Bern et al. demonstrate that acute down-regulation of self-MHC-I in vivo can induce NK cell tolerance to missing-self, depending on the extent of MHC-I deficiency. Additionally, inflammation promotes missing-self reactivity in response to MHC-I down-regulation.
Abstract
Natural killer (NK) cells are innate lymphocytes that are thought to kill cells that down-regulate MHC class I (MHC-I) through “missing-self” recognition. NK cells from B2m−/− mice that lack surface MHC-I, however, are not autoreactive as predicted by the missing-self hypothesis. As a result, it is unclear if MHC-I down-regulation in vivo induces NK cell reactivity or tolerance to missing-self. Here, we generated a floxed B2m mouse to acutely down-regulate MHC-I in vivo in a host that normally expresses MHC-I. Global down-regulation of MHC-I induced NK cell hyporesponsiveness and tolerance to missing-self without overt missing-self reactivity. In contrast, down-regulation of MHC-I on a small fraction of hematopoietic cells triggered missing-self reactivity. Surprisingly, down-regulation of MHC-I only on CD4+ T cells predominately induced tolerance to missing-self without resetting NK cell responsiveness. In this setting, inflammation triggered substantial missing-self reactivity. These results show that MHC-I down-regulation can induce either NK cell tolerance or killing in vivo and that inflammation promotes missing-self reactivity.
Introduction
Natural killer (NK) cells are innate lymphoid cells that control viral infections and tumors through cytotoxicity and production of cytokines such as IFN-γ (Orr and Lanier, 2010). According to the “missing-self” hypothesis, NK cells complement T cell immunity by killing infected and transformed cells that down-regulate MHC-I to evade MHC-I–restricted T cells (Kärre et al., 1986). NK cells recognize MHC-I through germline-encoded MHC-I–specific inhibitory receptors, such as mouse Ly49 receptors (Karlhofer et al., 1992) that prevent NK cell activation via cytoplasmic immunoreceptor tyrosine-based inhibitory motifs (Long et al., 2013). Loss of MHC-I, i.e., missing-self, relieves inhibitory signals, allowing NK cell activation; however, the requirements for missing-self reactivity in vivo are incompletely understood. A better understanding of this process will inform efforts to improve cancer immunotherapies that use NK cells and missing-self recognition (Daher and Rezvani, 2018).
Evidence for the missing-self hypothesis comes from studies showing that NK cells kill MHC-I–deficient tumor cell lines and T cell blasts in vitro (Kärre et al., 1986; Höglund et al., 1991; Liao et al., 1991) and adoptively transferred MHC-I–deficient cells in vivo (Kärre et al., 1986; Bix et al., 1991). However, it has long been recognized that NK cells from MHC-I–deficient mice, such as mice that lack β2-microglobulin (B2m−/−), are not autoreactive as predicted by the missing-self hypothesis (Bix et al., 1991; Liao et al., 1991). In contrast, NK cells from B2m−/− mice are unable to reject MHC-I–deficient grafts in vivo (Bix et al., 1991) and exhibit defective killing of MHC-I–deficient T cell blasts in vitro (Höglund et al., 1991; Liao et al., 1991). These results suggest that NK cells from MHC-I–deficient mice are tolerant to missing-self; however, the mechanisms that establish NK cell self-tolerance in MHC-I–deficient mice remain poorly understood.
Early studies proposed that NK cells maintain self-tolerance by expressing at least one MHC-I–specific inhibitory receptor that binds self-MHC-I (Valiante et al., 1997). Some NK cells in WT mice, however, can establish self-tolerance without expressing any known self-MHC-I–specific inhibitory receptors (Fernandez et al., 2005). Moreover, although the Ly49 repertoire is altered in MHC-I–deficient mice (Salcedo et al., 1997), the receptor repertoire model, based on known receptors, is unable to explain how NK cells establish self-tolerance in the absence of MHC-I. As a result, receptor repertoire development may contribute to NK cell self-tolerance, but it is likely that additional tolerance mechanisms exist.
More recent studies have suggested that NK cell self-tolerance is achieved through alterations in NK cell functionality rather than receptor repertoire (Fernandez et al., 2005; Kim et al., 2005). NK cells from MHC-I–deficient mice are hyporesponsive to stimulation through antibody-mediated cross-linking of their activation receptors (Fernandez et al., 2005; Kim et al., 2005). Additionally, NK cells from WT mice that lack self-MHC-I–specific inhibitory receptors are similarly hyporesponsive (Fernandez et al., 2005; Kim et al., 2005). Also, inactivating mutations in the immunoreceptor tyrosine-based inhibitory motifs of self-MHC-I–specific inhibitory Ly49 receptors render NK cells hyporesponsive (Kim et al., 2005; Bern et al., 2017). These results have been used to argue that self-MHC-I–specific inhibitory receptors “license” or “educate” NK cells to become responsive to triggering through their activation receptors (Kim et al., 2005). NK cells from MHC-I–deficient mice have thus been proposed to be self-tolerant because they are unlicensed or uneducated (Yokoyama and Kim, 2006); however, it is unclear if there are other contributors to NK cell tolerance.
Interestingly, NK cells can reset their educated phenotype to adapt to different MHC-I environments. Transfer of NK cells from MHC-I–deficient to –sufficient mice or up-regulation of MHC-I expression with an inducible MHC-I transgene improves NK cell responses to stimulation through activation receptors (Elliott et al., 2010; Joncker et al., 2010; Ebihara et al., 2013). In contrast, transfer of NK cells from WT to B2m−/− mice results in a loss of NK cell education (Joncker et al., 2010). Similarly, NK cells residing in MHC-I–deficient tumors adapt to the local MHC-I–deficient environment and become hyporesponsive (Ardolino et al., 2014). These results suggest that the educated NK cell phenotype is plastic, allowing NK cells to adapt to changing MHC-I environments, but this has only been evaluated with adoptive transfer of NK cells. It then becomes unclear if loss of MHC-I expression in a previously MHC-I–positive environment with educated NK cells, i.e., “acute” MHC-I down-regulation, induces killing or NK cell adaptation. As a result, it is not known when missing-self occurs in vivo.
NK cells from irradiation chimeras reconstituted with 1:1 mixtures of WT and B2m−/− fetal liver cells are tolerant of adoptively transferred MHC-I–deficient cells while remaining responsive to stimulation through their activation receptors (Wu and Raulet, 1997; Shifrin et al., 2016). These results suggest that NK cells in mixed WT:B2m−/− chimeras are tolerant to missing-self, perhaps in a manner related to the NK cell transfer and inducible MHC-I studies mentioned previously (Elliott et al., 2010; Joncker et al., 2010; Ebihara et al., 2013). Interestingly, tolerance to missing-self in chimeric mice is broken by infection with murine CMV (MCMV) or treatment with cytokines (Sun and Lanier, 2008; Shifrin et al., 2016). However, it remains unknown if this form of tolerance is restricted to mixed chimeras in which NK cells are chronically exposed to missing-self targets or if it more generally applies to other settings. Additionally, it is unclear if inflammatory cytokines are required for missing-self recognition in general or if they are only required to break the form of tolerance that develops in mixed chimeras.
Here, we generated mice in which B2m could be inducibly deleted to directly study the response of NK cells that developed in an MHC-I–sufficient setting to acute down-regulation of MHC-I in vivo. MHC-I down-regulation was found to induce multiple NK cell responses besides missing-self reactivity that depended on the context. Importantly, the inflammatory environment in which MHC-I was down-regulated was found to regulate NK cell missing-self reactivity.
Results
Generation of conditional B2m knockout mice
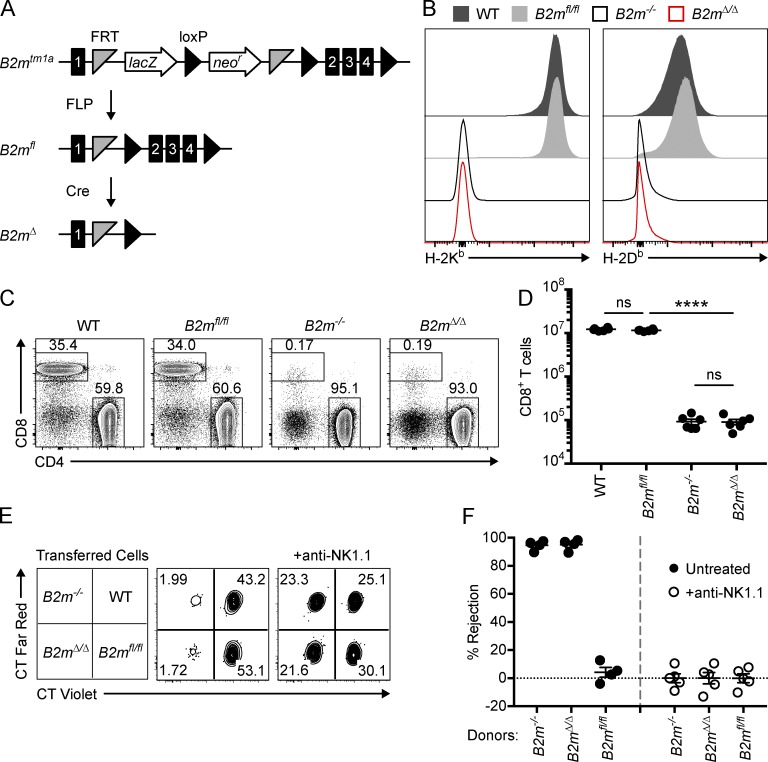
To develop a mouse in which MHC-I expression could be down-regulated, we generated floxed B2m mice. C57BL/6 embryonic stem (ES) cells were obtained with a B2m knockout first allele with conditional potential (B2mtm1a; Fig. 1 A). The HEPD0673_4_D09 ES cell clone was micro-injected into Albino B6 blastocysts so that germline transmission could be assessed by coat color. Mice containing the germline-transmitted B2mtm1a allele were confirmed by PCR and bred to a FLP deleter strain to remove the lacZ and neor genes flanked by FLT recognition target sites to generate the floxed B2m allele (B2mfl; Fig. S1, A–D).
Figure 1.
Conditional deletion of B2m leads to loss of surface MHC-I. (A) The targeted B2mtm1a allele is depicted before (top) and after (middle) removal of the LacZ and neomycin resistance (neor) genes by FLP recombinase. Germline-expressed Cre recombinase was used to generate the B2mΔ allele (bottom). (B) Representative histograms of H-2Kb and H-2Db expression on total lymphocytes from spleens of WT, B2mfl/fl, B2m−/−, and B2mΔ/Δ mice. (C) Representative dot plots showing the percentage of T cells (CD19− CD3+) that express CD4 or CD8. Data in B and C are representative of two independent experiments with three mice per group. (D) Total splenic CD8+ T cell number in WT, B2mfl/fl, B2m−/−, and B2mΔ/Δ mice (n = 6 mice per group). Data in D are combined from two independent experiments. (E and F) Splenocytes from WT, B2mfl/fl, B2m−/−, and B2mΔ/Δ mice were labeled with CFSE and differentially labeled with CT violet and CT far red as indicated. Labeled cells were injected i.v. into WT recipient mice, and donor cells were recovered from spleens of recipients after 2 d. (E) Representative dot plots showing the relative percentages of transferred cells (CFSE+) recovered from the spleens of WT recipient mice that were depleted of NK cells with anti-NK1.1 (right) or undepleted (middle). (F) Percentage of NK cell–specific rejection of donor cells by WT recipient mice (n = 4–5 recipient mice per group). Data in F are representative of two independent experiments with four to five recipient mice per group that received the same mix of donor cells in each experiment. Statistical significance was calculated by one-way ANOVA with Bonferroni’s multiple comparisons test. Each symbol in D and F represents an individual mouse. Error bars indicate mean ± SEM; ****, P < 0.0001; ns, not significant.
To validate the B2mfl allele, floxed B2m mice were bred with CMV-Cre transgenic mice for ubiquitous expression of Cre to induce germline deletion of B2m (B2mΔ; Fig. 1 A and Fig. S1, A–D). Splenocytes from B6 (WT) and B2mfl/fl mice both expressed surface H-2Kb and H-2Db (Fig. 1 B). In contrast, the original germline B2m knockout (B2m−/−) and the B2mΔ/Δ mouse both lacked surface expression of MHC-I (Fig. 1 B). In addition, B2mΔ/Δ mice were deficient in CD8+ T cells (Fig. 1, C and D), consistent with impaired positive selection of cytotoxic T cells as originally described in B2m−/− mice (Koller et al., 1990; Zijlstra et al., 1990). Furthermore, splenocytes from B2mΔ/Δ mice were rejected after transfer into WT mice to a similar extent as B2m−/− splenocytes, and this rejection was abrogated by depletion of NK cells with the anti-NK1.1 antibody (Fig. 1, E and F), as previously described (Öberg et al., 2004). Collectively, these data indicate that B2mfl/fl mice express MHC-I and that Cre mediates B2m deletion, resulting in cells from B2mΔ/Δ mice that lack surface MHC-I and are targets for missing-self recognition.
NK cell missing-self reactivity is not observed after global down-regulation of MHC-I
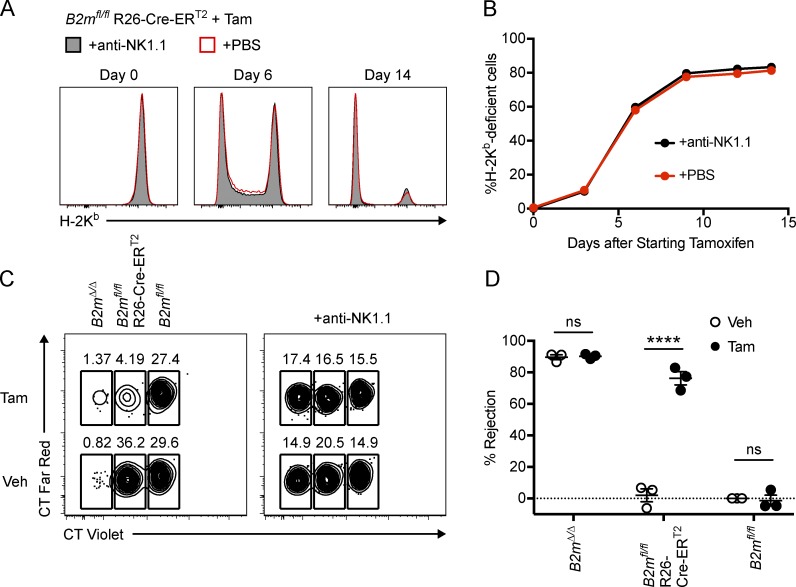
To study the in vivo NK cell response to acute down-regulation of MHC-I, floxed B2m mice were bred to Rosa26-Cre-ERT2 (R26-Cre-ERT2) mice that express a ubiquitous tamoxifen-inducible Cre. Tamoxifen treatment of B2mfl/fl R26-Cre-ERT2 mice induced genetic deletion of B2m as assessed by PCR (Fig. S1, E and F). We expected that tamoxifen treatment of B2mfl/fl R26-Cre-ERT2 mice would induce the accumulation of MHC-I–deficient cells only when NK cells were depleted. Surprisingly, tamoxifen induced the accumulation of a substantial population of MHC-I–deficient cells in B2mfl/fl R26-Cre-ERT2 mice even in the presence of NK cells (Fig. 2, A and B). In addition, depletion of NK cells with the anti-NK1.1 antibody did not lead to any observed increase in MHC-I–deficient cells (Fig. 2, A and B). These data suggest that global down-regulation of MHC-I does not induce overt NK cell missing-self reactivity as predicted by the missing-self hypothesis (Kärre et al., 1986).
Figure 2.
Global down-regulation of MHC-I does not induce overt NK cell missing-self reactivity. (A) Representative histograms showing H-2Kb expression on CD45+ lymphocytes from peripheral blood of B2mfl/fl R26-Cre-ERT2 mice treated with tamoxifen starting on day 0. Mice were injected i.p. with anti-NK1.1 antibody to deplete NK cells or with PBS control as indicated. (B) The percentage of H-2Kb–deficient CD45+ cells that accumulate in the blood of tamoxifen-treated B2mfl/fl R26-Cre-ERT2 mice over time (n = 4 mice per group). Similar results to those in A and B were observed in a second experiment with three mice per group. (C and D) B2mfl/fl, B2mfl/fl R26-Cre-ERT2, and B2mΔ/Δ mice were treated with tamoxifen or vehicle control on days 0–4, and splenocytes were harvested and labeled with CFSE plus CT violet and CT far red as indicated on day 16. Labeled splenocytes were i.v. injected into WT recipients, and recovery of donor cells was analyzed after 2 d. (C) Representative dot plots showing the relative percentages of transferred cells (CFSE+) recovered from the spleens of WT recipient mice that were depleted of NK cells with anti-NK1.1 (right) or undepleted (left). (D) NK cell–specific rejection of the indicated donor cells combined from three independent experiments in which donor cells were injected on day 14 or 16 (each symbol represents an average of four to five recipient mice injected with the same mix of donor cells; two-way ANOVA with Bonferroni’s multiple comparisons test). CT far red labeling was inverted in one of the replicates in D. Error bars indicate mean ± SEM; ****, P < 0.0001; ns, not significant. Tam, tamoxifen; Veh, vehicle.
To investigate the reasons why NK cell reactivity was not observed after global down-regulation of MHC-I, we first used conventional assays to test whether cells from B2mfl/fl R26-Cre-ERT2 mice fail to become targets for missing-self recognition by NK cells after tamoxifen treatment (Fig. 2, C and D). B2mfl/fl, B2mfl/fl R26-Cre-ERT2, and B2mΔ/Δ mice were treated with either tamoxifen or vehicle control (corn oil), and splenocytes from these mice were subsequently transferred into WT recipients. NK cell–specific rejection was quantified by calculating the relative recovery of donor cell populations normalized to NK cell–depleted recipient mice. Splenocytes from B2mfl/fl R26-Cre-ERT2 mice were rejected by NK cells only when the donor mice were treated with tamoxifen (Fig. 2, C and D). Control B2mfl/fl splenocytes were not rejected when donor mice received tamoxifen. In contrast, B2mΔ/Δ splenocytes were strongly rejected by NK cells, regardless of whether the donor mice received tamoxifen or corn oil. These data show that the MHC-I–deficient cells induced by tamoxifen in B2mfl/fl R26-Cre-ERT2 mice become targets for missing-self recognition by NK cells upon adoptive transfer. However, NK cells in tamoxifen-treated B2mfl/fl R26-Cre-ERT2 mice appear to remain tolerant to these potential missing-self targets.
NK cells adapt to global down-regulation of MHC-I
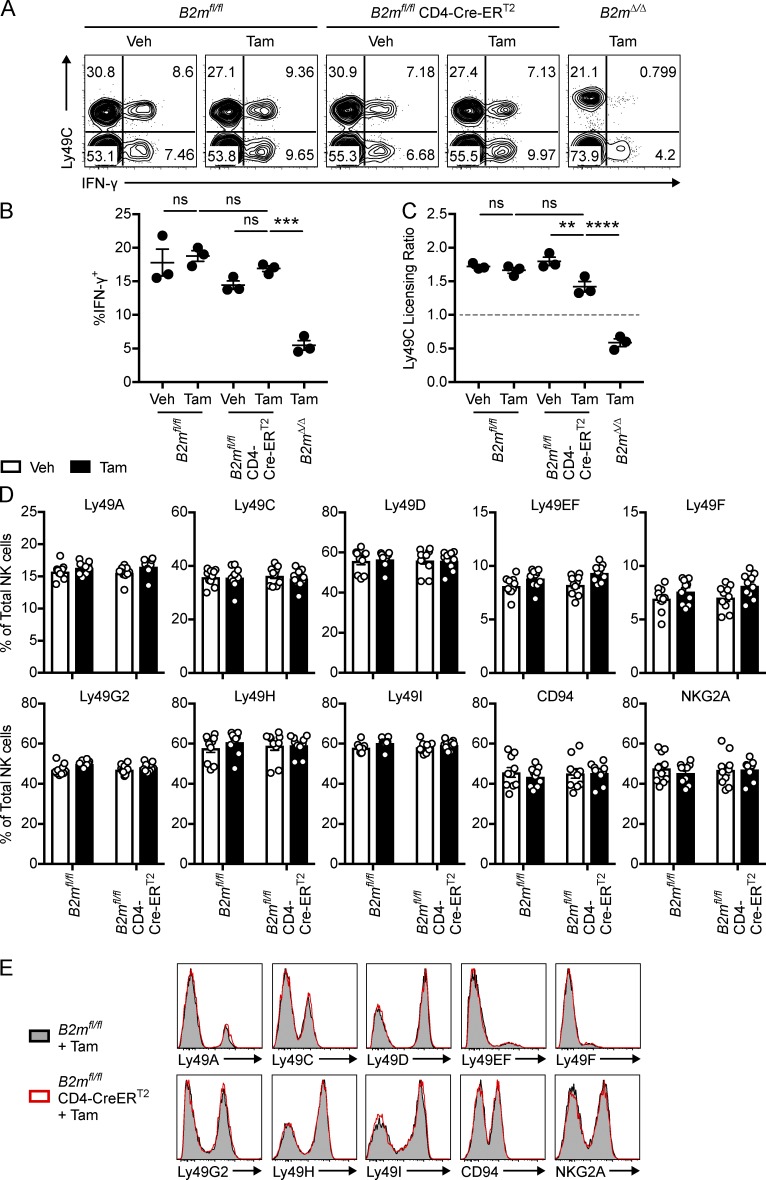
A previous attempt to generate a mouse with inducible deletion of an H-2Dd transgene driven by Mx1-Cre or CMV-Cre-ER was confounded by baseline leakiness of Cre expression that induced NK cell tolerance to H-2Dd–deficient cells in the absence of tamoxifen (Ioannidis et al., 2001). To test if there was similar leakiness of Cre in our B2mfl/fl R26-Cre-ERT2 mice, we performed an in vivo cytotoxicity assay in B2mfl/fl and B2mfl/fl R26-Cre-ERT2 mice that had been pretreated with vehicle control or tamoxifen (Fig. 3 A). NK cells from B2mfl/fl R26-Cre-ERT2 mice treated with vehicle control robustly rejected B2mΔ/Δ splenocytes, although to a slightly lower extent than NK cells from B2mfl/fl mice (Fig. 3, B and C). In contrast, NK cells from tamoxifen-treated B2mfl/fl R26-Cre-ERT2 mice were unable to reject B2mΔ/Δ splenocytes (Fig. 3, B and C). These results demonstrate that NK cells from B2mfl/fl R26-Cre-ERT2 mice are initially capable of rejecting MHC-I–deficient cells; however, induced global MHC-I down-regulation results in NK cell tolerance to missing-self.
Figure 3.
Global down-regulation of MHC-I induces loss of NK cell licensing. (A) B2mfl/fl and B2mfl/fl R26-Cre-ERT2 mice were treated with tamoxifen or vehicle control followed by i.v. injection of labeled donor splenocytes as indicated. (B) Representative histograms showing the relative percentages of transferred B2mfl/fl and B2mΔ/Δ cells (CFSE+ CT far redlow) recovered from the spleens of recipient mice after 2 d. B2mfl/fl and B2mΔ/Δ cells were differentially labeled with CT violet as indicated. (C) Summary of NK cell–specific rejection of B2mΔ/Δ donor cells (n = 4–5 mice per group; two-way ANOVA with Bonferroni’s multiple comparisons test). Data in C are combined from two independent experiments. Similar results were seen with WT and B2m−/− donor splenocytes (CFSE+ CT far redhigh) that were cotransferred in these experiments. (D–G) B2mfl/fl, B2mfl/fl R26-Cre-ERT2, and B2mΔ/Δ mice were treated with tamoxifen or vehicle control on days 0–4, and splenocytes were stimulated on day 14 with plate-bound anti-NK1.1 antibody or PMA and ionomycin. (D) Representative dot plots showing percentage of NK cells (CD3− CD19− NKp46+) that express Ly49C and IFN-γ after stimulation with plate-bound anti-NK1.1. (E) Percentage of NK cells that express IFN-γ after stimulation with plate-bound anti-NK1.1 (n = 3–4 mice per group; one-way ANOVA with Bonferroni’s multiple comparisons test). (F) Relative production of IFN-γ by Ly49C+ and Ly49C− NK cells after stimulation with anti-NK1.1 is quantified by an Ly49C licensing ratio (see Materials and methods; n = 3–4 mice per group; one-way ANOVA with Bonferroni’s multiple comparisons test). (G) Percentage of NK cells that express IFN-γ after stimulation with PMA and ionomycin (n = 3–4 mice per group). Data in D–G are representative of two independent experiments with three to four mice per group. Each symbol in C and E–G represents an individual mouse. Error bars indicate mean ± SEM; ***, P < 0.001; ****, P < 0.0001; ns, not significant. Tam, tamoxifen; Veh, vehicle.
Importantly, we did not observe any changes in NK cell number after global down-regulation of MHC-I in B2mfl/fl R26-Cre-ERT2 mice (Fig. S2 A), and we observed only minor changes in NK cell maturation based on CD11b, CD27, and KLRG1 expression (Fig. S2, B and C). Additionally, the NK cell inhibitory receptor repertoire was relatively unchanged by global down-regulation of MHC-I besides subtle changes in expression of Ly49C, Ly49F, and Ly49G2 (Fig. S2 D). Thus, it seems unlikely that global down-regulation of MHC-I induces NK cell tolerance through impacting NK cell development or the receptor repertoire.
Previous adoptive transfer studies have shown that WT NK cells can reset their responsiveness to stimulation through activation receptors when placed in an MHC-I–deficient environment (Joncker et al., 2010). To test if NK cells adapt to global loss of MHC-I, we treated B2mfl/fl and B2mfl/fl R26-Cre-ERT2 mice with tamoxifen or vehicle control and subsequently stimulated splenocytes with plate-bound anti-NK1.1 antibody as previously described (Kim et al., 2005; Jonsson and Yokoyama, 2010). IFN-γ production was analyzed as a measure of NK cell responsiveness, and an Ly49C licensing ratio was calculated to measure the preferential capacity of Ly49C+ NK cells to respond to stimulation (see Materials and methods). As expected, only a small percentage of NK cells from B2mΔ/Δ mice produced IFN-γ after stimulation (Fig. 3, D and E), which corresponded to an Ly49C licensing ratio <1 (Fig. 3, D and F). In contrast, a large fraction of control NK cells from B2mfl/fl mice produced IFN-γ after anti-NK1.1 stimulation, regardless of whether or not the mouse was pretreated with tamoxifen (Fig. 3, D and E). Additionally, Ly49C+ NK cells from B2mfl/fl mice were preferentially responsive to stimulation through NK1.1, as indicated by an Ly49C licensing ratio >1 (Fig. 3, D and F). These baseline data show that NK cells from B2mΔ/Δ mice are unlicensed, while NK cells from B2mfl/fl mice are licensed as previously shown for B2m−/− and WT NK cells (Kim et al., 2005).
However, NK cells from tamoxifen-treated B2mfl/fl R26-Cre-ERT2 mice exhibited reduced IFN-γ production in response to anti-NK1.1 stimulation (Fig. 3, D and E). Additionally, NK cells from tamoxifen-treated B2mfl/fl R26-Cre-ERT2 mice exhibited an Ly49C licensing ratio <1 (Fig. 3, D and F). In contrast, control NK cells from B2mfl/fl R26-Cre-ERT2 mice pretreated with vehicle control produced IFN-γ at levels similar to B2mfl/fl mice. Importantly, NK cells from tamoxifen-treated B2mfl/fl R26-Cre-ERT2 mice produced IFN-γ at levels similar to controls in response to stimulation with PMA and ionomycin, which bypasses activation receptor triggering (Fig. 3 G). These data indicate that NK cells remain capable of producing IFN-γ after global MHC-I down-regulation, but they become hyporesponsive to stimulation through activation receptors, i.e., they become unlicensed. Thus global down-regulation of MHC-I induces NK cell tolerance to missing-self through resetting NK cell education.
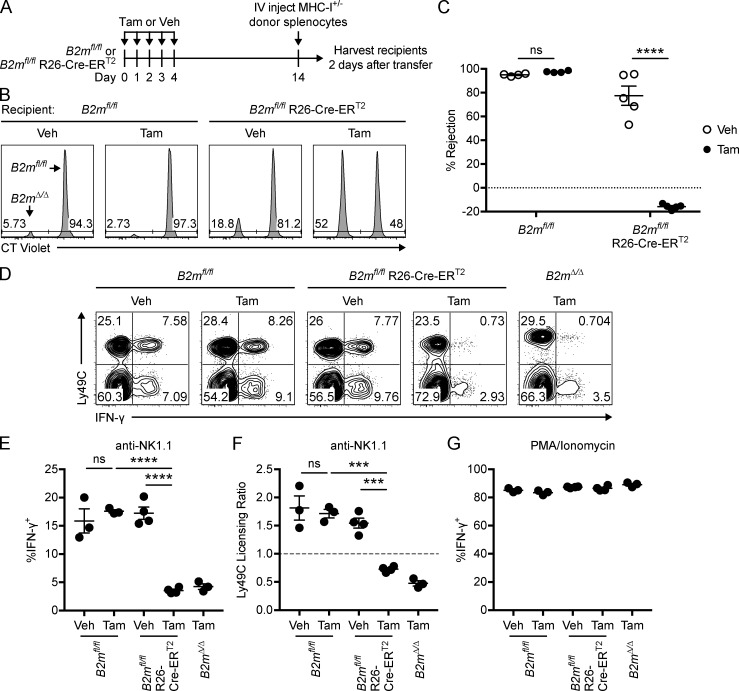
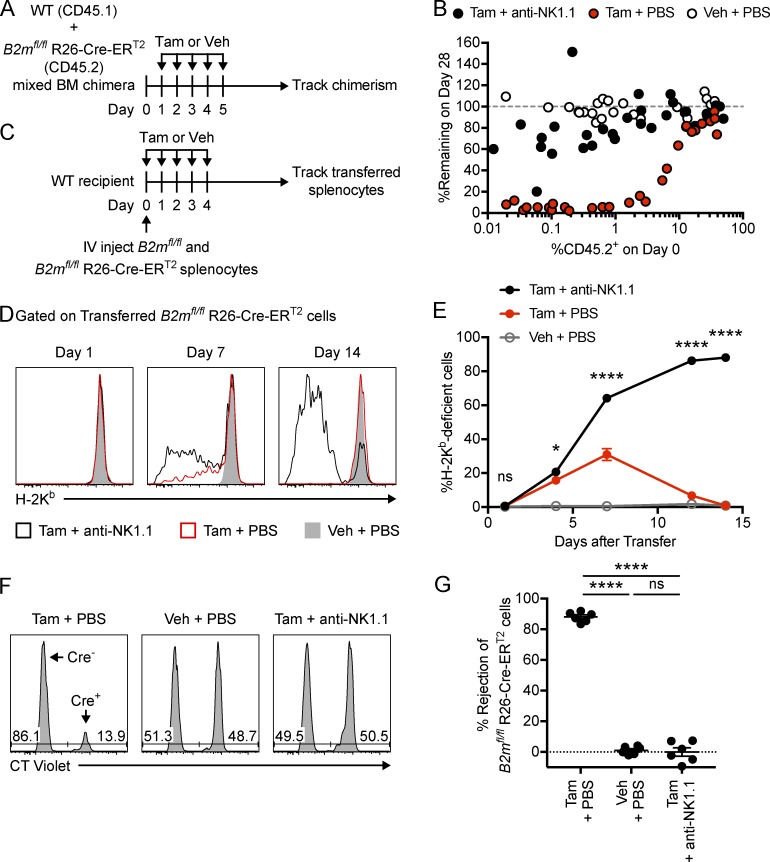
Missing-self is observed after down-regulation of MHC-I on a small population of cells
Early studies showed that NK cell–mediated rejection of hematopoietic grafts could be overcome by large doses of transferred cells (Cudkowicz and Bennett, 1971; Murphy et al., 1987; Koh et al., 2005). To test if the effect of MHC-I down-regulation depends on the number of missing-self targets, we generated mixed bone marrow (BM) chimeras with different ratios of donor BM to produce chimeric mice in which variable percentages of hematopoietic cells were reconstituted from B2mfl/fl R26-Cre-ERT2 BM (Fig. 4, A and B). Before tamoxifen treatment, we determined the degree of chimerism and found that tamoxifen induced a loss of B2mfl/fl R26-Cre-ERT2 hematopoietic cells that was abrogated by NK cell depletion (Fig. 4 B). However, this missing-self reactivity was overcome when the percentage of B2mfl/fl R26-Cre-ERT2 hematopoietic cells was increased (Fig. 4 B). In contrast, B2mfl/fl R26-Cre-ERT2 hematopoietic cell frequencies remained relatively constant in vehicle-treated chimeras (Fig. 4 B). These results suggest that MHC-I down-regulation results in missing-self reactivity when MHC-I deficiency extends only to a limited number of cells.
Figure 4.
Down-regulation of MHC-I is sufficient for missing-self recognition of a limited number of cells. (A) Mixed BM chimeras were generated by reconstituting irradiated WT (CD45.1) mice with different ratios of BM from WT (CD45.1) and B2mfl/fl R26-Cre-ERT2 (CD45.2) mice. After 18–21 wk, chimeras were treated with tamoxifen or vehicle control starting on day 1, and peripheral blood was analyzed weekly from day 0 to 28. Chimeras were injected with anti-NK1.1 to deplete NK cells or with PBS on day −2 and every 7 d after. (B) Percentage of B2mfl/fl R26-Cre-ERT2 cells remaining in the blood on day 28 relative to day 0. Percentage of CD45.2+ cells was assessed within all CD45.1 and/or CD45.2+ lymphocytes. (C) Splenocytes from B2mfl/fl and B2mfl/fl R26-Cre-ERT2 mice were labeled with CT far red and differentially labeled with CT violet and i.v. injected into WT recipient mice (20 × 106 of each donor). Recipient mice were subsequently treated with tamoxifen or vehicle for 5 d. Recipient mice were injected i.p. with anti-NK1.1 to deplete NK cells or with PBS control. (D) Representative histograms showing H-2Kb expression on transferred B2mfl/fl R26-Cre-ERT2 cells (CT far red+ CT violethigh) from blood of recipient mice over time. (E) Percentage of transferred B2mfl/fl R26-Cre-ERT2 cells that are H-2Kb–deficient in the blood of recipient mice over time (n = 6 mice per group; two-way repeated measure ANOVA with Bonferroni’s multiple comparisons test). Asterisks in E indicate statistical significance between the tamoxifen-treated groups treated with anti-NK1.1 or PBS. (F) Representative histograms showing the relative percentages of transferred cells (CT far red+) recovered from the spleens of recipient mice on day 14. B2mfl/fl (Cre−), and B2mfl/fl R26-Cre-ERT2 (Cre+) donor cells were distinguished by CT violet labeling as indicated. (G) Percentage of NK cell–specific rejection of B2mfl/fl R26-Cre-ERT2 donor cells by WT recipient mice treated as indicated (n = 6 mice per group; one-way ANOVA with Bonferroni’s multiple comparisons test). Data in B, E, and G are combined from two independent experiments in which all recipients within an experiment received the same mix of donor BM or splenocytes. Each symbol in B and G represents an individual recipient mouse. Error bars indicate mean ± SEM; *, P < 0.05; ****, P < 0.0001; ns, not significant. Tam, tamoxifen; Veh, vehicle.
To test if tamoxifen was similarly sufficient to induce missing-self on a small population of transferred cells, we adoptively transferred splenocytes from B2mfl/fl R26-Cre-ERT2 mice into WT recipients that were subsequently treated with tamoxifen or vehicle control (Fig. 4 C). Remarkably, tamoxifen treatment resulted in only a small population of H-2Kb–deficient transferred cells (Fig. 4, D and E). However, depletion of NK cells with anti-NK1.1 dramatically increased the percentage of H-2Kb–deficient transferred cells (Fig. 4 D and E), indicating NK cell–mediated selection for residual MHC-I+ cells with tamoxifen treatment in mice not receiving anti-NK1.1 depletion. To further validate this interpretation, we directly compared cotransferred B2mfl/fl R26-Cre-ERT2 splenocytes and control B2mfl/fl splenocytes after tamoxifen treatment and found selective loss of B2mfl/fl R26-Cre-ERT2 splenocytes that was abrogated with NK cell depletion (Fig. 4, F and G). These data indicate that down-regulation of MHC-I on transferred cells is sufficient to induce missing-self recognition by NK cells. Because tamoxifen did not induce overt NK cell reactivity in B2mfl/fl R26-Cre-ERT2 mice (Fig. 2, A and B), these results together indicate that MHC-I down-regulation is capable of inducing distinct NK cell responses, cytotoxicity, or adaptation that depends on the dose of missing-self targets.
To further investigate how NK cells respond to intermediate doses of missing-self targets, we bred B2mfl/fl mice to CD4-Cre-ERT2 transgenic mice to facilitate tamoxifen-induced deletion only in CD4+ T cells (Aghajani et al., 2012). CD4+ T cells were chosen as target cells for the following reasons: CD4+ T cells constitute a substantially smaller cell population compared with those targeted by the R26-Cre-ERT2, CD4+ T cells have been suggested to be targets for NK cell cytotoxicity (Lu et al., 2007; Waggoner et al., 2012), and the CD4-Cre-ERT2 does not target NK cells (Fig. 5, A and B). Treatment of B2mfl/fl CD4-Cre-ERT2 mice with tamoxifen induced a subtle but reproducible reduction in the percentage of CD4+ T cells; however, we did not observe a statistically significant reduction in CD4+ T cell number compared with tamoxifen-treated B2mfl/fl mice (Fig. 5, C–E). These results suggest that down-regulation of MHC-I on CD4+ T cells leads to low-level missing-self recognition, similar to what was observed when ∼10% of hematopoietic cells down-regulated MHC-I in mixed BM chimeras (Fig. 4 B). However, the majority of CD4+ T cells that down-regulated H-2Kb in response to tamoxifen were not rejected by NK cells (Fig. 5, A–E), suggesting that NK cells establish tolerance to the remaining MHC-I–deficient CD4+ T cells. To test this idea, we assessed whether B2mfl/fl CD4-Cre-ERT2 mice pretreated with tamoxifen were capable of rejecting transferred B2mΔ/Δ cells. B2mfl/fl CD4-Cre-ERT2 mice treated with vehicle control were able to reject B2mΔ/Δ splenocytes to a similar degree as B2mfl/fl controls (Fig. 5, F and G), indicating that B2mfl/fl CD4-Cre-ERT2 NK cells were not tolerant to missing-self at baseline. However, tamoxifen treatment abrogated the ability of NK cells in B2mfl/fl CD4-Cre-ERT2 mice to reject B2mΔ/Δ splenocytes (Fig. 5, F and G). These results show that down-regulation of MHC-I only on CD4+ T cells induces NK cell tolerance toward missing-self.
Figure 5.
Minimal NK cell reactivity is observed after down-regulation of MHC-I on CD4+ T cells. B2mfl/fl and B2mfl/fl CD4-Cre-ERT2 mice were treated with tamoxifen or vehicle control on days 0–4, and spleens were harvested for flow cytometry on day 14. (A) Percentage of NK cells (CD45+ CD19− CD3− NK1.1+ NKp46+), B cells (CD45+ CD3− CD19+), CD8+ T cells (CD45+ CD19− CD3+ CD4− CD8+), and CD4+ T cells (CD45+ CD19− CD3+ CD8− CD4+) that express H-2Kb after treatment with tamoxifen (closed bars) or vehicle (open bars; n = 10 mice per group). (B) Representative histograms showing H-2Kb expression on the indicated cell types. (C) Representative dot plots showing the percentage of splenic T cells (CD45+ CD19− CD3+) that express CD4 or CD8. (D) Percentage of splenic T cells that express CD4 (n = 10 mice per group). (E) Number of CD4+ splenic T cells (n = 10 mice per group). Data in A, D, and E are combined from three independent experiments. (F and G) B2mfl/fl and B2mfl/fl CD4-Cre-ERT2 mice were treated with tamoxifen or vehicle control on days 0–4 followed by i.v. injection of labeled donor splenocytes on day 14. Recipient spleens were harvested after 2 d to analyze donor cell recovery. (F) Representative histograms showing the relative percentages of transferred cells (CFSE+) recovered from the spleens of recipient mice. (G) Summary of NK cell–specific rejection of B2mΔ/Δ donor cells (n = 4–6 mice per group). Data in G is combined from two independent experiments in which all recipients within an experiment received the same mix of donor cells. Statistical significance was calculated by two-way ANOVA with Bonferroni’s multiple comparisons test. Each symbol in A, D, E, and G represents an individual mouse. Error bars indicate mean ± SEM; **, P < 0.01; ****, P < 0.0001; ns, not significant. Tam, tamoxifen; Veh, vehicle.
NK cell tolerance to MHC-I–deficient CD4+ T cells is not due to education
To further investigate the mechanism by which NK cells establish tolerance to MHC-I–deficient CD4+ T cells in tamoxifen-treated B2mfl/fl CD4-Cre-ERT2 mice, we stimulated splenocytes from B2mfl/fl, B2mfl/fl CD4-Cre-ERT2, and B2mΔ/Δ mice with plate-bound anti-NK1.1. A higher percentage of NK cells from tamoxifen-treated B2mfl/fl CD4-Cre-ERT2 mice produced IFN-γ after anti-NK1.1 stimulation, as compared with B2mΔ/Δ mice (Fig. 6, A and B). Importantly, NK cells from tamoxifen-treated B2mfl/fl CD4-Cre-ERT2 mice produced IFN-γ at similar levels to B2mfl/fl mice and vehicle controls. This was accompanied by an Ly49C licensing ratio >1 in tamoxifen-treated B2mfl/fl CD4-Cre-ERT2 mice, indicating that NK cells remained licensed through Ly49C (Fig. 6 C). We did notice a subtle reduction in the Ly49C licensing ratio comparing vehicle- and tamoxifen-treated B2mfl/fl CD4-Cre-ERT2 mice in some experiments, but this is of unclear significance because the licensing ratio was still >1 (Fig. 6 C). Together, these data show that NK cells remain responsive to stimulation through activation receptors after down-regulation of MHC-I on CD4+ T cells. These results suggest that MHC-I down-regulation on CD4+ T cells does not induce the same resetting of NK cell education that was seen after global down-regulation of MHC-I. This suggests that distinct mechanisms establish NK cell tolerance to missing-self in B2mfl/fl R26-Cre-ERT2 and B2mfl/fl CD4-Cre-ERT2 mice treated with tamoxifen.
Figure 6.
NK cell responsiveness and receptor repertoire are not affected by MHC-I down-regulation on CD4+ T cells. B2mfl/fl, B2mfl/fl CD4-Cre-ERT2, and B2mΔ/Δ mice were treated with tamoxifen or vehicle control on days 0–4, and splenocytes were harvested on day 14 for anti-NK1.1 stimulation and receptor repertoire analysis. (A) Representative dot plots showing the percentage of total NK cells (CD45+ CD3− CD19− NKp46+) that express Ly49C and IFN-γ after stimulation with plate-bound anti-NK1.1. (B) Summary of the percentage of total NK cells that express IFN-γ after stimulation with plate-bound anti-NK1.1 (n = 3 mice per group; one-way ANOVA with Bonferroni’s multiple comparisons test). (C) The Ly49C licensing ratio for total NK cells after stimulation with anti-NK1.1 (n = 3 mice per group; one-way ANOVA with Bonferroni’s multiple comparisons test). Data in B and C are representative of three independent experiments with three to four mice per group. (D) The percentage of NK cells (CD3− CD19− NK1.1+ NKp46+) that express the indicated receptor (n = 10 mice per group). Data in D are combined from three independent experiments. (E) Representative histograms showing expression of the indicated receptor on NK cells from B2mfl/fl and B2mfl/fl CD4-Cre-ERT2 mice treated with tamoxifen. Each symbol in B–D represents an individual mouse. Error bars indicate mean ± SEM; ** P < 0.01; *** P < 0.001; ****, P < 0.0001; ns, not significant. Tam, tamoxifen; Veh, vehicle.
We next tested if NK cells from tamoxifen-treated B2mfl/fl CD4-Cre-ERT2 mice establish tolerance to MHC-I–deficient CD4+ T cells through skewing of the inhibitory receptor repertoire. We observed similar expression of Ly49 receptors and CD94/NKG2A on NK cells from tamoxifen-treated B2mfl/fl CD4-Cre-ERT2 mice compared with vehicle-treated or B2mfl/fl mice (Fig. 6, D and E). Additionally, we did not observe any alterations in NK cell number or maturation based on expression of CD11b, CD27, and KLRG1 in tamoxifen-treated B2mfl/fl CD4-Cre-ERT2 mice (Fig. S3). Notably, we also did not detect up-regulation of the activation marker CD69 or markers of T cell exhaustion such as PD-1, LAG-3, TIM-3, or CTLA-4 (Wherry and Kurachi, 2015) in response to MHC-I down-regulation on CD4+ T cells (Fig. S4 E). These data suggest that NK cell tolerance to missing-self after MHC-I down-regulation on CD4+ T cells does not result from mechanisms analogous to T cell anergy, clonal deletion, or exhaustion, which render T cells hyporesponsive or alter the T cell receptor repertoire (Xing and Hogquist, 2012; Schietinger and Greenberg, 2014).
Robust missing-self reactivity occurs in the context of infection
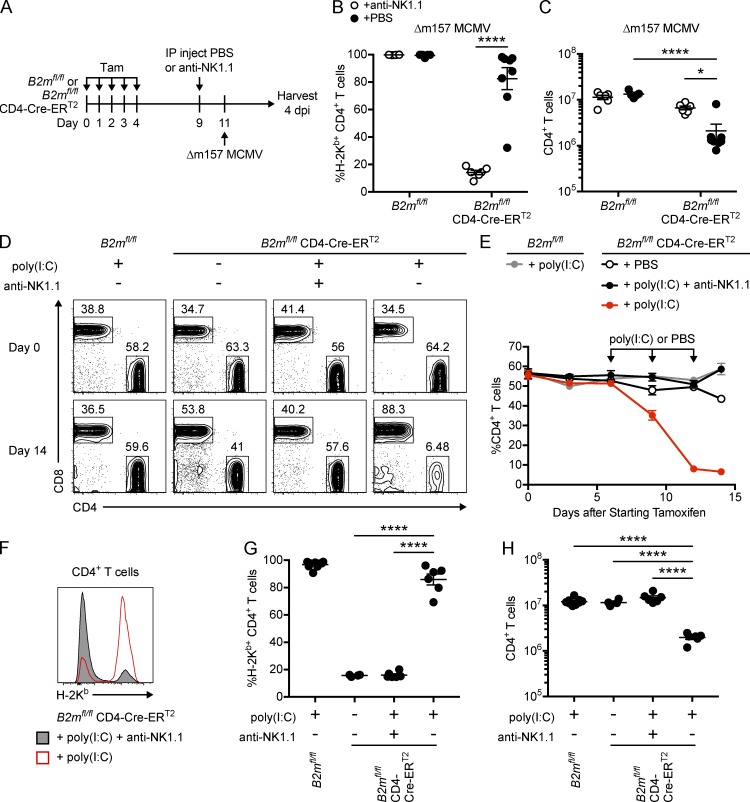
Previous studies have shown that MCMV infection breaks NK cell tolerance to missing-self in mixed WT:B2m−/− chimeras (Sun and Lanier, 2008; Shifrin et al., 2016). This led us to hypothesize that viral infection induces NK cell missing-self reactivity in response to acute down-regulation of MHC-I as well. To test this idea, B2mfl/fl CD4-Cre-ERT2 mice were treated with tamoxifen and subsequently infected with Δm157 MCMV (Fig. 7 A). Mice were infected with Δm157 instead of WT MCMV to exclude the effects of Ly49H-mediated cytotoxicity and so that NK cells could be depleted without significantly increasing the viral burden (Brown et al., 2001; Bubić et al., 2004; Parikh et al., 2015), which could have confounded previous experiments with mixed chimeras (Sun and Lanier, 2008). In the context of NK cell depletion, CD4+ T cells from tamoxifen-treated B2mfl/fl CD4-Cre-ERT2 mice were mostly MHC-I deficient, even in the context of infection (Fig. 7 B). Remarkably, however, a high percentage of CD4+ T cells in undepleted B2mfl/fl CD4-Cre-ERT2 mice treated with tamoxifen and infected with Δm157 MCMV expressed MHC-I (Fig. 7 B). This selection for MHC-I–positive cells was accompanied by a reduction in the number of splenic CD4+ T cells in tamoxifen-treated B2mfl/fl CD4-Cre-ERT2 mice infected with Δm157 MCMV compared with NK cell–depleted mice and B2mfl/fl controls (Fig. 7 C). These results together suggest that in the context of Δm157 MCMV infection, NK cells robustly reject CD4+ T cells that down-regulate MHC-I.
Figure 7.
MCMV infection and poly(I:C) induce missing-self reactivity toward MHC-I–deficient CD4+ T cells. (A–C) B2mfl/fl and B2mfl/fl CD4-Cre-ERT2 mice were treated with tamoxifen on days 0–4, injected with PBS or anti-NK1.1 antibody on day 9, and infected with 5 × 103 PFU of Δm157 MCMV on day 11. Splenocytes were harvested 4 d after infection for flow cytometry as depicted in A. Tam, tamoxifen; Veh, vehicle. (B) The percentage of CD4+ T cells (CD45+ CD19− CD3+ CD8− CD4+) that express H-2Kb in the spleens of mice 4 d after infection (n = 5–8 mice per group; two-way ANOVA with Bonferroni’s multiple comparisons test). (C) The number of splenic CD4+ T cells 4 d after infection (n = 5–8 mice per group; two-way ANOVA with Bonferroni’s multiple comparisons test). (D–H) B2mfl/fl and B2mfl/fl CD4-Cre-ERT2 mice were treated with tamoxifen on days 0–4, followed by i.p. injection of poly(I:C) or PBS on days 6, 9, and 12. Mice were injected with anti-NK1.1 to deplete NK cells or with PBS control on day −2 and every 7 d after. (D) Representative dot plots showing CD4 and CD8 expression on peripheral blood T cells (CD45+ CD19− CD3+) on days 0 and 14. (E) Percentage of blood T cells that express CD4 over time (n = 4–7 mice per group). (F) Representative histogram showing H-2Kb expression on day 14 splenic CD4+ T cells from anti-NK1.1– or PBS-treated B2mfl/fl CD4-Cre-ERT2 mice treated with tamoxifen and poly(I:C). (G) Percentage of splenic CD4+ T cells that express H-2Kb on day 14 (n = 4–9 mice per group; one-way ANOVA with Bonferroni’s multiple comparisons test). (H) Number of splenic CD4+ T cells on day 14 (n = 4–9 mice per group; one-way ANOVA with Bonferroni’s multiple comparisons test). Data in B, C, E, G, and H are combined from two independent experiments. Each symbol in B, C, G, and H represents an individual mouse. Error bars indicate mean ± SEM; *, P < 0.05; ****, P < 0.0001.
Pattern recognition receptor agonist stimulation drives NK cell reactivity toward missing-self
Previous studies have shown that cytotoxic control of MCMV requires cytokine stimulation of NK cells to promote expression of perforin and granzyme B (Fehniger et al., 2007; Lucas et al., 2007; Parikh et al., 2015), but it is unclear if cytokines play a similar role in NK cell self-tolerance. We hypothesized that NK cell missing-self reactivity in tamoxifen-treated B2mfl/fl CD4-Cre-ERT2 mice during viral infection might be regulated by inflammatory cytokines instead of NK cell education. To test the role of inflammation in missing-self recognition in vivo, B2mfl/fl CD4-Cre-ERT2 mice were treated with tamoxifen followed by PBS or poly(I:C), an adjuvant that signals through the pattern recognition receptors (PRRs) TLR3 and MDA5 to induce production of inflammatory cytokines that activate NK cells (Akazawa et al., 2007; McCartney et al., 2009). Poly(I:C) thus mimics aspects of the inflammatory environment induced by MCMV infection, which also triggers MyD88-dependent responses (Krug et al., 2004). Remarkably, poly(I:C) induced rapid loss of CD4+ T cells from the peripheral blood of B2mfl/fl CD4-Cre-ERT2, but not B2mfl/fl mice treated with tamoxifen (Fig. 7, D and E). This rapid loss of CD4+ T cells was not observed in B2mfl/fl CD4-Cre-ERT2 mice treated with tamoxifen and PBS control (Fig. 7, D and E). Importantly, depletion of NK cells with anti-NK1.1 completely abrogated the loss of CD4+ T cells induced by poly(I:C) (Fig. 7, D and E). These data indicate that poly(I:C) induces NK cell missing-self reactivity in tamoxifen-treated B2mfl/fl CD4-Cre-ERT2 mice.
To test if poly(I:C) acts through potentiating missing-self recognition, expression of MHC-I was analyzed on residual CD4+ T cells from the spleens of B2mfl/fl CD4-Cre-ERT2 mice treated with tamoxifen and poly(I:C) (Fig. 7, F and G). Tamoxifen induced down-regulation of H-2Kb on ∼80% of splenic CD4+ T cells from B2mfl/fl CD4-Cre-ERT2 mice treated with PBS control. Remarkably, a substantially higher percentage of CD4+ T cells from B2mfl/fl CD4-Cre-ERT2 mice treated with tamoxifen and poly(I:C) expressed H-2Kb compared with mice that did not receive poly(I:C) (Fig. 7, F and G). In contrast, depletion of NK cells with anti-NK1.1 completely abrogated this effect (Fig. 7, F and G), indicating that poly(I:C) induced an NK cell–dependent selection for MHC-I+ cells that was accompanied by an NK cell–dependent reduction in splenic CD4+ T cell number in B2mfl/fl CD4-Cre-ERT2 mice treated with tamoxifen and poly(I:C) (Fig. 7 H). These data show that poly(I:C) induces NK cell reactivity toward MHC-I–deficient CD4+ T cells, indicating that a PRR agonist can regulate NK cell reactivity toward missing-self.
To test if poly(I:C) promotes killing of MHC-I–deficient CD4+ T cells by affecting the NK cell or the target cell, short-term killing of adoptively transferred B2mΔ/Δ CD4+ T cells was assessed using poly(I:C)-treated donor or recipient mice (Fig. S4, A and B). Subtle rejection of CD4+ T cells from PBS-treated B2mΔ/Δ mice was observed during a 3-h in vivo cytotoxicity assay in PBS-treated recipient mice. CD4+ T cells from poly(I:C)-treated B2mΔ/Δ donor mice were rejected only slightly more than those from PBS-treated B2mΔ/Δ donors. In contrast, poly(I:C)-treated recipient mice exhibited substantially greater rejection of B2mΔ/Δ CD4+ T cells compared with PBS-treated recipients (Fig. S4, A and B). Additionally, poly(I:C) was found to up-regulate granzyme B expression in NK cells from tamoxifen-treated B2mfl/fl CD4-Cre-ERT2 mice (Fig. S4, C and D), similar to what has previously been shown in infected and poly(I:C)-treated WT mice (Fehniger et al., 2007; Lucas et al., 2007; Parikh et al., 2015). Together, these results suggest that poly(I:C) promotes missing-self reactivity in tamoxifen-treated B2mfl/fl CD4-Cre-ERT2 mice at least in part by enhancing NK cell cytotoxic capacity.
IL-15–IL-15Rα-Fc complex promotes missing-self reactivity
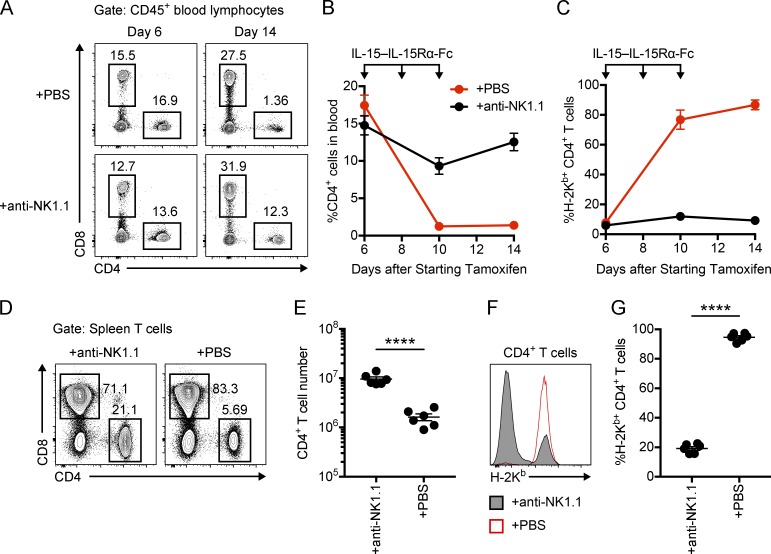
Previous studies have suggested that NK cell expression of cytotoxic mediators during viral infection depends on trans-presentation of IL-15 (Fehniger et al., 2007; Lucas et al., 2007). To test if IL-15 is sufficient to enhance NK cell missing-self reactivity, tamoxifen-treated B2mfl/fl CD4-Cre-ERT2 mice were treated with IL-15–IL-15Rα-Fc complex in a manner similar to previous studies (Rubinstein et al., 2006; Shifrin et al., 2016). IL-15–IL-15Rα-Fc complex was found to induce a substantial reduction in CD4+ T cells that was abrogated by NK cell depletion (Fig. 8, A and B; and Fig. 8, D and E). Importantly, the residual CD4+ T cells in IL-15–IL-15Rα-Fc complex–treated B2mfl/fl CD4-Cre-ERT2 mice were predominately H-2Kb+ unless mice were depleted of NK cells (Fig. 8 C; and Fig. 8, F and G). These results show that IL-15–IL-15Rα-Fc complex is sufficient to promote missing-self recognition of MHC-I–deficient CD4+ T cells by NK cells.
Figure 8.
IL-15 agonist induces missing-self reactivity toward MHC-I–deficient CD4+ T cells. B2mfl/fl CD4-Cre-ERT2 mice were treated with tamoxifen on days 0–4 and i.p. injected with IL-15 + IL-15Rα-Fc (0.6 µg + 3 µg) on days 6, 8, and 10. Mice were injected with anti-NK1.1 or PBS on days 4, 5, and 12. (A) Representative dot plots showing CD4 and CD8 expression on CD45+ blood lymphocytes. (B) Percentage of CD45+ blood lymphocytes that express CD4 over time. (C) Percentage of blood CD4+ T cells (CD45+ CD8− CD4+) that express H-2Kb over time. (D) Representative dot plot showing CD4 and CD8 expression by day 14 spleen T cells (CD45+ CD19− NK1.1− CD1d-Tet− CD3+). (E) Number of splenic CD4+ T cells on day 14. (F) Representative histogram showing H-2Kb expression on day 14 splenic CD4+ T cells. (G) Percentage of splenic CD4+ T cells that express H-2Kb on day 14. Data in B, C, E, and G are combined from two independent experiments (n = 6 mice per group; unpaired t test). Each symbol in E and G represents an individual mouse. Error bars indicate mean ± SEM; ****, P < 0.0001.
Discussion
Here, we show that down-regulation of MHC-I in vivo in a host that otherwise expressed MHC-I normally does not automatically lead to NK cell–mediated rejection, as predicted by the missing-self hypothesis. Instead, while we acknowledge that the timing of MHC-I down-regulation induced by tamoxifen in our experiments appears not to be instantaneous, the “acute” or induced loss of MHC-I can result in multiple distinct NK cell responses. Global down-regulation of MHC-I was found to induce NK cell tolerance to missing-self by resetting NK cell education. In contrast, down-regulation of MHC-I on a fraction of hematopoietic cells in mixed BM chimeras, on transferred cells, or on CD4+ T cells could trigger missing-self recognition and cytotoxicity. Additionally, CD4+ T cell–specific down-regulation of MHC-I was able to induce NK cell tolerance to missing-self without evidence of an alteration in the capacity of NK cells to be triggered through their activation receptors. In this setting, viral infection, PRR agonist stimulation, and cytokine stimulation could drive robust NK cell reactivity toward missing-self. Thus, our findings suggest that inflammation is important for breaking NK cell self-tolerance, despite the generally accepted paradigm based on the missing-self hypothesis alone.
The missing-self hypothesis predicts that down-regulation of MHC-I is sufficient to trigger NK cell cytotoxicity (Ljunggren and Kärre, 1990). In strong support of missing-self, host NK cells have been shown to kill adoptively transferred cells that were rendered MHC-I deficient by germline deletion of B2m (Bix et al., 1991), which was recapitulated here when MHC-I deficiency was induced on transferred splenocytes. In contrast, adoptive transfer of WT NK cells into MHC-I–deficient mice induced NK cells to adapt and become tolerant to missing-self (Joncker et al., 2010). These functionally opposing outcomes raise the question: When does missing-self attack occur versus when does NK cell tolerance to missing-self occur instead? Our results show that acute down-regulation of MHC-I in vivo can induce both NK cell killing and tolerance in different contexts.
Acute down-regulation of MHC-I in mixed BM chimeras and on CD4+ T cells was sufficient to induce missing-self recognition and killing, but this appeared to be limited to only a small number of MHC-I–deficient cells. In contrast, we did not find any evidence for MHC-I–deficient cell depletion when we induced ubiquitous down-regulation of MHC-I. These results could be due to enhanced susceptibility of certain cell types to NK cell lysis compared with others. Alternatively, it is possible that we did not observe missing-self reactivity after global MHC-I down-regulation because NK cells became overwhelmed or exhausted by the large dose of missing-self targets. However, NK cells did not appear to be hyporesponsive to activation stimuli after MHC-I down-regulation on CD4+ T cells. Additionally, NK cells did not up-regulate markers of T cell exhaustion (Wherry and Kurachi, 2015) after down-regulation of MHC-I on CD4+ T cells. As a result, we favor the explanation that NK cells respond differently to missing-self depending on the dose of MHC-I–deficient cells. This is also supported by differences in NK cell activation receptor responses after down-regulation of MHC-I on all cells compared with only CD4+ T cells. In any case, an inflammatory environment, such as that induced by systemic MCMV infection, PRR agonist (poly[I:C]) administration, or simply cytokine (IL-15–IL-15Rα-Fc complex) treatment, promoted NK cell cytotoxicity toward most MHC-I–deficient CD4+ T cells. This mechanism ensures that robust NK cell killing of MHC-I–deficient “self” cells primarily occurs in an inflammatory environment such as during an infection.
Our laboratory previously proposed that NK cell self-tolerance is established through a licensing (education) process that ensures that only NK cells that express self-MHC-I–specific inhibitory receptors become fully functional and capable of killing through missing-self recognition (Kim et al., 2005). As a result, we proposed that NK cells from B2m−/− mice were self-tolerant because they were unlicensed and hypofunctional, rather than because of changes in inhibitory receptor expression (Yokoyama and Kim, 2006). If licensing fully explains NK cell self-tolerance in B2m−/− mice, however, then acute down-regulation of MHC-I in a mouse that contains licensed NK cells should induce NK cell missing-self reactivity. In contrast, we found here that NK cells remained self-tolerant after global down-regulation of MHC-I in B2mfl/fl R26-Cre-ERT2 mice. Global down-regulation of MHC-I was found to only induce subtle changes in NK cell inhibitory receptor expression, which suggests that self-tolerance was not maintained through receptor repertoire skewing. In contrast, global down-regulation of MHC-I was found to “unlicense” NK cells, which presumably contributed to maintenance of self-tolerance. Surprisingly, down-regulation of MHC-I only on CD4+ T cells induced a slight loss of target cells, but NK cells did not alter their education and remained predominately self-tolerant in this setting as well. These data suggest that other mechanisms besides receptor repertoire skewing and NK cell education may contribute to NK cell tolerance to missing-self in settings where NK cells are exposed to missing-self targets but remain educated, similar to what has been suggested with mixed chimeras (Shifrin et al., 2016). Notably, this form of NK cell self-tolerance was readily broken by inflammatory stimuli in B2mfl/fl CD4-Cre-ERT2 mice. These results suggest that a lack of inflammation at steady-state contributes to NK cell self-tolerance in B2m−/− mice in addition to the lack of NK cell education.
Previous studies have used adoptive transfer of WT NK cells into B2m−/− mice to suggest that NK cells adapt to changing MHC-I environments by resetting their education (Joncker et al., 2010). However, these studies were limited because MHC-I expression was only altered globally with the host B2m−/− mouse, which differs from settings of infection and tumorigenesis in which MHC-I down-regulation occurs on only a subpopulation of cells. Additionally, previous studies have suggested that T cells from B2m−/− mice can reject WT cells (Zijlstra et al., 1992; Apasov and Sitkovsky, 1993; Glas et al., 1994), which necessitated irradiation or T cell depletion of recipient mice in these transfer experiments (Joncker et al., 2010), which could have influenced the results. Here, we used the B2mfl/fl R26-Cre-ERT2 mouse to globally down-regulate MHC-I without impairing T cell development. Our results show that global down-regulation of MHC-I leads to loss of NK cell education, which is in agreement with prior adoptive transfer experiments (Joncker et al., 2010). Notably, it is unclear if NK cells become uneducated in response to global MHC-I down-regulation because they become over-activated and anergic or because of a reeducation process that is independent of activation signals. Surprisingly, down-regulation of MHC-I only on CD4+ T cells did not induce a loss of NK cell education. Our results may suggest that the dose of the MHC-I–deficient cells influences whether MHC-I down-regulation induces loss of NK cell education or not. Alternatively, MHC-I expression by only specific cell types may be required to educate NK cells. Breeding the floxed B2m mouse to strains that express cell type–specific Cre will be valuable for distinguishing between these possibilities and for identifying specific cell types required to educate NK cells.
Previous studies using transgenic mice with mosaic H-2Dd expression (Johansson et al., 1997) and WT:B2m−/− mixed fetal liver chimeras (Wu and Raulet, 1997) have suggested that chronic exposure to MHC-I–deficient cells dominantly induces tolerance to missing-self. Our results show that tamoxifen treatment of B2mfl/fl R26-Cre-ERT2 and B2mfl/fl CD4-Cre-ERT2 mice also induces tolerance to adoptively transferred MHC-I–deficient targets. However, acute down-regulation of MHC-I on a small percentage of cells in mixed BM chimeras led to missing-self reactivity. These results extend previous findings by showing that acute down-regulation of MHC-I can also dominantly induce tolerance to missing-self, though we further show that this tolerance, at least in the acute setting, is dependent on the percentage of MHC-I–deficient cells in the host.
Because cytokines are known to stimulate NK cells to express perforin and granzyme B for cytotoxic control of MCMV (Fehniger et al., 2007; Parikh et al., 2015), we hypothesize that inflammation induces NK cell autoreactivity by enhancing the cytotoxic capacity of NK cells. Consistent with this idea, we observed that poly(I:C) induced elevated granzyme B expression in NK cells from mice with MHC-I–deficient CD4+ T cells. However, future studies with B2mfl/fl CD4-Cre-ERT2 mice bred to Gzmb−/− mice will be required to definitively determine the role of cytotoxic mediators in inflammation-induced missing-self recognition of MHC-I–deficient CD4+ T cells. Moreover, future studies will be needed to determine whether inflammation induces missing-self reactivity through additional mechanisms, as well as through effects of inflammation on NK cell number, trafficking, and receptor repertoire and through effects of inflammation on other cell types. This NK cell tolerance to missing-self in B2mfl/fl CD4-Cre-ERT2 mice that can be broken by inflammation resembles T cell immunological ignorance, in which potentially autoreactive T cells are prevented from causing overt autoimmunity likely due in part to a lack of appropriate activation signals, rather than anergy or clonal deletion (Ohashi et al., 1991; Oldstone et al., 1991). Notably, the dose of self-antigen is thought to determine whether potentially autoreactive T cells remain ignorant or become tolerant (Kurts et al., 1999). Similarly, our results point to a role for the dose of MHC-I–deficient cells in determining whether NK cells ignore missing-self targets or adapt to them by becoming uneducated.
If cytokine stimulation is required for NK cells to kill MHC-I–deficient targets, however, it still remains unclear how NK cells are capable of rejecting MHC-I–deficient transferred cells without an inflammatory stimulus (Öberg et al., 2004). It is possible that the process of harvesting single cell suspensions from MHC-I–deficient mice induces stress signals and dead cells that trigger low-level inflammation in recipient mice. However, missing-self reactivity was also observed toward a small number of cells in mixed BM chimeras that were performed months after the BM transfer. Alternatively, perhaps basal levels of inflammation are sufficient to stimulate NK cells to kill a small number of missing-self targets. Intriguingly, IFNAR1−/− and IFNAR2−/− mice have been shown to be susceptible to MHC-I–deficient RMA/s tumors, suggesting that cytokine stimulation is essential for NK cell–mediated control of classical missing-self tumors (Swann et al., 2007). Additionally, it has long been acknowledged that in vitro NK cell cytotoxicity of MHC-I–deficient targets requires pretreating mice with poly(I:C) (Djeu et al., 1979; Liao et al., 1991) or activating NK cells in culture with cytokines such as IL-2 (Grimm et al., 1982; Rosenstein et al., 1984). Collectively with our results, it seems likely that cytokine signaling in NK cells is generally required for missing-self reactivity; however, future studies will be needed to test if NK cells respond directly or indirectly to specific cytokines to kill nontransformed cells that are rendered acutely MHC-I–deficient in vivo, as in our studies.
Previous studies with WT:B2m−/− mixed chimeras have suggested that infection or inflammation can break NK cell tolerance to missing-self (Sun and Lanier, 2008; Shifrin et al., 2016). It is unclear from these studies, however, if NK cells start out tolerant to missing-self in these chimeras, because in one study (Sun and Lanier, 2008), B2m−/− cells were gradually lost from the chimeras over time, and in a second study (Shifrin et al., 2016), B2m−/− cells were found to be stable in the chimeras. Additionally, it is unclear if this presumed tolerance to missing-self is unique to settings in which NK cells are chronically exposed to MHC-I–deficient cells or if it applies to settings in which MHC-I is down-regulated acutely. Here we show that NK cells can remain tolerant to missing-self in response to acute MHC-I down-regulation. Additionally, our results extend the previous findings from mixed chimeras by showing that inflammation plays a critical role in triggering NK cell missing-self reactivity toward self cells that acutely down-regulate MHC-I.
MHC-I–specific inhibitory receptors on NK cells are an appealing target for tumor immunotherapy; however, recent clinical trials failed to show efficacy of anti-KIR2D checkpoint blockade in patients with smoldering multiple myeloma (Korde et al., 2014). Antibody blockade of MHC-I–specific inhibitory receptors is predicted to induce NK cell missing-self recognition by mimicking the effects of MHC-I down-regulation. Our results, however, show that down-regulation of MHC-I alone does not induce substantial missing-self reactivity in vivo. In contrast, MHC-I down-regulation has the potential to induce NK cell tolerance through loss of education, which has been proposed to explain in part the lack of efficacy of anti-KIR2D checkpoint blockade (Carlsten et al., 2016). Notably, our results suggest that inflammation is needed to observe substantial NK cell reactivity toward missing-self targets in vivo. These results suggest that combination therapy with anti-KIR2D and an inflammatory stimulus or IL-15 agonist (Romee et al., 2018) may improve the efficacy of checkpoint blockade of NK cells as others have proposed previously (Vahlne et al., 2010; Ardolino et al., 2014), but also suggest that these combination therapies may have the potential to induce side-effects from NK cell–mediated autoreactivity.
Materials and methods
Mice
C57BL/6 (B6) and B6-Ly5.1 (B6.SJL-PtprcaPepcb/BoyCrCrl) mice were purchased from Charles River Laboratories. B2m−/− (B6.129P2-B2mtm1Unc/J), Albino B6 (B6(Cg)-Tyrc-2J/J), R26-Cre-ERT2 (B6.129-Gt(ROSA)26Sortm1(cre/ERT2)Tyj/J), and CD4-Cre-ERT2 (B6(129X1)-Tg(Cd4-cre/ERT2)11Gnri/J) mice were purchased from The Jackson Laboratory. FLP transgenic mice (C57BL/6-Tg(CAGGS-Flpe)2ARTE) were purchased from Artemis Pharmaceuticals. CMV-Cre mice (Schwenk et al., 1995) on the B6 background were provided by Marco Colonna (Washington University in St. Louis, St. Louis, MO). Control B2m−/− mice used in experiments were obtained from The Jackson Laboratory, either directly or subsequently bred in-house. B6 and B6-Ly5.1 mice used in experiments were obtained directly from Charles River Laboratories. All other experimental and control mice were bred in-house at Washington University in St. Louis. Mice were 8–14-wk old at the start of experiments unless otherwise stated. Male and/or female mice were used in individual experiments without blinding or randomization. Animal protocols were approved by the Institutional Animal Care and Use Committee at Washington University in St. Louis.
Development of floxed B2m mice
Three JM8A3.N1 (C57BL/6N-Atm1Brd) ES cell clones (HEPD0673_4_D09, HEPD0673_4_G10, and HEPD0673_4_H09) carrying the targeted allele B2mtm1a(EUCOMM)Hmgu (B2mtm1a) were purchased from the European Conditional Mouse Mutagenesis Consortium (EUCOMM). Clone HEPD0673_4_D09 was confirmed to be correctly targeted by Southern blot analysis and was micro-injected into Albino B6 blastocysts by the Transgenic, Knockout, and Micro-Injection Core at Washington University in St. Louis. Chimeric mice were bred to Albino B6 to identify germline transmission by coat color. Mice containing the germline-transmitted B2mtm1a allele were subsequently bred to FLP transgenic mice to generate the B2mtm1c (B2mfl) allele. The albino and FLP alleles were removed through breeding. Mice carrying the B2mfl allele were bred to CMV-Cre transgenic mice to generate the B2mtm1d (B2mΔ) allele with a germline deletion of B2m. The CMV-Cre transgene was subsequently bred out before experiments with B2mΔ/Δ mice, except in one replicate of the experiment in Fig. 1 (E and F), in which the B2mΔ/Δ donor cells were heterozygous for CMV-Cre. Excision by FLP and CMV-Cre were confirmed by PCR (Fig. S1, A–D) with Phusion High-Fidelity PCR Master Mix (New England Biolabs) with the following primers: primer a (5′-CAGCTCTCAGCACTGGATCA-3′), primer b (5′-GAACTTCGGAATAGGAACTTCG-3′), primer c (5′-CACCGTGCACCATCTTACAT-3′), and primer d (5′-AGAGCCCTCACCACTTCTCA-3′). Genomic DNA was isolated from mice of varied ages for PCR in Fig. S1 (B–D).
Antibodies and flow cytometry
The following were purchased from BD Biosciences: anti-Ly49G2 (4D11), anti-Ly49F (HBF-719), and streptavidin PE. The following were purchased from eBioscience: Fixable Viability Dye eFluor 506, Fixable Viability Dye eFluor 780, anti–IFN-γ (XMG1.2), anti-NKp46 (29A1.4), anti-NK1.1 (PK136), anti-CD19 (eBio1D3), anti-CD3e (145-2C11), anti-CD11b (M1/70), anti-CD45 (30-F11), anti-CD45.1 (A20), anti-CD45.2 (104), anti-CD4 (RM4-5), anti-CD8b (eBioH35-17.2), anti-CD8a (53–6.7), anti–CTLA-4 (UC10-4B9), anti–LAG-3 (eBioC9B7W), anti-Ly49H (3D10), anti-Ly49E/F (CM4), anti–TIM-3 (RMT3-23), anti-Ly49D (eBio4E5), anti-CD69 (H1.2F3), anti-Ly49G2 (eBio4D11), anti-NKG2AB6 (16a11), anti-CD94 (18d3), and anti-CD27 (LG.7F9). The following were purchased from BioLegend: streptavidin APC, anti-KLRG1 (2F1), anti–PD-1 (29F.1A12), anti-NK1.1 (PK136), anti–H-2Kb (AF6-88.5), and anti–H-2Db (KH95). The following was purchased from Abcam: anti-Ly49I (YLI-90). The following was purchased from Thermo Fisher: anti-Ly49F (HBF-719). The following was purchased from Jackson ImmunoResearch: Alexa Fluor 647–conjugated Goat anti-Mouse IgG-γ, Fc subclass 3 specific (anti-mouse IgG3). PBS-57–loaded mouse CD1d tetramer (CD1d-tet) conjugated to PE was obtained from the National Institutes of Health Tetramer Core Facility. PK136 antibody (anti-NK1.1) was purified in our laboratory from supernatants of the PK136 hybridoma (American Type Culture Collection) or purchased from Leinco Technologies. NK cell depletion was performed by i.p. injection of 200 µg of purified PK136 antibody. Mice were excluded from analysis if PK136 injection was shown to be unsuccessful by flow cytometry for NK cells in the blood after the first injection or in the spleen. JR9 antibody (anti-Ly49A) was purified in our laboratory from supernatants of the JR9 hybridoma that was provided by Jacques Roland (Pasteur Institute, Paris, France). 4LO3311 antibody (anti-Ly49C) was purified in our laboratory from supernatants of the 4LO3311 hybridoma that was provided by Suzanne Lemieux (Institut National de la Recherche Scientifique, Institut Armand-Frappier, Laval, Quebec, Canada). Anti-Ly49A and anti-Ly49C antibodies were conjugated in our laboratory to FITC or to biotin using the EZ-Link Sulfo-NHS-LC-LC-biotin kit (Thermo Fisher). Ly49C staining was performed with either anti–Ly49C-biotin followed by fluorophore-conjugated streptavidin or purified anti-Ly49C followed by anti-mouse IgG3–Alexa Fluor 647. Blocking of Fc receptors was performed with supernatants of the 2.4G2 (anti–Fc-γRII/III) hybridoma (American Type Culture Collection). Surface staining for flow cytometry was performed on ice in either 2.4G2 supernatant or staining buffer (PBS with 1% BSA and 0.01% Sodium Azide). Cells were gated on lymphocytes with exclusion of doublets and exclusion of dead cells with Fixable Viability Dye (BD Biosciences) or forward scatter and side scatter (only in some in vivo cytotoxicity experiments). CD45+ cells throughout the text refers to cells stained with either the anti-CD45 or anti-CD45.2 antibody. CD8+ cells throughout the text refers to cells stained with either the anti-CD8a or anti-CD8b antibodies. Samples were analyzed using a FACSCanto (BD Biosciences) or a FACSAria Fusion (BD Biosciences). Data were analyzed using FlowJo v9.9.6 or v10.4.2 (Tree Star).
Tamoxifen treatment of mice
Mice were administered tamoxifen (Sigma-Aldrich) by oral gavage (4 mg/d for five consecutive days) in 200 µl of corn oil (Sigma-Aldrich) as according to published protocols (Anastassiadis et al., 2010). In most experiments, NK cells were depleted from a subset of mice by i.p. injection of 200 µg anti-NK1.1 (PK136) antibody on day −2 and every 7 d after for the duration of the experiment unless stated otherwise. When indicated, 100 µg of poly(I:C) high molecular weight (InvivoGen) in 0.9% NaCl or a PBS control was administered to mice by i.p. injection.
In vivo cytotoxicity assay
Mice used for donor splenocytes in in vivo cytotoxicity assays were 8–18 wk old at the time of transfer. Donor splenocytes were harvested and labeled in vitro with different combinations of CFSE (Life Technologies), CellTrace violet (CT violet; Thermo Fisher), and CT far red (Thermo Fisher). For experiments with six donor cell populations, all donor cells were labeled with 2.5 µM CFSE, and donors were differentially labeled with CT violet (5, 1, or 0.2 µM) and CT far red (1 or 0.04 µM). For experiments with four donor cell populations, all donor cells were labeled with 2.5 µM CFSE, and donors were differentially labeled with CT violet (5 or 0.2 µM) and CT far red (1 or 0.04 µM). For experiments with three donor cell populations, all donor cells were labeled with 2.5 µM CFSE, and donors were differentially labeled with CT violet (5 µM, 1 µM, or 0.2 µM). For experiments with two donor cell populations, all donor cells were labeled with either 2.5 µM CFSE or with 0.2 µM CT far red, depending on the experiment. The two donor cell populations were differentially labeled with CT violet (5 or 0.2 µM). In some experiments, four donor cell populations were injected, but only two were displayed in the figure for clarity. Recipient mice were injected i.v. with 2 × 106 of each donor cell except for Fig. 4 (D–G), in which 20 × 106 of each donor cell was injected. Spleens from recipient mice were harvested 2 d after transfer of donor cells except for Fig. 4 (D–G) and Fig. S4 (A and B). NK cell–specific rejection was calculated by gating on transferred cells and excluding dead cells either by Fixable Viability Dye (BD Biosciences) or forward scatter and side scatter. Rejection was quantified as %Rejection = [1 − (Target/Control)/(Target/Control)Average(NK depleted)] × 100, where the target was the donor cell of interest, and the control was a WT or B2mfl/fl donor cell population depending on the experiment. In Fig. 2 D, %Rejection was calculated using vehicle-treated B2mfl/fl donor cells as the control. The ratio of target to control cells was normalized to the average ratio recovered from NK cell–depleted mice to calculate rejection by NK cells.
In vitro splenocyte stimulations
Splenocytes were stimulated in vitro as previously described (Kim et al., 2005; Jonsson and Yokoyama, 2010). In brief, 5 × 106 splenocytes in R10 media were plated into 24-well culture plates that that were precoated with anti-NK1.1 (PK136; 1 µg/ml) or that contained 0.5 µg/ml PMA (Sigma-Aldrich) and 4 µg/ml ionomycin (Sigma-Aldrich). Cells were incubated for a total of 7 h at 37°C. GolgiPlug (BD Biosciences) was added to the wells after the first hour. After stimulation, cells were stained with fixable viability dye and subsequently with antibodies to surface antigens. Cells were then fixed and permeabilized using the Fixation/Permeabilization Solution kit (BD Cytofix/Cytoperm) to stain for intracellular IFN-γ. Fixable viability dye was used to gate on viable cells in all experiments. The Ly49C licensing ratio was calculated as follows (Jonsson and Yokoyama, 2010): Ly49C licensing ratio = [(%Ly49C+IFN-γ+)/(%Ly49C+)]/[(%Ly49C−IFN-γ+)/(%Ly49C−)].
MCMV infections
Infections were performed with 5 × 103 PFU of Δm157 MCMV, which was previously shown to differ from the WT1 MCMV strain by a single nucleotide (Cheng et al., 2010; Parikh et al., 2015). Mice were infected by i.p. injection of salivary gland propagated virus in 200 µl PBS.
Cytokine treatments
Murine IL-15 was purchased from PeproTech. Mouse IL-15R α Fc chimera protein (IL-15Rα-Fc) was purchased from R&D Systems. IL-15 and IL-15Rα-Fc were complexed by incubating a mixture of 6.67 µg/ml IL-15 and 33.3 µg/ml IL-15Rα-Fc in PBS for 30 min at 37°C before injection. Mice were injected i.p. with 0.6 µg IL-15 and 3 µg IL-15Rα-Fc in 200 µl PBS every other day for a total of three injections. This dose of IL-15–IL-15Rα-Fc was chosen based on previous studies (Rubinstein et al., 2006; Shifrin et al., 2016). After IL-15–IL-15Rα-Fc complex treatment, splenic CD4+ T cells were gated as CD45+ CD19− NK1.1− CD1d-Tet− CD3+ CD8− CD4+ to exclude NKT cells because NKT cells have been shown to robustly expand in response to IL-15–IL-15Rα-Fc complex (Stoklasek et al., 2006; Guo et al., 2015) and because MHC-I down-regulation was inefficient in CD4+ NKT cells in both PBS-treated and anti-NK1.1–treated groups in these experiments (data not shown).
Mixed BM chimeras
B6-Ly5.1 mice were irradiated with 950 rad by either an x-ray or gamma irradiator. Irradiated mice were injected i.v. with mixtures of BM from WT B6-Ly5.1 (CD45.1) and B2mfl/fl R26-Cre-ERT2 (CD45.2) mice. BM mixtures contained 0.5, 1, 5, 25, and 50% BM from B2mfl/fl R26-Cre-ERT2 mice. Chimeras were given antibiotic (sulfamethoxazole/trimethoprim) water for ∼1 mo after irradiation. Experiments on mixed BM chimeras started 18–21 wk after reconstitution. Blood was collected weekly for 4 wk to assess chimerism, but data are shown only for the final day (day 28) for simplicity. The percentage of B2mfl/fl R26-Cre-ERT2 (CD45.2) cells remaining on day 28 is calculated as %Remaining = [(%CD45.2+/%CD45.1+)Day28/(%CD45.2+/%CD45.1+)Day0] × 100. Chimerism was assessed within Live CD45.1 and/or CD45.2+ lymphocytes.
Statistics
Statistical analysis was performed using Prism 7 (GraphPad). P values were calculated using one-way ANOVA with Bonferroni’s multiple comparisons test, two-way ANOVA with Bonferroni’s multiple comparisons test, or unpaired t test. Asterisks indicate statistical significance as follows: ****, P < 0.0001; ***, P < 0.001; **, P < 0.01; *, P < 0.05; ns, not significant.
Online supplemental material
Fig. S1 shows genetic deletion with PCR within the B2m gene in B2mΔ/Δ and tamoxifen-treated B2mfl/fl R26-Cre-ERT2 mice. Fig. S2 shows NK cell number, maturation, and receptor repertoire in B2mfl/fl R26-Cre-ERT2 mice treated with vehicle or tamoxifen. Fig. S3 shows NK cell number and maturation in B2mfl/fl CD4-Cre-ERT2 mice treated with vehicle or tamoxifen. Fig. S4 shows that poly(I:C) enhances NK cell killing of transferred B2mΔ/Δ splenocytes and enhances granzyme B expression in NK cells.
Supplementary Material
Acknowledgments
We thank J. Michael White (Transgenic, Knockout, and Micro-Injection Core at Washington University) for blastocyst injections of B2mtm1a ES cells and Andrea Lin for technical assistance.
This work was supported by National Institutes of Health grants R01AI129545 and R01AI131680 (to W.M. Yokoyama) and F30DK112466 (to M.D. Bern).
The authors declare no competing financial interests.
Author contributions: M.D. Bern and W.M. Yokoyama designed the research. M.D. Bern, B.A. Parikh., L. Yang, D.L. Beckman, and J. Poursine-Laurent performed the experiments. M.D. Bern and W.M. Yokoyama analyzed the data and wrote the paper.
References
- Aghajani K., Keerthivasan S., Yu Y., and Gounari F.. 2012. Generation of CD4CreER(T2) transgenic mice to study development of peripheral CD4-T-cells. Genesis. 50:908–913. 10.1002/dvg.22052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akazawa T., Ebihara T., Okuno M., Okuda Y., Shingai M., Tsujimura K., Takahashi T., Ikawa M., Okabe M., Inoue N., et al. 2007. Antitumor NK activation induced by the Toll-like receptor 3-TICAM-1 (TRIF) pathway in myeloid dendritic cells. Proc. Natl. Acad. Sci. USA. 104:252–257. 10.1073/pnas.0605978104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastassiadis K., Glaser S., Kranz A., Berhardt K., and Stewart A.F.. 2010. A practical summary of site-specific recombination, conditional mutagenesis, and tamoxifen induction of CreERT2. Methods Enzymol. 477:109–123. 10.1016/S0076-6879(10)77007-5 [DOI] [PubMed] [Google Scholar]
- Apasov S., and Sitkovsky M.. 1993. Highly lytic CD8+, alpha beta T-cell receptor cytotoxic T cells with major histocompatibility complex (MHC) class I antigen-directed cytotoxicity in beta 2-microglobulin, MHC class I-deficient mice. Proc. Natl. Acad. Sci. USA. 90:2837–2841. 10.1073/pnas.90.7.2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardolino M., Azimi C.S., Iannello A., Trevino T.N., Horan L., Zhang L., Deng W., Ring A.M., Fischer S., Garcia K.C., and Raulet D.H.. 2014. Cytokine therapy reverses NK cell anergy in MHC-deficient tumors. J. Clin. Invest. 124:4781–4794. 10.1172/JCI74337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bern M.D., Beckman D.L., Ebihara T., Taffner S.M., Poursine-Laurent J., White J.M., and Yokoyama W.M.. 2017. Immunoreceptor tyrosine-based inhibitory motif-dependent functions of an MHC class I-specific NK cell receptor. Proc. Natl. Acad. Sci. USA. 114:E8440–E8447. 10.1073/pnas.1713064114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bix M., Liao N.S., Zijlstra M., Loring J., Jaenisch R., and Raulet D.. 1991. Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature. 349:329–331. 10.1038/349329a0 [DOI] [PubMed] [Google Scholar]
- Brown M.G., Dokun A.O., Heusel J.W., Smith H.R., Beckman D.L., Blattenberger E.A., Dubbelde C.E., Stone L.R., Scalzo A.A., and Yokoyama W.M.. 2001. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science. 292:934–937. 10.1126/science.1060042 [DOI] [PubMed] [Google Scholar]
- Bubić I., Wagner M., Krmpotić A., Saulig T., Kim S., Yokoyama W.M., Jonjić S., and Koszinowski U.H.. 2004. Gain of virulence caused by loss of a gene in murine cytomegalovirus. J. Virol. 78:7536–7544. 10.1128/JVI.78.14.7536-7544.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsten M., Korde N., Kotecha R., Reger R., Bor S., Kazandjian D., Landgren O., and Childs R.W.. 2016. Checkpoint Inhibition of KIR2D with the Monoclonal Antibody IPH2101 Induces Contraction and Hyporesponsiveness of NK Cells in Patients with Myeloma. Clin. Cancer Res. 22:5211–5222. 10.1158/1078-0432.CCR-16-1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T.P., Valentine M.C., Gao J., Pingel J.T., and Yokoyama W.M.. 2010. Stability of murine cytomegalovirus genome after in vitro and in vivo passage. J. Virol. 84:2623–2628. 10.1128/JVI.02142-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudkowicz G., and Bennett M.. 1971. Peculiar immunobiology of bone marrow allografts. II. Rejection of parental grafts by resistant F 1 hybrid mice. J. Exp. Med. 134:1513–1528. 10.1084/jem.134.6.1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher M., and Rezvani K.. 2018. Next generation natural killer cells for cancer immunotherapy: the promise of genetic engineering. Curr. Opin. Immunol. 51:146–153. 10.1016/j.coi.2018.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djeu J.Y., Heinbaugh J.A., Holden H.T., and Herberman R.B.. 1979. Augmentation of mouse natural killer cell activity by interferon and interferon inducers. J. Immunol. 122:175–181. [PubMed] [Google Scholar]
- Ebihara T., Jonsson A.H., and Yokoyama W.M.. 2013. Natural killer cell licensing in mice with inducible expression of MHC class I. Proc. Natl. Acad. Sci. USA. 110:E4232–E4237. 10.1073/pnas.1318255110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J.M., Wahle J.A., and Yokoyama W.M.. 2010. MHC class I-deficient natural killer cells acquire a licensed phenotype after transfer into an MHC class I-sufficient environment. J. Exp. Med. 207:2073–2079. 10.1084/jem.20100986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger T.A., Cai S.F., Cao X., Bredemeyer A.J., Presti R.M., French A.R., and Ley T.J.. 2007. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity. 26:798–811. 10.1016/j.immuni.2007.04.010 [DOI] [PubMed] [Google Scholar]
- Fernandez N.C., Treiner E., Vance R.E., Jamieson A.M., Lemieux S., and Raulet D.H.. 2005. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 105:4416–4423. 10.1182/blood-2004-08-3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glas R., Ohlén C., Höglund P., and Kärre K.. 1994. The CD8+ T cell repertoire in beta 2-microglobulin-deficient mice is biased towards reactivity against self-major histocompatibility class I. J. Exp. Med. 179:661–672. 10.1084/jem.179.2.661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm E.A., Mazumder A., Zhang H.Z., and Rosenberg S.A.. 1982. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J. Exp. Med. 155:1823–1841. 10.1084/jem.155.6.1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Luan L., Rabacal W., Bohannon J.K., Fensterheim B.A., Hernandez A., and Sherwood E.R.. 2015. IL-15 Superagonist-Mediated Immunotoxicity: Role of NK Cells and IFN-γ. J. Immunol. 195:2353–2364. 10.4049/jimmunol.1500300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglund P., Ohlén C., Carbone E., Franksson L., Ljunggren H.G., Latour A., Koller B., and Kärre K.. 1991. Recognition of beta 2-microglobulin-negative (beta 2m-) T-cell blasts by natural killer cells from normal but not from beta 2m- mice: nonresponsiveness controlled by beta 2m- bone marrow in chimeric mice. Proc. Natl. Acad. Sci. USA. 88:10332–10336. 10.1073/pnas.88.22.10332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis V., Zimmer J., Beermann F., and Held W.. 2001. Cre recombinase-mediated inactivation of H-2Dd transgene expression: evidence for partial missing self-recognition by Ly49A NK cells. J. Immunol. 167:6256–6262. 10.4049/jimmunol.167.11.6256 [DOI] [PubMed] [Google Scholar]
- Johansson M.H., Bieberich C., Jay G., Kärre K., and Höglund P.. 1997. Natural killer cell tolerance in mice with mosaic expression of major histocompatibility complex class I transgene. J. Exp. Med. 186:353–364. 10.1084/jem.186.3.353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joncker N.T., Shifrin N., Delebecque F., and Raulet D.H.. 2010. Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment. J. Exp. Med. 207:2065–2072. 10.1084/jem.20100570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson A.H., and Yokoyama W.M.. 2010. Assessing licensing of NK cells. Methods Mol. Biol. 612:39–49. 10.1007/978-1-60761-362-6_4 [DOI] [PubMed] [Google Scholar]
- Karlhofer F.M., Ribaudo R.K., and Yokoyama W.M.. 1992. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 358:66–70. 10.1038/358066a0 [DOI] [PubMed] [Google Scholar]
- Kärre K., Ljunggren H.G., Piontek G., and Kiessling R.. 1986. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 319:675–678. 10.1038/319675a0 [DOI] [PubMed] [Google Scholar]
- Kim S., Poursine-Laurent J., Truscott S.M., Lybarger L., Song Y.J., Yang L., French A.R., Sunwoo J.B., Lemieux S., Hansen T.H., and Yokoyama W.M.. 2005. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 436:709–713. 10.1038/nature03847 [DOI] [PubMed] [Google Scholar]
- Koh C.Y., Welniak L.A., and Murphy W.J.. 2005. Lack of correlation between an assay used to determine early marrow allograft rejection and long-term chimerism after murine allogeneic bone marrow transplantation: effects of marrow dose. Biol. Blood Marrow Transplant. 11:252–259. 10.1016/j.bbmt.2005.01.006 [DOI] [PubMed] [Google Scholar]
- Koller B.H., Marrack P., Kappler J.W., and Smithies O.. 1990. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science. 248:1227–1230. 10.1126/science.2112266 [DOI] [PubMed] [Google Scholar]
- Korde N., Carlsten M., Lee M.J., Minter A., Tan E., Kwok M., Manasanch E., Bhutani M., Tageja N., Roschewski M., et al. 2014. A phase II trial of pan-KIR2D blockade with IPH2101 in smoldering multiple myeloma. Haematologica. 99:e81–e83. 10.3324/haematol.2013.103085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug A., French A.R., Barchet W., Fischer J.A.A., Dzionek A., Pingel J.T., Orihuela M.M., Akira S., Yokoyama W.M., and Colonna M.. 2004. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 21:107–119. 10.1016/j.immuni.2004.06.007 [DOI] [PubMed] [Google Scholar]
- Kurts C., Sutherland R.M., Davey G., Li M., Lew A.M., Blanas E., Carbone F.R., Miller J.F., and Heath W.R.. 1999. CD8 T cell ignorance or tolerance to islet antigens depends on antigen dose. Proc. Natl. Acad. Sci. USA. 96:12703–12707. 10.1073/pnas.96.22.12703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao N.S., Bix M., Zijlstra M., Jaenisch R., and Raulet D.. 1991. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science. 253:199–202. 10.1126/science.1853205 [DOI] [PubMed] [Google Scholar]
- Ljunggren H.G., and Kärre K.. 1990. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol. Today. 11:237–244. 10.1016/0167-5699(90)90097-S [DOI] [PubMed] [Google Scholar]
- Long E.O., Kim H.S., Liu D., Peterson M.E., and Rajagopalan S.. 2013. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu. Rev. Immunol. 31:227–258. 10.1146/annurev-immunol-020711-075005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Ikizawa K., Hu D., Werneck M.B., Wucherpfennig K.W., and Cantor H.. 2007. Regulation of activated CD4+ T cells by NK cells via the Qa-1-NKG2A inhibitory pathway. Immunity. 26:593–604. 10.1016/j.immuni.2007.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M., Schachterle W., Oberle K., Aichele P., and Diefenbach A.. 2007. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 26:503–517. 10.1016/j.immuni.2007.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney S., Vermi W., Gilfillan S., Cella M., Murphy T.L., Schreiber R.D., Murphy K.M., and Colonna M.. 2009. Distinct and complementary functions of MDA5 and TLR3 in poly(I:C)-mediated activation of mouse NK cells. J. Exp. Med. 206:2967–2976. 10.1084/jem.20091181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy W.J., Kumar V., and Bennett M.. 1987. Acute rejection of murine bone marrow allografts by natural killer cells and T cells. Differences in kinetics and target antigens recognized. J. Exp. Med. 166:1499–1509. 10.1084/jem.166.5.1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öberg L., Johansson S., Michaëlsson J., Tomasello E., Vivier E., Kärre K., and Höglund P.. 2004. Loss or mismatch of MHC class I is sufficient to trigger NK cell-mediated rejection of resting lymphocytes in vivo - role of KARAP/DAP12-dependent and -independent pathways. Eur. J. Immunol. 34:1646–1653. 10.1002/eji.200424913 [DOI] [PubMed] [Google Scholar]
- Ohashi P.S., Oehen S., Buerki K., Pircher H., Ohashi C.T., Odermatt B., Malissen B., Zinkernagel R.M., and Hengartner H.. 1991. Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 65:305–317. 10.1016/0092-8674(91)90164-T [DOI] [PubMed] [Google Scholar]
- Oldstone M.B., Nerenberg M., Southern P., Price J., and Lewicki H.. 1991. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 65:319–331. 10.1016/0092-8674(91)90165-U [DOI] [PubMed] [Google Scholar]
- Orr M.T., and Lanier L.L.. 2010. Natural killer cell education and tolerance. Cell. 142:847–856. 10.1016/j.cell.2010.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh B.A., Piersma S.J., Pak-Wittel M.A., Yang L., Schreiber R.D., and Yokoyama W.M.. 2015. Dual Requirement of Cytokine and Activation Receptor Triggering for Cytotoxic Control of Murine Cytomegalovirus by NK Cells. PLoS Pathog. 11:e1005323 10.1371/journal.ppat.1005323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romee R., Cooley S., Berrien-Elliott M.M., Westervelt P., Verneris M.R., Wagner J.E., Weisdorf D.J., Blazar B.R., Ustun C., DeFor T.E., et al. 2018. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood. 131:2515–2527. 10.1182/blood-2017-12-823757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein M., Yron I., Kaufmann Y., and Rosenberg S.A.. 1984. Lymphokine-activated killer cells: lysis of fresh syngeneic natural killer-resistant murine tumor cells by lymphocytes cultured in interleukin 2. Cancer Res. 44:1946–1953. [PubMed] [Google Scholar]
- Rubinstein M.P., Kovar M., Purton J.F., Cho J.H., Boyman O., Surh C.D., and Sprent J.. 2006. Converting IL-15 to a superagonist by binding to soluble IL-15Ralpha. Proc. Natl. Acad. Sci. USA. 103:9166–9171. 10.1073/pnas.0600240103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo M., Diehl A.D., Olsson-Alheim M.Y., Sundbäck J., Van Kaer L., Kärre K., and Ljunggren H.G.. 1997. Altered expression of Ly49 inhibitory receptors on natural killer cells from MHC class I-deficient mice. J. Immunol. 158:3174–3180. [PubMed] [Google Scholar]
- Schietinger A., and Greenberg P.D.. 2014. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 35:51–60. 10.1016/j.it.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk F., Baron U., and Rajewsky K.. 1995. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 23:5080–5081. 10.1093/nar/23.24.5080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifrin N.T., Kissiov D.U., Ardolino M., Joncker N.T., and Raulet D.H.. 2016. Differential Role of Hematopoietic and Nonhematopoietic Cell Types in the Regulation of NK Cell Tolerance and Responsiveness. J. Immunol. 197:4127–4136. 10.4049/jimmunol.1402447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoklasek T.A., Schluns K.S., and Lefrançois L.. 2006. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J. Immunol. 177:6072–6080. 10.4049/jimmunol.177.9.6072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.C., and Lanier L.L.. 2008. Cutting edge: viral infection breaks NK cell tolerance to “missing self”. J. Immunol. 181:7453–7457. 10.4049/jimmunol.181.11.7453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann J.B., Hayakawa Y., Zerafa N., Sheehan K.C., Scott B., Schreiber R.D., Hertzog P., and Smyth M.J.. 2007. Type I IFN contributes to NK cell homeostasis, activation, and antitumor function. J. Immunol. 178:7540–7549. 10.4049/jimmunol.178.12.7540 [DOI] [PubMed] [Google Scholar]
- Vahlne G., Lindholm K., Meier A., Wickström S., Lakshmikanth T., Brennan F., Wilken M., Nielsen R., Romagné F., Wagtmann N.R., et al. 2010. In vivo tumor cell rejection induced by NK cell inhibitory receptor blockade: maintained tolerance to normal cells even in the presence of IL-2. Eur. J. Immunol. 40:813–823. 10.1002/eji.200939755 [DOI] [PubMed] [Google Scholar]
- Valiante N.M., Uhrberg M., Shilling H.G., Lienert-Weidenbach K., Arnett K.L., D’Andrea A., Phillips J.H., Lanier L.L., and Parham P.. 1997. Functionally and structurally distinct NK cell receptor repertoires in the peripheral blood of two human donors. Immunity. 7:739–751. 10.1016/S1074-7613(00)80393-3 [DOI] [PubMed] [Google Scholar]
- Waggoner S.N., Cornberg M., Selin L.K., and Welsh R.M.. 2012. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 481:394–398. 10.1038/nature10624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry E.J., and Kurachi M.. 2015. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15:486–499. 10.1038/nri3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M.F., and Raulet D.H.. 1997. Class I-deficient hemopoietic cells and nonhemopoietic cells dominantly induce unresponsiveness of natural killer cells to class I-deficient bone marrow cell grafts. J. Immunol. 158:1628–1633. [PubMed] [Google Scholar]
- Xing Y., and Hogquist K.A.. 2012. T-cell tolerance: central and peripheral. Cold Spring Harb. Perspect. Biol. 4:a006957 10.1101/cshperspect.a006957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama W.M., and Kim S.. 2006. How do natural killer cells find self to achieve tolerance? Immunity. 24:249–257. 10.1016/j.immuni.2006.03.006 [DOI] [PubMed] [Google Scholar]
- Zijlstra M., Bix M., Simister N.E., Loring J.M., Raulet D.H., and Jaenisch R.. 1990. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 344:742–746. 10.1038/344742a0 [DOI] [PubMed] [Google Scholar]
- Zijlstra M., Auchincloss H. Jr., Loring J.M., Chase C.M., Russell P.S., and Jaenisch R.. 1992. Skin graft rejection by beta 2-microglobulin-deficient mice. J. Exp. Med. 175:885–893. 10.1084/jem.175.4.885 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.