Abstract
Invasive Burmese pythons (Python bivittatus Kuhl, 1820) have introduced a lung parasite, Raillietiella orientalis, (Hett, 1915) from the python’s native range in Southeast Asia to its introduced range in Florida, where parasite spillover from pythons to two families and eight genera of native snakes has occurred. Because these novel host species present a diversity of ecological and morphological traits, and because these parasites attach to their hosts with hooks located on their cephalothorax, we predicted that R. orientalis would exhibit substantial, host-associated phenotypic plasticity in cephalothorax shape. Indeed, geometric morphometric analyses of 39 parasites from five host species revealed significant variation among host taxa in R. orientalis cephalothorax shape. We observed differences associated with host ecology, where parasites from semi-aquatic and aquatic snakes exhibited the greatest morphological similarity. Morphological analyses of R. orientalis recovered from invasive pythons, native pit vipers, and terrestrial snakes each revealed distinct shapes. Our results suggest R. orientalis can exhibit significant differences in morphology based upon host species infected, and this plasticity may facilitate infection with this non-native parasite in a wide array of novel squamate host species.
Introduction
Non-native species can harbor parasites and pathogens capable of infecting native taxa within their introduced range, a process known as parasite spillover [1–3]. For species with indirect lifestyles, potential obstacles to parasite spillover include low host density and lack of an appropriate intermediate host [4]. When a parasite species with an indirect life cycle successfully establishes, it may remain host-specific, infecting only the non-native host with which it was introduced, so long as an appropriate intermediate host is present [5]. This may result from low host susceptibility among potential definitive hosts within the parasite’s invaded range, a lack of appropriate intermediate hosts to allow transfer to potential hosts, or from founder effects resulting in reduced plasticity preventing transfer pathways present in the indigenous range of the parasite [6]. Despite the complexity of indirect parasite life cycles, some parasites co-introduced with non-native hosts have demonstrated an ability to infect novel hosts native to the introduced range [7, 8], often due to a combination of a parasite’s phenotypic plasticity among hosts [9–12] and the immunological naivety of a new host, rendering the host unable to deter infection by the novel parasite [13, 14].
Burmese pythons (Python bivittatus), native to Southeast Asia, have become established in southern Florida [15, 16] where they have co-introduced a lung parasite (Raillietiella orientalis: Raillietiellidae) previously unknown from North America [3]. Raillietiella orientalis has spilled over into the assemblage of native Floridian snakes, where the parasites have higher prevalence and intensity, achieve larger body size, and have populations dominated by reproductive females (Miller et al., in review). Within its native Asian distribution, R. orientalis infects snakes of at least four families, likely because a variety of intermediate hosts can be used to complete the indirect life cycle of this parasite [17]. Thus, the diverse network of intermediate and definitive hosts of the parasite in its native range appears to be replicated in Florida [3].
The diversity of definitive hosts for R. orientalis, and the associated differences in the lungs of these hosts that vary significantly in form and function [18], may result in concomitant variation of the cephalothorax morphology of this parasite, since the cephalothorax contains the hook structures that allow attachment within the host’s lung. Paradoxically, all R. orientalis sampled from Florida thus far are a single species with limited genetic diversity [3], suggesting that any morphological adaptations that facilitate attachment to such a wide range of hosts will take the form of morphological (i.e., phenotypic) plasticity. We used geometric morphometric analyses, which have been used to demonstrate phenotypic variability among populations of other parasite species [19–22], to investigate plastic responses of R. orientalis to phylogenetically and ecologically divergent native host snakes in southern Florida. We used these data to aid in understanding the mechanism that allows this parasite to successfully infect a wide range of native host taxa (Miller et al., in review).
Materials and methods
Burmese pythons were collected from their introduced range in southern Florida (Miami-Dade and Monroe Counties) during 2009–2015. Pythons were collected by hand during road surveys, opportunistic encounters, and through a collaborative removal effort between the United States Geological Survey and the National Park Service. Pythons were euthanized in accordance with AVMA guidelines by captive bolt gun and frozen within 24 hours. Native snakes were salvaged as road-kill during road surveys conducted in locations sympatric with pythons (see methods in [3]). Nocturnal road surveys were conducted consecutively, and salvaged native snakes were judged to be less than 24 hr post mortem upon collection and were immediately frozen. Snakes were dissected and pentastomes were collected and stored in 95% ethanol per standard preservation methods [23]. Methods of collection and preservation of snakes and parasites were comparable, respectively; Therefore, we assume potential fixation effects occurred in an unbiased fashion within and among hosts allowing us to interpret variation among hosts as indicating biological differences and not fixation effects. Pentastomes were cleared using an 80% phenol solution for 12–24 hours prior to being photographed. Each pentastome was placed on its dorsum on a microscope slide with a cover slip affixed on top of the specimen. Only adult female pentastomes were used, with sex determined based upon the presence (male) or absence (female) of copulatory spicules. Photographs of the ventral hooks and oral cadre (i.e. chitinised structure on mouth) of each parasite were taken at 2x magnification using a Nikon Eclipse Ni-E microscope and a Nikon DS-Fi2 camera; a 1000 μm scale was added to each image using Nikon NIS-Elements AR imaging software.
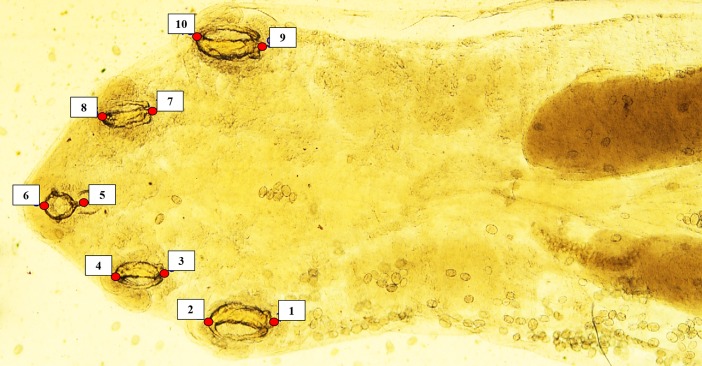
Ten homologous landmarks were designated on each photograph to examine placement of the oral cadre and hooks used to grasp the host’s lung tissue during feeding (Fig 1); points 1, 3, 7, and 9 were at the insertion of each hook; points 2, 4, 8, and 10 were at the anterior-most point of the curve of each hook; point 5 was at the peg-like extension of the oral cadre near the pharynx; and point 6 was at the anterior-most point of the oral cadre. Landmarks from each photo were digitized and a tps file containing X, Y coordinates for each landmark was prepared using tpsDig2 software [24]. We used host species as a classifier variable and a Procrustes ANOVA was used to test for differences in parasite cephalothorax shape among host taxa. Canonical variates analyses (CVA) was used to visualize separation among parasites in multivariate space. Confidence ellipses (95%) were assigned to all ordinations. Centroid size variation among parasites was analyzed to assess the effect of variation in pentastome length on hook arrangement. Morphological variation of hook structure in R. orientalis was compared to a consensus specimen and was visualized in Cartesian space via transformation grids. All analyses were performed using MORPHOJ software [25].
Fig 1. Whole mount of Raillietiella orientalis showing placement of homologous landmarks.
Ten landmarks were used in geometric morphometric analyses to examine variation in hook and oral cadre morphology among host taxa. Pentastome samples were cleared in a phenol solution prior to analyses. The R. orientalis pentastome shown was collected from a snake, Nerodia clarkii, native to Florida.
Results
A subsample of snake hosts collected were used in analyses to ensure the number of R. orientalis obtained from individuals of each host species met requirements for geometric morphometric analysis in which the total sample size minus the number of groups is greater than the number of landmarks [26]. Hosts of R. orientalis included five snake species, four of which were native to Florida (Agkistrodon piscivorus Gloyd, 1969: Viperidae; Coluber constrictor Linnaeus, 1758: Colubridae; Nerodia clarkii Kennicott, 1860: Colubridae; and Thamnophis sirtalis Linnaeus, 1758: Colubridae) and one snake that is a Florida invasive (Python bivittatus: Pythonidae). Thirty-nine R. orientalis specimens were examined from the five host species (Table 1).
Table 1. Sample sizes of snake hosts and pentastomes (Raillietiella orientalis) collected from southern Florida.
The number of host individuals and the total number of parasites examined per host are shown for each host species. Snake species native to Florida included Agkistrodon piscivorus, Coluber constrictor, Nerodia clarkii, and Thamnophis sirtalis; R. orientalis collected from invasive Burmese pythons (Python bivittatus) were examined for comparison.
| Host species | Number of host individuals | Number of R. orientalis |
|---|---|---|
| Python bivittatus | 5 | 10 |
| Agkistrodon piscivorus | 2 | 4 |
| Coluber constrictor | 2 | 6 |
| Nerodia clarkii | 3 | 14 |
| Thamnophis sirtalis | 3 | 5 |
| Total | 15 | 39 |
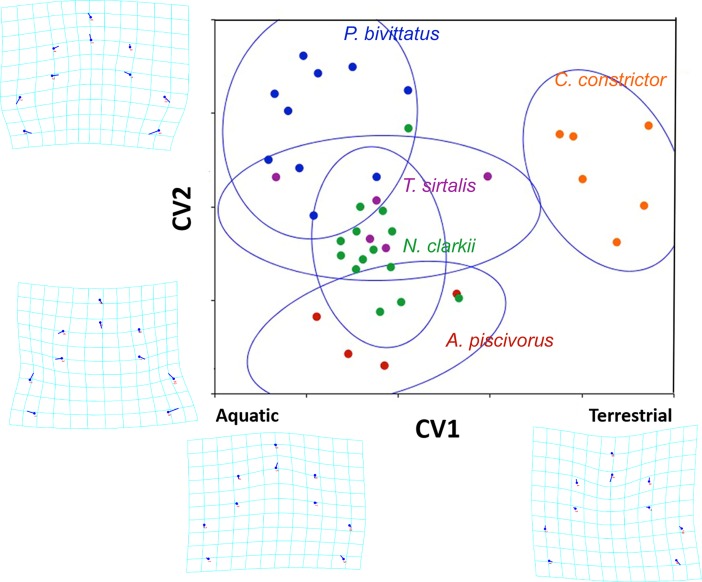
Parasite hooks and oral cadre morphology showed significant shape variation (Procrustes F = 2.27; df = 64; P = 0.0007). Centroid size variation was not significant (F = 2.16; df = 4; P = 0.09). Canonical variance analysis (CVA) separated terrestrial (C. constrictor) from aquatic (all other species) snakes along axis CV1(x) and separated pythonids (P. bivittatus) from pit vipers along axis CV2(y) (Fig 2). The total variation explained by both axes was 80.50%, with CV1(x) and CV2(y) accounting for 53.72% and 26.78% of the total variation, respectively.
Fig 2. Canonical variance analysis plot depicting the relative separation of Raillietiella orientalis among snake hosts.
Circles represent R. orientalis specimens obtained from their respective snake host (Python bivittatus = blue; Thamnophis sirtalis = purple; Nerodia clarkii = green; Coluber constrictor = orange; Agkistrodon piscivorus = red). Axis [CV1(x)] was replaced by a perceived biological axis based on ecological variation among hosts. Confidence ellipses (95%) are shown for each centroid. Wire frame grids are shown at the ends of each axis to provide context on variation in head morphology of R. orientalis along axes.
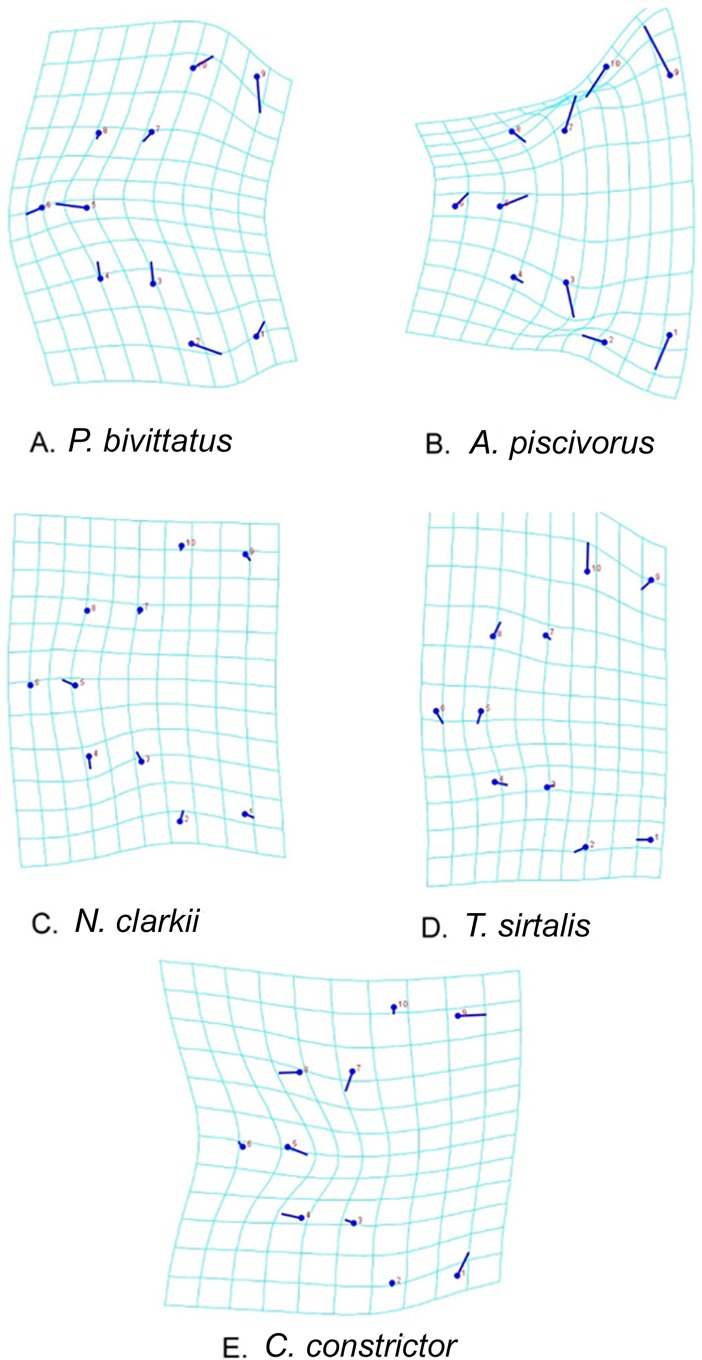
Significant variation in pentastome cephalothorax morphology occurred in length of the oral cadre and the extension of the oral cadre near the pharynx, hook length, and rotation of the hooks (Fig 3). Raillietiella orientalis samples collected from pythons had the shortest oral cadre, parallel anterior hooks, and posterior hooks that rotated away from the midline of the body compared to R. orientalis collected from other snake hosts. Those from A. piscivorus exhibited wider placement of the posterior hooks as well as compression along the anteroposterior axis, causing the oral cadre to align with the anterior hooks laterally. The anterior point of each hook also turned medially, a feature unique to R. orientalis within this host. Raillietiella orientalis recovered from N. clarkii and T. sirtalis did not differ from each other in morphology, exhibiting comparable anteroposterior compression to A. piscivorus, but with hooks that were parallel along the body axis or turned slightly in a lateral direction. However, increased lateral rotation of the hooks was observed in T. sirtalis compared to N. clarkii. Raillietiella orientalis infecting C. constrictor exhibited the most compression along the long axis of the body, the longest oral cadre parallel anterior hooks and laterally-rotated posterior hooks.
Fig 3. Transformation grids showing host-specific changes in hook and oral cadre morphology of Raillietiella orientalis.
Morphological variation of R. orientalis among host species is shown by lines originating from a circle. Circles indicate the position of the average specimen. The direction of the line relative to a circle indicates specific variation in morphology among species relative to a consensus specimen.
Discussion
All pentastomes used in this study were identified as R. orientalis based on phylogenetic analyses of the COI and 18S genes [3]. However, geometric morphometric analyses revealed morphologically distinct parasites according to their host taxa. The greatest similarities observed were exhibited by R. orientalis collected from N. clarkii and T. sirtalis, hosts that are morphologically, ecologically, and phylogenetically similar [27]. Raillietiella orientalis recovered from P. bivittatus, A. piscivorus, and C. constrictor each occupied distinct regions of morphological space, with C. constrictor parasites displaying the greatest distinction from other host taxa in hook and oral cadre morphology.
The relationship of parasite morphology and host taxa observed on the CVA ordination plot is best explained by the functional group of the host taxa, with aquatic and semi-aquatic snakes (A. piscivorus, N. clarkii, T. sirtalis, and P. bivittatus) separated from a terrestrial snake host (C. constrictor) along axis CV1(x). Aquatic snakes may share similar lung morphology due to selective pressures (e.g. hydrostatic pressure and respiratory demands) experienced in an aquatic environment [28]. Ecological similarity amidst aquatic snakes may promote development of analogous oral cadre and hook arrangements in R. orientalis adults.
Significant morphological groupings such as those we observed are likely to have one of two explanations: (1) host immune response affects pentastome development, or (2) pentastomes exhibit morphological plasticity to take advantage of diverse hosts. Host-induced morphological variation has been documented by numerous studies, but little in the way of an explanation has materialized [9, 11, 12, 29–34]. A series of experimental infections performed on calves with the nematode Ostertagia ostertagi demonstrated hosts that had been previously infected, or were older, with a better-developed immune system, contained parasites with significantly underdeveloped vulval flaps [31–33]. Further study revealed that parasites were more likely to be underdeveloped if their predecessors had arrested development [31]. However, the strongest factor affecting parasite development was the hosts’ immune response.
Variation of R. orientalis morphology among host species is not likely a result of host immune response, as R. orientalis is highly competent (i.e. able to reproduce) in snake hosts from both aquatic and terrestrial functional groups. If a host immune response elicited a change in oral cadre and hook morphology, it would be assumed that altering morphology would mitigate infection and limit the success of the parasite within the host. To the contrary, R. orientalis exhibits higher prevalence, infection intensity, fecundity, and greater size in snakes native to Florida versus the pythons from which they were introduced which has facilitated the spread of this parasite to native Florida snakes northward of the current geographic range of invasive Burmese pythons (Miller et al., in review). Therefore, in addition to an ability of R. orientalis to exploit immunologically naïve hosts, this parasite can alter its morphology in ways that suggest optimization of its capacity to attach to novel taxa within its invaded range.
Other pentastome species have demonstrated significant variation according to their developmental stage. Raillietiella indica was considered a distinct species based entirely on morphology, but genetic work elucidated that it is an early instar of Raillietiella frenatus [9]. Because females included in our study were adults and centroid size variation was found to be insignificant (i.e. variation in the length of R. orientalis among hosts did not account for the observed taxon-specific parasite hook morphology), ontogenetic variation among pentastomes does not explain the morphological groupings of R. orientalis recovered.
While effort to minimize fixation effects were taken, the potential for fixation error remains a possibility [35, 36]. If a fixation error was present, these effects would be expected to be distributed in an unbiased fashion among parasites within and among hosts, allowing us to describe biological effects despite any fixation effects that may linger. Moreover, wire frame grids, added to our CVA analysis, support that significant morphological variation of R. orientalis among host taxa observed in this study are unlikely to be influenced by the potential for muscle contraction or distortion of morphological traits during fixation.
Parasites use cues from their microenvironment to alter traits and behaviors that maximize fitness [34]. Morphological modification in a parasite is a mechanism known to decrease host specificity and, as a result, increases rates of infection [29]. Morphologically distinct phenotypes have been documented for numerous parasite species and much of this phenotypic variation has been linked to host taxa [12, 29, 30, 37, 38]. For many taxa, morphologically distinct parasites assumed to represent several species have been resolved into a single species under molecular analyses, showing that parasites exhibit less co-evolution with a specific host than previously believed and instead optimize the ability to utilize a variety of hosts [10, 39], (but see [40]). Raillietiella orientalis demonstrates this phenomenon. Within their native distribution in Asia, known definitive hosts include snakes from diverse families, including Pythonidae, Colubridae, Elapidae, and Viperidae [17]. Within its introduced Florida range, R. orientalis has been documented to infect snakes from two families and eight genera (Miller et al., in review), along with two genera of non-indigenous lizards (MAM, pers. comm.). Within its introduced Australian range, in addition to native snakes, introduced cane toads (Rhinella marina) have been infected by this parasite [9, 41]. Remarkable phenotypic plasticity may allow R. orientalis to infect such a variety of hosts where it has been introduced. Because these hosts are not co-evolved with R. orientalis, their efficacy to resist or ameliorate infection may be reduced (naïve host syndrome, [13]), allowing this non-native pentastome to maximize resource use from its novel host. In addition to the capability of R. orientalis to infect diverse taxa, the species’ demonstrated ability to alter its morphology to successfully infect hosts increases its potential for negatively impacting a multitude of hosts.
Supporting information
A TPS datafile is provided including all Raillietiella orientalis examined in geometric morphometric analyses.
(PDF)
The catalog number is provided for Raillietiella orientalis pentastomes examined using geometric morphometric analyses. All R. orientalis specimens were deposited to the Auburn University Museum of Natural History (AUM). The host species of each parasite is provided. Pentastomes from the same host share the same catalog number with different individual parasites identified by letter.
(PDF)
Acknowledgments
We thank Haruka Wada for use of equipment and software to photograph pentastomes. We thank Robert N. Reed for providing additional parasite and host samples and John M. Kinsella for assistance with morphological identification of pentastomes. Collection was conducted under permits obtained from the National Park Service (permit number EVER-2015-SCI-0053) by MAM. This study was approved by the Auburn University Institutional Animal Care and Use Committee (IACUC) and all research was conducted in compliance with IACUC protocol # 2013–2386. Any use of trade, firm, or product names are for descriptive purposes only and does not imply endorsement by the U.S. Government.
Data Availability
All relevant data are in the paper and its Supporting Information files.
Funding Statement
Funded by Auburn University Center for Environmental Studies at the Urban-Rural Interface (https://cws.auburn.edu/CESURI/); to MAM; The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Auburn University Dissertation Fellowship Grants (http://www.auburn.edu/); to MAM. Any use of trade, firm, or product names are for descriptive purposes only and does not imply endorsement by the U.S. Government.
References
- 1.Dunn AM, Torchin ME, Hatcher MJ, Kotanen PM, Blumenthal DM, Byers JE, et al. Indirect effects of parasites in invasions. Funct Ecol. 2012;26: 1262–1274. [Google Scholar]
- 2.Lymbery AJ, Morine M, Kanani HG, Beatty SJ, Morgan DL. Co-invaders: The effects of alien parasites on native hosts. Int J Parasitol Parasites Wildl. 2014;3: 171–177. 10.1016/j.ijppaw.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller MA., Kinsella JM., Snow RW., Hayes MM., Falk BG., Reed RN. et al. Parasite spillover: Indirect effects of invasive Burmese pythons. Ecol Evol. 2018;8: 830–840. 10.1002/ece3.3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paterson RA, Pritchard DW, Dick JTA, Alexander ME, Hatcher MJ, Dunn AM. Predator cue studies reveal strong trait-mediated effects in communities despite variation in experimental design. Anim Behav. 2013;86: 1301–1313. [Google Scholar]
- 5.Dubey S, Shine R. Origin of the parasites of an invading species, the Australian cane toad (Bufo marinus): are the lungworms Australian or American? Mol Ecol. 2008;17: 4418–4424. 10.1111/j.1365-294X.2008.03922.x [DOI] [PubMed] [Google Scholar]
- 6.Vandame R, Colin ME, Morand S, Otero-Colina G. Levels of compatibility in a new host-parasite association: Apis mellifera/Varroa jacobsoni. Can J Zool. 2000;78: 2037–2044. [Google Scholar]
- 7.Hanselmann R, Rodríguez A, Lampo M, Fajardo-Ramos L, Aguirre AA, Kilpatrick AM, et al. Presence of an emerging pathogen of amphibians in introduced bullfrogs Rana catesbeiana in Venezuela. Biol Conserv. 2004;120: 115–119. [Google Scholar]
- 8.Dobson A, Foufopoulos F. Emerging infectious pathogens of wildlife. Philos T R Soc B. 2001;356: 1001–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelehear C, Spratt DM, Dubey S, Brown GP, Shine R. 2011. Using combined morphological, allometric and molecular approaches to identify species of the genus Raillietiella (Pentastomida). PloS One. 2011;6: e24936 10.1371/journal.pone.0024936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheang CC, Tsang LM, Chu KH, Cheng IJ, Chan BK. Host-specific phenotypic plasticity of the turtle barnacle Chelonibia testudinaria: a widespread generalist rather than a specialist. PloS One. 2013;8: e57592 10.1371/journal.pone.0057592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Léon GP. Host-induced morphological variability in adult Posthodiplostomum minimum (Digenea: Neodiplostomidae). J Parasitol. 1995;81: 818–820. [PubMed] [Google Scholar]
- 12.Huber JT, Rajakulendran VK. Redescription of and host-induced antennal variation in Anaphes iole Girault (Hymenoptera: Mymaridae), an egg parasite of Miridae (Hemiptera) in North America. Can Entomol. 1988;120: 893–901. [Google Scholar]
- 13.Mastitsky SE, Karatayev AY, Burlakova LE, Molloy DP. Parasites of exotic species in invaded areas: does lower diversity mean lower epizootic impact? Divers Distrib. 2010;16: 798–803. [Google Scholar]
- 14.Prenter J, MacNeil C, Dick JTA, Dunn AM. Roles of parasites in animal invasions. Trends Ecol Evol. 2004;19: 385–390. 10.1016/j.tree.2004.05.002 [DOI] [PubMed] [Google Scholar]
- 15.Meshaka WE, Loftus WF, Steiner T. The herpetofauna of Everglades National Park. Florida Scientist. 2000;6: 84–103. [Google Scholar]
- 16.Snow RW, Krysko KL, Enge KM, Oberhofer L, Warren-Bradley A, Wilkins L. Introduced populations of Boa constrictor (Boidae) and Python molurus molurus (Pythonidae) in southern Florida In: Henderson RW, Powell R, editors. The Biology of Boas and Pythons. Eagle Mountain: Eagle Mountain Publishing; 2007. [Google Scholar]
- 17.Christoffersen ML, De Assis JE. A systematic monograph of the recent Pentastomida, with a compilation of their hosts. Zool Med Leiden. 2013;87: 1–206. [Google Scholar]
- 18.Perry SF. Reptilian lungs: functional anatomy and evolution Vol. 79 Springer Science & Business Media: 2013. [PubMed] [Google Scholar]
- 19.Hugot JP, Baylac M. Comparative landmark analysis of various Oxyuridae parasites of primates and rodents In: Marcus LF, Cort M, Loy A, Naylor GJP, Slice DE (editors). Springer: Advances in Morphometrics; 1996. [Google Scholar]
- 20.Hugot J, Baylac M. Shape patterns of genital papillae in pinworms (Enterobiinae, Oxyurida, Nematoda) parasite of primates: a landmark analysis. Infect Genet Evol. 2007;7: 168–179. 10.1016/j.meegid.2006.08.001 [DOI] [PubMed] [Google Scholar]
- 21.Vignon M, Sasal P. The use of geometric morphometrics in understanding shape variability of sclerotized haptoral structures of monogeans (Platyhelminthes) with insights into biogeographic variability. Parasitol Int. 2010;59: 183–191. 10.1016/j.parint.2010.01.006 [DOI] [PubMed] [Google Scholar]
- 22.Kmentová N, Gelnar M, Mendlová M, Van Steenberge M, Koblmüller S, Vanhove MP. Reduced host-specificity in a parasite infecting non-littoral Lake Tanganyika cichlids evidenced by intraspecific morphological and genetic diversity. Scientific reports. 2016;6: 39605 10.1038/srep39605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pritchard MH, Kruse G. The collection and preservation of animal parasites. University of Nebraska Press, Lincoln NE, 1982; p. 61. [Google Scholar]
- 24.Rohlf FJ. tpsdig2 Stony Brook NY: Department of Ecology and Evolution, State; 2004. [Google Scholar]
- 25.Klingenberg CP. MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Resour. 2011;11: 353–7. 10.1111/j.1755-0998.2010.02924.x [DOI] [PubMed] [Google Scholar]
- 26.Zelditch ML, Swiderski DL, Sheets HD. Geometric morphometrics for biologists: a primer. Academic Press; 2012. [Google Scholar]
- 27.Gibbons JW, Dorcas ME. North American watersnakes: a natural history. University of Oklahoma Press; 2004. [Google Scholar]
- 28.Lillywhite HB. Circulatory adaptations of snakes to gravity. Am Zool. 1987;27: 81–95. [Google Scholar]
- 29.Downes BJ. Host induced morphology in mites: implications for host parasite coevolution. Syst Zool. 1990;39: 162–168. [Google Scholar]
- 30.Blankespoor HD. Host induced variation in Plagiorchis noblei Park, 1936 (Plagiorchiidae: Trematoda). Am Midl Nat. 1974;92: 415–433. [Google Scholar]
- 31.Michel JF, Lancaster MB, Hong C. Host induced effects on the vulval flap of Ostertagia ostertagi. Int J Parasitol. 1972;2: 305–317. [DOI] [PubMed] [Google Scholar]
- 32.Michel JF, Lancaster MB, Hong C. The development and symmetry of the vulval flap of Ostertagia ostertagi. Int J Parasitol. 1972;2: 297–304. [DOI] [PubMed] [Google Scholar]
- 33.Michel JF, Lancaster MB, Hong C. The effect of genetic factors on the vulval flap of Ostertagia ostertagi. Int J Parasitol. 1976;6: 83–86. [DOI] [PubMed] [Google Scholar]
- 34.Mideo N, Reece SE. Plasticity in parasite phenotypes: evolutionary and ecological implications for disease. Future Microbiol. 2012;7: 17–24. 10.2217/fmb.11.134 [DOI] [PubMed] [Google Scholar]
- 35.Fruciano C. Measurement error in geometric morphometrics. Dev Genes Evol. 2016;226: 139–158. 10.1007/s00427-016-0537-4 [DOI] [PubMed] [Google Scholar]
- 36.Bakkes DK. Evaluation of measurement error in rotational mounting of larval Rhipicephalus (Acari: Ixodida: Ixodidae) species in geometric morphometrics. Zoomorphology. 2017;136: 403–10. [Google Scholar]
- 37.Perkins SL, Martinsen ES, Falk BG. Do molecules matter more than morphology? Promises and pitfalls in parasites. Parasitology. 2011;138: 1664–74. 10.1017/S0031182011000679 [DOI] [PubMed] [Google Scholar]
- 38.Hildebrand J, Adamczyk M, Laskowski Z, Zaleśny G. Host-dependent morphology of Isthmiophora melis (Schrank, 1788) Luhe, 1909 (Digenea, Echinostomatinae)–morphological variation vs. molecular stability. Parasit Vectors. 2015;8: 481 10.1186/s13071-015-1095-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zardus JD, Lake DT, Frick MG, Rawson PD. Deconstructing an assemblage of “turtle” barnacles: species assignments and fickle fidelity in Chelonibia. Mar Biol. 2014;161: 45–59. [Google Scholar]
- 40.de León GP, Nadler SA. What we don't recognize can hurt us: a plea for awareness about cryptic species. J Parasitol. 2010; 96: 453–464. 10.1645/GE-2260.1 [DOI] [PubMed] [Google Scholar]
- 41.Kelehear C, Spratt DM, O’Meally D, Shine R. Pentastomids of wild snakes in the Australian tropics. Int J Parasitol Parasites Wildl. 2014; 3: 20–31. 10.1016/j.ijppaw.2013.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A TPS datafile is provided including all Raillietiella orientalis examined in geometric morphometric analyses.
(PDF)
The catalog number is provided for Raillietiella orientalis pentastomes examined using geometric morphometric analyses. All R. orientalis specimens were deposited to the Auburn University Museum of Natural History (AUM). The host species of each parasite is provided. Pentastomes from the same host share the same catalog number with different individual parasites identified by letter.
(PDF)
Data Availability Statement
All relevant data are in the paper and its Supporting Information files.