Benoit and Poüs highlight new work from Lindeboom et al. revealing how CLASP reorients microtubule networks in plants growing toward light.
Abstract
Microtubule reorientation into a longitudinal network during the phototropic response in Arabidopsis thaliana depends on their severing by katanin at crossovers. Lindeboom et al. (2019. J. Cell Biol. https://doi.org/10.1083/jcb.201805047) show that at newly generated plus ends, the anti-catastrophe activity of CLASP is essential for further growth.
Microtubules (MTs) are polarized and dynamic polymers of tubulin, with plus ends able to alternate between growth and shrinking. This process, which is called dynamic instability, intrinsically depends on the presence or loss of a tubulin GTP cap at MT plus ends. It is highly regulated by several MT-associated proteins (MAPs) of the plus end–tracking protein (+TIP) family. These regulators comprise polymerases and depolymerases, which add or remove tubulin subunits, and regulators of transitions that promote disassembly (catastrophes) or interrupt it, making regrowth possible (rescues). Also, important regulation mechanisms involve the stabilization of both MT ends and the creation of new free ends thanks to MT severing. In animal cells, MT arrays are most frequently built around MT organizing centers (MTOCs), mainly the centrosomes and the Golgi complex. They may also self-organize into acentrosomal arrays, thanks to nucleation on preexisting MTs, detachment from the MTOCs, or more distal severing. Acentrosomal organization is frequent, if not exclusive, in epithelial cells along the apico-basal polarity axis, in neurites (both in dendrites and in the axon), in multinucleated skeletal muscle cells, in the oocyte germ cells before meiosis, in unicellular yeast, and in plant cells.
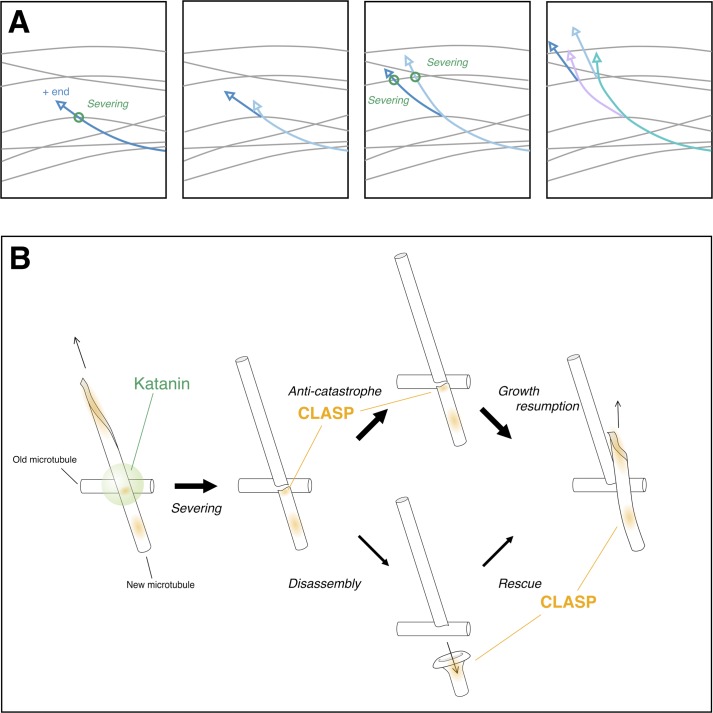
Due to their highly dynamic nature, MTs are a good means for cells to sense, organize, and signal in response to normal or stressful environmental cues. The phototropic response, which makes plants grow toward light, involves the creation of an auxin gradient and reorientation of the MT array to promote aligned cellulose fibril deposition and consecutive anisotropic growth. In the hypocotyl of Arabidopsis thaliana, the cortical cells undergo such MT reorientation within minutes in response to blue light. Starting from an orientation perpendicular to the main growth axis, the MT network undergoes a complete remodeling to switch to a longitudinal array. To achieve such a transformation, cells locally cut their network to rebuild it in a new orientation, thanks to the katanin-mediated severing of MTs at steep angle crossings, in a way that preferentially affects the younger MT (the crosser; 1). The two MTs that originate from the severed MT grow in a direction roughly perpendicular to the MT that was crossed, allowing the amplification of the longitudinal network (Fig. 1 A).
Figure 1.
MT reorientation in response to blue light. (A) MT reorientation results from the severing (green circles) of MTs (colored arrows) that crossed transversal MTs (gray). Longitudinal network amplification results from the protection against disassembly of both the newly created minus and plus ends. (B) Katanin (green) concentrated at MT crossover cuts the youngest MT, and then CLASP (orange), although less concentrated than at other MT dynamic hot spots, prevents the disassembly of the newly created plus end, allowing its elongation (top path). Alternatively, a depolymerizing plus end may be rescued thanks to the presence of CLASP (bottom path).
In this issue, Lindeboom et al. show that the MT network amplification that follows katanin-mediated severing relies on the conserved +TIP CLASP, which mainly protects newly created plus ends against disassembly (an anti-catastrophe effect), in addition to its rescue-promoting function away from crossings (Fig. 1 B). Although CLASP is less abundant at MT crossovers than at potential rescue sites and bent MT regions, it plays a determinant role in preventing the loss of severed MTs. Two other +TIPs were analyzed in this study: EB1 seems only important to reduce severing waiting time at crossovers, whereas SPIRAL1 does not participate at all in MT array reorientation, although it contributes to a lesser extent than CLASP in the control of rescues.
The advance made here by Lindeboom et al. (2) in understanding the mechanisms that control MT array reorientation in phototropism raises questions about MAP recruitment and interplay at MT crossovers and about the coordination between severing, protection against disassembly, and array amplification. The determinants of CLASP recruitment along the MT body and at crossovers, and its functions at each location, have to be better understood. Recent in vitro studies with mammalian CLASP2 showed that the second TOG domain of the protein suppresses catastrophes when targeted to MT plus ends, most likely by preventing the loss of the GTP cap, while it stimulates rescues behind the MT tip (3). CLASP2 protects both leading and lagging protofilaments against catastrophes during the periods of tip repair (3), which suggests that it might also recognize regions with lattice damage and defects and mark future potential rescue sites that frequently occur at MT crossovers (4, 5). In hypocotyl cortical cells, limited amounts of CLASP at crossovers could result from competition with the active recruitment of katanin. This is reminiscent of the situation in pavement cells of the cotyledon, in which SPIRAL2 and augmin protect MT crossovers from severing by katanin and prevent MT alignment (6, 7). Of note, in the hypocotyl, newly created minus ends are prevented from disassembly thanks to SPIRAL2, which tracks both minus and plus ends and also concentrates at MT crossovers (8, 9). Concerning katanin recruitment and target recognition, its ability to bind to MT defects (10) could explain why it is enriched at MT crossovers, but not why it selectively cuts the youngest MT.
Like crossovers, MT bundling and bending segments represent regions where the MTs are stressed due to the encounter of obstacles (like other MTs or membranes). Stress during elongation may cause subtle defects like a shift in the number of protofilaments, which may locally prevent GTP hydrolysis. Localized lattice damage of various origins may also occur during or after elongation, with the loss of tubulin subunits and their replacement by GTP-tubulin (4). The regions that present such alterations may thus transiently display a tubulin conformation that resembles the GTP caps (GTP islands). Such “MT dynamic hot spots” in the lattice frequently resist disassembly and function as rescue sites. Thanks to the coordinated recruitment of Hot Spot-Associated Proteins, which include at least katanin and some +TIPs (SPIRAL2 and CLASP in plants; EB3, CLIP-170, and CLASP in mammalian cells; 1, 3, 4, 5, 8, 9), MT dynamic hot spots are key locations where cells record morphological or stress-induced information. This information will control the future dynamic behavior of the MT network, pointing out the importance of hot spots in self-organization, both in centrosomal and acentrosomal arrays. In conclusion, the new work by Lindeboom et al. (2) provides an excellent basis for further exploring the mechanisms that determine how MT hotspots may control reorientation of the MT network by CLASP during phototropism.
Acknowledgments
The authors declare no competing financial interests.
References
- 1.Lindeboom J.J., et al. 2013. Science. 342:1245533–1245533. [DOI] [PubMed] [Google Scholar]
- 2.Lindeboom J.J., et al. J. Cell Biol. 2019 doi: 10.1083/jcb.201805047. [DOI] [Google Scholar]
- 3.Aher A., et al. 2018. Dev. Cell. 46:40–58.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aumeier C., et al. 2016. Nat. Cell Biol. 18:1054–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Forges H., et al. 2016. Curr. Biol. 26:3399–3406. [DOI] [PubMed] [Google Scholar]
- 6.Wightman R., et al. 2013. Curr. Biol. 23:1902–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang G., et al. 2018. Curr. Biol. 28:1311–1317.e3. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura M., et al. 2018. J. Cell Biol. 217:915–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan Y., et al. 2018. Curr. Biol. 28:987–994.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Díaz-Valencia J.D., et al. 2011. Biophys. J. 100:2440–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]