol 183, 6500–6512 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bourgeois-Daigneault MC & Thibodeau J Autoregulation of MARCH1 expression by dimerization and autoubiquitination. J. Immunol 188, 4959–4970 (2012). [DOI] [PubMed] [Google Scholar]
- 131.Furuta K, Walseng E & Roche PA Internalizing MHC class II-peptide complexes are ubiquitinated in early endosomes and targeted for lysosomal degradation. Proc. Natl Acad. Sci. USA 110, 20188–20193 (2013).In this study, we show that peptide–MHC class II molecules that are being internalized from the plasma membrane are targets of MARCH1-mediated ubiquitylation and lysosomal degradation.
- 132.Tze LE et al. CD83 increases MHC II and CD86 on dendritic cells by opposing IL-10-driven MARCH1-mediated ubiquitination and degradation. J. Exp. Med 208, 149–165 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Klein E et al. CD83 localization in a recycling compartment of immature human monocyte-derived dendritic cells. Int. Immunol 17, 477–487 (2005). [DOI] [PubMed] [Google Scholar]
- 134.Bourgeois-Daigneault MC & Thibodeau J Identification of a novel motif that affects the conformation and activity of the MARCH1 E3 ubiquitin ligase. J. Cell Sci 126, 989–998 (2013). [DOI] [PubMed] [Google Scholar]
- 135.Ma JK, Platt MY, Eastham-Anderson J, Shin JS & Mellman I MHC class II distribution in dendritic cells and B cells is determined by ubiquitin chain length. Proc. Natl Acad. Sci. USA 109, 8820–8827 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.MacGurn JA, Hsu PC & Emr SD Ubiquitin and membrane protein turnover: from cradle to grave. Annu. Rev. Biochem 81, 231–259 (2012). [DOI] [PubMed] [Google Scholar]
- 137.Tortorella D, Gewurz BE, Furman MH, Schust DJ & Ploegh HL Viral subversion of the immune system. Annu. Rev. Immunol 18, 861–926 (2000). [DOI] [PubMed] [Google Scholar]
- 138.Lapaque N et al. Salmonella regulates polyubiquitination and surface expression of MHC class II antigens. Proc. Natl Acad. Sci. USA 106, 14052–14057 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wilson JE, Katkere B & Drake JR Francisella tularensis induces ubiquitin-dependent major histocompatibility complex class II degradation in activated macrophages. Infect. Immun 77, 4953–4965 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hunt D et al. Francisella tularensis elicits IL-10 via a PGE2-inducible factor, to drive macrophage MARCH1 expression and class II down-regulation. PLoS ONE 7, e37330 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ferrari G, Langen H, Naito M & Pieters J A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell 97, 435–447 (1999). [DOI] [PubMed] [Google Scholar]
- 142.Ramachandra L, Noss E, Boom WH & Harding CV Processing of Mycobacterium tuberculosis antigen 85B involves intraphagosomal formation of peptide-major histocompatibility complex II complexes and is inhibited by live bacilli that decrease phagosome maturation. J. Exp. Med 194, 1421–1432 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gutierrez MG et al. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 119, 753–766 (2004). [DOI] [PubMed] [Google Scholar]
- 144.Lilley BN & Ploegh HL Viral modulation of antigen presentation: manipulation of cellular targets in the ER and beyond. Immunol. Rev 207, 126–144 (2005). [DOI] [PubMed] [Google Scholar]
- 145.Tomazin R et al. Cytomegalovirus US2 destroys two components of the MHC class II pathway, preventing recognition by CD4+ T cells. Nature Med. 5, 1039–1043 (1999). [DOI] [PubMed] [Google Scholar]
- 146.Hegde NR et al. Inhibition of HLA-DR assembly, transport, and loading by human cytomegalovirus glycoprotein US3: a novel mechanism for evading major histocompatibility complex class II antigen presentation. J. Virol 76, 10929–10941 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Odeberg J, Plachter B, Branden L & Soderberg-Naucler C Human cytomegalovirus protein pp65 mediates accumulation of HLA-DR in lysosomes and destruction of the HLA-DR α-chain. Blood 101, 4870–4877 (2003). [DOI] [PubMed] [Google Scholar]
- 148.Temme S, Eis-Hubinger AM, McLellan AD & Koch N The herpes simplex virus-1 encoded glycoprotein B diverts HLA-DR into the exosome pathway. J. Immunol 184, 236–243 (2010). [DOI] [PubMed] [Google Scholar]
- 149.Toussaint H et al. Human immunodeficiency virus type 1 nef expression prevents AP-2-mediated internalization of the major histocompatibility complex class II-associated invariant chain. J. Virol 82, 8373–8382 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Stumptner-Cuvelette P et al. Human immunodeficiency virus-1 Nef expression induces intracellular accumulation of multivesicular bodies and major histocompatibility complex class II complexes: potential role of phosphatidylinositol 3-kinase. Mol. Biol. Cell 14, 4857–4870 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zuo J et al. Epstein–Barr virus evades CD4+ T cell responses in lytic cycle through BZLF1-mediated downregulation of CD74 and the cooperation of vBcl-2. PLoS Pathog. 7, e1002455 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ressing ME et al. Epstein–Barr virus gp42 is posttranslationally modified to produce soluble gp42 that mediates HLA class II immune evasion. J. Virol 79, 841–852 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]