Abstract
Flavonoids are secondary metabolites of plants that often have medical applications. The influences of different sample drying pretreatments on flavonoids and antioxidant activity of ferns have not studies. Dryopteris erythrosora leaves used to analyze flavonoid alterations resulting from drying pretreatments. The total flavonoid content of D. erythrosora leaves exposed to different pretreatments was significantly different. The total flavonoid content of samples initially air-dried in shade and then oven-dried at 75°C were the highest (7.6%), while samples initially dried at 75°C had the lowest content (2.17%). Antioxidant activities of D. erythrosora leaves with different pretreatments varied. Group B first air-dried in the shade and then oven-dried at 75°C and group C first air-dried in the sun and then oven-dried at 75°C, both showed relatively stronger antioxidant activity. The best pretreatment for preserving the flavonoids was to first dry the plant material in the shade and then complete the drying process in an oven at 75°C. It was tentatively identified 22 flavonoids among the four different pretreatments by HPLC-ESI-TOF-MS.
Introduction
Flavonoids are plant secondary metabolites with medical applications [1]. Some factors influenced flavonoid levels, such as harvest time [2–4], shade netting, planting time [5], development [6], and using the light transmittance paper bags [7]. Sample processing can influence the quantity and quality of bioactive compounds [8–10]. For example, the flavonoid content of fresh mulberry leaves was highest and the content in leaves that were oven-dried at 100–105°C was lowest [11]. Flavonoid content, DPPH scavenging activity, and reducing power of Salvia officinalis L. leaves dried in the shade were higher than levels in leaves oven-dried at 65°C [12]. Paramignya trimera dried in an oven at 25°C had a higher flavonoid content than samples dried in an oven at 100°C [8]. Flavonoid yields differed in Belamcanda chinensis dried at temperatures ranging from 40°C to 120°C [13]. These studies demonstrated that drying conditions could alter the flavonoid content and biological activity of plant flavonoids.
However, the manner in which different drying pretreatments affect flavonoid levels remains unclear. Ferns generally have high flavonoid contents but there is little information on the effects of drying pretreatments on flavonoid levels. Therefore, Dryopteris erythrosora (Eaton) O. Ktze. (Dryopteridaceae) used for the analysis of flavonoids. The aims of this study were to: (I) assess the effects of different drying pretreatments on the flavonoids and the antioxidant activity of D. erythrosora leaves, and (II) determine the best drying pretreatment for conserving fern flavonoids and antioxidant activity.
Materials and methods
Plant materials
D. erythrosora, living in a partial shade habitat, were collected from Shanghai Sheshan National Forest Park in April 2017. The coordinates of the Shanghai Sheshan National Forest are E: 121°11'27.27"; N: 31° 5'47.41." The plants identified by Prof. Jianguo Cao. Voucher specimens deposited in the STC of the College of Life & Environmental Science, Shanghai Normal University.
Chemicals
The chemicals used were the same as in a previous report [14]. Rutin (purity > 99.0%), 2,2-Diphenyl-1-picrylhydrazyl (DPPH), 2,2’-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), Nitrotetrazolium blue chloride (NBT), phenazine methosulfate (PMS), nicotinamide adenine dinucleotide (NADH), 5, 5’-dithiobis-(2-nitrobenzoic acid) (DTNB) and 2,4,6-tri-2-pyridyl-s-triazine (TPTZ) were purchased from Sigma Co. (Shanghai, China). Acetonitrile was purchased from Thermo Fisher Scientific (Shanghai, China).
Preparation of plant extracts
Fresh leaves of D. erythrosora were randomly separated into four groups and then exposed to four treatments. Group A leaves cleaned and frozen in liquid nitrogen were pulverized. Group B leaves were initially dried in the shade about one day then oven-dried at 75°C for 48 h, before grinding them to a powder. Group C leaves were initially dried in the sun (on top of absorbent old newspapers), then oven-dried at 75°C for 48 h, then ground to a powder. Group D leaves were cleaned then initially oven-dried at 75°C for 48 h prior to grinding. Ground samples were passed through an 80-mesh screen.
Powders (1.0 g) from groups B, C, and D were separately added to 60% ethanol (25 mL) and disposed with ultrasound machine-assist (20 min) and then water-bath at 50°C for 2 h. The extraction process repeated twice, and the mixture was filtered using a vacuum suction filter pump with the solution volume maintained at 50 mL. To obtain the same dry weight, 3.3 g samples from group A ground in a mortar and then added to 60% ethanol (25 mL) for extraction. Extraction was prepared as the above.
One portion of the extract used for determining the total flavonoid content and antioxidant activity. The other portion was extracted by petroleum ether (PE), dichloromethane (DCM), ethyl acetate (EtOAc), and n-butanol (nBuOH) for HPLC-ESI-TOF-MS analysis of flavonoids.
Determination of total flavonoids content
A colorimetric assay was used for determining flavonoid content. First, gradient concentration rutins were successively added to 5% NaNO2 for 6 min, 5% Al(NO3)3 for 6 min, 4% NaOH for 12 min, and then the optical density (OD) of the mixture was recorded at 510 nm. Second, the linear equation (y = A+Bx) of rutin was plotted using Origin 7.5 software. Third, we determined the optical density (OD) of the gradient concentration extracts. The total flavonoid content was calculated as follows: total flavonoid content (%) = [(OD1+OD2+OD3)/3-A]/B*10/2*Volume/1000*100% [14].
Antioxidant activity
DPPH assay
The DPPH free radical scavenging activity assay was based on information in a previous study [14]. Briefly, 1 mL DPPH (0.1 mM in ethanol) and extracts with a gradient of concentrations were mixed. After incubation for 30 min, the absorbance value was measured at 517 nm. A 60% methanol sample was used as the control group. The DPPH free radical scavenging activity was calculated using the following formula: DPPH free radical scavenging activity (%) = (1-Asample 517/Acontrol 517)*100. The experiments were performed in triplicate (RSD < 5.0%).
ABTS assay
The ABTS assay of the extracts was previously described [14]. Briefly, 150 μL extracts with gradient concentration and 3 mL of appropriately diluted ABTS solutions were mixed. After incubating for 6 min, the absorbance value at 734 nm was determined. The ABTS free radical scavenging activity was calculated by: ABTS free radical scavenging activity (%) = (1-Asample 734/Acontrol 734)*100. The experiments were performed in triplicate (RSD < 5.0%).
Superoxide anion (O2-) scavenging activity
Determination of superoxide anion (O2-) scavenging activity was the same as described in a previous study [14]. In brief, 1 mL extracts with gradient concentrations were mixed with sodium phosphate buffer and added to 1 mL NBT (150 μM), 1mL NADH (468 μM), and 1 mL PMS (60 μM) in turns, incubating at 25°C for 5 min. The absorbance at 560 nm was then determined. Superoxide anion (O2-) scavenging activity was calculated by: Superoxide anion (O2-) scavenging activity (%) = (1-Asample 560/Acontrol 560)*100. The experiments were performed in triplicate (RSD < 5.0%).
Reducing power assay
The reducing power assay was reported previously [14]. The mixture, which included 1 mL extract with gradient concentration, 2.5 mL phosphate buffer, and 2.5 mL potassium ferricyanide, was maintained in a water bath at 50°C for 20 min, then 10% TCA was added to terminate the reaction. After centrifugation, 50% of the supernatant was mixed with 2.5 mL of distilled water and 0.5 mL of 0.1% ferric chloride. The remaining 50% of the supernatant was mixed with 3 mL of distilled water as the control group. Optical density at 700 nm reflected the reducing power. The experiments were performed in triplicate with similar results (RSD < 5.0%).
FRAP assay
The FRAP assay was described in a previous report [14]. The FRAP reagent was made up with TPTZ (10 mM) in HCl solution (40 mM) and FeCl3 (20 mM) in 250 mL acetate buffer (pH 3.6). The FRAP reagent was used immediately after preparation. A gradient concentration of extracts was added to the FRAP reagent. After 4 min, the optical density of the mixture at 593 nm was determined. The calibration curves of Fe2+ were used to calculate the results. The FRAP reagent with distilled water was used as the control group. The experiments were performed in triplicate with similar results (RSD < 5.0%).
Flavonoid analysis of D. erythrosora leaves with different drying pretreatments using HPLC-ESI-TOF-MS
Chromatographic separation was performed on an Agilent 1100 HPLC system (USA Agilent Technologies), equipped with a binary pump, a microdegasser, Hi-performance well-plate auto sampler, thermostat column compartment, and diode-array detector (DAD). UV spectra were recorded between 190 and 400 nm, and the UV detector was set at 254 nm. Separation was performed on a SHISEIDO MG-C18 (1003.3 mm; i.d. 3.0 mm) column using a gradient elution [methanol (A)/ water (0.1%HCOOH)(B)].
Extractions of petroleum ether (PE), dichloromethane (DCM), ethyl acetate (EtOAc), and n-butanol (nBuOH) were each diluted 10 times. The gradient program was 0–15 min, 15–45% A; 15–25 min, 45–55% A; 25–35 min, 55–90% A; the flow rate maintained at 0.4 mL/min, and the sample injection volume was 10 μL. The column temperature was set at 25°C. All of the MS experiments conducted on an Agilent 6220 Time-of-Flight mass spectrometer (TOF) equipped with an electrospray ionization (ESI) interface (Agilent Technologies, USA). Both the auxiliary and nebulizer gases were nitrogen with flow rates of 10 L/min. The MS analysis was performed in both positive and negative scan modes under the following operation parameters: dry gas temperature = 350°C, voltage = 160V and the nebulizer pressure = 45 psi. Full scan data acquisition and dependent scan event data acquisition were performed from m/z 100–1200.
Results and discussion
Total flavonoid content of D. erythrosora leaves with different drying pretreatments
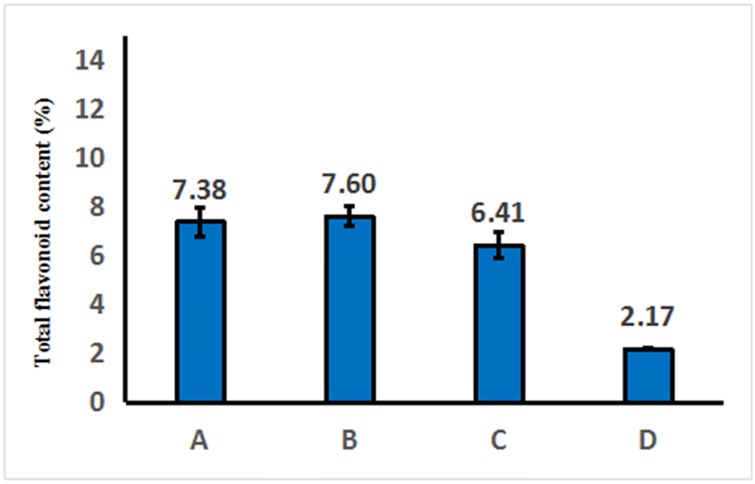
Datas from the standard curve of Rutin were list in S1 Table. The total flavonoid contents of D. erythrosora leaves with different drying pretreatments were 7.38% (Group A), 7.6% (Group B), 6.41% (Group C), and 2.17% (Group D), respectively (Fig 1). The total flavonoid content of extracts from group B, which were first dried about one day then completely oven-dried at 75°C was the highest. The flavonoid content from group D, dried at 75°C in oven directly after cleaning, was the lowest.
Fig 1. Total flavonoid content of D. erythrosora leaves with different drying pretreatments.
Group A: liquid nitrogen freezing-grinding directly; Group B: shade-drying directly; Group C: sun-drying directly; Group D: direct heating at 75°C.
The process of drying in the shade produced a slow rate of water loss.which might result in the increasing of total flavonoid content. Sun-drying produced a faster rate of water loss, ending flavonoid metabolism. Full sunlight exposure can influence flavonoid metabolites in leaves [15–16], which results in a decreased flavonoid content. Samples from group A, which were ground with liquid nitrogen, showed the total flavonoid content similar to the live samples. We speculate that water and sunlight are the main factors affecting total flavonoid content in leaf samples. In addition, flavonoids are heat sensitive [17]. Heating at 75°C directly can destroy enzyme activity and block the synthesis pathway of flavonoids. This may be the reason why the total flavonoid content of Group D was the lowest.
Antioxidant activity
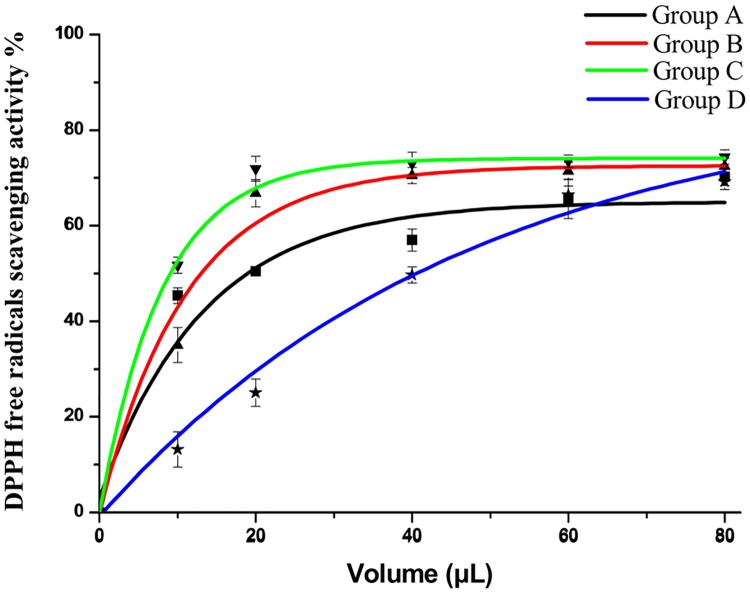
The DPPH free radical scavenging activities of D. erythrosora leaf extracts with different pretreatments were shown in Fig 2. When the doses were from 0–80 μL, the DPPH free radical scavenging potential increased. Group C was higher than group B. Fewer than 10 μL of the extracts could scavenge about 50% of the free radicals. However, group D was significantly lower than group C. The IC 50 of groups A, B, C, and D were 0.6 μg/mL, 0.38 μg/mL, 0.25 μg/mL, and 0.35 μg/mL, respectively. The DPPH free radical scavenging activity of the extracts was arranged as group C>group B>group A>group D.
Fig 2. DPPH free radicals scavenging activity of D. erythrosora leaves with different drying pretreatments.
Group A: liquid nitrogen freezing-grinding directly; Group B: shade-drying directly; Group C: sun-drying directly; Group D: heating at 75°C directly.
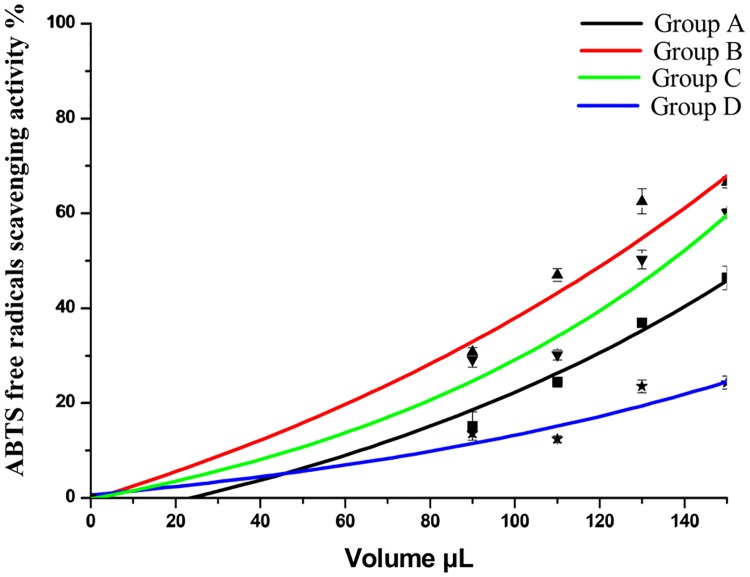
The ABTS free radical scavenging activities are shown in Fig 3. When the volume was between 0–150 μL, the ABTS free radical scavenging potential increased. Group B was the highest and group D the lowest. A 120 μL amount of the extract scavenged about 50% of the free radicals. The IC 50 of groups A, B, and C were 4.62 μg/mL, 3.72 μg/mL, and 3.52 μg/mL, respectively. The ABTS free radical scavenging potential of the groups were: B> C> A> D
Fig 3. ABTS free radicals scavenging activity of D. erythrosora leaves with different drying pretreatments.
Group A: liquid nitrogen freezing-grinding directly; Group B: shade-drying directly; Group C: sun-drying directly; Group D: heating at 75°C directly.
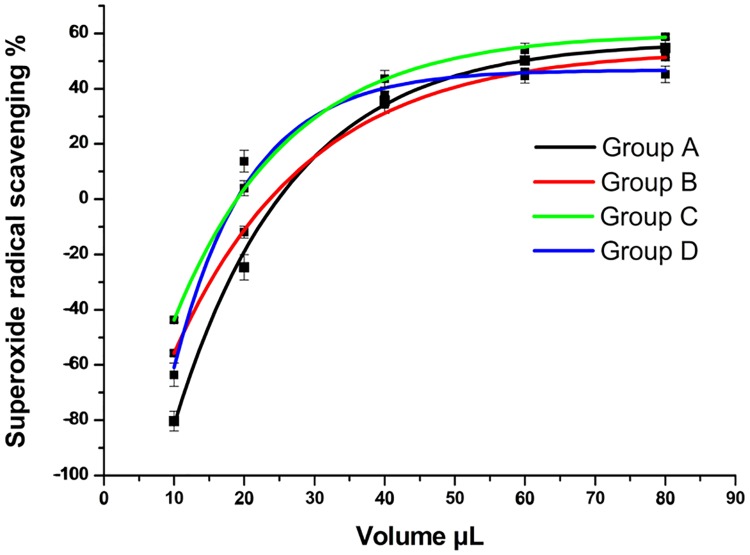
The superoxide anion scavenging activity showed in Fig 4. In the range from 10–90 μL, the superoxide anion scavenging potential increased. The samples that were first dried in the sun and then oven-dried at 75°C had relatively stronger superoxide scavenging activities. The IC 50 values of groups A, B, and C were 1.81 μg/mL, 2.28 μg/mL, and 1.25 μg/mL, respectively.
Fig 4. Superoxide radicals scavenging activity of D. erythrosora leaves with different drying pretreatments.
Group A: liquid nitrogen freezing-grinding directly; Group B: shade-drying directly; Group C: sun-drying directly; Group D: heating at 75°C directly.
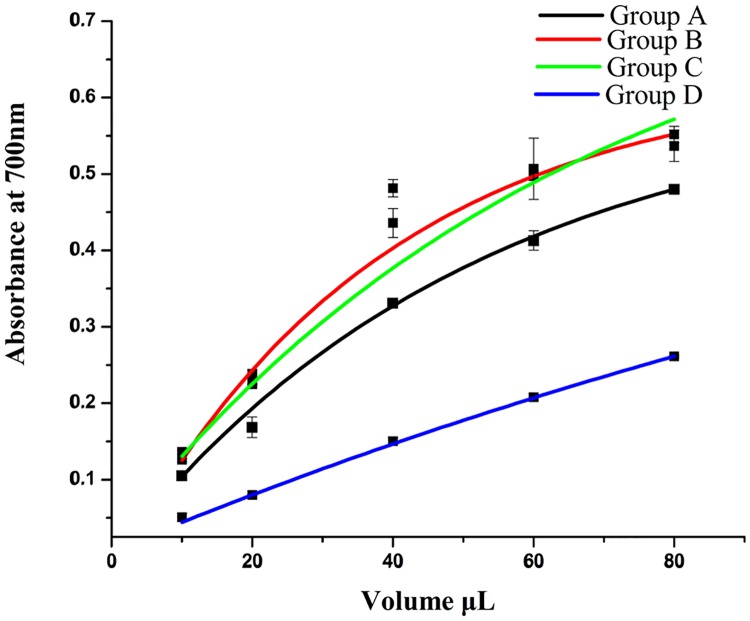
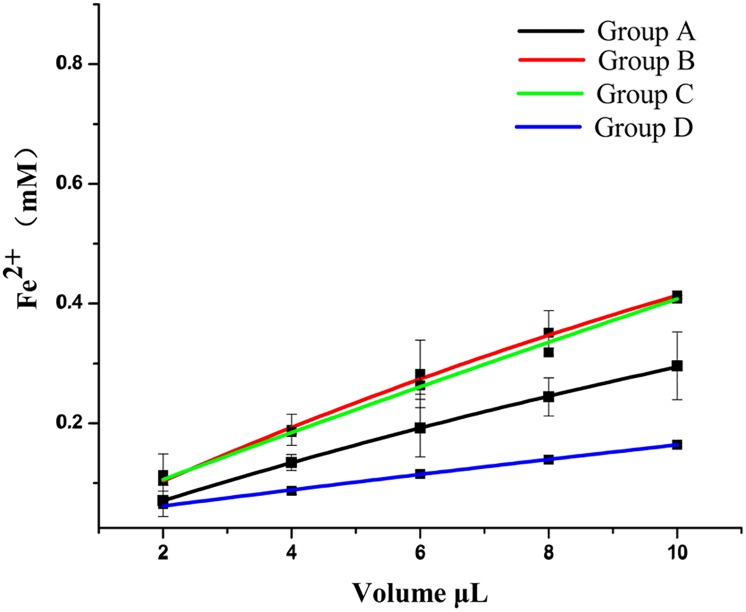
The FRAP assay and reducing power assay results are shown in Figs 5 and 6. Extracts from D. erythrosora leaves with different drying pretreatments possessed both antioxidant and reductive activity of Fe3+. With increased volume, the activity increased. The activity level between groups was: B> C> A> D.
Fig 5. Reducing power of D. erythrosora leaves with different drying pretreatments.
Group A: liquid nitrogen freezing-grinding directly; Group B: shade-drying directly; Group C: sun-drying directly; Group D: heating at 75°C directly.
Fig 6. The FRAP of D. erythrosora leaves with different drying pretreatments.
Group A: liquid nitrogen freezing-grinding directly; Group B: shade-drying directly; Group C: sun-drying directly; Group D: heating at 75°C directly.
These results illustrated that different drying pretreatments altered the antioxidant activities of D. erythrosora leaves. Except for the superoxide anion (O2-) scavenging assay, groups B and C both had stronger antioxidant activity than group D, which might be related to the flavonoid contents.
Flavonoids analysis of D. erythrosora leaves with different drying pretreatments using HPLC-ESI-TOF-MS
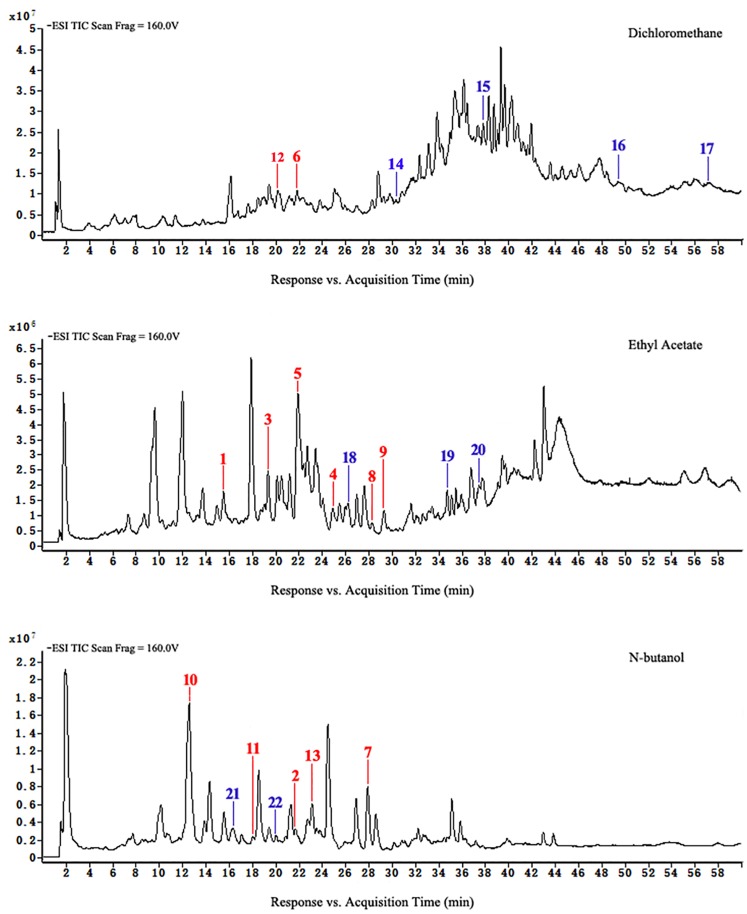
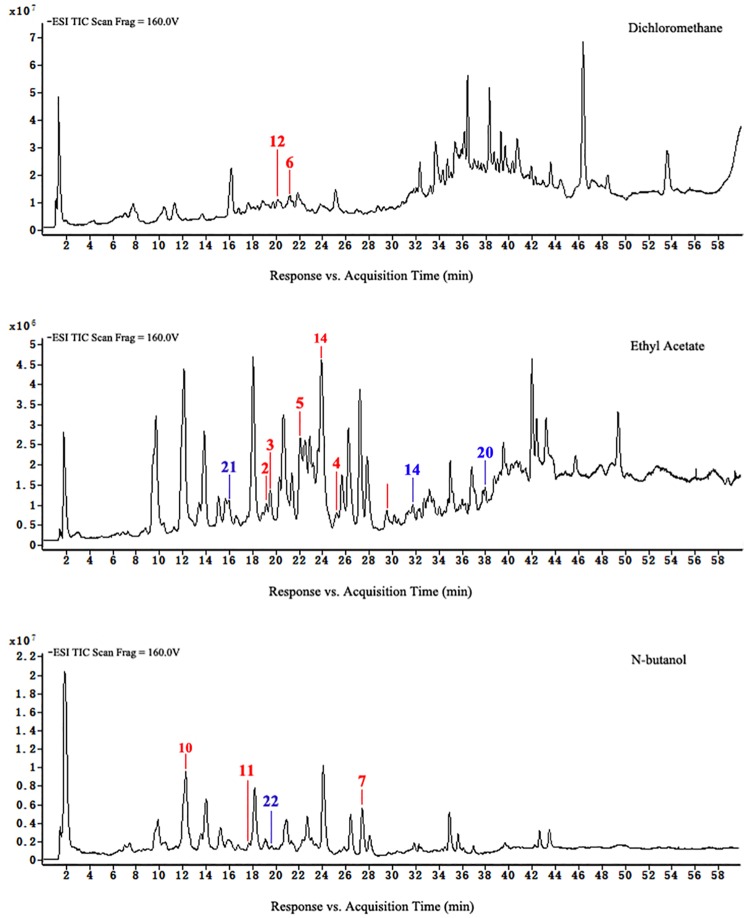
HPLC-ESI-TOF-MS was used for the qualitative analysis of flavonoids of D. erythrosora leaves. Comparing the data with known chromatograms and mass spectral data, a total of 22 peaks were tentatively identified as flavonoids (Table 1). There were 8 flavonols, 4 flavones, 3 chalcones, 2 flavanols, 2 flavanones, 1 homoisoflavones, 1 isoflavone, and 1 isoflavanone in the mass spectrometry-total ions chromatogram (MS-TIC) of extracts from D. erythrosora leaves with different drying pretreatments in the negative ion mode. The main flavonoids of D. erythrosora leaves were flavone and flavonols, and this result is consistent with previous studies [18–22].
Table 1. Analysis of D. erythrosora leaves with different pretreatments by HPLC-ESI-TOF-MS.
| No | Rt | Formula | M/Dalton | M/MFG | m/z [M-H]- | ppm | UV/nm | Identification | Compound type | A ×107 | B ×107 | C ×107 | D ×107 | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 15.53a | C17H16O8 | 348.0847 | 348.0845 | 347.0774 | -0.46 | 245, 280 | (+)-catechin-8-acetic acid | flavanol | 0.14 | 1.61 | ND | ND | [27] |
| 2. | 19.00a | C20H18O11 | 434.086 | 434.0849 | 433.0793 | -2.45 | 245, 270 | Quercetin-3-O-β-D-xylopyranoside | flavonol | 2.00 | 1.32 | 0.21 | ND | [28] |
| 3. | 19.37a | C21H20O13 | 480.0903 | 480.09 | 479.0831 | -0.82 | 245, 270 | Myricetin 3-O-glucoside | flavonol | 0.19 | 1.95 | 0.73 | ND | [29] |
| 4. | 20.35a | C27H30O15 | 594.1591 | 594.1585 | 593.1518 | -1.05 | 270, 287 | Scutellarein 7-O-glucobioside | flavone | 0.15 | 1.05 | 0.29 | 1.68 | [30] |
| 5. | 21.97a | C20H30O16 | 610.1541 | 610.1534 | 609.1468 | -1.18 | 258, 355 | Rutin | flavonol | 2.23 | 7.11 | 1.22 | ND | [31] |
| 6. | 22.79a | C27H30O14 | 578.1632 | 578.1636 | 577.1559 | 0.62 | 245, 268 | Apigenin 7-O-rutinoside | flavone | 4.67 | 3.54 | 4.02 | 0.73 | [32] |
| 7. | 26.96a | C20H18O9 | 402.0957 | 402.0951 | 401.0885 | -1.61 | 255, 320 | Apigenin-C-pentoside | flavone | 4.08 | 11.96 | 8.05 | 0.69 | [33] |
| 8. | 28.26a | C17H18O7 | 334.1058 | 334.1053 | 333.098 | -1.61 | 255, 280 | 10-O-Methylhematoxylol B/10-O-methylepihematoxylol B | Homoisoflavonoid | 1.03 | 0.27 | ND | 0.24 | [34] |
| 9. | 29.27a | C26H26O11 | 514.1476 | 514.1475 | 513.1402 | -0.21 | 255, 280 | Koreanoside B | flavonol | 1.03 | 1.10 | 0.72 | ND | [35] |
| 10. | 12.50b | C32H36O18 | 708.1898 | 707.1902 | 707.1822 | 0.9 | 240, 295, 330 | Kaempferide 3-Rhamnoside-7-(6"-Succinylglucose) | flavonol | 19.40 | 42.74 | 21.25 | ND | [36] |
| 11. | 17.95b | C26H28O14 | 564.1487 | 564.1479 | 563.1414 | -1.35 | 255, 335 | Apigenin-6-C-ara-8-C-glu | flavone | 0.90 | 0.56 | 0.28 | ND | [37] |
| 12. | 22.69b | C21H20O12 | 464.0962 | 464.0955 | 463.0889 | -1.54 | 255, 355 | Isocarthamidin-7-O-glucuronide | flavonol | 0.87 | 2.01 | 2.13 | ND | [38,39] |
| 13. | 24.50b | C20H18O10 | 418.0896 | 418.09 | 417.0823 | 0.95 | 245, 330 | Kaempferol 3-O-α-L-arabinopyranoside | flavonol | 18.14 | 3.62 | 4.98 | 0.39 | [40] |
| 14. | 30.35c | C30H30O15 | 630.159 | 630.1585 | 629.1517 | -0.82 | 250, 270 | 2S-5,7,2',5'-Tetrahydroxy-6-methoxyflavanone | flavanone | ND | 1.00 | 0.26 | ND | [41] |
| 15. | 37.80c | C22H26O9 | 434.1581 | 434.1577 | 433.1508 | -0.89 | 250, 285 | 2'-Hydroxy-2,3,4,5,4',5',6'-heptamethoxychalcone | chalcone | ND | 4.68 | ND | ND | [42] |
| 16. | 49.55c | C23H28O9 | 448.1723 | 448.1733 | 447.1660 | 2.31 | 250 | 2,3,4,5,2',4',5',6'-Octamethoxychalcone | chalcone | ND | 5.65 | ND | 1.37 | [43] |
| 17. | 57.21c | C30H34O11 | 570.2096 | 570.2101 | 569.2023 | 0.9 | 250 | (-)-5,7-O-Dimethyl-3′,4′,5′-O-trimethylepigallocatechin-3-O-(3′′,4′′,5′′-O-trimethyl) gallate | flavanol | ND | 2.84 | ND | ND | [44] |
| 18. | 26.18d | C25H24O12 | 516.127 | 516.1268 | 515.1198 | -0.5 | 260, 330 | Formononetin-7-O-(6''-malonylglucoside) | isoflavone | ND | 0.47 | ND | ND | [45] |
| 19. | 34.73d | C24H26O13 | 522.1375 | 522.1373 | 521.1302 | -0.35 | 255, 280 | (2S)-5-Hydroxy-7,8,6'-trimethoxyflavanone-2'-O-b-D-glucuronide | flavanone | ND | 0.86 | ND | ND | [46,47] |
| 20. | 37.43d | C26H30O13 | 550.1689 | 550.1686 | 549.1616 | -0.51 | 255 | Isoliquiritin apioside | chalcone | ND | 0.46 | 0.20 | ND | [48,49] |
| 21. | 16.35e | C25H24O13 | 532.122 | 532.1217 | 531.1147 | -0.51 | 243, 285 | Biochanin A-7-O-glucoside-6”-O-malonate | isoflavanone | ND | 4.08 | 0.31 | ND | [50] |
| 22. | 19.9e | C27H30O17 | 626.1493 | 626.1483 | 625.1421 | -1.64 | 245, 270 | Quercetin-O-dihexoside | flavonol | ND | 0.84 | 0.38 | ND | [51] |
a: total ion chromatograms of ethyl acetate of extracts from group A;
b: total ion chromatograms of n-butanol of extracts from group A;
c: total ion chromatograms of dichloromethane of extracts from group B;
d: total ion chromatograms of ethyl acetate of extracts from group B;
e: total ion chromatograms of n-butanol of extracts from group B
Chalcones and isoflavones were absent in group A but present in groups B and C. This is the first report of these compounds in the Dryopteridaceae. This result showed chalcones and isoflavones could not be synthetized during natural growth of D. erythrosora leaves but could result from the stresses of sun-drying or shade-drying. Except for anthocyanin and its derivatives, all of the flavonoid types in the flavonoid synthesis pathway could be found [23]. We concluded that the flavonoid metabolic pathways of ferns are similar to spermatophytes and that the metabolic pathways are closely related to the stress response.
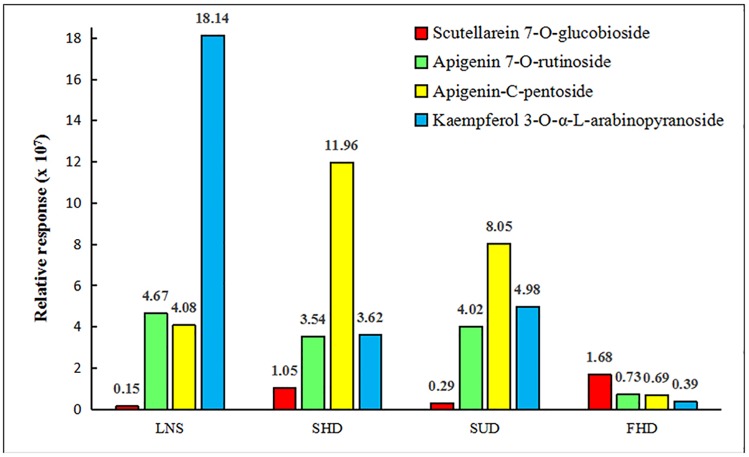
Loss of flavonoids from group D was the greatest. Figs 7 to 10 show that scutellarein 7-O-glucobioside, apigenin 7-O-rutinoside, apigenin-C-pentoside, and kaempferol 3-O-α-L-arabinopyranoside are common flavonoids. This suggests that the flavonoid biosynthesis of the four components was unaffected by the drying pretreatments. However, the contents of the four flavonoids significantly changed (Fig 11). We used Group A samples (frozen-ground in liquid nitrogen) as the standard and found that the content of scutellarein 7-O-glucobioside increased. The largest amounts of these compounds were from group D. This result indicated that heating directly accelerated the synthesis of scutellarein 7-O-glucobioside.
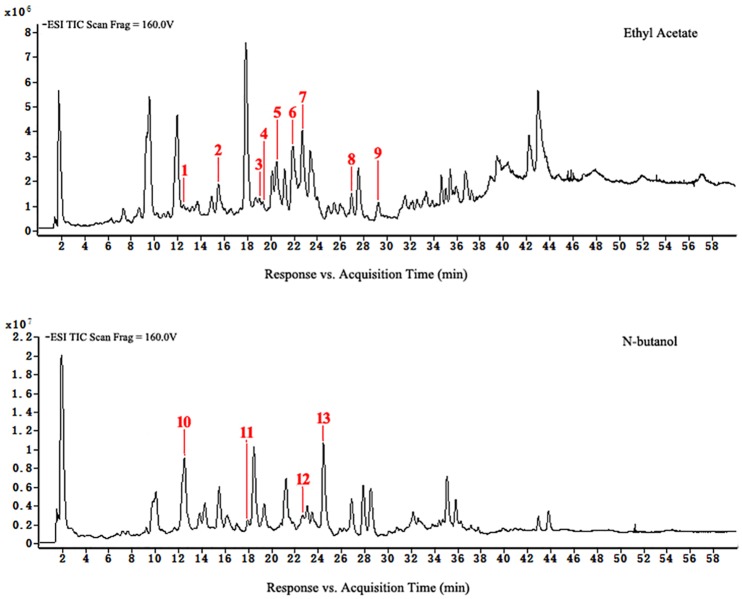
Fig 7. HPLC-ESI-TOF-MS total ion chromatograms of ethyl acetate and n-butanol of extracts from group A.
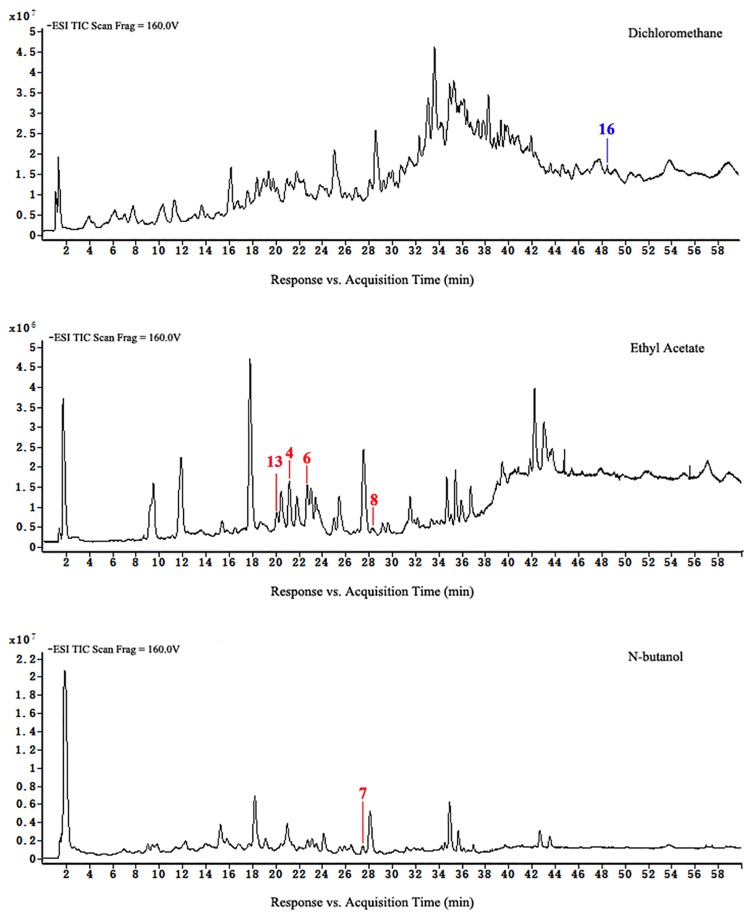
Fig 10. HPLC-ESI-TOF-MS total ion chromatograms of dichloromethane, ethyl acetate, and n-butanol of extracts from group D.
Fig 11. The common flavonoids with four different drying pretreatments (LNS: Group A; SHD: Group B; SUD: Group C; and FHD: Group D).
Fig 8. HPLC-ESI-TOF-MS total ion chromatograms of dichloromethane, ethyl acetate, and n-butanol of extracts from group B.
Fig 9. HPLC-ESI-TOF-MS total ion chromatograms of dichloromethane, ethyl acetate, and n-butanol of extracts from group C.
The contents of apigenin 7-O-rutinoside and kaempferol 3-O-α-L-arabinopyranoside both decreased, and the lowest content was in group D. This demonstrated that the two compounds might decompose or transformed with direct heating. In addition, the amounts of apigenin-C-pentoside of groups B and C increased, but decreased in group D. This suggests that temperature might be the main factor influencing the synthesis of apigenin-C-pentoside.
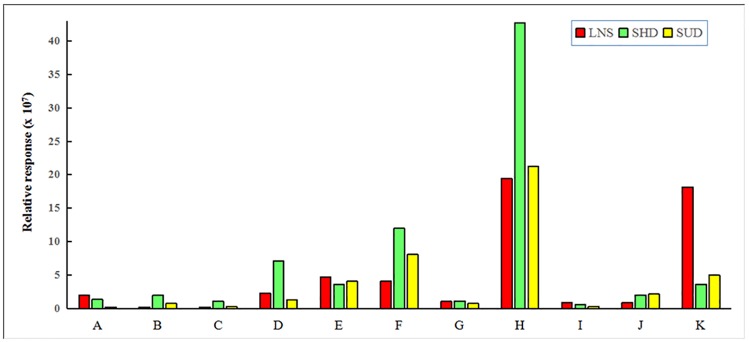
Among groups A, B, and C, 11 common flavonoids were found. The contents of these flavonoids also changed (Fig 12). The key difference in the drying pretreatments between groups B and C was the dehydration rate, which resulted in a myricetin 3-O-glucoside, kaempferide 3-rhamnoside-7-(6"-succinylglucose), and isocarthamidin-7-O-glucuronide increase but a reduction in quercetin-3-O-β-D-xylopyranoside and apigenin-6-C-ara-8-C-glu. The content of koreanoside B changed little, indicating that the synthesis of koreanoside B was not influenced. The decreased rutin may have resulted from the decomposition or the transformation.
Fig 12. The common flavonoids with three different drying prtreatments (LNS: Group A; SHD: Group B; and SUD: Group C).
A: Quercetin-3-O-β-D-xylopyranoside; B: Myricetin 3-O-glucoside; C: Scutellarein 7-O-glucobioside; D: Rutin; E: Apigenin 7-O-rutinoside; F: Apigenin-C-pentoside; G: Koreanoside B; H: Kaempferide 3-Rhamnoside-7-(6"-Succinylglucose); I: Apigenin-6-C-ara-8-C-glu; J: Isocarthamidin-7-O-glucuronide; K: Kaempferol 3-O-α-L-arabinopyranoside.
The number of flavonoids was greatest in group B. Compared to group A, group B had 9 additional flavonoids and the contents of 8 common compounds increased. The levels of kaempferide 3-rhamnoside-7-(6"-succinylglucose) were 2× greater than group A.
High light irradiance can influence the biosynthesis of dihydroxy B-ring-substituted flavonoids [24–26]. In this study, the effects of light irradiance were not obvious. Among the different drying pretreaments, the loss of flavonoids in group D was the greatest (67% lost).
Conclusion
This study examined the influences of different drying pretreatments of D. erythrosora leaves on total flavonoid contents, antioxidant activity, and flavonoid ingredients. The main conclusions were: a) The total flavonoids contents, antioxidant activities, and flavonoid ingredients in leaves with different drying pretreatments varied, and samples that were first dried in the shade then oven-dried at 75°C had the highest flavonoid content and strongest antioxidant activities. b) The pretreatment of rapid oven drying in a 75°C oven produced the greatest loss of flavonoids. The most successful pretreatment for conserving leaf flavonoids was the initial shade drying followed by oven drying at 75°C. Freezing and grinding in liquid nitrogen followed by filtering was also a useful technique.
Supporting information
(DOCX)
Acknowledgments
The work was sponsored by Shanghai Engineering Research Center of Plant Germplasm Resources (No. 17DZ2252700). The authors had permission from Shanghai Forestry Bureau to collect specimens.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funded by Grant numbers: 17DZ2252700. URLs of Shanghai Engineering Research Center of Plant Germplasm Resources: http://pgdc.shnu.edu.cn. Quanxi Wang received the funding.
References
- 1.Delmas D, Xiao JB, EDITORIAL (Hot Topic: Natural polyphenols properties: Chemopreventive and chemosensitizing activities). Anti-Cancer Agent Med. 2012; 12: 835. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Cao JG, Dai XL, Xiao JB, Wu YH, Wang QX. Total flavonoid concentrations of bryophytes from Tianmu Mountain, Zhejiang Province (China): Phylogeny and ecological factors. PloS one. 2017a; 12: e0173003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, Wang ML, Cao JG, Wu YH, Xiao JB, Wang QX. Analysis of flavonoids and antioxidants in extracts of ferns from Tianmu Mountain in Zhejiang Province (China). Ind Crop Prod. 2017b; 97: 137–145. [Google Scholar]
- 4.Xie YH, Zheng YX, Dai XL, Wang QX, Cao JG, Xiao JB. Seasonal dynamics of total flavonoid contents and antioxidant activity of Dryopteris erythrosora. Food Chem. 2015; 186: 113–118. 10.1016/j.foodchem.2014.05.024 [DOI] [PubMed] [Google Scholar]
- 5.Bergquist SÅM, Gertsson UE, Nordmark LYG, Olsson M. Effects of shade nettings, sowing time and storage on baby spinach flavonoids. J Sci Food Agric. 2007, 87: 2464–2471. [Google Scholar]
- 6.Lister CE, Lancaster JE, Walker JRL. Developmental changes in enzymes biosynthesis in the skins of red and green apple cultivars. J Sci Food Agric. 1996; 71: 330. [Google Scholar]
- 7.Xu HX, Chen JW, Xie M. Effect of different light transmittance paper bags on fruit quality and antioxidant capacity in loquat. J Sci Food Agric. 2010; 90: 1783–1788. 10.1002/jsfa.4012 [DOI] [PubMed] [Google Scholar]
- 8.Nguyen VT, Pham NMQ, Vuong QV, Bowyer MC, Altena IA, Scarlett CJ. Phytochemical retention and antioxidant capacity of xao tam phan (Paramignya trimera) root as prepared by different drying methods. Dry Technol. 2016; 34: 324–334. [Google Scholar]
- 9.Ma CH, Yang L, Li W, Yue JQ, Li J, Zu YG. Ultrasound-assisted extraction of arabinogalactan and dihydroquercetin simultaneously from Larix gmelinii as a pretreatment for pulping and papermaking. PloS one, 2014; 9(12): e114105 10.1371/journal.pone.0114105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ledesma-Escobar CA, Priego-Capote F, Luque de Castro MD. Comparative study of the effect of sample pretreatment and extraction on the determination of flavonoids from lemon (Citrus limon). PloS one. 2016; 11(1): e0148056 10.1371/journal.pone.0148056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia ZH, Tang MC, Wu JM. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999; 64: 555–559. [Google Scholar]
- 12.Hamrouni-Sellami I, Rahali FZ, Rebey IB, Bourgou S, Limam F, Marzouk B. Total phenolics, flavonoids, and antioxidant activity of sage (Salvia officinalis L.) plants as affected by different drying methods. Food Bioprocess Tech. 2013; 6: 806–817. [Google Scholar]
- 13.Zhu Y, Pu BQ, Xie GY, Tian M, Xu FY, Qin MJ. Dynamic changes of flavonoids contents in the different parts of rhizome of Belamcanda chinensis during the thermal drying process. Molecules. 2014; 19: 10440–10454. 10.3390/molecules190710440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Cao JG, Wu YH, Wang QX, Xiao JB. Flavonoids, antioxidant potential, and acetylcholinesterase inhibition activity of the extracts from the gametophyte and archegoniophore of Marchantia polymorpha L. Molecule. 2016; 21: 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agati G, Galardi C, Gravano E, Romani A, Tattini M. Flavonoid distribution in tissues of Phillyrea latifolia as estimated by microspectrofluorometry and multispectral fluorescence microimaging. Photochem Photobiol. 2002; 76: 350–360. [DOI] [PubMed] [Google Scholar]
- 16.Tattini M, Gravano E, Pinelli P, Mulinacci N, Romani A. Flavonoids accumulate in leaves and glandular trichomes of Phillyrea latifolia exposed to excess solar radiation. New Phytol. 2000; 148: 69–77. [DOI] [PubMed] [Google Scholar]
- 17.Chaaban H, Ioannou I, Chebil L, Slimane M, Gérardin C, Paris C. Effect of heat processing on thermal stability and antioxidant activity of six flavonoids. J Food Process Pres. 2017; 41: e13203. [Google Scholar]
- 18.Hiraoka A. Flavonoid patterns in athyriaceae and dryopteridaceae. Biochem Syst Ecol. 1978; 6: 171–175. [Google Scholar]
- 19.Min BS, Tomiyama M, Ma CM, Nakamura N, Hattori M. Kaempferol acetylrhamnosides from the rhizome of Dryopteris crassirhizoma and their inhibitory effects on three different activities of human immunodeficiency virus-1 reverse transcriptase. Chem Pharm Bull. 2001; 49: 546–550. [DOI] [PubMed] [Google Scholar]
- 20.Imperato F. Kempferol 3-O-(acetylrutinoside), a new flavonoid and two new fern constituents, quercetin 3-O-(acetylglucoside) and 3-O-(acetylrutinoside). Am Fern J. 2006; 96: 93–95. [Google Scholar]
- 21.Imperato F. A new flavonoid, quercetin 3-O-(X″-acetyl-X″-cinnomoyl -glucoside) and a new fern consituent quescetin 3-O-(glucosylrhamnoside) from Dryopteris villarii. Am Fern J. 2007a; 97: 124–126. [Google Scholar]
- 22.Imperato F. Three new flavonoid glycosides, kaempferol 3-O-(caffeoylrhamnoside), apigenin 4’-O- (caffeoylglucoside) and 4’-O-(feruloylglucoside) from Dryopteris villarii. Am Fern J. 2007b; 97: 233–236. [Google Scholar]
- 23.Brenda WS. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001; 126: 485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agati G, Biricolti S, Guidi L, Ferrini F, Fini A, Tattini M. The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves. J Plant Physiol. 2011; 168: 204–212. 10.1016/j.jplph.2010.07.016 [DOI] [PubMed] [Google Scholar]
- 25.Ryan KG, Markham KR, Bloor SJ, Bradley JM, Mitchell KA, Jordan BR. UV-B radiation induces increase in quercetin: kaempferol ratio in wild-type and transgenic lines of Petunia. Photochem Photobiol. 1998; 68: 323–330. [Google Scholar]
- 26.Ryan KG, Swinny EE, Markham KR, Winefield C. Flavonoid gene expression and UV photoprotection in transgenic and mutant Petunia leaves. Phytochemistry. 2002; 59: 23–32. [DOI] [PubMed] [Google Scholar]
- 27.Li SP, Zhai XY, Wang TM, Ma W, Hu J, Wang SS, Li N, Wang KJ. New flavonoids and triterpenoids from the roots of Rosa laevigata. J Mex Chem Soc. 2014; 58: 369–372. [Google Scholar]
- 28.Wang L, Wu Y, Bei Q, Shi K, Wu Z. Fingerprint profiles of flavonoid compounds from different Psidium guajava leaves and their antioxidant activities. J Sep Sci. 2017; 40: 3817–3829. 10.1002/jssc.201700477 [DOI] [PubMed] [Google Scholar]
- 29.Van DM JD, Joubert E, Manley M, De BD, Malherbe CJ, Gelderblom WC. In Vitro Hepatic Biotransformation of Aspalathin and Nothofagin, Dihydrochalcones of Rooibos (Aspalathus linearis), and Assessment of Metabolite Antioxidant Activity. J Agric Food Chem. 2010; 58: 2214–2220. 10.1021/jf903917a [DOI] [PubMed] [Google Scholar]
- 30.Denikeeva MF, Litvinenko VI, Borodin LI. Flavonoid compounds of Scutellaria przewalskii. Chem Nat Comp. 1973; 6: 532. [Google Scholar]
- 31.Fedosov AI, Kyslychenko AA, Gudzenko AV, Semenchenko OM, Kyslychenko VS. The determination of phenolic compounds in garlic extracts by HPLC GC/MS technique. Der Pharma Chemica. 2016; 8: 118–124. [Google Scholar]
- 32.Guvenalp Z, Ozbek H, Karadayi M, Gulluce M, Kuruuzum-Uz A, Salih B, Demirezer O. Two antigenotoxic chalcone glycosides from Mentha longifolia subsp. Longifolia. Pharm Biol. 2015; 53: 888–896. 10.3109/13880209.2014.948633 [DOI] [PubMed] [Google Scholar]
- 33.Jin M, Zhang XH, Zhao MC, Deng M, Du YH, Zhou Y, et al. Integrated genomics-based mapping reveals the genetics underlying maize flavonoid biosynthesis. BMC Plant Biol. 2017; 17: 17 10.1186/s12870-017-0972-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin LG, Xie H, Wang YT, Ding J, Ye Y. Chemical constituents from the heartwood of Haematoxylon campechianum as protein tyrosine kinase inhibitors. Chem Biodivers. 2014; 11: 776–783. 10.1002/cbdv.201300183 [DOI] [PubMed] [Google Scholar]
- 35.Li HM, Zhou C, Chen CH, Li RT, Lee KH. Flavonoids isolated from heat-processed epimedium koreanum and their anti-HIV-1 activities. Helv Chim Acta. 2015; 98: 1177–1187. [Google Scholar]
- 36.KT, Woncheol Y, Gunho J, Sunlim K, Youngup K. Relationship of transformation efficiency and metabolites induced in korean soybean cotyledons treated with Sonication. Korean J Crop Sci. 2013; 58: 119–127. [Google Scholar]
- 37.Islam MN, Downey F, Ng KY. Comprehensive profiling of flavonoids in Scutellaria incana L. using LC-Q-TOF-MS. Acta Chromatogr. 2013; 25: 555–569. [Google Scholar]
- 38.Tomimori T, Imoto Y, Miyaichi Y. Studies on the constituents of Scutellaria species. XIII. On the flavonoid constituents of the root of Scutellaria rivularis Wall. Chem Pharm Bull. 1990; 38: 3488–3490. [Google Scholar]
- 39.Ren Q, Xia T, Quan XG, Ding L, Wang HY. Antileukemic activity of the chemical constituents from Scutellaria barbata D. Don. Acta Chromatogr. 2017; 3: 399–413. [Google Scholar]
- 40.Zhao Q, Liu F, Li QJ, Chen WP. Chemical constituents from flowers of Rosa chinensis. Chin Tradit Herbal Drugs. 2012; 43: 1484–1488. [Google Scholar]
- 41.Miyaichi Y, Imoto Y, Tomimori T. Studies on the nepalese crude drugs. IX.: on the flavonoid constituents of the root of Scutellaria scandens BUCH.-HAM. ex D. DON. Chem Pharm Bull. 1988; 36: 2371–2376. [Google Scholar]
- 42.Miyaichi Y, Kizu H, Tomimori T, Liu C. Studies on the constituents of Scutellaria species. XI.: on the flavonoid constituents of the aerial parts of Scutellaria indica L. Chem Pharm Bull. 1989; 37: 794–797. [Google Scholar]
- 43.Utenova BT, Malterud KE, Rise F. Antioxidant activity of O-protected derivatives of (-)-epigallocatechin-3-gallate: inhibition of soybean and rabbit 15-lipoxygenases. Arkivoc. 2007; 27: 6–16. [Google Scholar]
- 44.Beck AB, Knox JR. The acylated isoflavone glycosides from subterranean clover and red clover. Aust J Chem. 1971; 24: 1509–1518. [Google Scholar]
- 45.Li W, Koike K, Asada Y, Hirotani M, Rui H, Yoshikawa T, Nikaido T. Flavonoids from Glycyrrhiza pallidiflora hairy root cultures. Phytochemistry. 2002; 60: 351–355. [DOI] [PubMed] [Google Scholar]
- 46.Miyaichi Y, Imoto Y, Tomimori T, Lin C. Studies on the constituents of scutellaria species. IX. on the flavonoid constituents of the root of Scutellaria indica L. Chem Pharm Bull. 1987; 35: 3720. [Google Scholar]
- 47.Nikaido T, Ohmoto T, Kinoshita T, Sankawa U, Monache FD, Botta B, et al. Inhibition of adenosine 3',5'-cyclic monophosphate phosphodiesterase by flavonoids. III. Chem Pharm Bull. 1989; 37: 1392–1395. [DOI] [PubMed] [Google Scholar]
- 48.Dai JY, Yang JL, Li C. Transport and metabolism of flavonoids from Chinese herbal remedy Xiaochaihu-tang across human intestinal Caco-2 cell monolayers. Acta Pharmacol Sin. 2008; 29: 1086–1093. 10.1111/j.1745-7254.2008.00850.x [DOI] [PubMed] [Google Scholar]
- 49.Atsuko TA, Kyoko IS, Naoki SU. Determination of the plant origin of licorice oil extract, a natural food additive, by principal component analysis based on chemical components. J Food Hyg Soc Jpn. 2015; 56: 217–227. [DOI] [PubMed] [Google Scholar]
- 50.Taujenis L, Padarauskas A, Mikaliuniene J, Cesevičiene J, Lemežiene N. Identification of isoflavones and their conjugates in red clover by liquid chromatography coupled with DAD and MS detectors. Chemija. 2015; 26: 107–112. [Google Scholar]
- 51.Li PL, Su WW, Yun S, Liao YQ, Liao YY, Liu H, et al. Toward a scientific understanding of the effectiveness, material basis and prescription compatibility of a Chinese herbal formula Dan-hong injection. Sci Rep-UK. 2017; 7: 46266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.