A role for the gut microbiome in facilitating microglial maturation and shaping microglial physiology has emerged in recent years. This review highlights evidence demonstrating the various mechanisms by which the gut microbiota can influence microglia in both homeostatic and disease conditions.
Abstract
Microglia, the resident immune cells in the brain, are essential for modulating neurogenesis, influencing synaptic remodeling, and regulating neuroinflammation by surveying the brain microenvironment. Microglial dysfunction has been implicated in the onset and progression of several neurodevelopmental and neurodegenerative diseases; however, the multitude of factors and signals influencing microglial activity have not been fully elucidated. Microglia not only respond to local signals within the brain but also receive input from the periphery, including the gastrointestinal (GI) tract. Recent preclinical findings suggest that the gut microbiome plays a pivotal role in regulating microglial maturation and function, and altered microbial community composition has been reported in neurological disorders with known microglial involvement in humans. Collectively, these findings suggest that bidirectional crosstalk between the gut and the brain may influence disease pathogenesis. Herein, we discuss recent studies showing a role for the gut microbiome in modulating microglial development and function in homeostatic and disease conditions and highlight possible future research to develop novel microbial treatments for disorders of the brain.
Introduction
Microglia are tissue-resident macrophages that make up ∼5–15% of total brain cells and have several well-defined functions in the central nervous system (CNS; Pelvig et al., 2008). During early development, microglia actively regulate neuronal cell numbers and synaptic refinement, ultimately shaping neural circuitry (Sierra et al., 2010; Paolicelli et al., 2011; Wynn et al., 2013). To sustain brain homeostasis, microglia constantly survey their microenvironment through the dynamic extension and retraction of their processes (Davalos et al., 2005; Nimmerjahn et al., 2005). Upon sensing signals of infection or injury, microglia transition from a homeostatic surveillance state to an activated state, facilitating antimicrobial or tissue repair programs that restore homeostasis (Saijo and Glass, 2011).
In addition to important roles in brain development and homeostasis, recent genetic studies provide evidence that microglia contribute to the pathogenesis of several neurodegenerative and neurodevelopmental disorders (Salter and Stevens, 2017). However, environmental factors and mechanisms shaping the developmental, homeostatic, and pathogenic program of microglia remain poorly understood. Within the CNS, microglial activity is governed in part by cytokines and chemokines, neurotransmitters, and other molecules that regulate signaling pathways that influence various brain functions (Xavier et al., 2014). Once thought to be shielded from the circulatory system by the blood–brain barrier (BBB), microglial activity is now known to be influenced by factors originating outside the CNS, including the gut. Sophisticated crosstalk between the CNS and the gut microbiome (known as the gut–brain axis) is critical for several facets of CNS physiology, including microglial development and function (Mayer et al., 2015; Fung et al., 2017). Recent studies provide important insights into the role of gut microbiota in microglial maturation, identity, and function, both in steady state conditions and in diseases associated with elevated microglial activation. These findings have sparked a new field in microbiology focused on identifying and mapping direct and indirect interactions between the gut microbiota and microglia.
In this review, we will highlight mechanisms by which the gut–brain axis regulates microglial identity and function during development and aging. We then discuss gut–brain communication pathways and how perturbations in the healthy gut microbiota (i.e., dysbiosis) could potentially lead to microglial dysfunction. Finally, we will highlight possible interactions of the microbiome and microglia in the context of neurodevelopmental and neurodegenerative disorders exemplified by autism spectrum disorder (ASD) and Parkinson’s disease (PD), respectively.
Microglia during development and adulthood
Microglia maturation
Microglia were first discovered in the early 20th century by Pío del Río-Hortega, who pioneered exploration of microglial morphology and function (Sierra et al., 2016). Until recently, the origins and precise lineage of microglia have been subjects of significant debate in the biomedical research community. The analogous function and structure between microglia and macrophages inspired the hypothesis that these cells share a common lineage. However, the advent of new methods to study cellular lineages, including genetic tracing, transgenics, and fate-mapping analyses, defined distinct developmental trajectories and ontogenies between these two cell populations (Ginhoux et al., 2010).
Microglial development is thought to be precisely orchestrated by an intrinsic genetic program and environmental cues (Fig. 1). This process begins in the yolk sac around embryonic day 7.5 (E7.5) as microglia emerge from erythromyeloid progenitor cells, which are hematopoietic precursor cells of the mesoderm (Alliot et al., 1999; Ginhoux et al., 2010; Kierdorf et al., 2013; Gomez Perdiguero et al., 2015; Hoeffel et al., 2015; Sheng et al., 2015). The maturation and differentiation of erythromyeloid progenitors into microglia within the yolk sac requires several transcription factors, including RUNX1, JUN, PU.1, and IRF8, the expression of which coincides with that of microglial markers, including CX3CR1, CD11b, and F4/80 (Ginhoux et al., 2010; Matcovitch-Natan et al., 2016). At E8.5, microglia become mobile and begin to migrate from the embryonic yolk sac to the brain. This process of brain colonization precedes the formation of the BBB, which eventually shields microglia from potentially toxic peripheral influences throughout both fetal development and adulthood (Obermeier et al., 2013). Once in the brain, microglia are broadly distributed at varying densities and maintain a stable rate of proliferation depending on the stage of host development (Askew et al., 2017). The capacity for microglia to self-renew in their local environment, independent of hematopoietic progenitor cells circulating the bloodstream, is a defining feature of these innate immune cells (Gomez Perdiguero et al., 2015).
Figure 1.
Gut microbiota influences microglial development and maturation. (A) Microglial maturation states can be described in three primary phases: early, pre-, and adult microglia. Each phase of development can be defined by expression of a subset of genes that correspond to a core set of microglial functions. Early and premicroglia have two main functions during early brain development: synaptic remodeling and subsequent shaping of neural circuitry and regulating the number of neurons through mechanisms of programmed cell death (PCD). A few weeks after birth, microglia transition to the “adult microglia” stage, in which they constantly survey their immediate surroundings and actively maintain homeostatic conditions. In the presence of tissue damage or an immune stimulus, microglia activate pro- and anti-inflammatory signaling cascades to clear pathogens and repair tissue damage to restore brain health. Recent evidence suggests that prenatal and postnatal inputs from the gut microbiota are critical for microglial maturation and function. (B) In SPF mice, a diverse gut microbiota promotes microglial development and maturation. Microglial development appears arrested in GF mice, as supported by high expression of genes characteristic of early and premicroglia in microglia from adult GF mice. This arrest in microglial maturation impedes their ability to initiate a sufficient immune response during infection. EMP, erythromyeloid progenitor.
Early microglia identity and function
Transcriptomic studies suggest that after populating the brain, microglia undergo a stepwise maturation program in parallel to brain development, from early microglia (until E14) to premicroglia (E14 to the first weeks after birth) and finally adult microglia (Fig. 1; Matcovitch-Natan et al., 2016). Early microglia and premicroglia gene expression signatures are associated with cellular development, growth, and proliferation, whereas genes enriched in adult microglia are associated with immune signaling pathways (Matcovitch-Natan et al., 2016; Thion et al., 2018).
In addition to innate immune cell functions that are characteristic of all tissue-resident macrophages, recent evidence has elucidated additional developmental and homeostatic functions of microglia that are specific to the nervous system. During the early stages of brain development, early microglia and premicroglia phagocytose excess neurons and release neurotrophic and neurotoxic factors, thereby controlling the relative ratio of neurogenesis to apoptosis to ensure that numbers of neurons are maintained within a defined range (Sierra et al., 2010; Cunningham et al., 2013). In addition to dictating neuronal density, microglia supply a steady stream of neurotrophic factors (such as nerve growth factor [NGF], brain-derived neurotrophic factor [BDNF], and insulin-like growth factor 1 [IGF-1]) that promote neuronal survival and differentiation of neural progenitors (Gomes et al., 2013; Ueno et al., 2013).
Microglia also play a role in establishing and shaping neural circuitry during postnatal stages of development, which has implications for cognitive function and social behavior (Paolicelli et al., 2011). Synaptic remodeling resulting in the removal of excess synapses eliminates redundancies in neural circuitry and improves efficiency in neural crosstalk. In a remarkable parallel to macrophage recognition of pathogens, this process has been shown to depend on several complement proteins (C1q and C3) that tag extraneous synapses for microglial engulfment (Stevens et al., 2007; Schafer et al., 2012). Furthermore, high-resolution microscopy has confirmed a physical, albeit transient, interaction between microglia and synapses on neighboring neurons (Tremblay et al., 2010).
Adult microglia identity and function
In mice, microglia transition to an adult phenotype a few weeks after birth. The adult microglia transcriptome overlaps with, but is distinct from, other tissue-resident macrophages and is characterized by expression of microglial-specific markers, including Sall1, P2ry12, Gpr84, and Tmem119 (Hickman et al., 2013; Butovsky et al., 2014; Gosselin et al., 2014; Matcovitch-Natan et al., 2016). While microglia are heavily involved in shaping the neuronal and synaptic landscape during early development, they are more actively involved in homeostasis and immune surveillance during later developmental stages and adulthood, as suggested by up-regulation of genes involved in immune regulation (Matcovitch-Natan et al., 2016). Systematic analyses of human microglial gene expression from postmortem and surgical tissues indicate broad similarities between human and mouse microglial gene expression but also significant differences, particularly with regard to expression of genes associated with the pathogenesis of neurodevelopmental and neurodegenerative diseases (Galatro et al., 2017; Gosselin et al., 2017).
Adult microglia are a morphologically dynamic population of cells; they display a wide spectrum of structural and molecular phenotypes that reflect the status of their extracellular environment at a given time. Depending on the surrounding microenvironment, microglia can exist in a “surveying” or “active” state. Under steady-state conditions, surveying microglia have a ramified morphology with a small cell body and many long extended processes that are used to continuously scan and assess the health of cells in close proximity—a process critical for maintaining homeostasis in the absence of pathology (Nimmerjahn et al., 2005; Torres-Platas et al., 2014). Upon insult to brain tissue, microglia swiftly activate, retracting their processes and transitioning to an amoeboid morphology with an enlarged cell body (Nimmerjahn et al., 2005; Torres-Platas et al., 2014). Depending on the nature of the insult, microglia can initiate pro- or anti-inflammatory signaling cascades. Activation of pro-inflammatory signaling pathways causes microglia to release pro-inflammatory cytokines (e.g., IL-6, IL-12, IL-1β, and TNF-α) and reactive species (e.g., nitric oxide and reactive oxygen species) into their surrounding environment to suppress and fight off invading pathogens (Franco and Fernández-Suárez, 2015; Tang and Le, 2016). Conversely, activation of anti-inflammatory pathways allows microglia to mitigate and repair damage caused by the initial immune stimulus and the pro-inflammatory response. Activation of these pathways triggers release of anti-inflammatory cytokines (e.g., IL-4, IL-10, and TGF-β) and neurotrophic factors that prevent development of chronic inflammation and allow microglia to maintain their neuroprotective and wound-healing properties (Franco and Fernández-Suárez, 2015). Maintaining tight control over microglial activation states is critical for CNS health, given the risk of pathology that is associated with heightened neuroinflammation.
In addition to their innate immune and homeostatic functions, microglia have been implicated in the pathogenesis of a broad spectrum of neurodegenerative and behavioral diseases, including PD, Alzheimer’s disease (AD), multiple sclerosis (MS), schizophrenia, and ASD (Vargas et al., 2005; Hirsch et al., 2012; Frick et al., 2013; Bilimoria and Stevens, 2015). In each case, there is evidence of immune activation, suggesting a role for microglia-driven inflammation as an etiologic factor (Perry and Holmes, 2014; Norden et al., 2015). Of particular interest, recent genetic studies have identified a large number of coding and noncoding risk alleles for neurodegenerative and behavioral diseases that affect genes highly or preferentially expressed in microglia (Lambert et al., 2013; Welter et al., 2014). Thus far, the risk alleles that have been identified are largely nonoverlapping across diseases and affect genes involved in diverse cellular processes, implying complex and poorly understood mechanisms linking microglia to neurodegeneration and behavioral disorders.
Gut–brain axis influences microglia development and function
Until recently, the microbiome did not spark much attention among neuroscientists. However, recent work characterizing the extensive communication between the gut and the brain has demonstrated an active role for gut bacteria in governing several aspects of CNS physiology.
The ∼100 trillion microorganisms that reside in the digestive tract, and the wide assortment of metabolites they produce, are critical for maintaining health (Lloyd-Price et al., 2016). Within this complex community in humans are >1,000 bacterial species (Frank and Pace, 2008). Initial microbial colonization of the gut happens at birth and is heavily influenced by the mode of delivery (cesarean section versus vaginal birth; Dominguez-Bello et al., 2010; Rodríguez et al., 2015). In the first few years of life, the gut microbiota is relatively less diverse and less stable compared with that of adults, with an abundance of Proteobacteria and Actinobacteria (Palmer et al., 2007; Rodríguez et al., 2015). By the age of 5, the gut microbiota stabilizes and begins to resemble that of an adult, with members of Bacteroidetes and Firmicutes becoming most abundant (Eckburg et al., 2005).
Advances in sequencing technologies and bioinformatic tools to study the gut microbiome have contributed to a greater appreciation of its diversity, plasticity, and paramount role in a multitude of physiological functions. Along with preserving the integrity of the intestinal–epithelial barrier along the gastrointestinal (GI) tract, gut bacteria are critical for the development and maturation of the host’s innate and adaptive immune systems, nutrient absorption, host metabolism, and protection against foreign invaders (Hooper et al., 2001; Bäckhed et al., 2004; Geuking et al., 2011; Round et al., 2011). Indeed, the functions of the gut microbiota extend beyond the physical borders of the digestive tract in which they reside. The diverse repertoire of metabolites and signaling molecules produced by gut bacteria enter the systemic circulation, facilitating the molecular crosstalk between host and microbes throughout the body (Martinez et al., 2017).
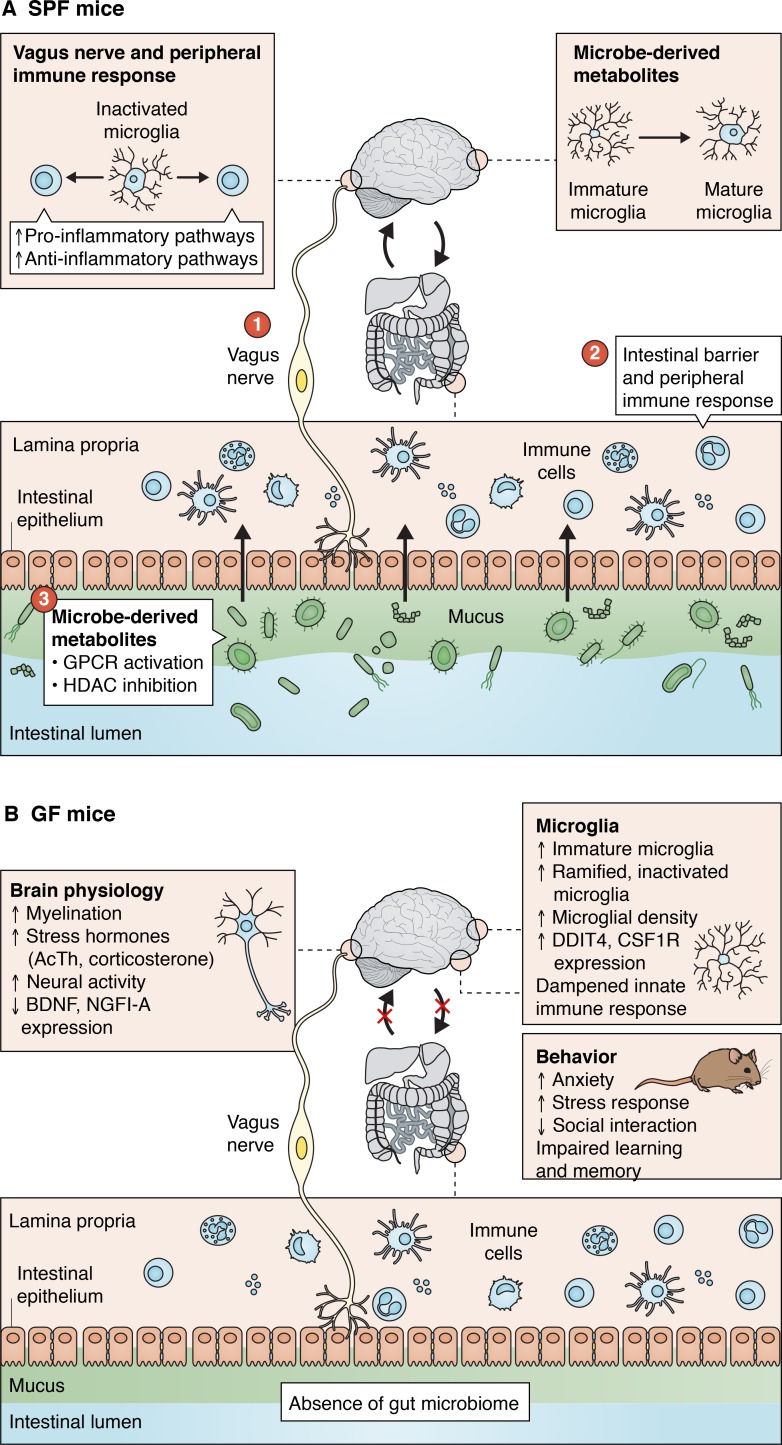
Communication between gut microbes and the CNS is mediated by a combination of immune, enteric, and neural pathways that provide physical and chemical connections between the CNS and the periphery, and several experimental paradigms have been used to demonstrate that gut microbes influence many facets of CNS physiology (Mayer et al., 2015; Fung et al., 2017; Yoo and Mazmanian, 2017). Germ-free (GF; i.e., lacking all commensal and pathogenic microbes) mice, antibiotics, fecal microbiota transplant (FMT), and pre-/probiotics have demonstrated a role for gut bacteria in neurotransmitter signaling, synaptic plasticity, myelination, and neurogenesis (Diaz Heijtz et al., 2011; Neufeld et al., 2011; Ogbonnaya et al., 2015; Hoban et al., 2016). Additionally, the absence of gut microbes causes deficits in cognition and social interaction, further supporting the role of gut microbes in higher-order brain functioning (Neufeld et al., 2011; Luczynski et al., 2016).
The gut microbiota affects various cells in the CNS, including microglia. Indeed, recent studies have demonstrated that microglia are sensitive to factors produced by the gut microbiota. Striking differences in the structure and function of microglia derived from specific pathogen–free (SPF) and GF mice have been observed, both at the genetic and morphological level (Erny et al., 2015). Since then, new work has defined additional factors and pathways by which gut microbes influence microglial maturation and function within the CNS.
Maternal microbiota shapes prenatal microglial maturation and function
While the womb is likely a sterile environment, new findings suggests that signals from maternal gut microbes may shape the developmental trajectory of fetal microglia close to the time of birth (Thion et al., 2018). At E14.5, embryonic microglia from offspring of GF dams display minor differences in gene expression compared with their SPF counterparts, whereas microglia collected close to birth (E18.5) demonstrate marked differences in gene expression, chromatin accessibility, morphology, and regional distribution (Thion et al., 2018). For example, microglia from E18.5 embryos from GF mothers show an increased density in various brain regions, with increased numbers of microglia exhibiting a ramified morphology, indicative of a resting state. Altered microglial morphology and density, as well as attenuated inflammatory responses, are also observed in offspring of GF dams immediately after birth, a time when microglia typically exhibit an activated phenotype (Castillo-Ruiz et al., 2018).
Interestingly, sex-related differences have been observed with regard to the influence of the maternal gut microbiota on microglial function in offspring. In male offspring of GF dams, disruption of the transcriptomic profile and morphology of microglia was greatest during the embryonic phase of development, and differentially regulated genes were mostly associated with metabolic and translational pathways (Thion et al., 2018). In female offspring, by contrast, disruption was most notable in adults, and differentially expressed genes were primarily involved in immune and transcriptional signaling (Thion et al., 2018). This suggests that input of maternal gut microbes may have a greater impact on early microglia and premicroglia in male offspring than in females. These trends might help to explain the inherent functional differences in microglia from intact male versus female mice, as well as the sex variations in incidence rates of several neurological disorders (Schwarz et al., 2012; Hanamsagar et al., 2015). The heterogeneity of microglial behavior in response to both intrinsic and extrinsic factors provides further evidence of their complexity, with the gut microbiome representing a key contributing factor in microglial development and function.
Diverse gut microbiota is a prerequisite for adult microglial maturation and function
Consistent with patterns observed prenatally, microglia from adult GF mice, lacking constant postnatal input from gut microbiota, show distinct differences in density and morphology compared with those derived from SPF mice (Erny et al., 2015). Morphological variations in microglia from adult GF mice include increased cell volume, dendrite length, segment number, and branch points. Microglia from adult GF mice also display differential expression of genes associated with microglial maturation, including down-regulation of genes that regulate cell activation and immune system defense pathways, such as Mapk8, IL-1α, Ly86, Jak3, and Stat1, all of which are normally highly expressed in adult microglia (Fig. 1; Erny et al., 2015; Matcovitch-Natan et al., 2016). Concurrently, genes highly expressed in microglia during early developmental stages that promote cell proliferation and survival, including Csf1r and Ddit4, are aberrantly up-regulated in microglia isolated from adult GF mice.
The immature gene expression profile resulting from the absence of gut microbiota contributes to the inability of microglia to properly respond to immunostimulants such as LPS and the lymphocytic choriomeningitis virus, both of which failed to elicit an appropriate activation response in microglia from GF mice (Erny et al., 2015). The dampened immune response to both LPS and lymphocytic choriomeningitis virus included a relative decrease in microglial expression of genes encoding pro-inflammatory cytokines (e.g., IL-1β, IL-6, and TNF-α) and a reduction in microglia with an activated, amoeboid morphology. These findings suggest that the gut microbiota is likely imperative for an adequate microglia-mediated immune response against pathogens invading the CNS. Taken together, the gene expression profile and behavior of microglia from GF mice or under microbiota-depleted conditions is reminiscent of an immature microglial phenotype, indicating that input from gut microbiota is required for microglia to progress to later stages of cellular maturation and adequately fulfill their role in immune surveillance.
To examine the extent to which microbial colonization influences microglial physiology, Erny et al. (2015) characterized microglia from mice co-colonized with Bacteroides distasonis, Lactobacillus salivarius, and Clostridium cluster XIV. Microglia from mice with such a limited microbial complexity displayed a genetic signature and morphology similar to that observed in microglia from GF mice. However, recolonization of those mice with a more diverse microbial community facilitated the transition to a mature microglial phenotype typically found in adult SPF animals. Thus, the presence of a complex and diverse microbial community, rather than exposure to gut bacteria per se, appears to be a prerequisite for proper microglial development and function.
Investigations into the temporal window for microbe-mediated regulation of microglial maturation has revealed the need for constant input from a diverse gut microbiota. This claim is supported by the conversion of microglia from adult SPF mice into an immature phenotype following antibiotic administration to deplete the microbiota. With the exception of indiscernible differences in microglial density, microglia isolated from antibiotic-treated SPF mice exhibit a microglial gene expression profile and morphology nearly identical to those derived from GF mice (Erny et al., 2015; Thion et al., 2018). These findings suggest that microglia are highly sensitive to perturbations in the gut microbial community during adulthood and require continual input from a complex gut microbiota to maintain homeostasis in the adult.
Gut–brain communication pathways: Vagal transmission and systemic circulation
Despite the many unanswered questions regarding the intersection between gut microbiota and microglial physiology, there is evidence that pathways that collectively integrate the gut–brain axis influence microglial function in both homeostatic and disease conditions (Fig. 2). Gut–brain communication may influence microglia via two routes: the vagus nerve and the circulatory system.
Figure 2.
Gut–brain communication pathways. Communication between the gut microbiota and the CNS encompasses several conduits along neural, enteric, and immune pathways. (A) Proper microglial maturation and behavior is dependent on crosstalk along the gut–brain axis. Information about the state of peripheral inflammation and GI health is received in the CNS via vagal afferents that innervate the GI tract and can influence microglial activation and neuroinflammation. Fine-tuning of the intestinal barrier by gut microbiota and their interactions with gut immune cells modulates peripheral inflammation and can trigger downstream inflammatory responses in the CNS. BBB-permeable bacterial metabolites, including SCFAs, modulate microglial maturation through mechanisms that are yet to be determined. (B) The absence of gut microbes in GF mice confers a variety of physiological abnormalities in neural and microglial behavior in the CNS, resulting in heightened anxiety, stress, hyperactivity, and other behavioral symptoms. BDNF, brain-derived neurotrophic factor; HDAC, histone deacetylase.
The vagus nerve
Thousands of sensory and motor fibers from the vagus nerve connect the gut and the brainstem and serve as a conduit for neural signals. Communication through the vagus nerve is essential for signals mediating satiety, stress, and mood, and these signals are governed by changes in enteric neuron activity and the behavior of gut microbes (Goehler et al., 2005; Forsythe et al., 2014; Browning et al., 2017). Given their close physical proximity, symbiotic and pathogenic gut bacteria can directly interact with and activate the vagus nerve, thereby exerting effects upstream to the CNS. Oral inoculation with the pathogen Campylobacter jejuni or intraduodenal injection of the probiotic strain Lactobacillus johnsonii are sufficient to induce activation of vagal sensory neurons innervating the GI tract, as well as neurons in the brainstem (Goehler et al., 2005; Tanida et al., 2005). Additionally, metabolites and neuroactive substances produced by microbes activate chemoreceptors located at vagal nerve endings (Hara et al., 1999; Raybould, 2010). Indeed, the anxiolytic effects of administration of the probiotic species Lactobacillus rhamnosus and Bifidobacterium longum is absent in vagotomized mice, providing strong evidence that gut–vagal nerve interactions regulate social behavior (Wang et al., 2002; Bercik et al., 2011; Bravo et al., 2011).
Communication between intestinal microbes and vagal afferents also appears to influence microglia and the level of inflammation in the CNS (Forsythe et al., 2014). In addition to interacting with gut microbes, vagal nerves interact extensively with different components of the peripheral immune system, continuously monitoring the inflammatory state of the gut (Borovikova et al., 2000; Miao et al., 2004). Upon sensing a change in inflammation, such as increased production of pro-inflammatory cytokines, vagal afferents relay this information to the CNS and can ultimately influence the level of neuroinflammation (Goehler et al., 1999). Concurrently, vagal efferent nerves relay information about the immune status of the brain back to the gut, with increased CNS inflammation feeding back to inhibit further release of peripheral pro-inflammatory cytokines through acetylcholine-mediated signaling (Wang et al., 2003; Goehler et al., 2005). Effective vagal nerve signaling is critical for sending appropriate signals to microglia in order to modulate levels of neuroinflammation. Electrical stimulation of the vagus nerve in the presence of an immune challenge in the periphery has downstream effects on microglial behavior, including up-regulation of anti-inflammatory pathways in the brain (Frasch et al., 2016; Meneses et al., 2016; Kaczmarczyk et al., 2017). Vagus nerve stimulation combined with LPS challenge has also been reported to decrease microglial production of the pro-inflammatory cytokines IL-6, IL-1β, and TNFα in the brain, an effect no longer observed following vagotomy (Meneses et al., 2016). These findings support the role of the vagus nerve as a physical conduit between gut microbial activity and neuroinflammation.
Regulation of the intestinal barrier and peripheral immune response
The presence of bacteria along the GI tract is critical for the maintenance of the intestinal barrier, which facilitates the exchange of nutrients, water, and electrolytes and prevents the passage of harmful substances and pathogens from the intestinal lumen into the bloodstream (Jakobsson et al., 2015). By altering expression levels of tight junction proteins along the epithelial wall, and thus the level of bacterial infiltration in the mucosal layer, the gut microbiota can fine-tune the level of intestinal permeability (Karczewski et al., 2010; Alaish et al., 2013). The regulation of the intestinal barrier by gut microbiota shapes their role as mediators of the intestinal and peripheral immune response. Decreased strength of the intestinal barrier due to dysbiosis and other factors permits entry of pathogenic, immune-stimulating, and neuroactive substances into the systemic circulation (Kelly et al., 2015). Once in the circulation, these substances activate a pro-inflammatory immune response mediated by peripheral T cells and macrophages and compromise the integrity of the BBB (Rochfort et al., 2014). Increased circulation of BBB-permeable pro-inflammatory cytokines and neurotoxic compounds may contribute to heightened microglial activation and production of pro-inflammatory cytokines in the brain (Qin et al., 2008; Riazi et al., 2008).
Along with affecting the level of permeability along the intestinal tract, gut microbiota can influence the state of peripheral inflammation through interactions with nearby immune cells. Approximately 70–80% of immune cells in the human body are found in the gut, allowing for direct gut–immune cell interactions (Vighi et al., 2008). When microbe-associated molecular patterns produced by pathogenic invaders bind to pattern recognition receptors, such as TLRs, on host cells, they influence the production of both pro- and anti-inflammatory cytokines (Fung et al., 2017). The circulation and subsequent entry of these cytokines into the brain acts locally on CNS cells, including microglia, that express the appropriate cytokine receptors, thereby influencing the state of inflammation in the brain. Indeed, increased intestinal inflammation driven by either LPS or bacterial infection correlates with elevated levels of microglial activation and release of pro-inflammatory cytokines (Riazi et al., 2008; Henry et al., 2009). These studies provide further confirmation of the intimate link between peripheral inflammation, influenced in part by the gut microbiota, and microglial activation and neuroinflammation.
Bacterial-derived neuroactive substances
Microbe-derived neuroactive metabolites are additional contributors to gut–brain crosstalk. Circulation of microbe-derived neurotransmitters, including acetylcholine (Lactobacillus), GABA (Bifidobacteria and Lactobacillus), and serotonin (Enterococcus and Streptococcus), can potentially influence microglial activation through direct and indirect means (Komatsuzaki et al., 2005; Yano et al., 2015; Pokusaeva et al., 2017). Studies have demonstrated that 90% of serotonin required for the regulation of mood, behavior, sleep, and several other functions within the CNS and GI tract is produced in the gut. Binding of serotonin to 5-HT receptors on microglia induces release of cytokine-carrying exosomes, providing another mechanism for gut-induced modulation of neuroinflammation (Glebov et al., 2015). Another microbial metabolite that influences microglia activity is tryptophan, a serotonin precursor. Metabolism of tryptophan by activated microglia produces the neurotoxin quinolinic acid, an N-methyl-D-aspartate agonist implicated in several neurological conditions including Huntington’s disease and depression (Feng et al., 2017). In a recent study using the experimental autoimmune encephalomyelitis (EAE) mouse model of MS, peripheral metabolism of dietary tryptophan by the gut microbiota was shown to generate metabolites that dampen the ability of microglia to induce pro-inflammatory responses in astrocytes, thereby ameliorating disease (Rothhammer et al., 2018). These findings provide further confirmation of the role of gut microbiota in influencing behavioral and physiological functions previously thought to be exclusively controlled by local factors in the brain.
Short-chain fatty acids (SCFAs) are metabolic byproducts of bacterial dietary fiber fermentation that can enter the systemic circulation and cross the BBB (Conn et al., 1983). Among the most abundantly produced SCFAs are acetic acid, propionic acid, and butyric acid, which collectively make up ∼95% of SCFAs synthesized in the gut (Cook and Sellin, 1998). SCFAs can exert physiological effects in the CNS via two primary mechanisms: activation of G protein–coupled receptors (GPCRs) expressed in the liver, spleen, and large intestine and inhibition of histone deacetylases (Kimura et al., 2011; Tan et al., 2014b). SCFAs have been shown to activate sympathetic nervous system activity and alleviate intestinal inflammation, and altered SCFA production has been demonstrated in a variety of neuropathologies such as PD and ASD (Smith et al., 2013; MacFabe, 2015; Unger et al., 2016).
More recently, the effects of SCFAs have been extended to microglia. Supplementation of drinking water with a mixture of three primary SCFAs (acetic acid, propionic acid, and butyric acid) rescued the immature genetic and morphological phenotype of microglia from GF mice (Erny et al., 2015). However, the exact SCFA signaling pathways that modulate microglial maturation have yet to be fully elucidated. SPF mice lacking the free fatty acid receptor 2 (FFAR2), a GPCR required for SCFA signaling in the gut, exhibited a microglial phenotype similar to that observed in GF mice (Erny et al., 2015). The absence of FFAR2 expression on microglia suggests that SCFAs may influence microglial maturation through signals that originate in the GI tract. However, exactly how this signal propagates to the CNS and governs microglial behavior is poorly understood. In addition to GPCR signaling, the ability of SCFAs to permeate the BBB and infiltrate the CNS suggests potential direct influences on microglia. Indeed, treatment of microglial cells in vitro with various SCFAs, including valproic acid and butyric acid, elevates levels of acetylation of histone H3 (Chen et al., 2007). This suggests that the ability of SCFAs to influence microglial behavior in vivo might occur through a combination of GPCR signaling and histone deacetylase inhibition. Together, these studies highlight the potential of gut-derived metabolites to enter the systemic circulation and exert their effects on microglia in the CNS.
Microglial dysfunction and microbial dysbiosis in disease
Given their wide spectrum of physiological functions and myriad roles in development and homeostasis, microglia are believed to be involved in the pathogenesis of several neurodevelopmental and neurodegenerative disorders. However, the factors and signals within the brain environment and the periphery that modulate microglial function and response during disease are not fully understood. The recent increase in research into gut–brain communication has created a new narrative for how the microbiome may shape microglial identity and function and how the microbiome, via microglia, may modulate the pathogenesis of neurological diseases. Accordingly, understanding the gut–brain axis will provide the foundation for potential novel therapeutic approaches in the years ahead.
Because of our increased awareness of the gut–brain axis, it has become clear that various neurological diseases, once thought to originate exclusively in the brain, are influenced by peripheral factors. The possible involvement of the microbiota in neurodevelopmental and neurodegenerative diseases stems from two primary observations. First is the critical role of gut-derived factors in regulating microglial function in the healthy state, which suggests that signals originating from the gut microbiota might also drive a pathogenic microglial phenotype that promotes disease. Second, dysbiosis is observed in several neurological conditions in which microglial dysfunction is thought to be a contributing factor to disease development, including ASD and PD (Table 1; Hsiao et al., 2013; Sampson et al., 2016). This dysbiosis is potentially sufficient to markedly disrupt microglial function and subsequently facilitate disease pathogenesis. Here, we discuss an emerging role for the gut–brain axis in ASD and PD, where most work has been done to date.
Table 1. Neuropathologies characterized by both microglial dysfunction and microbial dysbiosis.
| Neuropathology | Categorization | Hallmarks of microglial dysfunction | Hallmarks of microbial dysbiosis | References |
|---|---|---|---|---|
| ASD | Neurodevelopmental | Elevated microglial activation and release of pro-inflammatory cytokines in several brain regions. | 23–70% of individuals with ASD report GI symptoms (e.g., constipation and abdominal bloating). | De Angelis et al., 2013; Hsiao et al., 2013; Kang et al., 2013; Wang et al., 2013; De Rubeis et al., 2014; Gupta et al., 2014; Hsiao, 2014; Zhan et al., 2014; Koyama and Ikegaya, 2015; Martínez-Cerdeño, 2017 |
| Synaptic and neural circuitry dysfunction found in postmortem brain tissue from individuals with ASD. | Increased Clostridium and Lactobacillus and decreased Bacteroidetes and Bifidobacterium found in fecal samples collected from children with ASD. | |||
| Mice lacking microglia during early stages of postnatal development demonstrate cognitive and behavioral hallmarks reminiscent of ASD, in addition to abnormal neuronal signaling. | Decreased SCFA levels in ASD patients compared to healthy controls. | |||
| Monocolonization of GF mice with Bacteroides fragilis attenuates cognitive and GI defects in mice. | ||||
| Schizophrenia | Neuropsychiatric | Increased microglial activity observed in PET scan of schizophrenic patients. | Risk factors for schizophrenia involve disruptions to gut microbial community, including maternal infection, premature delivery, cesarean section delivery, and young-age viral infection. | West et al., 2006; Severance et al., 2010, 2015; Shaw, 2010; Diaz Heijtz et al., 2011; Miller et al., 2011; Monji et al., 2013; Hercher et al., 2014; Na et al., 2014; Yolken and Dickerson, 2014; Castro-Nallar et al., 2015; Reisinger et al., 2015; Bloomfield et al., 2016; Sekar et al., 2016; van Kesteren et al., 2017 |
| Elevated pro-inflammatory cytokine release (IL-2, IL-6, IL-8, and TNF-α) and neuroinflammation in the CNS. | High levels of colitis and GI dysfunction in schizophrenic patients. | |||
| Elevated microglial density in temporal cortex of schizophrenic patients. | GF and MIA mice display schizophrenic-like behaviors (e.g., decreased sociability and anhedonia). | |||
| Microglia-mediated disruptions in white matter structure and volume in the prefrontal cortex. | Oropharyngeal microbiota of schizophrenic patients is less diverse than controls and enriched in Lactobacilli, Bifidobacterium, and Eubacterium and depleted in Neisseria and Haemophilus. | |||
| Abnormal synaptic remodeling by microglia disrupts neural circuitry in schizophrenic patients due to increased expression of complement proteins C3 and C4. | Schizophrenic patients demonstrate dysregulation of several metabolic pathways regulated by the gut microbiota. | |||
| MDD | Neuropsychiatric | Postmortem analysis of human brain tissue reveals elevated microglial activation and density in MDD patients. | High concurrence between GI disorders, such as IBS and MDD. | Benton et al., 2007; Bailey et al., 2011; Dinan and Cryan, 2013; Brites and Fernandes, 2015; Yirmiya et al., 2015; Marin et al., 2017 |
| Increased microglial secretion of exosomes carrying pro-inflammatory cytokines in individuals with MDD. | Probiotic supplementation of Lactobacillus casei improved mood in patients with depression. | |||
| Chronic stress, a partial contributor to/risk factor for depression, is attributed to increased microglia-driven neuroinflammation. | Mouse model of MDD exhibiting high levels of stress has increased levels of Clostridium and reduced levels of Lactobacillus and Bacteroides. | |||
| Precise role of heightened neuroinflammation in the brain in MDD remains poorly understood. | ||||
| PD | Neurodegenerative | High levels of microglial activation found in the substantia nigra in brain tissue from PD patients. | >80% of PD patients report GI dysfunction (e.g., increased intestinal permeability, constipation, and nausea) 10–20 yr prior to onset of motor symptoms. | McGeer et al., 1988; Akiyama and McGeer, 1989; Gerhard et al., 2006; Kim and Joh, 2006; Watson et al., 2012; Fasano et al., 2013; Tan et al., 2014a; Keshavarzian et al., 2015; Scheperjans et al., 2015; Machado et al., 2016; Poirier et al., 2016; Sampson et al., 2016; Unger et al., 2016; Zhang et al., 2017 |
| PET scans from 11 PD patients reveal widespread microglial activation in the basal ganglia and the temporal and frontal cortex that exceeds the level of activation found in healthy controls. | Microbiota of PD patients demonstrate increased levels of Enterobacteriaceae and decreased levels of Bacteroidetes and Prevotellaceae. | |||
| α-Synuclein aggregates trigger microglial activation in the substantia nigra. | Concentrations of SCFAs (acetate, propionate, and butyrate) were lower in fecal samples collected from PD patients. | |||
| Microglial release of pro-inflammatory cytokines and neurotoxic factors is a contributing factor to dopaminergic cell death. | SIBO was observed in 25–54.5% of patients. | |||
| Heightened microglial activation observed in several Parkinsonian-like transgenic mice (α-synuclein overexpression) and toxin-induced mouse models (MPTP, 6-OHDA, and rotenone). | Misfolding and aggregation of α-synuclein may begin in enteric neurons that innervate the gut. | |||
| GF mice overexpressing α-synuclein demonstrate attenuated motor and GI symptoms compared to their SPF counterparts. | ||||
| AD | Neurodegenerative | PET scans and postmortem analysis of brain tissue from AD patients reveal elevated microglial activation correlating with severity of disease in several brain regions (hippocampus, entorhinal cortex, and parietal cortex). | The absence of a microbiota in a GF mouse model of AD reduces aggregation of amyloid beta, microglial activation, and neuroinflammation. | Xiang et al., 2006; Koenigsknecht-Talboo et al., 2008; Shie et al., 2009; Ferrarelli, 2015; Hamelin et al., 2016; Hong et al., 2016; Minter et al., 2016; Harach et al., 2017; Keren-Shaul et al., 2017; Ho et al., 2018 |
| Microglia were found to drive propagation of tau protein. | Reduction of microbial diversity following antibiotic administration reduced amyloid beta pathology and microglial activation in AD mice. | |||
| Microglia aggregation surrounds amyloid beta plaques. | Microbiota of APPPS1 transgenic mice have a higher Bacteroidetes/Firmicutes ratio compared to WT mice along with reduced levels of Verrucomicrobia. | |||
| Neurodegeneration occurs partially in response to microglia-driven chronic inflammation. | In vitro administration of several SCFAs (valeric acid, propionic acid, and butyric acid) obstructs aggregation of amyloid beta protein. | |||
| Neuroprotective microglia subtype recently identified operating through a TREM2-mediated signaling pathway. | ||||
| Complement protein (C1q), involved in mediating microglial synaptic remodeling, is upregulated in AD mouse models. | ||||
| ALS | Neurodegenerative | PET scans from ALS patients demonstrate high levels of microglial activation in the motor cortex and prefrontal cortex. | Small pilot study finds decreased microbial diversity in five ALS patients characterized by intestinal inflammation, low Firmicutes/Bacteroidetes ratio, and low SCFA levels. | Turner et al., 2004; Beers et al., 2006; Zhao et al., 2010, 2013; Gerber et al., 2012; Wu et al., 2015; Geloso et al., 2017; Rowin et al., 2017; Zhang et al., 2017 |
| Microglial release of pro-inflammatory cytokines and neurotoxic factors (TNF-α and IL-1β) increases as disease progresses. | G93 ALS mice expressing mutant SOD1 protein have lower expression of intestinal epithelial tight junction proteins and subsequent disruption to the intestinal barrier. | |||
| Microglia expressing mutated Cu,Zn superoxide dismutase (SOD1), a familial ALS gene, accelerates loss of motor neurons and disease progression, while WT microglia conferred neuroprotective effects. | G93 mice have a varying gut microbiota composition compared to healthy control mice with reduced levels of Escherichia coli, Fermicus, and butyrate-producing bacteria. | |||
| The neuroprotective role of anti-inflammatory microglia found in early stages of ALS is lost as increased levels of pro-inflammatory microglial activity drive neurodegeneration. | Drinking water supplemented with the SCFA butyrate improved intestinal barrier function and life expectancy in a G93 ALS mouse model. | |||
| Secretion of mutated SOD1 protein into extracellular space triggers microglial dysfunction and activation. | ||||
| MS | Autoimmune/ neurodegenerative | Colocalization of activated microglia and areas of demyelination and inflammatory lesion in MS patients and EAE mice. | Patients with MS have high levels of intestinal permeability. | Yacyshyn et al., 1996; Benveniste, 1997; Heppner et al., 2005; Sun et al., 2006; Piccio et al., 2007; Yokote et al., 2008; Frischer et al., 2009; Napoli and Neumann, 2010; Lee et al., 2011; Vogel et al., 2013; Miyake et al., 2015; Cekanaviciute et al., 2017; Gao et al., 2017; Kosmidou et al., 2017; Luo et al., 2017 |
| Activated microglia produce reactive oxygen species that contribute to oxidative stress and heightened neuronal injury, neurodegeneration, and demyelination. | High concurrence of inflammatory bowel disease and MS. | |||
| Inhibiting microglial activation prevented the onset of EAE in mice and decreased the presence of CNS lesions. | Dysbiosis found in MS patients (n = 20) characterized by depleted levels of Bacteroides and Prevotella and enriched levels of Bifidobacterium and Streptococcus compared to healthy controls. | |||
| Microglia-mediated remyelination is impaired in MS patients. | Patients (n = 31) with MS have an altered microbiota composition compared to age- and gender-matched controls, with increased levels of Pseudomonas and Mycoplana. | |||
| Activation of microglia during the early stage of disease facilitates recruitment of T cells from the periphery. | Monocolonization of GF mice with different species enriched in MS patients (Akkermansia muciniphila and Parabacteroides distasonis) influenced differentiation of regulatory T cells and lymphocytes. | |||
| Subsets of microglia with activated TNFR2 and TREM2 signaling demonstrate a neuroprotective role in EAE mice. | Development and severity of EAE is lower in GF mice and antibiotic-treated mice compared to SPF mice, as shown by an attenuated release of pro-inflammatory cytokines. | |||
| Whether microglial-driven neuroinflammation is a cause or consequence of neurodegeneration in MS remains unclear. |
IBS, irritable bowel syndrome; MDD, major depressive disorder; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; PET, positron emission tomography; SIBO, small intestinal bacterial overgrowth.
ASD
Children with ASD present with a wide range of cognitive and behavioral symptoms, including delayed language development, impaired social and communication skills, and repetitive behavior, depending on where they lie on the spectrum of disease severity (Plauche Johnson, 2004). In addition, symptoms of gut dysfunction such as irritable bowel syndrome, chronic diarrhea, and/or constipation are found in 23–70% of patients with ASD (Chaidez et al., 2014). The high prevalence of GI comorbidities among ASD patients and the correlation between the level of GI distress and severity of ASD symptoms has prompted studies investigating whether the development and/or progression of ASD has microbial origins. Cross-sectional studies comparing the gut microbiota composition between healthy and ASD individuals have revealed an altered microbiota profile in patients with ASD, with several studies reporting increased levels of Clostridium and Lactobacillus along with elevated levels of SCFAs, including propionic and butyric acid (Wang et al., 2012, 2013; De Angelis et al., 2013; Kang et al., 2013). However, given the small sample size in these pilot studies, further studies with larger cohorts are warranted.
While the etiology of ASD is complex and incompletely determined, microglia may influence the course of the disease. A collective consequence of microglial dysfunction is stunted neuronal development and immature brain circuitry, which could ultimately manifest in the ASD behavioral phenotypes. Postmortem analysis of brain tissue collected from ASD patients show perturbations in microglial immune surveillance and synaptic remodeling, with evidence for heightened microglial activation, including increased expression of MHC II, elevated levels of pro- and anti-inflammatory cytokines, and increased microglial density (Vargas et al., 2005; Morgan et al., 2010; Voineagu et al., 2011; Gupta et al., 2014; Lee et al., 2017). Impaired synaptic remodeling by microglia might contribute to the increased density of dendritic spines and excitatory synapses found in the brains of patients with ASD (Martínez-Cerdeño, 2017). Findings from animal models also support a possible role for microglia in ASD. Mice lacking the fractalkine receptor Cx3cr1 demonstrate a temporary reduction in the number of microglia during early postnatal development, as well as increased synaptic density, immature brain circuitry, and signal transmission deficits that persist into adulthood (Paolicelli et al., 2011; Zhan et al., 2014). Abnormal neural circuitry due to the absence of microglia during a critical window of brain development in these mice is associated with behavioral deficits similar to those seen in individuals with ASD (Zhan et al., 2014).
Other preclinical models have provided insight into the role of the microbiota and microglia in driving the pathology of ASD (Needham et al., 2018). The development of the maternal immune activation (MIA) model was motivated by the increased incidence rate of ASD in children whose mothers suffered from a severe infection during certain stages of pregnancy. In the MIA model, offspring of mice injected with polyinosinic-polycytidylic acid (poly(I:C)), a synthetic viral mimic that activates TLR3, demonstrate core symptoms of ASD, including repetitive behaviors, communication deficits, and decreased sociability (Malkova et al., 2012). These MIA offspring also exhibit increased intestinal permeability and intestinal inflammation, two GI symptoms commonly found in children with ASD (Hsiao et al., 2013; Chaidez et al., 2014). Provision of IL-17 promoting segmented filamentous bacteria to MIA mothers further enhances behavioral abnormities in MIA offspring (Kim et al., 2017). While the gut microbiota contributes to ASD symptomatology in both mice and humans, it also has protective effects. Similar to its ameliorative effect in colitis, oral administration of Bacteroides fragilis to offspring of MIA mice at weaning rescues the integrity of the intestinal barrier, reduces anxiety, and improves communication and repetitive behaviors (Hsiao et al., 2013). Similarly, provision of Lactobacillus reuteri to offspring of dams fed a high-fat diet attenuates their social deficits, further supporting a role for the gut microbiota in influencing outcomes of neurodevelopmental disorders (Buffington et al., 2016).
While commensal symbiotic bacteria are critical for proper microglial maturation, induction of infection in pregnant dams can have disruptive effects on the progression of microglial development in offspring (Pratt et al., 2013; Matcovitch-Natan et al., 2016; Mattei et al., 2017). Microglia from young MIA offspring show increased expression of inflammation-related genes typically associated with an adult microglial phenotype and down-regulation of genes expressed during earlier developmental stages (e.g., Pu.1 and Irf8), suggesting that maternal inflammation disrupts the timeline of normal microglial maturation (Matcovitch-Natan et al., 2016). Adult MIA offspring exhibit heightened microglial activation, as noted by increased levels of IL-6 and TNF-α (Pratt et al., 2013; Mattei et al., 2017). However, the differences in the microglial transcriptome seen in offspring from MIA versus healthy dams decline as mice reach adulthood. These findings indicate that the effects of maternal inflammation on microglial development and behavior in offspring may be restricted to a narrow developmental window, after which microglia revert to a relatively normal phenotype in adulthood. While the MIA model has limitations in terms of replicating the complex symptomatology of ASD, the model faithfully recapitulates abnormalities in both microglial behavior and GI function that are frequently observed in patients. Replication of microbiome effects on microglia in additional animal models would help strengthen the gut–brain link to neurodevelopmental disorders.
Studies of ASD and other neurodevelopmental disorders provide evidence for a potential pathogenic role for both dysbiosis and microglial dysfunction, and suggests that microglia may link early-life dysbiosis to long-lasting neurological abnormalities. Perturbations in the composition of gut microbes during early developmental stages due to maternal infection, mode of delivery, antibiotic use, and early-age infections increase an individual’s predisposition to atypical behavioral patterns. Given the requirement for constant input from the gut microbiota for normal microglial development and function, it is plausible that microbial effects on neural development and behavior may occur through changes in microglial activity (Erny et al., 2015; Thion et al., 2018). New evidence to further support this link will provide a greater appreciation for the role of a healthy gut microbiome in normal microglial and cognitive development.
PD
PD is the second most common neurodegenerative disorder and is defined by the presence of motor symptoms including bradykinesia, rigidity, and resting tremor (Postuma et al., 2015). Pathological hallmarks of PD include death of dopaminergic neurons of the substantia nigra pars compacta and intraneuronal accumulation of the α-synuclein protein (Goedert et al., 2013). A complex interplay of genetic and environmental factors is thought to drive the pathogenesis of PD, eventually leading to mitochondrial and autophagic dysfunction (Shulman et al., 2011).
One prevailing theory to explain synucleinopathies is that progressive alterations in the microenvironment of microglia, such as increased deposition of α-synuclein, can change microglial behavior and function (Zhang et al., 2005; Su et al., 2008). These changes may ultimately trigger a neuropathogenic microglial phenotype that facilitates and accelerates PD pathology. Heightened microglial activation results in the release of pro-inflammatory cytokines (e.g., IL-6 and TNF-α) and a variety of other neurotoxic compounds into their immediate extracellular environment (Zhang et al., 2005; Kim and Joh, 2006). This in turn could compromise neuronal function and eventually lead to synaptic degeneration and neuronal death. The sustained activation of microglia due to external cues from both misfolded α-synuclein and damaged neurons likely instigates a cycle of chronic inflammation that further perpetuates the death of dopaminergic neurons and accelerates progression of the disease (Zhang et al., 2005; Kim and Joh, 2006).
Although PD is predominantly classified as a brain disorder affecting neurons of the nigrostriatal pathway, some believe that pathology originates in the gut. According to Braak’s hypothesis, aggregation of α-synuclein spreads from the enteric nervous system to the brain via the vagus nerve in cases of sporadic PD (Braak et al., 2004; Rietdijk et al., 2017). Evidence for this theory is supported by both preclinical and clinical evidence demonstrating the presence of α-synuclein deposits in enteric neurons of the gut before the detection of misfolded α-synuclein in the CNS (Braak et al., 2004; Bencsik et al., 2014). While this pattern of α-synuclein spreading is not observed in all cases of sporadic PD, vagotomy may be associated with reduced risk of developing PD in humans, potentially implicating peripheral influences on disease development (Svensson et al., 2015; Liu et al., 2017).
Given the possible crosstalk between the gut microbiota and microglia, the composition of intestinal bacteria may modulate disease pathogenesis. Nonmotor symptoms, including chronic constipation and GI distress, precede motor symptoms in up to 80% of PD patients (O’Sullivan et al., 2008; Poirier et al., 2016; Unger et al., 2016). Moreover, differences in the gut microbiota composition, bacterial load, and levels of bacterial metabolites have been observed in patients with PD when compared with healthy individuals (Hill-Burns et al., 2017). Studies show altered abundance of certain bacterial strains in patients with PD, changes that may correlate with severity of motor deficits (Scheperjans et al., 2015; Mertsalmi et al., 2017). While interindividual variation is high, PD patients often exhibit increased levels of Enterobacteriaceae and decreased levels of Bacteroidetes and Prevotellaceae (Keshavarzian et al., 2015; Unger et al., 2016). Small intestinal bacterial overgrowth is an additional defining disease feature in 25–54.5% of PD patients (Fasano et al., 2013; Tan et al., 2014a). The shift in microbial communities may contribute to the elevated levels of peripheral inflammation and intestinal permeability that are frequently seen in PD patients and might drive misfolding of α-synuclein in the gut and its retrograde propagation to the CNS.
Support for the notion that the microbiota may drive the pathogenesis of PD was provided by studies in GF mice that overexpress human α-synuclein. These mice display reduced motor deficits, GI dysfunction, and microglial activation when compared with mice with an intact gut microbiota (Sampson et al., 2016). This observation suggests that the gut microbiota, along with genetic predisposition, may be required for disease onset and/or progression. Feeding these GF transgenic mice a mixture of three SCFAs up-regulated microglial activation and induced motor deficits similar to those observed in colonized animals (Sampson et al., 2016). In another recent study, FMT from healthy mice into mice injected with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a toxin-induced model of PD, attenuated microglial activation and motor deficits and decreased SCFA levels, suggesting that active signals from the gut microbiota may influence PD outcomes (Sun et al., 2018). Interestingly, a recent study showed that overall fecal SCFA concentrations were reduced in patients with PD compared with controls, while some specific SCFAs were relatively increased (Unger et al., 2016). The current uncertainties in the role of SCFAs in various PD models may stem from concentration- and region-dependent effects of SCFAs on host physiology. Despite these preliminary findings, precisely how the gut microbiota and microbial metabolites influence motor symptoms and neuroinflammation in PD remains poorly understood.
The presence of both microbial dysbiosis and microglial dysfunction has been characterized in behavioral (schizophrenia and depression), neuroinflammatory (MS), and neurodegenerative (AD and amyotrophic lateral sclerosis [ALS]) disorders (Table 1). Whether gut microbiota directly or indirectly affect microglia in these conditions remains largely unknown. Gradual changes in microbiota composition have been observed as normal features of aging, including changes in microbial resilience, stability, and diversity, which are features that occur alongside changes in microglial physiology with age (Zapata and Quagliarello, 2015; Buford, 2017). Similar to neurodevelopmental disorders, this trend suggests that microbiota-driven changes in microglial behavior might have a larger role in the onset or progression of neurodegenerative disorders than previously thought (Buford, 2017). Studies investigating changes in microbiome composition and microglial function in the healthy and diseased brain over time will provide additional insights into the nature of gut–brain interactions during the aging process.
Future directions and challenges
Tremendous progress has been made recently in elucidating and characterizing the distinct components and signals of the gut–brain axis. However, the studies to date likely only represent an initial glimpse into the functional interplay between the gut microbiome, microglia, and neurodevelopmental and neurodegenerative disorders. The advent of new tools, such as advanced next-generation sequencing methods used to study and characterize the microbiome and microglia, will facilitate further identification and characterization of mechanisms by which gut microbiota influence microglial maturation and function (Bennett et al., 2016). For example, the application of single-cell sequencing to study microglia has also paved the way for potential identification of unique microglial subsets with neuroprotective roles in the context of neurodegenerative disease (Keren-Shaul et al., 2017). These studies have shifted the narrative from the notion of exclusively pathogenic microglia to one of a more nuanced mixture of microglial subsets, including some with neuroprotective properties, enabling a greater appreciation of the multifaceted role microglia might play in driving neuropathological phenotypes and potentially accelerating the development of microglia-targeted therapies.
One of the outstanding questions is how changes in the gut microbiome might lead to an altered microglial phenotype and eventually to impaired brain homeostasis. It is unclear whether the observed disease-associated alterations in microbial communities are a cause or consequence of altered brain function and whether interventions targeting the microbiome can restore microglial function and lead to beneficial effects in neurodevelopmental and neurodegenerative diseases. Signals originating from the gut microbiota and transmitted to the brain have the potential to alleviate or exacerbate disease pathogenesis, changes that may operate through gut-mediated changes in microglial behavior. Thus, continued exploration of the intersection of microbiology, immunology, and neurobiology holds immense therapeutic promise. Several different microbiome-targeted approaches, including prebiotic, probiotic, and FMT strategies, have shown promising results for a variety of GI conditions in preclinical and clinical models and could be extended to pathologies involving microglia in the near future (Fond et al., 2015; Winek et al., 2016). Further investment in gut–brain axis research may catalyze the potential of harnessing the gut microbiome for development of innovative, noninvasive, and effective therapeutic strategies for disorders of the brain.
Acknowledgments
We would like to thank members of the Mazmanian and Glass laboratory for their critical review of this manuscript and thoughtful insight and discussion.
R. Abdel-Haq is supported by the U.S. Department of Defense and the Donna and Benjamin M. Rosen Bioengineering Center. Related work in the Glass laboratory is funded by the National Institutes of Health (grants AG057706 and NS096170). Related work in the Mazmanian laboratory is funded by the Heritage Medical Research Institute, the Simons Foundation (grant 322839), the U.S. Department of Defense (PD160030), and the National Institutes of Health (grants MH100556 and NS085910) to S.K. Mazmanian.
The authors declare no competing financial interests.
Author contributions: R. Abdel-Haq wrote the manuscript with support from J.C.M. Schlachetzki. J.C.M. Schlachetzki and C.K. Glass critically reviewed and edited the manuscript. S.K. Mazmanian contributed to the conception and design of the manuscript. All authors reviewed the manuscript before submission.
References
- Akiyama H., and McGeer P.L.. 1989. Microglial response to 6-hydroxydopamine-induced substantia nigra lesions. Brain Res. 489:247–253. 10.1016/0006-8993(89)90857-3 [DOI] [PubMed] [Google Scholar]
- Alaish S.M., Smith A.D., Timmons J., Greenspon J., Eyvazzadeh D., Murphy E., Shea-Donahue T., Cirimotich S., Mongodin E., Zhao A., et al. . 2013. Gut microbiota, tight junction protein expression, intestinal resistance, bacterial translocation and mortality following cholestasis depend on the genetic background of the host. Gut Microbes. 4:292–305. 10.4161/gmic.24706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alliot F., Godin I., and Pessac B.. 1999. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res. Dev. Brain Res. 117:145–152. 10.1016/S0165-3806(99)00113-3 [DOI] [PubMed] [Google Scholar]
- Askew K., Li K., Olmos-Alonso A., Garcia-Moreno F., Liang Y., Richardson P., Tipton T., Chapman M.A., Riecken K., Beccari S., et al. . 2017. Coupled Proliferation and Apoptosis Maintain the Rapid Turnover of Microglia in the Adult Brain. Cell Reports. 18:391–405. 10.1016/j.celrep.2016.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed F., Ding H., Wang T., Hooper L.V., Koh G.Y., Nagy A., Semenkovich C.F., and Gordon J.I.. 2004. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA. 101:15718–15723. 10.1073/pnas.0407076101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey M.T., Dowd S.E., Galley J.D., Hufnagle A.R., Allen R.G., and Lyte M.. 2011. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav. Immun. 25:397–407. 10.1016/j.bbi.2010.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers D.R., Henkel J.S., Xiao Q., Zhao W., Wang J., Yen A.A., Siklos L., McKercher S.R., and Appel S.H.. 2006. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA. 103:16021–16026. 10.1073/pnas.0607423103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencsik A., Muselli L., Leboidre M., Lakhdar L., and Baron T.. 2014. Early and persistent expression of phosphorylated α-synuclein in the enteric nervous system of A53T mutant human α-synuclein transgenic mice. J. Neuropathol. Exp. Neurol. 73:1144–1151. 10.1097/NEN.0000000000000137 [DOI] [PubMed] [Google Scholar]
- Bennett M.L., Bennett F.C., Liddelow S.A., Ajami B., Zamanian J.L., Fernhoff N.B., Mulinyawe S.B., Bohlen C.J., Adil A., Tucker A., et al. . 2016. New tools for studying microglia in the mouse and human CNS. Proc. Natl. Acad. Sci. USA. 113:E1738–E1746. 10.1073/pnas.1525528113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton D., Williams C., and Brown A.. 2007. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur. J. Clin. Nutr. 61:355–361. 10.1038/sj.ejcn.1602546 [DOI] [PubMed] [Google Scholar]
- Benveniste E.N. 1997. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J. Mol. Med. (Berl.). 75:165–173. 10.1007/s001090050101 [DOI] [PubMed] [Google Scholar]
- Bercik P., Park A.J., Sinclair D., Khoshdel A., Lu J., Huang X., Deng Y., Blennerhassett P.A., Fahnestock M., Moine D., et al. . 2011. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 23:1132–1139. 10.1111/j.1365-2982.2011.01796.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilimoria P.M., and Stevens B.. 2015. Microglia function during brain development: New insights from animal models. Brain Res. 1617:7–17. 10.1016/j.brainres.2014.11.032 [DOI] [PubMed] [Google Scholar]
- Bloomfield P.S., Selvaraj S., Veronese M., Rizzo G., Bertoldo A., Owen D.R., Bloomfield M.A.P., Bonoldi I., Kalk N., Turkheimer F., et al. . 2016. Microglial Activity in People at Ultra High Risk of Psychosis and in Schizophrenia: An [(11)C]PBR28 PET Brain Imaging Study. Am. J. Psychiatry. 173:44–52. 10.1176/appi.ajp.2015.14101358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovikova L.V., Ivanova S., Zhang M., Yang H., Botchkina G.I., Watkins L.R., Wang H., Abumrad N., Eaton J.W., and Tracey K.J.. 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 405:458–462. 10.1038/35013070 [DOI] [PubMed] [Google Scholar]
- Braak H., Ghebremedhin E., Rüb U., Bratzke H., and Del Tredici K.. 2004. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 318:121–134. 10.1007/s00441-004-0956-9 [DOI] [PubMed] [Google Scholar]
- Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., and Cryan J.F.. 2011. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA. 108:16050–16055. 10.1073/pnas.1102999108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brites D., and Fernandes A.. 2015. Neuroinflammation and Depression: Microglia Activation, Extracellular Microvesicles and microRNA Dysregulation. Front. Cell. Neurosci. 9:476 10.3389/fncel.2015.00476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning K.N., Verheijden S., and Boeckxstaens G.E.. 2017. The Vagus Nerve in Appetite Regulation, Mood, and Intestinal Inflammation. Gastroenterology. 152:730–744. 10.1053/j.gastro.2016.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffington S.A., Di Prisco G.V., Auchtung T.A., Ajami N.J., Petrosino J.F., and Costa-Mattioli M.. 2016. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell. 165:1762–1775. 10.1016/j.cell.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buford T.W. 2017. (Dis)Trust your gut: the gut microbiome in age-related inflammation, health, and disease. Microbiome. 5:80 10.1186/s40168-017-0296-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O., Jedrychowski M.P., Moore C.S., Cialic R., Lanser A.J., Gabriely G., Koeglsperger T., Dake B., Wu P.M., Doykan C.E., et al. . 2014. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat. Neurosci. 17:131–143. 10.1038/nn.3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Ruiz A., Mosley M., George A.J., Mussaji L.F., Fullerton E.F., Ruszkowski E.M., Jacobs A.J., Gewirtz A.T., Chassaing B., and Forger N.G.. 2018. The microbiota influences cell death and microglial colonization in the perinatal mouse brain. Brain Behav. Immun. 67:218–229. 10.1016/j.bbi.2017.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Nallar E., Bendall M.L., Pérez-Losada M., Sabuncyan S., Severance E.G., Dickerson F.B., Schroeder J.R., Yolken R.H., and Crandall K.A.. 2015. Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ. 3:e1140 10.7717/peerj.1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekanaviciute E., Yoo B.B., Runia T.F., Debelius J.W., Singh S., Nelson C.A., Kanner R., Bencosme Y., Lee Y.K., Hauser S.L., et al. . 2017. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl. Acad. Sci. USA. 114:10713–10718. 10.1073/pnas.1711235114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaidez V., Hansen R.L., and Hertz-Picciotto I.. 2014. Gastrointestinal problems in children with autism, developmental delays or typical development. J. Autism Dev. Disord. 44:1117–1127. 10.1007/s10803-013-1973-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P.S., Wang C.-C., Bortner C.D., Peng G.-S., Wu X., Pang H., Lu R.-B., Gean P.-W., Chuang D.-M., and Hong J.-S.. 2007. Valproic acid and other histone deacetylase inhibitors induce microglial apoptosis and attenuate lipopolysaccharide-induced dopaminergic neurotoxicity. Neuroscience. 149:203–212. 10.1016/j.neuroscience.2007.06.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn A.R., Fell D.I., and Steele R.D.. 1983. Characterization of alpha-keto acid transport across blood-brain barrier in rats. Am. J. Physiol. 245:E253–E260. [DOI] [PubMed] [Google Scholar]
- Cook S.I., and Sellin J.H.. 1998. Review article: short chain fatty acids in health and disease. Aliment. Pharmacol. Ther. 12:499–507. 10.1046/j.1365-2036.1998.00337.x [DOI] [PubMed] [Google Scholar]
- Cunningham C.L., Martínez-Cerdeño V., and Noctor S.C.. 2013. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J. Neurosci. 33:4216–4233. 10.1523/JNEUROSCI.3441-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D., Grutzendler J., Yang G., Kim J.V., Zuo Y., Jung S., Littman D.R., Dustin M.L., and Gan W.-B.. 2005. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8:752–758. 10.1038/nn1472 [DOI] [PubMed] [Google Scholar]
- De Angelis M., Piccolo M., Vannini L., Siragusa S., De Giacomo A., Serrazzanetti D.I., Cristofori F., Guerzoni M.E., Gobbetti M., and Francavilla R.. 2013. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One. 8:e76993 10.1371/journal.pone.0076993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rubeis S., He X., Goldberg A.P., Poultney C.S., Samocha K., Cicek A.E., Kou Y., Liu L., Fromer M., Walker S., et al. UK10K Consortium . 2014. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 515:209–215. 10.1038/nature13772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Heijtz R., Wang S., Anuar F., Qian Y., Björkholm B., Samuelsson A., Hibberd M.L., Forssberg H., and Pettersson S.. 2011. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA. 108:3047–3052. 10.1073/pnas.1010529108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan T.G., and Cryan J.F.. 2013. Melancholic microbes: a link between gut microbiota and depression? Neurogastroenterol. Motil. 25:713–719. 10.1111/nmo.12198 [DOI] [PubMed] [Google Scholar]
- Dominguez-Bello M.G., Costello E.K., Contreras M., Magris M., Hidalgo G., Fierer N., and Knight R.. 2010. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA. 107:11971–11975. 10.1073/pnas.1002601107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckburg P.B., Bik E.M., Bernstein C.N., Purdom E., Dethlefsen L., Sargent M., Gill S.R., Nelson K.E., and Relman D.A.. 2005. Diversity of the human intestinal microbial flora. Science. 308:1635–1638. 10.1126/science.1110591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erny D., Hrabě de Angelis A.L., Jaitin D., Wieghofer P., Staszewski O., David E., Keren-Shaul H., Mahlakoiv T., Jakobshagen K., Buch T., et al. . 2015. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18:965–977. 10.1038/nn.4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A., Bove F., Gabrielli M., Petracca M., Zocco M.A., Ragazzoni E., Barbaro F., Piano C., Fortuna S., Tortora A., et al. . 2013. The role of small intestinal bacterial overgrowth in Parkinson’s disease. Mov. Disord. 28:1241–1249. 10.1002/mds.25522 [DOI] [PubMed] [Google Scholar]
- Feng W., Wang Y., Liu Z.-Q., Zhang X., Han R., Miao Y.-Z., and Qin Z.-H.. 2017. Microglia activation contributes to quinolinic acid-induced neuronal excitotoxicity through TNF-α. Apoptosis. 22:696–709. 10.1007/s10495-017-1363-5 [DOI] [PubMed] [Google Scholar]
- Ferrarelli L.K. 2015. Microglia spread tau. Sci. Signal. 8:ec329 10.1126/scisignal.aad8159 [DOI] [Google Scholar]
- Fond G., Boukouaci W., Chevalier G., Regnault A., Eberl G., Hamdani N., Dickerson F., Macgregor A., Boyer L., Dargel A., et al. . 2015. The “psychomicrobiotic”: Targeting microbiota in major psychiatric disorders: A systematic review. Pathol. Biol. (Paris). 63:35–42. 10.1016/j.patbio.2014.10.003 [DOI] [PubMed] [Google Scholar]
- Forsythe P., Bienenstock J., and Kunze W.A.. 2014. Vagal pathways for microbiome-brain-gut axis communication. Adv. Exp. Med. Biol. 817:115–133. 10.1007/978-1-4939-0897-4_5 [DOI] [PubMed] [Google Scholar]
- Franco R., and Fernández-Suárez D.. 2015. Alternatively activated microglia and macrophages in the central nervous system. Prog. Neurobiol. 131:65–86. 10.1016/j.pneurobio.2015.05.003 [DOI] [PubMed] [Google Scholar]
- Frank D.N., and Pace N.R.. 2008. Gastrointestinal microbiology enters the metagenomics era. Curr. Opin. Gastroenterol. 24:4–10. 10.1097/MOG.0b013e3282f2b0e8 [DOI] [PubMed] [Google Scholar]
- Frasch M.G., Szynkaruk M., Prout A.P., Nygard K., Cao M., Veldhuizen R., Hammond R., and Richardson B.S.. 2016. Decreased neuroinflammation correlates to higher vagus nerve activity fluctuations in near-term ovine fetuses: a case for the afferent cholinergic anti-inflammatory pathway? J. Neuroinflammation. 13:103 10.1186/s12974-016-0567-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick L.R., Williams K., and Pittenger C.. 2013. Microglial dysregulation in psychiatric disease. Clin. Dev. Immunol. 2013:608654 10.1155/2013/608654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischer J.M., Bramow S., Dal-Bianco A., Lucchinetti C.F., Rauschka H., Schmidbauer M., Laursen H., Sorensen P.S., and Lassmann H.. 2009. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 132:1175–1189. 10.1093/brain/awp070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung T.C., Olson C.A., and Hsiao E.Y.. 2017. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 20:145–155. 10.1038/nn.4476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatro T.F., Holtman I.R., Lerario A.M., Vainchtein I.D., Brouwer N., Sola P.R., Veras M.M., Pereira T.F., Leite R.E.P., Möller T., et al. . 2017. Transcriptomic analysis of purified human cortical microglia reveals age-associated changes. Nat. Neurosci. 20:1162–1171. 10.1038/nn.4597 [DOI] [PubMed] [Google Scholar]
- Gao H., Danzi M.C., Choi C.S., Taherian M., Dalby-Hansen C., Ellman D.G., Madsen P.M., Bixby J.L., Lemmon V.P., Lambertsen K.L., and Brambilla R.. 2017. Opposing Functions of Microglial and Macrophagic TNFR2 in the Pathogenesis of Experimental Autoimmune Encephalomyelitis. Cell Reports. 18:198–212. 10.1016/j.celrep.2016.11.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geloso M.C., Corvino V., Marchese E., Serrano A., Michetti F., and D’Ambrosi N.. 2017. The Dual Role of Microglia in ALS: Mechanisms and Therapeutic Approaches. Front. Aging Neurosci. 9:242 10.3389/fnagi.2017.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber Y.N., Sabourin J.-C., Rabano M., Vivanco M., and Perrin F.E.. 2012. Early functional deficit and microglial disturbances in a mouse model of amyotrophic lateral sclerosis. PLoS One. 7:e36000 10.1371/journal.pone.0036000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard A., Pavese N., Hotton G., Turkheimer F., Es M., Hammers A., Eggert K., Oertel W., Banati R.B., and Brooks D.J.. 2006. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol. Dis. 21:404–412. 10.1016/j.nbd.2005.08.002 [DOI] [PubMed] [Google Scholar]
- Geuking M.B., Cahenzli J., Lawson M.A.E., Ng D.C.K., Slack E., Hapfelmeier S., McCoy K.D., and Macpherson A.J.. 2011. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 34:794–806. 10.1016/j.immuni.2011.03.021 [DOI] [PubMed] [Google Scholar]
- Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S., Mehler M.F., Conway S.J., Ng L.G., Stanley E.R., et al. . 2010. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 330:841–845. 10.1126/science.1194637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glebov K., Löchner M., Jabs R., Lau T., Merkel O., Schloss P., Steinhäuser C., and Walter J.. 2015. Serotonin stimulates secretion of exosomes from microglia cells. Glia. 63:626–634. 10.1002/glia.22772 [DOI] [PubMed] [Google Scholar]
- Goedert M., Spillantini M.G., Del Tredici K., and Braak H.. 2013. 100 years of Lewy pathology. Nat. Rev. Neurol. 9:13–24. 10.1038/nrneurol.2012.242 [DOI] [PubMed] [Google Scholar]
- Goehler L.E., Gaykema R.P., Nguyen K.T., Lee J.E., Tilders F.J., Maier S.F., and Watkins L.R.. 1999. Interleukin-1beta in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems? J. Neurosci. 19:2799–2806. 10.1523/JNEUROSCI.19-07-02799.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehler L.E., Gaykema R.P.A., Opitz N., Reddaway R., Badr N., and Lyte M.. 2005. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav. Immun. 19:334–344. 10.1016/j.bbi.2004.09.002 [DOI] [PubMed] [Google Scholar]
- Gomes C., Ferreira R., George J., Sanches R., Rodrigues D.I., Gonçalves N., and Cunha R.A.. 2013. Activation of microglial cells triggers a release of brain-derived neurotrophic factor (BDNF) inducing their proliferation in an adenosine A2A receptor-dependent manner: A2A receptor blockade prevents BDNF release and proliferation of microglia. J. Neuroinflammation. 10:16 10.1186/1742-2094-10-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez Perdiguero E., Klapproth K., Schulz C., Busch K., Azzoni E., Crozet L., Garner H., Trouillet C., de Bruijn M.F., Geissmann F., and Rodewald H.-R.. 2015. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 518:547–551. 10.1038/nature13989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin D., Link V.M., Romanoski C.E., Fonseca G.J., Eichenfield D.Z., Spann N.J., Stender J.D., Chun H.B., Garner H., Geissmann F., and Glass C.K.. 2014. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. 159:1327–1340. 10.1016/j.cell.2014.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin D., Skola D., Coufal N.G., Holtman I.R., Schlachetzki J.C.M., Sajti E., Jaeger B.N., O’Connor C., Fitzpatrick C., Pasillas M.P., et al. . 2017. An environment-dependent transcriptional network specifies human microglia identity. Science. 356:eaal3222 10.1126/science.aal3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Ellis S.E., Ashar F.N., Moes A., Bader J.S., Zhan J., West A.B., and Arking D.E.. 2014. Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nat. Commun. 5:5748 10.1038/ncomms6748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelin L., Lagarde J., Dorothée G., Leroy C., Labit M., Comley R.A., de Souza L.C., Corne H., Dauphinot L., Bertoux M., et al. Clinical IMABio3 team . 2016. Early and protective microglial activation in Alzheimer’s disease: a prospective study using 18F-DPA-714 PET imaging. Brain. 139:1252–1264. 10.1093/brain/aww017 [DOI] [PubMed] [Google Scholar]
- Hanamsagar R., Bolton J., Alter M., and Bilbo S.. 2015. Microglia show sex-differences in gene expression patterns over development and following immune challenge: Relevance for sex-differences in neurodevelopmental disorders. Brain Behav. Immun. 49:e10–e11. 10.1016/j.bbi.2015.06.056 [DOI] [Google Scholar]
- Hara H., Haga S., Aoyama Y., and Kiriyama S.. 1999. Short-chain fatty acids suppress cholesterol synthesis in rat liver and intestine. J. Nutr. 129:942–948. 10.1093/jn/129.5.942 [DOI] [PubMed] [Google Scholar]
- Harach T., Marungruang N., Duthilleul N., Cheatham V., Mc Coy K.D., Frisoni G., Neher J.J., Fåk F., Jucker M., Lasser T., and Bolmont T.. 2017. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 7:41802 10.1038/srep41802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry C.J., Huang Y., Wynne A.M., and Godbout J.P.. 2009. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav. Immun. 23:309–317. 10.1016/j.bbi.2008.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner F.L., Greter M., Marino D., Falsig J., Raivich G., Hövelmeyer N., Waisman A., Rülicke T., Prinz M., Priller J., et al. . 2005. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat. Med. 11:146–152. 10.1038/nm1177 [DOI] [PubMed] [Google Scholar]
- Hercher C., Chopra V., and Beasley C.L.. 2014. Evidence for morphological alterations in prefrontal white matter glia in schizophrenia and bipolar disorder. J. Psychiatry Neurosci. 39:376–385. 10.1503/jpn.130277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman S.E., Kingery N.D., Ohsumi T.K., Borowsky M.L., Wang L.-C., Means T.K., and El Khoury J.. 2013. The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci. 16:1896–1905. 10.1038/nn.3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill-Burns E.M., Debelius J.W., Morton J.T., Wissemann W.T., Lewis M.R., Wallen Z.D., Peddada S.D., Factor S.A., Molho E., Zabetian C.P., et al. . 2017. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov. Disord. 32:739–749. 10.1002/mds.26942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch E.C., Vyas S., and Hunot S.. 2012. Neuroinflammation in Parkinson’s disease. Parkinsonism Relat. Disord. 18(Suppl 1):S210–S212. 10.1016/S1353-8020(11)70065-7 [DOI] [PubMed] [Google Scholar]
- Ho L., Ono K., Tsuji M., Mazzola P., Singh R., and Pasinetti G.M.. 2018. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Rev. Neurother. 18:83–90. 10.1080/14737175.2018.1400909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoban A.E., Stilling R.M., Ryan F.J., Shanahan F., Dinan T.G., Claesson M.J., Clarke G., and Cryan J.F.. 2016. Regulation of prefrontal cortex myelination by the microbiota. Transl. Psychiatry. 6:e774 10.1038/tp.2016.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffel G., Chen J., Lavin Y., Low D., Almeida F.F., See P., Beaudin A.E., Lum J., Low I., Forsberg E.C., et al. . 2015. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 42:665–678. 10.1016/j.immuni.2015.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S., Beja-Glasser V.F., Nfonoyim B.M., Frouin A., Li S., Ramakrishnan S., Merry K.M., Shi Q., Rosenthal A., Barres B.A., et al. . 2016. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 352:712–716. 10.1126/science.aad8373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper L.V., Wong M.H., Thelin A., Hansson L., Falk P.G., and Gordon J.I.. 2001. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 291:881–884. 10.1126/science.291.5505.881 [DOI] [PubMed] [Google Scholar]
- Hsiao E.Y. 2014. Gastrointestinal issues in autism spectrum disorder. Harv. Rev. Psychiatry. 22:104–111. 10.1097/HRP.0000000000000029 [DOI] [PubMed] [Google Scholar]
- Hsiao E.Y., McBride S.W., Hsien S., Sharon G., Hyde E.R., McCue T., Codelli J.A., Chow J., Reisman S.E., Petrosino J.F., et al. . 2013. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 155:1451–1463. 10.1016/j.cell.2013.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson H.E., Rodríguez-Piñeiro A.M., Schütte A., Ermund A., Boysen P., Bemark M., Sommer F., Bäckhed F., Hansson G.C., and Johansson M.E.V.. 2015. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 16:164–177. 10.15252/embr.201439263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarczyk R., Tejera D., Simon B.J., and Heneka M.T.. 2017. Microglia modulation through external vagus nerve stimulation in a murine model of Alzheimer’s disease. J. Neurochem. 10.1111/jnc.14284 [DOI] [PubMed] [Google Scholar]
- Kang D.-W., Park J.G., Ilhan Z.E., Wallstrom G., Labaer J., Adams J.B., and Krajmalnik-Brown R.. 2013. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One. 8:e68322 10.1371/journal.pone.0068322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karczewski J., Troost F.J., Konings I., Dekker J., Kleerebezem M., Brummer R.-J.M., and Wells J.M.. 2010. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 298:G851–G859. 10.1152/ajpgi.00327.2009 [DOI] [PubMed] [Google Scholar]
- Kelly J.R., Kennedy P.J., Cryan J.F., Dinan T.G., Clarke G., and Hyland N.P.. 2015. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 9:392 10.3389/fncel.2015.00392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren-Shaul H., Spinrad A., Weiner A., Matcovitch-Natan O., Dvir-Szternfeld R., Ulland T.K., David E., Baruch K., Lara-Astaiso D., Toth B., et al. . 2017. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell. 169:1276–1290.e17. 10.1016/j.cell.2017.05.018 [DOI] [PubMed] [Google Scholar]
- Keshavarzian A., Green S.J., Engen P.A., Voigt R.M., Naqib A., Forsyth C.B., Mutlu E., and Shannon K.M.. 2015. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 30:1351–1360. 10.1002/mds.26307 [DOI] [PubMed] [Google Scholar]
- Kierdorf K., Erny D., Goldmann T., Sander V., Schulz C., Perdiguero E.G., Wieghofer P., Heinrich A., Riemke P., Hölscher C., et al. . 2013. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 16:273–280. 10.1038/nn.3318 [DOI] [PubMed] [Google Scholar]
- Kim Y.S., and Joh T.H.. 2006. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson’s disease. Exp. Mol. Med. 38:333–347. 10.1038/emm.2006.40 [DOI] [PubMed] [Google Scholar]
- Kim S., Kim H., Yim Y.S., Ha S., Atarashi K., Tan T.G., Longman R.S., Honda K., Littman D.R., Choi G.B., and Huh J.R.. 2017. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature. 549:528–532. 10.1038/nature23910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura I., Inoue D., Maeda T., Hara T., Ichimura A., Miyauchi S., Kobayashi M., Hirasawa A., and Tsujimoto G.. 2011. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA. 108:8030–8035. 10.1073/pnas.1016088108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsknecht-Talboo J., Meyer-Luehmann M., Parsadanian M., Garcia-Alloza M., Finn M.B., Hyman B.T., Bacskai B.J., and Holtzman D.M.. 2008. Rapid microglial response around amyloid pathology after systemic anti-Abeta antibody administration in PDAPP mice. J. Neurosci. 28:14156–14164. 10.1523/JNEUROSCI.4147-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsuzaki N., Shima J., Kawamoto S., Momose H., and Kimura T.. 2005. Production of γ-aminobutyric acid (GABA) by Lactobacillus paracasei isolated from traditional fermented foods. Food Microbiol. 22:497–504. 10.1016/j.fm.2005.01.002 [DOI] [Google Scholar]
- Kosmidou M., Katsanos A.H., Katsanos K.H., Kyritsis A.P., Tsivgoulis G., Christodoulou D., and Giannopoulos S.. 2017. Multiple sclerosis and inflammatory bowel diseases: a systematic review and meta-analysis. J. Neurol. 264:254–259. 10.1007/s00415-016-8340-8 [DOI] [PubMed] [Google Scholar]
- Koyama R., and Ikegaya Y.. 2015. Microglia in the pathogenesis of autism spectrum disorders. Neurosci. Res. 100:1–5. 10.1016/j.neures.2015.06.005 [DOI] [PubMed] [Google Scholar]
- Lambert J.-C., Ibrahim-Verbaas C.A., Harold D., Naj A.C., Sims R., Bellenguez C., DeStafano A.L., Bis J.C., Beecham G.W., Grenier-Boley B., et al. Cohorts for Heart and Aging Research in Genomic Epidemiology . 2013. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 45:1452–1458. 10.1038/ng.2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A.S., Azmitia E.C., and Whitaker-Azmitia P.M.. 2017. Developmental microglial priming in postmortem autism spectrum disorder temporal cortex. Brain Behav. Immun. 62:193–202. 10.1016/j.bbi.2017.01.019 [DOI] [PubMed] [Google Scholar]
- Lee Y.K., Menezes J.S., Umesaki Y., and Mazmanian S.K.. 2011. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA. 108(Suppl 1):4615–4622. 10.1073/pnas.1000082107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Fang F., Pedersen N.L., Tillander A., Ludvigsson J.F., Ekbom A., Svenningsson P., Chen H., and Wirdefeldt K.. 2017. Vagotomy and Parkinson disease: A Swedish register-based matched-cohort study. Neurology. 88:1996–2002. 10.1212/WNL.0000000000003961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Price J., Abu-Ali G., and Huttenhower C.. 2016. The healthy human microbiome. Genome Med. 8:51 10.1186/s13073-016-0307-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczynski P., McVey Neufeld K.-A., Oriach C.S., Clarke G., Dinan T.G., and Cryan J.F.. 2016. Growing up in a Bubble: Using Germ-Free Animals to Assess the Influence of the Gut Microbiota on Brain and Behavior. Int. J. Neuropsychopharmacol. 19:pyw020 10.1093/ijnp/pyw020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C., Jian C., Liao Y., Huang Q., Wu Y., Liu X., Zou D., and Wu Y.. 2017. The role of microglia in multiple sclerosis. Neuropsychiatr. Dis. Treat. 13:1661–1667. 10.2147/NDT.S140634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFabe D.F. 2015. Enteric short-chain fatty acids: microbial messengers of metabolism, mitochondria, and mind: implications in autism spectrum disorders. Microb. Ecol. Health Dis. 26:28177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado V., Zöller T., Attaai A., and Spittau B.. 2016. Microglia-Mediated Neuroinflammation and Neurotrophic Factor-Induced Protection in the MPTP Mouse Model of Parkinson’s Disease-Lessons from Transgenic Mice. Int. J. Mol. Sci. 17:E151 10.3390/ijms17020151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova N.V., Yu C.Z., Hsiao E.Y., Moore M.J., and Patterson P.H.. 2012. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav. Immun. 26:607–616. 10.1016/j.bbi.2012.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin I.A., Goertz J.E., Ren T., Rich S.S., Onengut-Gumuscu S., Farber E., Wu M., Overall C.C., Kipnis J., and Gaultier A.. 2017. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci. Rep. 7:43859 10.1038/srep43859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez K.B., Leone V., and Chang E.B.. 2017. Microbial metabolites in health and disease: Navigating the unknown in search of function. J. Biol. Chem. 292:8553–8559. 10.1074/jbc.R116.752899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Cerdeño V. 2017. Dendrite and spine modifications in autism and related neurodevelopmental disorders in patients and animal models. Dev. Neurobiol. 77:393–404. 10.1002/dneu.22417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matcovitch-Natan O., Winter D.R., Giladi A., Vargas Aguilar S., Spinrad A., Sarrazin S., Ben-Yehuda H., David E., Zelada González F., Perrin P., et al. . 2016. Microglia development follows a stepwise program to regulate brain homeostasis. Science. 353:aad8670 10.1126/science.aad8670 [DOI] [PubMed] [Google Scholar]
- Mattei D., Ivanov A., Ferrai C., Jordan P., Guneykaya D., Buonfiglioli A., Schaafsma W., Przanowski P., Deuther-Conrad W., Brust P., et al. . 2017. Maternal immune activation results in complex microglial transcriptome signature in the adult offspring that is reversed by minocycline treatment. Transl. Psychiatry. 7:e1120 10.1038/tp.2017.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer E.A., Tillisch K., and Gupta A.. 2015. Gut/brain axis and the microbiota. J. Clin. Invest. 125:926–938. 10.1172/JCI76304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer P.L., Itagaki S., Boyes B.E., and McGeer E.G.. 1988. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 38:1285–1291. 10.1212/WNL.38.8.1285 [DOI] [PubMed] [Google Scholar]
- Meneses G., Bautista M., Florentino A., Díaz G., Acero G., Besedovsky H., Meneses D., Fleury A., Del Rey A., Gevorkian G., et al. . 2016. Electric stimulation of the vagus nerve reduced mouse neuroinflammation induced by lipopolysaccharide. J. Inflamm. (Lond.). 13:33 10.1186/s12950-016-0140-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertsalmi T.H., Aho V.T.E., Pereira P.A.B., Paulin L., Pekkonen E., Auvinen P., and Scheperjans F.. 2017. More than constipation - bowel symptoms in Parkinson’s disease and their connection to gut microbiota. Eur. J. Neurol. 24:1375–1383. 10.1111/ene.13398 [DOI] [PubMed] [Google Scholar]
- Miao F.J.-P., Green P.G., and Levine J.D.. 2004. Mechanosensitive duodenal afferents contribute to vagal modulation of inflammation in the rat. J. Physiol. 554:227–235. 10.1113/jphysiol.2003.056804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B.J., Buckley P., Seabolt W., Mellor A., and Kirkpatrick B.. 2011. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol. Psychiatry. 70:663–671. 10.1016/j.biopsych.2011.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minter M.R., Zhang C., Leone V., Ringus D.L., Zhang X., Oyler-Castrillo P., Musch M.W., Liao F., Ward J.F., Holtzman D.M., et al. . 2016. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci. Rep. 6:30028 10.1038/srep30028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake S., Kim S., Suda W., Oshima K., Nakamura M., Matsuoka T., Chihara N., Tomita A., Sato W., Kim S.-W., et al. . 2015. Dysbiosis in the Gut Microbiota of Patients with Multiple Sclerosis, with a Striking Depletion of Species Belonging to Clostridia XIVa and IV Clusters. PLoS One. 10:e0137429 10.1371/journal.pone.0137429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monji A., Kato T.A., Mizoguchi Y., Horikawa H., Seki Y., Kasai M., Yamauchi Y., Yamada S., and Kanba S.. 2013. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 42:115–121. 10.1016/j.pnpbp.2011.12.002 [DOI] [PubMed] [Google Scholar]
- Morgan J.T., Chana G., Pardo C.A., Achim C., Semendeferi K., Buckwalter J., Courchesne E., and Everall I.P.. 2010. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol. Psychiatry. 68:368–376. 10.1016/j.biopsych.2010.05.024 [DOI] [PubMed] [Google Scholar]
- Na K.-S., Jung H.-Y., and Kim Y.-K.. 2014. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 48:277–286. 10.1016/j.pnpbp.2012.10.022 [DOI] [PubMed] [Google Scholar]
- Napoli I., and Neumann H.. 2010. Protective effects of microglia in multiple sclerosis. Exp. Neurol. 225:24–28. 10.1016/j.expneurol.2009.04.024 [DOI] [PubMed] [Google Scholar]
- Needham B.D., Tang W., and Wu W.-L.. 2018. Searching for the gut microbial contributing factors to social behavior in rodent models of autism spectrum disorder. Dev. Neurobiol. 78:474–499. 10.1002/dneu.22581 [DOI] [PubMed] [Google Scholar]
- Neufeld K.M., Kang N., Bienenstock J., and Foster J.A.. 2011. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 23:255–264: e119. 10.1111/j.1365-2982.2010.01620.x [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A., Kirchhoff F., and Helmchen F.. 2005. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 308:1314–1318. 10.1126/science.1110647 [DOI] [PubMed] [Google Scholar]
- Norden D.M., Muccigrosso M.M., and Godbout J.P.. 2015. Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology. 96(Pt A):29–41. 10.1016/j.neuropharm.2014.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan S.S., Williams D.R., Gallagher D.A., Massey L.A., Silveira-Moriyama L., and Lees A.J.. 2008. Nonmotor symptoms as presenting complaints in Parkinson’s disease: a clinicopathological study. Mov. Disord. 23:101–106. 10.1002/mds.21813 [DOI] [PubMed] [Google Scholar]
- Obermeier B., Daneman R., and Ransohoff R.M.. 2013. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 19:1584–1596. 10.1038/nm.3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbonnaya E.S., Clarke G., Shanahan F., Dinan T.G., Cryan J.F., and O’Leary O.F.. 2015. Adult Hippocampal Neurogenesis Is Regulated by the Microbiome. Biol. Psychiatry. 78:e7–e9. 10.1016/j.biopsych.2014.12.023 [DOI] [PubMed] [Google Scholar]
- Palmer C., Bik E.M., DiGiulio D.B., Relman D.A., and Brown P.O.. 2007. Development of the human infant intestinal microbiota. PLoS Biol. 5:e177 10.1371/journal.pbio.0050177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli R.C., Bolasco G., Pagani F., Maggi L., Scianni M., Panzanelli P., Giustetto M., Ferreira T.A., Guiducci E., Dumas L., et al. . 2011. Synaptic pruning by microglia is necessary for normal brain development. Science. 333:1456–1458. 10.1126/science.1202529 [DOI] [PubMed] [Google Scholar]
- Pelvig D.P., Pakkenberg H., Stark A.K., and Pakkenberg B.. 2008. Neocortical glial cell numbers in human brains. Neurobiol. Aging. 29:1754–1762. 10.1016/j.neurobiolaging.2007.04.013 [DOI] [PubMed] [Google Scholar]
- Perry V.H., and Holmes C.. 2014. Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 10:217–224. 10.1038/nrneurol.2014.38 [DOI] [PubMed] [Google Scholar]
- Piccio L., Buonsanti C., Mariani M., Cella M., Gilfillan S., Cross A.H., Colonna M., and Panina-Bordignon P.. 2007. Blockade of TREM-2 exacerbates experimental autoimmune encephalomyelitis. Eur. J. Immunol. 37:1290–1301. 10.1002/eji.200636837 [DOI] [PubMed] [Google Scholar]
- Plauche Johnson C. 2004. Early clinical characteristics of children with autism. In Autistic Spectrum Disorders in Children. Gupta V., editor. CRC Press, Boca Raton, FL. pp. 83–121. 10.1201/9780203026229.ch5 [DOI] [Google Scholar]
- Poirier A.-A., Aubé B., Côté M., Morin N., Di Paolo T., and Soulet D.. 2016. Gastrointestinal Dysfunctions in Parkinson’s Disease: Symptoms and Treatments. Parkinsons Dis. 2016:6762528 10.1155/2016/6762528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokusaeva K., Johnson C., Luk B., Uribe G., Fu Y., Oezguen N., Matsunami R.K., Lugo M., Major A., Mori-Akiyama Y., et al. . 2017. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol. Motil. 29:e12904 10.1111/nmo.12904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma R.B., Berg D., Stern M., Poewe W., Olanow C.W., Oertel W., Obeso J., Marek K., Litvan I., Lang A.E., et al. . 2015. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30:1591–1601. 10.1002/mds.26424 [DOI] [PubMed] [Google Scholar]
- Pratt L., Ni L., Ponzio N.M., and Jonakait G.M.. 2013. Maternal inflammation promotes fetal microglial activation and increased cholinergic expression in the fetal basal forebrain: role of interleukin-6. Pediatr. Res. 74:393–401. 10.1038/pr.2013.126 [DOI] [PubMed] [Google Scholar]
- Qin L., He J., Hanes R.N., Pluzarev O., Hong J.-S., and Crews F.T.. 2008. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J. Neuroinflammation. 5:10 10.1186/1742-2094-5-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybould H.E. 2010. Gut chemosensing: interactions between gut endocrine cells and visceral afferents. Auton. Neurosci. 153:41–46. 10.1016/j.autneu.2009.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisinger S., Khan D., Kong E., Berger A., Pollak A., and Pollak D.D.. 2015. The poly(I:C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacol. Ther. 149:213–226. 10.1016/j.pharmthera.2015.01.001 [DOI] [PubMed] [Google Scholar]
- Riazi K., Galic M.A., Kuzmiski J.B., Ho W., Sharkey K.A., and Pittman Q.J.. 2008. Microglial activation and TNFalpha production mediate altered CNS excitability following peripheral inflammation. Proc. Natl. Acad. Sci. USA. 105:17151–17156. 10.1073/pnas.0806682105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietdijk C.D., Perez-Pardo P., Garssen J., van Wezel R.J.A., and Kraneveld A.D.. 2017. Exploring Braak’s Hypothesis of Parkinson’s Disease. Front. Neurol. 8:37 10.3389/fneur.2017.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochfort K.D., Collins L.E., Murphy R.P., and Cummins P.M.. 2014. Downregulation of blood-brain barrier phenotype by proinflammatory cytokines involves NADPH oxidase-dependent ROS generation: consequences for interendothelial adherens and tight junctions. PLoS One. 9:e101815 10.1371/journal.pone.0101815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez J.M., Murphy K., Stanton C., Ross R.P., Kober O.I., Juge N., Avershina E., Rudi K., Narbad A., Jenmalm M.C., et al. . 2015. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 26:26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V., Borucki D.M., Tjon E.C., Takenaka M.C., Chao C.-C., Ardura-Fabregat A., de Lima K.A., Gutiérrez-Vázquez C., Hewson P., Staszewski O., et al. . 2018. Microglial control of astrocytes in response to microbial metabolites. Nature. 557:724–728. 10.1038/s41586-018-0119-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round J.L., Lee S.M., Li J., Tran G., Jabri B., Chatila T.A., and Mazmanian S.K.. 2011. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 332:974–977. 10.1126/science.1206095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowin J., Xia Y., Jung B., and Sun J.. 2017. Gut inflammation and dysbiosis in human motor neuron disease. Physiol. Rep. 5:e13443 10.14814/phy2.13443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo K., and Glass C.K.. 2011. Microglial cell origin and phenotypes in health and disease. Nat. Rev. Immunol. 11:775–787. 10.1038/nri3086 [DOI] [PubMed] [Google Scholar]
- Salter M.W., and Stevens B.. 2017. Microglia emerge as central players in brain disease. Nat. Med. 23:1018–1027. 10.1038/nm.4397 [DOI] [PubMed] [Google Scholar]
- Sampson T.R., Debelius J.W., Thron T., Janssen S., Shastri G.G., Ilhan Z.E., Challis C., Schretter C.E., Rocha S., Gradinaru V., et al. . 2016. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell. 167:1469–1480.e12. 10.1016/j.cell.2016.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D.P., Lehrman E.K., Kautzman A.G., Koyama R., Mardinly A.R., Yamasaki R., Ransohoff R.M., Greenberg M.E., Barres B.A., and Stevens B.. 2012. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 74:691–705. 10.1016/j.neuron.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F., Aho V., Pereira P.A.B., Koskinen K., Paulin L., Pekkonen E., Haapaniemi E., Kaakkola S., Eerola-Rautio J., Pohja M., et al. . 2015. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 30:350–358. 10.1002/mds.26069 [DOI] [PubMed] [Google Scholar]
- Schwarz J.M., Sholar P.W., and Bilbo S.D.. 2012. Sex differences in microglial colonization of the developing rat brain. J. Neurochem. 120:948–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar A., Bialas A.R., de Rivera H., Davis A., Hammond T.R., Kamitaki N., Tooley K., Presumey J., Baum M., Van Doren V., et al. Schizophrenia Working Group of the Psychiatric Genomics Consortium . 2016. Schizophrenia risk from complex variation of complement component 4. Nature. 530:177–183. 10.1038/nature16549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance E.G., Dickerson F.B., Halling M., Krivogorsky B., Haile L., Yang S., Stallings C.R., Origoni A.E., Bossis I., Xiao J., et al. . 2010. Subunit and whole molecule specificity of the anti-bovine casein immune response in recent onset psychosis and schizophrenia. Schizophr. Res. 118:240–247. 10.1016/j.schres.2009.12.030 [DOI] [PubMed] [Google Scholar]
- Severance E.G., Prandovszky E., Castiglione J., and Yolken R.H.. 2015. Gastroenterology issues in schizophrenia: why the gut matters. Curr. Psychiatry Rep. 17:27 10.1007/s11920-015-0574-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. 2010. Increased urinary excretion of a 3-(3-hydroxyphenyl)-3-hydroxypropionic acid (HPHPA), an abnormal phenylalanine metabolite of Clostridia spp. in the gastrointestinal tract, in urine samples from patients with autism and schizophrenia. Nutr. Neurosci. 13:135–143. 10.1179/147683010X12611460763968 [DOI] [PubMed] [Google Scholar]
- Sheng J., Ruedl C., and Karjalainen K.. 2015. Most Tissue-Resident Macrophages Except Microglia Are Derived from Fetal Hematopoietic Stem Cells. Immunity. 43:382–393. 10.1016/j.immuni.2015.07.016 [DOI] [PubMed] [Google Scholar]
- Shie F.-S., Nivison M., Hsu P.-C., and Montine T.J.. 2009. Modulation of microglial innate immunity in Alzheimer’s disease by activation of peroxisome proliferator-activated receptor gamma. Curr. Med. Chem. 16:643–651. 10.2174/092986709787458399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman J.M., De Jager P.L., and Feany M.B.. 2011. Parkinson’s disease: genetics and pathogenesis. Annu. Rev. Pathol. 6:193–222. 10.1146/annurev-pathol-011110-130242 [DOI] [PubMed] [Google Scholar]
- Sierra A., Encinas J.M., Deudero J.J.P., Chancey J.H., Enikolopov G., Overstreet-Wadiche L.S., Tsirka S.E., and Maletic-Savatic M.. 2010. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 7:483–495. 10.1016/j.stem.2010.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A., de Castro F., Del Río-Hortega J., Rafael Iglesias-Rozas J., Garrosa M., and Kettenmann H.. 2016. The “Big-Bang” for modern glial biology: Translation and comments on Pío del Río-Hortega 1919 series of papers on microglia. Glia. 64:1801–1840. 10.1002/glia.23046 [DOI] [PubMed] [Google Scholar]
- Smith P.M., Howitt M.R., Panikov N., Michaud M., Gallini C.A., Bohlooly-Y M., Glickman J.N., and Garrett W.S.. 2013. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 341:569–573. 10.1126/science.1241165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B., Allen N.J., Vazquez L.E., Howell G.R., Christopherson K.S., Nouri N., Micheva K.D., Mehalow A.K., Huberman A.D., Stafford B., et al. . 2007. The classical complement cascade mediates CNS synapse elimination. Cell. 131:1164–1178. 10.1016/j.cell.2007.10.036 [DOI] [PubMed] [Google Scholar]
- Su X., Maguire-Zeiss K.A., Guiliano R., Prifti L., Venkatesh K., and Federoff H.J.. 2008. Synuclein activates microglia in a model of Parkinson's disease. Neurobiol. Aging. 29:1690–1701. 10.1016/j.neurobiolaging.2007.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M.-F., Zhu Y.-L., Zhou Z.-L., Jia X.-B., Xu Y.-D., Yang Q., Cui C., and Shen Y.-Q.. 2018. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav. Immun. 70:48–60. 10.1016/j.bbi.2018.02.005 [DOI] [PubMed] [Google Scholar]
- Sun S.-W., Liang H.-F., Trinkaus K., Cross A.H., Armstrong R.C., and Song S.-K.. 2006. Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn. Reson. Med. 55:302–308. 10.1002/mrm.20774 [DOI] [PubMed] [Google Scholar]
- Svensson E., Horváth-Puhó E., Thomsen R.W., Djurhuus J.C., Pedersen L., Borghammer P., and Sørensen H.T.. 2015. Vagotomy and subsequent risk of Parkinson’s disease. Ann. Neurol. 78:522–529. 10.1002/ana.24448 [DOI] [PubMed] [Google Scholar]
- Tan A.H., Mahadeva S., Thalha A.M., Gibson P.R., Kiew C.K., Yeat C.M., Ng S.W., Ang S.P., Chow S.K., Tan C.T., et al. . 2014a Small intestinal bacterial overgrowth in Parkinson’s disease. Parkinsonism Relat. Disord. 20:535–540. 10.1016/j.parkreldis.2014.02.019 [DOI] [PubMed] [Google Scholar]
- Tan J., McKenzie C., Potamitis M., Thorburn A.N., Mackay C.R., and Macia L.. 2014b Chapter Three - The Role of Short-Chain Fatty Acids in Health and Disease. In Advances in Immunology. Alt F.W., editor. Academic Press; pp. 91–119. [DOI] [PubMed] [Google Scholar]
- Tang Y., and Le W.. 2016. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 53:1181–1194. 10.1007/s12035-014-9070-5 [DOI] [PubMed] [Google Scholar]
- Tanida M., Yamano T., Maeda K., Okumura N., Fukushima Y., and Nagai K.. 2005. Effects of intraduodenal injection of Lactobacillus johnsonii La1 on renal sympathetic nerve activity and blood pressure in urethane-anesthetized rats. Neurosci. Lett. 389:109–114. 10.1016/j.neulet.2005.07.036 [DOI] [PubMed] [Google Scholar]
- Thion M.S., Low D., Silvin A., Chen J., Grisel P., Schulte-Schrepping J., Blecher R., Ulas T., Squarzoni P., Hoeffel G., et al. . 2018. Microbiome Influences Prenatal and Adult Microglia in a Sex-Specific Manner. Cell. 172:500–516.e16. 10.1016/j.cell.2017.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Platas S.G., Comeau S., Rachalski A., Bo G.D., Cruceanu C., Turecki G., Giros B., and Mechawar N.. 2014. Morphometric characterization of microglial phenotypes in human cerebral cortex. J. Neuroinflammation. 11:12 10.1186/1742-2094-11-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay M.-È., Lowery R.L., and Majewska A.K.. 2010. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 8:e1000527 10.1371/journal.pbio.1000527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M.R., Cagnin A., Turkheimer F.E., Miller C.C.J., Shaw C.E., Brooks D.J., Leigh P.N., and Banati R.B.. 2004. Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11C](R)-PK11195 positron emission tomography study. Neurobiol. Dis. 15:601–609. 10.1016/j.nbd.2003.12.012 [DOI] [PubMed] [Google Scholar]
- Ueno M., Fujita Y., Tanaka T., Nakamura Y., Kikuta J., Ishii M., and Yamashita T.. 2013. Layer V cortical neurons require microglial support for survival during postnatal development. Nat. Neurosci. 16:543–551. 10.1038/nn.3358 [DOI] [PubMed] [Google Scholar]
- Unger M.M., Spiegel J., Dillmann K.-U., Grundmann D., Philippeit H., Bürmann J., Faßbender K., Schwiertz A., and Schäfer K.-H.. 2016. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat. Disord. 32:66–72. 10.1016/j.parkreldis.2016.08.019 [DOI] [PubMed] [Google Scholar]
- van Kesteren C.F.M.G., Gremmels H., de Witte L.D., Hol E.M., Van Gool A.R., Falkai P.G., Kahn R.S., and Sommer I.E.C.. 2017. Immune involvement in the pathogenesis of schizophrenia: a meta-analysis on postmortem brain studies. Transl. Psychiatry. 7:e1075 10.1038/tp.2017.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas D.L., Nascimbene C., Krishnan C., Zimmerman A.W., and Pardo C.A.. 2005. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 57:67–81. 10.1002/ana.20315 [DOI] [PubMed] [Google Scholar]
- Vighi G., Marcucci F., Sensi L., Di Cara G., and Frati F.. 2008. Allergy and the gastrointestinal system. Clin. Exp. Immunol. 153(Suppl 1):3–6. 10.1111/j.1365-2249.2008.03713.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel D.Y.S., Vereyken E.J.F., Glim J.E., Heijnen P.D., Moeton M., van der Valk P., Amor S., Teunissen C.E., van Horssen J., and Dijkstra C.D.. 2013. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J. Neuroinflammation. 10:35 10.1186/1742-2094-10-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineagu I., Wang X., Johnston P., Lowe J.K., Tian Y., Horvath S., Mill J., Cantor R.M., Blencowe B.J., and Geschwind D.H.. 2011. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 474:380–384. 10.1038/nature10110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Yu M., Ochani M., Amella C.A., Tanovic M., Susarla S., Li J.H., Wang H., Yang H., Ulloa L., et al. . 2003. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 421:384–388. 10.1038/nature01339 [DOI] [PubMed] [Google Scholar]
- Wang L., Christophersen C.T., Sorich M.J., Gerber J.P., Angley M.T., and Conlon M.A.. 2012. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig. Dis. Sci. 57:2096–2102. 10.1007/s10620-012-2167-7 [DOI] [PubMed] [Google Scholar]
- Wang L., Christophersen C.T., Sorich M.J., Gerber J.P., Angley M.T., and Conlon M.A.. 2013. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol. Autism. 4:42 10.1186/2040-2392-4-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Wang B.-R., Zhang X.-J., Xu Z., Ding Y.-Q., and Ju G.. 2002. Evidences for vagus nerve in maintenance of immune balance and transmission of immune information from gut to brain in STM-infected rats. World J. Gastroenterol. 8:540–545. 10.3748/wjg.v8.i3.540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson M.B., Richter F., Lee S.K., Gabby L., Wu J., Masliah E., Effros R.B., and Chesselet M.-F.. 2012. Regionally-specific microglial activation in young mice over-expressing human wildtype alpha-synuclein. Exp. Neurol. 237:318–334. 10.1016/j.expneurol.2012.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welter D., MacArthur J., Morales J., Burdett T., Hall P., Junkins H., Klemm A., Flicek P., Manolio T., Hindorff L., and Parkinson H.. 2014. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 42(Database issue, D1):D1001–D1006. 10.1093/nar/gkt1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J., Logan R.F., Hubbard R.B., and Card T.R.. 2006. Risk of schizophrenia in people with coeliac disease, ulcerative colitis and Crohn’s disease: a general population-based study. Aliment. Pharmacol. Ther. 23:71–74. 10.1111/j.1365-2036.2006.02720.x [DOI] [PubMed] [Google Scholar]
- Winek K., Dirnagl U., and Meisel A.. 2016. The Gut Microbiome as Therapeutic Target in Central Nervous System Diseases: Implications for Stroke. Neurotherapeutics. 13:762–774. 10.1007/s13311-016-0475-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Yi J., Zhang Y.-G., Zhou J., and Sun J.. 2015. Leaky intestine and impaired microbiome in an amyotrophic lateral sclerosis mouse model. Physiol. Rep. 3:e12356 10.14814/phy2.12356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn T.A., Chawla A., and Pollard J.W.. 2013. Macrophage biology in development, homeostasis and disease. Nature. 496:445–455. 10.1038/nature12034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier A.L., Menezes J.R.L., Goldman S.A., and Nedergaard M.. 2014. Fine-tuning the central nervous system: microglial modelling of cells and synapses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369:20130593 10.1098/rstb.2013.0593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z., Haroutunian V., Ho L., Purohit D., and Pasinetti G.M.. 2006. Microglia activation in the brain as inflammatory biomarker of Alzheimer’s disease neuropathology and clinical dementia. Dis. Markers. 22:95–102. 10.1155/2006/276239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacyshyn B., Meddings J., Sadowski D., and Bowen-Yacyshyn M.B.. 1996. Multiple sclerosis patients have peripheral blood CD45RO+ B cells and increased intestinal permeability. Dig. Dis. Sci. 41:2493–2498. 10.1007/BF02100148 [DOI] [PubMed] [Google Scholar]
- Yano J.M., Yu K., Donaldson G.P., Shastri G.G., Ann P., Ma L., Nagler C.R., Ismagilov R.F., Mazmanian S.K., and Hsiao E.Y.. 2015. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 161:264–276. 10.1016/j.cell.2015.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R., Rimmerman N., and Reshef R.. 2015. Depression as a microglial disease. Trends Neurosci. 38:637–658. 10.1016/j.tins.2015.08.001 [DOI] [PubMed] [Google Scholar]
- Yokote H., Miyake S., Croxford J.L., Oki S., Mizusawa H., and Yamamura T.. 2008. NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora. Am. J. Pathol. 173:1714–1723. 10.2353/ajpath.2008.080622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R., and Dickerson F.. 2014. THE MICROBIOME-THE MISSING LINK IN THE PATHOGENESIS OF SCHIZOPHRENIA. Schizophr. Res. 153:S16 10.1016/S0920-9964(14)70050-7 [DOI] [Google Scholar]
- Yoo B.B., and Mazmanian S.K.. 2017. The Enteric Network: Interactions between the Immune and Nervous Systems of the Gut. Immunity. 46:910–926. 10.1016/j.immuni.2017.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata H.J., and Quagliarello V.J.. 2015. The microbiota and microbiome in aging: potential implications in health and age-related diseases. J. Am. Geriatr. Soc. 63:776–781. 10.1111/jgs.13310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y., Paolicelli R.C., Sforazzini F., Weinhard L., Bolasco G., Pagani F., Vyssotski A.L., Bifone A., Gozzi A., Ragozzino D., and Gross C.T.. 2014. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 17:400–406. 10.1038/nn.3641 [DOI] [PubMed] [Google Scholar]
- Zhang W., Wang T., Pei Z., Miller D.S., Wu X., Block M.L., Wilson B., Zhang W., Zhou Y., Hong J.-S., and Zhang J.. 2005. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 19:533–542. 10.1096/fj.04-2751com [DOI] [PubMed] [Google Scholar]
- Zhang Y.-G., Wu S., Yi J., Xia Y., Jin D., Zhou J., and Sun J.. 2017. Target Intestinal Microbiota to Alleviate Disease Progression in Amyotrophic Lateral Sclerosis. Clin. Ther. 39:322–336. 10.1016/j.clinthera.2016.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Beers D.R., Henkel J.S., Zhang W., Urushitani M., Julien J.-P., and Appel S.H.. 2010. Extracellular mutant SOD1 induces microglial-mediated motoneuron injury. Glia. 58:231–243. 10.1002/glia.20919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Beers D.R., and Appel S.H.. 2013. Immune-mediated mechanisms in the pathoprogression of amyotrophic lateral sclerosis. J. Neuroimmune Pharmacol. 8:888–899. 10.1007/s11481-013-9489-x [DOI] [PMC free article] [PubMed] [Google Scholar]