The value and social challenges of vaccination.
Abstract
Vaccines have made a key, cost-effective contribution to the prolongation of life expectancy and quality. Here we summarize challenges facing vaccinology and immunology at the level of society, scientific innovation, and technology in a global health perspective. We argue that vaccines represent a safety belt and life insurance for humankind.
“…but there was as yet no cause for the sort of alarm that had been displayed by parents, ‘justifiably enough,’ twenty-eight years earlier, during the largest outbreak of the disease ever reported—the 1916 polio epidemic in the northeastern United States, when there had been more than 27,000 cases, with 6,000 deaths. In Newark there had been 1,360 cases and 363 deaths. Now even in a year with an average number of cases…” (Roth, 2010).
Introduction
Nemesis by Philip Roth provides a vivid account of the drama of poliomyelitis in western countries in the 20th century and, more in general, of the drama of infectious diseases in a prevaccine world. In a span of less than 100 years, humankind has experienced an unprecedented increase in life expectancy and quality of life. Philip Roth’s Nemesis is not part of the human experience anymore, and polio is potentially amenable to eradication thanks to vaccines (Rappuoli, 2013; Plotkin, 2014). In the developed world, life expectancy has increased from an average of 40 yr to over 80 yr, and remarkable progress has been made in the developing world as well. Vaccines have played a major role in this dramatic improvement, which is unprecedented in the history of humankind. Vaccines are the most effective health intervention, and it has been estimated that they will save ~25 million deaths over 10 yr from 2010 to 2020, which is equivalent to five lives saved per minute. In terms of cost-effectiveness, it is estimated that $1 invested in vaccination results in a $10–44 healthcare saving (Ozawa et al., 2016). In spite of the success of vaccination in preventing disease and its cost-effectiveness, vaccines are faced with growing opposition in developing countries. Here we will discuss reasons for the “vaccine paradox,” i.e., unprecedented benefit faced with increasing opposition and skepticism. Advances in technology and scientific challenges will be outlined. In addition, advances in vaccinology and immunology will be placed in a global health perspective. It is our tenet that vaccines represent a safety belt and an insurance for mankind.
The vaccine paradox
A discussion of the cognitive and social aspects which underlie the increasing popularity of anti-vaccine attitudes is beyond our competence and the scope of this essay. There are several reasons for the spread of vaccine skepticism. Vaccines are victims of their own success in that frightening infectious diseases such as Nemesis’s polio are no longer part of the human experience in the developed world. Fake news such as the connection between vaccination and autism are fostered by social media and enjoy credibility from lay people to parliament and government levels. A widespread belief that “natural” is good has led to the misconception that diseases such as measles are a sort of workout for the immune system, while the fact that pathogens are professionals at suppressing immunity is widely unappreciated. Finally, there is no perception of the fundamental concept that although we are immunologically unique as individuals, we are a community in terms of resistance to infectious agents (herd immunity; Fine et al., 2011). Given this general framework, we argue that it is our social responsibility as a scientific community to stand and promote understanding of the value of vaccination. It is refreshing in this perspective that national and international immunological societies (International Union of Immunological Societies) have engaged their members in educational activities with the lay public and have taken firm stands on policy issues (Mantovani and Santoni, 2018).
Harnessing innate immunity in vaccinology
Immunological memory has long been perceived as a distinguishing feature of adaptive versus innate immunity. This clear-cut distinction is now questioned at the level of lymphoid as well as myeloid cell–mediated innate immunity. Natural killer cells, the prototype of innate lymphoid cells, are imprinted by encounters with viruses such as cytomegalovirus, and memory natural killer cells are better effectors of antiviral resistance (Goodier et al., 2018). An adaptive component has also long been recognized as a part of the macrophage-mediated response to pathogens (Bowdish et al., 2007; Netea et al., 2016) and a more long-term shaping of the response of myeloid cells to microbial moieties has been described and variably referred to as memory, adaptive-innate, or trained immunity (Bowdish et al., 2007; Netea et al., 2016). Interestingly, the observation of increased resistance against unrelated pathogens following Bacillus Calmette Guérin vaccination has been a major issue in support of the concept of trained innate immunity. Next generation adjuvants should consider harnessing the power of the adaptive potential of lymphoid and myeloid cell–mediated innate immunity (Haks et al., 2017).
Global health
Vaccines are a cornerstone in the long and difficult construction of better global health. Here we will exemplify this general statement with examples related to child mortality, gender health inequality, and Mycobacterium tuberculosis as a paradigm of a global killer.
Approximately 1.5 million children die every year because they do not have access to conventional vaccines (Clemens et al., 2010; Mantovani and Santoni, 2018). Only 1 child out of 20 has access to all 11 World Health Organization–recommended vaccines (tetanus, pertussis, diphtheria, polio, measles, rubella, pneumococcus, rotavirus, Haemophilus influenzae type B, and hepatitis B), and almost 20 million children fail to get a full course of basic vaccines. To tackle this dismal situation, in 2000, a private-public initiative known as the Global Alliance for Vaccines and Immunizations (or Gavi, the Vaccine Alliance) was launched. Gavi’s mission was and still is to promote equal access to vaccines for people in the world’s poorest countries and to cut the lag time between the introduction of a new vaccine in the developed and in the underdeveloped world. A discussion of Gavi’s composition, long-term goals, strategies, and vaccine portfolio is beyond the scope of this essay (Clemens et al., 2010). Suffice it to say that Gavi is estimated to have averted over 7 million deaths and that an unprecedented number of children were immunized with the diphtheria-tetanus-pertussis vaccine in 2016. Further progress will need to address future challenges which range from “running the last mile” to reach the most remote village to the integration of the opportunities offered by digital technologies (Berkley, 2017).
The human papilloma virus (HPV) vaccine is now part of the Gavi armamentarium (Mantovani and Santoni, 2018). HPV has a death toll of over 200,000 women per year, mostly among the underprivileged in developed countries and in the developing world. Sharing HPV vaccine therefore offers an unprecedented opportunity to improve global female health. This global vision of immunity against cancer is even more relevant at a time of split perspectives. On the one hand, immunotherapy has been recently revisited as an important treatment modality for cancer therapy. On the other hand, underdeveloped countries are faced with the looming perspective of a dramatic increase in cancer incidence, a “forgotten epidemic” (Graff, 2017).
Vaccine improvement or generation for diseases such as HIV, tuberculosis, malaria, dengue, and influenza is a global health challenge. These “big five” claim a toll of ~3.5 million deaths per year. M. tuberculosis alone infects over one fourth of the human population and causes over 1 million deaths per year (Kaufmann, 2013). Unless a better vaccine is developed, it is unlikely that a substantial reduction of the tuberculosis disease burden will be obtained. Recent Phase 2b data suggest that a vaccine composed of the mycobacterial fusion protein M72 and the AS01 adjuvant can be protective and hopefully will represent a turning point in the fight against tuberculosis (Van Der Meeren et al., 2018).
Back to the future
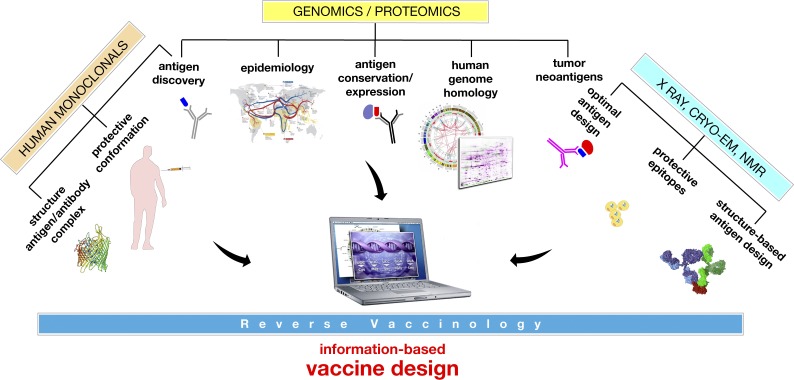
Since Edward Jenner developed the first vaccine against smallpox by taking the poxvirus from the pustules of cows, progress in conquering new diseases by vaccination happened when new technologies allowed the growth of new bacteria and viruses that could then be killed or attenuated and used as vaccines. During the last 40 years, a series of Nobel prize–winning discoveries in the fields of molecular biology, genomics, innate immunity, structural biology, and immunotherapy transformed vaccinology into a sophisticated multidisciplinary science which allowed the molecular design of new vaccines and enabled the licensure of vaccines that were technically impossible a few decades ago. Fig. 1 shows how the information coming from genomics, immunology, and structural biology can be integrated into modern vaccine design. Today, although we are still struggling to develop vaccines against some infectious diseases such as HIV, universal influenza, and chronic infections, we have technical solutions for most of the other diseases, and it is just remarkable how very recently adjuvants that integrate liposome delivery, toll-like receptor stimulation, and saponins enabled the development of a vaccine against herpes zoster that works in 90-yr-old people (Cunningham et al., 2016) and against tuberculosis (Van Der Meeren et al., 2018). This opens the field for the development of vaccines that can prolong healthy life in a society that is facing the problem of an aging population and for the conquest of a disease such as tuberculosis that infects one third of the human population, kills 1.7 million people annually, and represents the major burden in antimicrobial resistance. The pace of innovation in vaccinology continues, and vaccinology, fueled by the discovery of tumor neoantigens, checkpoint inhibitors, and the emerging evidence that latent viruses may be major contributors to neurodegenerative diseases, may soon contribute to the prevention and therapy of cancer and diseases such as Alzheimer’s and other neurodegenerative diseases.
Figure 1.
The design of new vaccines at the beginning of the third millennium. The design of innovative vaccines requires multidisciplinary approaches at the intersection of diverse disciplines such as epidemiology, immunology, genomics, and structural biology. NMR, nuclear magnetic resonance; Cryo-EM, Cryo-Electron Microscopy.
In conclusion, vaccines represent a land of opportunity for research in fundamental immunology, vaccinology sensu stricto, and social sciences. The latter is imperative to counter the spreading of anti-science attitudes, of which “no-vax” is the vanguard. Addressing the challenges and taking the opportunities briefly summarized here at the level of civil society and research, in a global health perspective, is imperative to fulfill the reality and potential of vaccines to serve as a safety belt and insurance policy for humankind at present and in the future.
Acknowledgments
A. Mantovani and R. Rappuoli were supported by the European Commission (FP7-HEALTH-2011-ADITEC-N°280873). A. Mantovani was supported by Fondazione Cariplo (2015-0564).
A. Mantovani has served and A. Santoni serves on the Board of Gavi, the Vaccine Alliance.
References
- Berkley S. 2017. Nature. 551:273 10.1038/d41586-017-05923-8 [DOI] [PubMed] [Google Scholar]
- Bowdish D.M., et al. 2007. Microbes Infect. 9:1680–1687. 10.1016/j.micinf.2007.09.002 [DOI] [PubMed] [Google Scholar]
- Clemens J., et al. 2010. Nat. Immunol. 11:1069–1072. 10.1038/ni1210-1069 [DOI] [PubMed] [Google Scholar]
- Cunningham A.L., et al. 2016. N. Engl. J. Med. 375:1019–1032. 10.1056/NEJMoa1603800 [DOI] [PubMed] [Google Scholar]
- Fine P., et al. 2011. Clin. Infect. Dis. 52:911–916. 10.1093/cid/cir007 [DOI] [PubMed] [Google Scholar]
- Goodier M.R., et al. 2018. Eur. J. Immunol. 48:50–65. 10.1002/eji.201646762 [DOI] [PubMed] [Google Scholar]
- Graff S. 2017. J. Natl. Cancer Inst. 109:djx169 10.1093/jnci/djx169 [DOI] [Google Scholar]
- Haks M.C., et al. 2017. Front. Immunol. 8:1563 10.3389/fimmu.2017.01563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S.H. 2013. Semin. Immunol. 25:172–181. 10.1016/j.smim.2013.04.006 [DOI] [PubMed] [Google Scholar]
- Mantovani A., and Santoni A.. 2018. Eur. J. Immunol. 48:12–14. 10.1002/eji.201870016 [DOI] [Google Scholar]
- Netea M.G., et al. 2016. Semin. Immunol. 28:317–318. 10.1016/j.smim.2016.07.002 [DOI] [PubMed] [Google Scholar]
- Ozawa S., et al. 2016. Health Aff. (Millwood). 35:199–207. 10.1377/hlthaff.2015.1086 [DOI] [PubMed] [Google Scholar]
- Plotkin S. 2014. Proc. Natl. Acad. Sci. USA. 111:12283–12287. 10.1073/pnas.1400472111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappuoli R. 2013. Semin. Immunol. 25:87–88. 10.1016/j.smim.2013.06.002 [DOI] [PubMed] [Google Scholar]
- Roth P. 2010. Nemesis. Houghton Mifflin Harcourt, Boston. [Google Scholar]
- Van Der Meeren O., et al. 2018. N. Engl. J. Med. 379:1621–1634. 10.1056/NEJMoa1803484 [DOI] [PMC free article] [PubMed] [Google Scholar]