Abstract
Concern that misinformation from direct-to-consumer marketing of largely unproven “biologic” treatments such as platelet-rich plasma and cell-based therapies may erode the public trust and the responsible investment needed to bring legitimate biological therapies to patients have resulted in calls to action from professional organizations and governing bodies. In response to substantial patient demand for biologic treatment of orthopaedic conditions, the American Academy of Orthopaedic Surgeons convened a collaborative symposium and established a consensus framework for improving and accelerating the clinical evaluation, use, and optimization of biologic therapies for musculoskeletal diseases. The economic and disease burden of musculoskeletal conditions is high. Of the various conditions discussed, knee osteoarthritis was identified as a “serious condition” associated with substantial and progressive morbidity and emerged as the condition with the most urgent need for clinical trial development. It was also recognized that stem cells have unique characteristics that are not met by minimally manipulated mixed cell preparations. The work group recommended that minimally manipulated cell products be referred to as cell therapy and that the untested and uncharacterized nature of these treatments be clearly communicated within the profession, to patients, and to the public. Minimum standards for product characterization and clinical research should also be followed. A framework for developing clinical trials related to knee OA was agreed upon. In addition to recommendations for development of high-quality multicenter clinical trials, another important recommendation was that physicians and institutions offering biologic therapies commit to establishing high-quality patient registries and biorepository-linked registries that can be used for postmarket surveillance and quality assessments.
The clinical use of biologics such as platelet-rich plasma (PRP) and cell-based therapies to treat orthopaedic complications has greatly outpaced the evidence. This phenomenon is due in part to the prevalence and seriousness of musculoskeletal conditions, in part due to the lack of satisfactory conventional treatment options, and in part due to widespread direct-to-consumer marketing of treatments that fall outside traditional regulatory pathways. To address these concerns, on February 15, 2018, through February 17, 2018, the American Academy of Orthopaedic Surgeons (AAOS) convened thought leaders from clinical medicine, research, and government at Stanford University for a “think tank” symposium on “Optimizing Clinical Use of Biologics in Orthopaedic Surgery.”1 Participants included academic and private practitioners, basic and clinical scientists from academia, patients, representatives from the AAOS, the National Institutes of Health (NIH), the American Orthopaedic Society for Sports Medicine, the Arthroscopy Association of North America, the International Cartilage Regeneration and Joint Preservation Society, and keynote speakers from the National Institutes of Standards and Technology, the Stanford Center for Innovative Study Design, and the FDA. The goals of the symposium were (1) to establish a clear, collective impact agenda for improving the clinical evaluation, use, and optimization of biologics in orthopaedics and (2) to develop a guidance document on clinically meaningful end points and outcome metrics to accelerate the evaluation of biologics for common orthopaedic conditions.
Musculoskeletal Diseases Include Serious Conditions for Which Conventional Treatments Are Lacking
Musculoskeletal pain and dysfunction attributable to trauma, obesity, and aging are a leading cause of physician visits, chronic pain, and disability in the United States.2 The economic burden of musculoskeletal diseases approaches $1 trillion annually in the United States, comprising approximately 7.4% of the gross domestic product.3 Although the disease burden is high, treatment options remain limited. Progression of serious conditions such as osteoarthritis (OA) that eventually fail conventional nonsurgical therapies lead to chronic pain, disability, and difficulty with self-care and activities of daily living. These circumstances make patients vulnerable to unsubstantiated claims in direct-to-consumer advertising.4,5
Misrepresentation of Uncharacterized and Unproven Minimally Manipulated Products as Stem Cells May Erode Public Trust and Compromise Development of Legitimate Cell Therapies
Public awareness of biologics thought to have regenerative potential has been accelerated by highly publicized use in professional athletes6 and by the national debate on embryonic stem (ES) cells. These circumstances, along with misrepresentation of uncharacterized, minimally manipulated cell preparations as “stem cells,” have led to a widespread clinical use of unproven biologic therapies.4,5
For decades, PRP served primarily as an intermediary in the manual preparation of life-saving platelet concentrates. Blood products have long been used clinically for a variety of needs where anticoagulated whole blood is centrifuged to separate it into plasma and packed red cell fractions. Manual preparation of a platelet concentrate involves collection of the PRP lying just above the white blood cell layer, followed by a second spin to permit further concentration of the platelets. Consequently, centrifuges have been an important fixture in hospitals and blood banks for decades.
This clinical history has set the stage for more recent widespread clinical use of PRP as a biologic therapy for musculoskeletal conditions. Furthermore, use of centrifuge-like devices and other mechanical methods to prepare minimally manipulated autologous cell preparations has been extended to fat, placenta, and many other tissues. These uncharacterized cell products have been marketed as stem cells and used to treat a long list of clinical conditions ranging from hair loss to retinopathy and, most commonly, orthopaedic applications.4 The high prevalence of painful and disabling orthopaedic conditions such as knee OA has also resulted in an exponential increase in the marketing of unproven biologics to relieve chronic pain.4,5
Concerns over misinformation from direct-to-consumer marketing of unproven treatments have led to recent calls to action from professional organizations including the National Academy of Sciences, the International Society for Cellular Therapy (ISCT), the American Association for the Advancement of Science, and the AAOS.7-9 Each of these groups recognizes the potential value of cell therapies and the risk that the current environment may erode the public trust and responsible investment that are needed to bring legitimate cellular and biological therapies to patients. This symposium directly addresses recent calls to action, particularly the need for clear standards in the nomenclature for cellular therapies and biologics, standards for measuring and reporting the composition of these therapies and their clinical outcomes, and the establishment of registries and clinical trial networks to accelerate rigorous assessment and optimization of regenerative therapies for musculoskeletal diseases. The consensus outcomes are summarized below.
Section I: Pathways to Improve Accountability for Biologics Currently in General Clinical Use
Recommendation 1: Define Terminology to Clearly Distinguish Uncharacterized Minimally Manipulated Autologous Cell Products From Rigorously Characterized, Culture-expanded and Purified Stem Cell and Progenitor Cell Populations
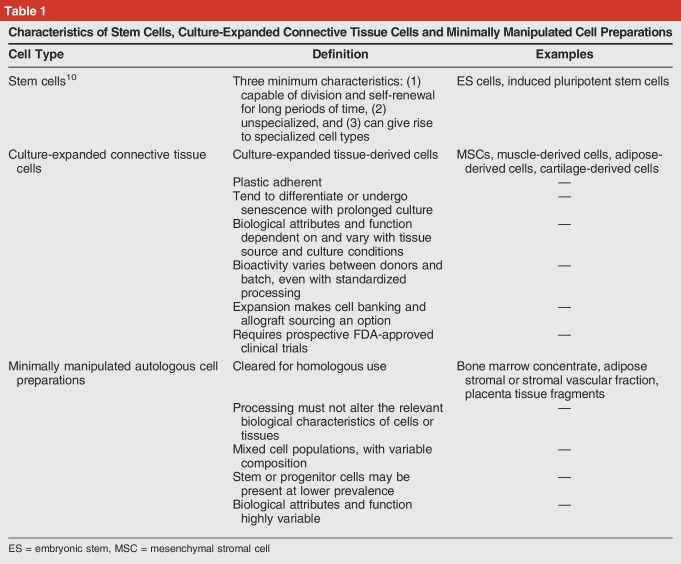
Stem cells have unique characteristics that are not met by minimally manipulated cell-based therapies being widely marketed in the United States (Table 1). The use of the term stem cells to describe minimally manipulated cell preparations is problematic and has created substantial confusion for patients, physicians, and the general public. As defined by the NIH,10 “Stem cells differ from other kinds of cells in the body. All stem cells have three general properties: they are capable of dividing and renewing themselves for long periods; they are unspecialized; and they can give rise to specialized cell types.” Prime examples of stem cells are the ES cells derived from early embryos or blastocysts with the ability to generate progeny that can differentiate into any tissue type. Use of ES cells is limited by ethical controversies and safety concerns.
Table 1.
Characteristics of Stem Cells, Culture-Expanded Connective Tissue Cells and Minimally Manipulated Cell Preparations
Virtually all current cell therapies offered in the United States for musculoskeletal conditions involve the transplantation of adult cells obtained through harvest and minimal manipulation of native tissues (eg, blood, bone marrow, fat). These tissues contain stem and progenitor cells. The concentration of these cells can be increased at the point of care using density separation or other means to improve efficacy in some settings.11 However, stem and progenitor cells are the least abundant cell type in these preparations. Depending on the tissue of origin, only one in one thousand to one in one million cells harvested from healthy tissues are stem or progenitor cells that are capable of differentiating into one or more connective tissues such as bone, cartilage, and fat.12-14 For adipose tissue, the potential stem and progenitor cells are thought to be pericytes embedded in the basement membrane of capillaries where enzymatic digestion is needed to release these cells.15 The efficacy of cell therapies is also dependent on cell source, processing technique, and setting. For example, bone marrow can be processed to increase the concentration of progenitors and improve bone or cartilage repair.7,11,16 However, bone marrow concentration has not consistently been shown to improve repair of osteochondral defects.17 Connective tissue progenitor cells are the heterogenous population of tissue-resident cells that can be activated to proliferate and to generate progeny that can be shown in vitro to differentiate into one or more connective tissues.12-14,16,18 For many indications, laboratory manipulation and culture expansion are needed to isolate and adequately enrich these cell populations.
Contributing to the confusion regarding stem cells, the substantial literature exists using the terminology of culture-expanded cells known as mesenchymal stem cell or mesenchymal stromal cell, both abbreviated as “MSC.”7,19,20 To improve clarity, the ISCT defined MSC to be mesenchymal “stromal” cells having the attributes of being plastic-adherent culture-expanded cells without hematopoietic cell markers that express specific cell surface markers (ie, CD73, CD90, and CD105) and that show the ability to differentiate into osteoblasts, adipocytes, and chondrocytes in vitro.7 Although there have been decades of promising in vitro and animal research exploring the capacity of culture-expanded MSC meeting these criteria to secrete immunomodulatory factors or contribute to new tissue formation, no MSC therapies have yet been cleared by the FDA for human clinical application to musculoskeletal diseases.
Recommendations
The consensus opinion is that the term stem cell has been overused to encompass uncharacterized minimally manipulated cell preparations, as well as tissue-derived culture-expanded cell populations. It is recommended that the use of minimally manipulated cell products and tissue-derived culture-expanded cells be referred to as cell therapy and that the untested and uncharacterized nature of these treatments be clearly understood by practitioners and clearly communicated within the profession, to patients, and to the public.
Future Directions
Expert opinion and consensus work groups can be convened to improve precision of terminology surrounding cell therapy. Establishment of standards and criteria for describing therapeutic cell populations will be needed for clear scientific and clinical communications.
Recommendation 2: Standardize Reporting Requirements
Examination of both minimally manipulated and culture-expanded preparations have identified the inherent variability of these products as a major hurdle to proper characterization and evaluation of their biological and clinical effects. Unlike conventional pharmaceuticals where a known concentration of a bioactive substance is administered to achieve a targeted biological effect, most biologics are complex mixtures of variable composition that are not easily assayed. This phenomenon is particularly evident for blood products such as PRP and for minimally manipulated autologous cell preparations where standards are lacking and where the biological status of the donor and the preparation methods vary widely.
As the most studied biologic used in orthopaedics, PRP composition is known to vary widely when blood from the same individual is obtained at different times of day or is prepared using systems from different manufacturers.21,22,23 Furthermore, growth factor and cytokine concentrations vary by donor age, health status, and sex.23,24 Similarly, progenitor and MSC populations isolated from a given donor also differ widely from one preparation to another and vary by age, sex, tissue source, harvest, and processing methods.12,13,14,R15,16,19,21,22,25,26,28 It is therefore necessary for scientific communications to become more rigorous and standardized in reporting these variables.9,12,13,16
Recommendations
It is recommended that Minimum Information for studies reporting Biologics (MIBO) checklists be used as a guide for study design and reporting29 (Tables 2 and 3). For PRP and cell-based therapies, the MIBO include specific items that reached a consensus among a panel of experts through the Delphi process.29 These proposed minimum requirements would facilitate clinical and experimental investigations into the mechanisms of action and efficacy in a broad range of diseases for PRP and cell-based therapies. Regarding MSC, the ISCT standard can be used to communicate whether the cells used meet the ISCT published standard.7
Table 2.
Minimum Reporting Standards for Clinical Studies Evaluating PRPa
Table 3.
Minimum Reporting Standards for Clinical Studies Evaluating Cell Therapiesa
Future Directions
Characterization of minimally prepared biologics using transcriptomic, proteomic, and metabolomic technologies, coupled with bioinformatic analysis, is needed for further refinement of standards. Furthermore, most of the several hundred platelet-harbored proteins and polypeptides have not been intensively studied in terms of their biologic activity. Experimental analysis of previously understudied and undiscovered platelet proteins may lead to discovery of new target proteins with specific functional roles. In addition, such studies may in fact show that certain “deleterious” components in PRP may be removed or neutralized to enhance the therapeutic benefit of PRP. For cell-based therapies, additional laboratory work to define progenitor subpopulations can be used to refine the description and understanding of the cell populations used. Refined use of nomenclature to distinguish between native stem and progenitor populations and culture-expanded cell populations will provide critically needed improvement to scientific and public communication.
Recommendation 3: Establish Registries for Postmarket Monitoring and Quality Assessments of Biologic Therapies
Registries provide opportunities to collect standardized data on clinical status and clinical outcomes for a variety of different interventions performed in the clinical setting to treat the same disease or condition. Data from joint replacement and other clinical registries also contribute to quality improvement initiatives and assessments. Furthermore, when used consistently, a well-organized and complete registry represents a large prospective cohort study. A registry can additionally be linked to a biorepository to capture and preserve clinical samples for selective future analysis. This design could be particularly powerful to understand the influence of variable PRP composition on clinical outcomes.
The orthopaedic community has established several registry models that could provide pathways for postmarket monitoring and quality control of the use of biologics in orthopaedics. These include registries from the scale of a single institution, an entire health system, to national and international registries. Several registry models have contributed important clinical data on practice patterns, provided early warning of potential issues related to a particular implant or treatment strategy, or show potential for contributing clinical evidence on the efficacy of PRP. These include the American Joint Replacement Registry,30 the Kaiser Registries,26 and the PRP Registry at the Veterans Hospital in Palo Alto, California.
To address the disconnect between the variable composition of PRP from different patients and clinical outcomes, a Biorepository-linked PRP Registry established at the Veterans Hospital in Palo Alto, CA, offers a model where patients receiving PRP injections for treatment of knee OA complete patient-reported outcomes (PROs) before treatment and at defined time points after treatment as part of the clinical care pathway. In parallel, a sample of the administered PRP is banked for patients consenting to federally funded research who additionally undergo functional and structural assessments of gait analysis and advanced quantitative MRI. This biorepository-linked registry supports correlation of PRP proteomics with PRO and quantitative clinical outcome metrics to evaluate potential mechanisms of action and clinical efficacy.
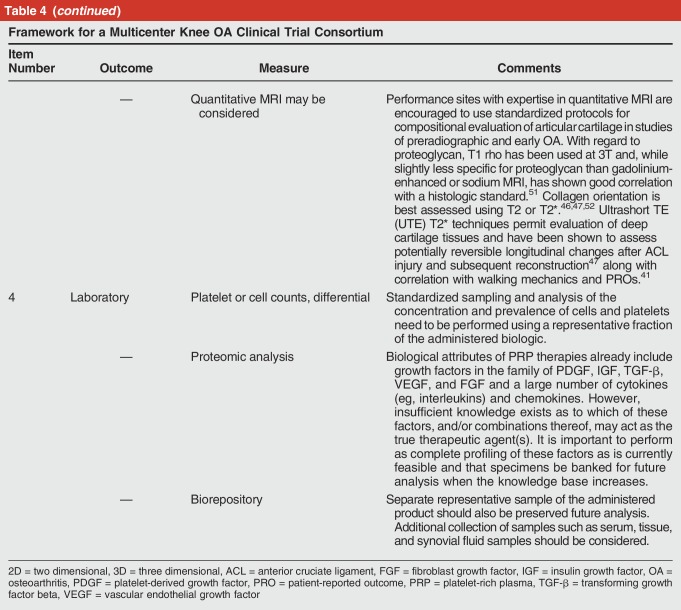
An effective biologics registry would require commitment from physicians, clinics, and hospitals to include all qualifying patients, appropriate incentives for physician and patient participation, and a mechanism for financial support of the human resources required to capture and report clinical baseline and outcomes data. For quality assessments, preparation technique, device used, and clinical laboratory data on the administered biologic will also need to be captured. Using PRP as an example, white blood cell and platelet counts in whole blood and in the administered PRP are the minimum data needed to determine whether the patient received leukocyte-rich or leukocyte-poor PRP and to what degree the platelets were concentrated by the device used.9,29,31 Similar minimal clinical laboratory test data would need to be established for cell-based treatments.9,12,13,16,29 Furthermore, tissue specimens may be also collected to assist in stratifying patient disease state, as well as for performing biomarker, molecular, and genomic analyses to synergize. These data may ultimately be required to define which patient populations are most likely to respond to therapy and to define the critical quality attributes of a cellular or biologic therapy.
Recommendations
It is recommended that physicians, clinics, and institutions offering biologic therapies commit to establishing high-quality patient registries that can be used for postmarket surveillance and quality assessments. The AAOS has expertise and processes in place to assist with registry development and implementation. The American Joint Replacement Registry is part of what will be a family of registries under the AAOS umbrella. Data sets can be customized for specific registries or for a biologics registry. It was recommended that further examination of the feasibility for establishing a national registry for postmarket surveillance and quality assessment of biologics be performed.
Future Directions
The collection and storage of biospecimens into a biorepository and collection of imaging outcome metrics necessitate standardized protocols, which further increases the expense and the complexity. For more immediate reliable generation of high-quality clinical data, it was the opinion of the work group that multicenter prospective clinical trials involving committed centers with appropriate volume and adequate follow-up, as well as willingness and ability to develop and maintain biorepositories and to follow standardized treatment, imaging, and outcomes data collection protocols, were needed.
Section II: Accelerating the Discovery, Development, and Delivery of 21st Century Cures
The 21st Century Cures Act was enacted in December 2016 with provisions to accelerate the development and translation of promising new therapies into clinical evaluation and use.32 This legislation increased funding for medical research, for combating the opioid epidemic, and included measures to streamline approval of new therapies for clinical trials. The law also provided a new expedited biologics product development program called Regenerative Medicine Advanced Therapy. Key elements of Regenerative Medicine Advanced Therapy include accelerated FDA approval for a regenerative medicine therapy that is intended to treat a serious or life-threatening disease or condition and that shows a potential to address unmet clinical needs for that disease or condition.
Recommendation 4: Designate Osteoarthritis as a Serious Medical Condition
The FDA has indicated that a serious disease or condition is one that is “associated with morbidity that has substantial impact on day-to-day functioning.”33 The designation of “whether a disease or condition is serious is a matter of clinical judgment,” based on its impact on survival, daily function, and the likelihood that such morbidity, if persistent or recurrent, has a high likelihood of progression if left untreated. In addition, “An unmet medical need is a condition whose treatment or diagnosis is not addressed adequately by available therapy.”
OA is a leading cause of disability worldwide for which disease-modifying treatments are lacking. Knee and hip OA reduces life expectancy with walking disability as a main risk factor. Studies show that the walking disability from OA exceeds that of heart disease.34 The Framingham study also showed more dependency with knee OA than with heart disease.35 OA has also been associated with an increased risk for premature death primarily from cardiovascular disease. In a propensity-matched landmark analysis to examine whether total joint arthroplasty of the hip and knee reduces the risk for serious cardiovascular events in patients with moderate-severe OA, Ravi et al36 showed that over a 7-year period, 8 total joints prevented 1 myocardial infarction.
Recommendations
On the basis of these data, the strength of the clinical evidence, and the group discussion, the consensus opinion is that OA meets all the criteria for designation as a serious condition with significant unmet clinical needs. The AAOS/NIH U-13 Biologics Symposium work group concurs with the Osteoarthritis Research Society International white paper entitled “Osteoarthritis: A Serious Disease.”37
Future Directions
Many other musculoskeletal conditions such as chronic tendinopathy, degenerative disk disease, and osteoporosis also have substantial and progressive negative impacts on daily function, morbidity, and mortality and should be further evaluated for designation as serious medical conditions.
Recommendation 5: Clarify, by Disease State, a Consensus Approach for Biological Markers of Interest and Clinical Trial Design
Using PRP treatment as a model, an important goal is to address the variability in outcomes by identifying the biologic targets for PRP. This is needed to more precisely choose the optimal PRP formulation to focus treatment for each specific tissue and to ultimately reduce this variability. As an example, for rotator cuff tendon repair, the primary targets are considered to be provision of signaling molecules that drive cellular differentiation to reform the organized structure of the enthesis.38 Further identification of biologic targets will require improved understanding of the underlying cellular and molecular mechanisms of tissue degeneration and repair for each disease state. Such mechanistic information may come from both animal and human studies. Although acute soft-tissue injury can be reproduced in animal models, it is difficult to simulate chronic conditions such as overuse tendinopathy and chronic, slowly-developing OA. Another important limitation of animal models is the inability to precisely control the mechanical loading environment that may also significantly vary from the human condition. Innovative studies in humans, using advanced imaging and limited biopsies, can be used to study the underlying biologic effects and thus help to identify the desired treatment targets.
In addition to defining the desired “biologic” targets (eg, cell proliferation, anti-inflammatory, antifibrotic effect), clinical outcome milestones are also important targets for PRP therapy. For example, for acute muscle injury, the primary goal may be prevention of reinjury rather than faster return to sport. For rotator cuff repair, the goal may be to decrease the rate of retear of the repaired tendon. Finally, mediators of pain/nociception have been advanced as therapeutic targets for the use of PRP and cell-based therapies to treat degenerative conditions such as tendinopathies and OA.
Once the biologic targets for a specific tissue are identified, steps can be taken to match the “ideal” PRP formulation to the tissue. For example, multiple randomized controlled trials and a meta-analysis have suggested that leukocyte-rich PRP is an efficacious treatment of lateral elbow tendinopathy,22,38 whereas leukocyte-poor PRP seems effective for treatment of symptomatic knee OA.39 In addition to identifying optimal PRP formulations, additional studies are needed to define the ideal dose and timing of PRP application to augment soft-tissue healing. For example, PRP may be more effective for rotator cuff repair if administered days to weeks after surgery, once a responding cell population is present, rather than just at the time of surgery. It is also likely that the particular PRP formulation should be tailored to specific time points in the healing process because the biologic targets are likely different at later healing phases.
It will be important to collect comprehensive demographic and clinical data from patients to allow later analyses of factors that may influence clinical outcome. In addition to standard demographic information (eg, age, sex), appropriate imaging should be used to allow quantitative grading of tissue structure and composition and to potentially provide insight into function. Adequate characterization of early stages of OA may require MRI for accurate staging. A sample of the treated tissue should be harvested for later analysis of tissue composition and microstructure, which could then be correlated with imaging characteristics, with the goal being to identify imaging biomarkers that predict outcome. Identification of imaging biomarkers in the treated tissue may also inform the choice of the type and dosing schedule of PRP. Ultimately, detailed transcriptomic and proteomic profiling of the affected tissue may contribute to a “precision medicine” approach to the use of PRP for soft-tissue injury.
It is further recommended that validated outcome measures for each specific tissue or anatomic region be identified. Where validated patient-reported instruments do not exist, the most promising metrics should be identified by consensus expert opinion, followed by validation as a research priority. In addition, the use of the NIH-funded Patient-Reported Outcomes Measurement Information System physical function instrument may be a suitable alternative.40 Additional functional metrics that provide quantitative data are also needed, such as gait analysis to measure functional impairment in knee OA.41 The use of wearable technologies may facilitate collection of these types of functional metrics.42
Finally, clinical trial design will require consideration of several important factors. An important factor in the design of a clinical trial for an acute soft-tissue injury is the timing from injury to treatment. The native, usually successful, healing response may be interrupted or delayed by a PRP administration if it is performed during the early inflammatory phase because PRP contains both anti- and pro-inflammatory factors. Furthermore, injection of saline as a placebo may dilute the naturally occurring hematoma at the injury site and may lead to a negative effect on healing. Standardized rehabilitation protocols should be defined and followed.
Robust statistical analyses will be required to study the interactions between intervention (ie, leukocyte-rich PRP, leukocyte-poor PRP), time point after injury, and injury grade or severity. Stratification should also be performed with regard to sex and age. Last, it will be important to consider identification and stratification by important metabolic and systemic factors that may affect treatment response, such as diabetes, rheumatologic conditions, and chronic use of anti-inflammatory or antifibrotic medications (ie, NSAIDs or losartan).
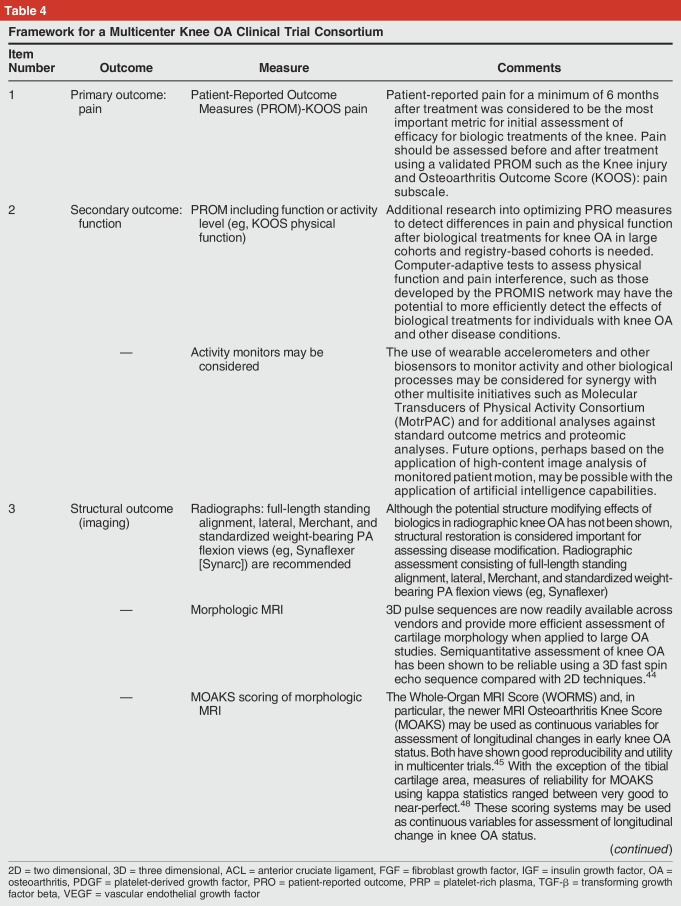
Recommendation 6: Establish the Framework for a Multicenter Knee Osteoarthritis Clinical Trial Consortium (Table 4)
Table 4.
Framework for a Multicenter Knee OA Clinical Trial Consortium
Of the conditions discussed, knee OA emerged as the clinical condition with the most urgent need for clinical trial development. Treatment of end-stage knee OA with knee replacement is already the largest single line item in the Medicare budget, and demand is expected to substantially increase year to year.43 The arthroplasty population treated for end-stage OA represents just a small fraction of the massive underlying demand for regenerative and biological treatments to reduce pain and to prevent or delay progression of early knee OA.
Safety Considerations
Treatment of knee OA with PRP and minimally manipulated autologous cells are already widely used in the United States. The existing studies do not show that these therapies are associated with substantial risk of harm.12,39 Where a proposed therapy does not present significant safety concerns, the focus can be directed toward phase II, III, and IV trials. For optimal evaluation of efficiency, prospective multicenter trials with randomization and placebo control are need. Given the prevalence of OA and the number of proposed biological treatments, randomization schemes with a 3:1 or 4:1 ratio of treatment groups to placebo will accelerate progress.
The Role of MRI in Characterizing Disease State
Although radiographs are helpful in assessing the knee mechanical axis and are reproducible for assessing joint space with appropriate technique, they are relatively insensitive to focal chondral defects and are inadequate for staging early disease. Because of its direct multiplanar acquisition, tomographic nature, and superior soft-tissue contrast, MRI is necessary to evaluate cartilage morphology and has shown superior reproducibility compared with arthroscopy.44,45 Recent advances in quantitative MR allow for assessment of cartilage relaxometry, targeting specific changes in proteoglycan content and collagen orientation, respectively, that improves the sensitivity of MRI for changes of early knee OA.
Future Directions
Characterization of the treated population with respect to clinical, structural, and biological attributes and disease state (eg, subtype, grade) is important. In addition to cell and protein composition, establishing specimen biorepositories will facilitate genomic and molecular analyses that can synergize with existing NIH areas of emphasis such as Helping to End Addiction Long-term, Molecular Transducers of Physical Activity Consortium, and precision medicine initiatives.
Consensus Knee Osteoarthritis Biologics Clinical Trial Design
For evaluation of knee OA treatments, the primary clinical research goals are to determine efficacy in relation to pain, function, and structure, with additional goals of evaluating cost-effectiveness if proven to be efficacious. Key elements from a federally funded pre-post observational trial in Veterans that influenced the consensus trial design include establishment of a biorepository, targeted biospecimen analysis, linkage of the resulting compositional data with clinical data, and PRO metrics along with the use of MRI to establish and stage OA disease and to assess structural outcomes.41,44,45,46,47,48 The MIBO checklists for PRP (Table 2) and cell therapy (Table 3) should be used as a guide for clinical study design and standardized reporting.29 Elements recommended for a knee OA clinical trial are summarized in Table 4.
Recommendation 7: Explore Accelerated Pathways for FDA Approval of New Drug Applications for Biologics to Treat Musculoskeletal Conditions
A patient panel highlighted the tremendous need and demand for effective treatments to restore function and alleviate musculoskeletal pain. This is particularly true for degenerative conditions such as OA and tendinopathy. The clinical history with minimally manipulated autogenous cell products and culture-expanded cells without genetic modifications for musculoskeletal indications suggest that these treatments can be considered “lower risk.”
Two international models for the use of culture-expanded MSC to treat orthopaedic complications were examined. In Japan, provisional approval is granted for a biologic that has been shown to be safe in a small sample of patients and with data showing a potential therapeutic effect.49 The manufacturer then has 7 years through postmarket studies to prove efficacy. If efficacy is not shown during postmarket surveillance, the product is withdrawn. In Chile, the government partnered with a private medical clinic to provide therapies based on culture-expanded bone MSC for a variety of musculoskeletal conditions. Data from this public-private partnership have demonstrated a low incidence of adverse effects and suggest therapeutic efficacy, most notably for OA.50
Recommendations
Patient demand and clinical need along with the international experience support exploration of new pathways developed through the 21st Century Cures Act to accelerate clinical evaluation of the use of autogenous cell sources and culture-expanded cell-based therapies to treat musculoskeletal conditions.32
Acknowledgments
The authors thank Fei Wang, PhD, the National Institute of Arthritis and Musculoskeletal and Skin Diseases collaborator who inspired and assisted with the conference and consensus statement development, and Erin Ransford, Manager, Research Advocacy, who assisted with all aspects of conference development and coordination. This symposium was funded by the American Academy of Orthopaedic Surgeons, the Stanford University Department of Orthopaedic Surgery, and NIH U-13 AR073668 (Chu).
Footnotes
Dr. Rodeo or an immediate family member serves as a paid consultant to the Joint Restoration Foundation and has stock or stock options held in Ortho RTI. Dr. Goodrich or an immediate family member is a member of a speakers' bureau or has made paid presentations on behalf of Allsource; serves as a paid consultant to Allsource; has stock or stock options held in ART; has received research or institutional support from Allsource; and serves as a board member, owner, officer, or committee member of the North American Veterinary Regenerative Medicine and the Orthopaedic Research Society. Dr. Huard or an immediate family member serves as a board member, owner, officer, or committee member of the Orthopaedic Research Society. Dr. Irrgang or an immediate family member serves as a board member, owner, officer, or committee member of the American Physical Therapy Association. Dr. LaPrade or an immediate family member has received royalties from Arthrex, Ossur, and Smith & Nephew; serves as a paid consultant to Arthrex, Ossur, and Smith & Nephew; has received research or institutional support from Arthrex, Smith & Nephew, Ossur, and Linvatec; and serves as a board member, owner, officer, or committee member of the American Orthopaedic Society for Sports Medicine and the International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine. Dr. Lattermann or an immediate family member serves as a paid consultant to Cartiheal, Novartis, Samumed, and Vericel; has stock or stock options held in Cocoon; has received research or institutional support from Smith & Nephew; and serves as a board member, owner, officer, or committee member of the International Cartilage Repair Society and the German-speaking Arthroscopy Society. Dr. Mandelbaum or an immediate family member has received royalties from Arthrex; serves as a paid consultant to Arthrex, DePuy, Exatech; and serves as a board member, owner, officer, or committee member of the CONCACAF Medical Committee and the Kerlan Jobe Institute. Dr. Mao or an immediate family member serves as an unpaid consultant to Mitogen and has stock or stock options held in Mitogen. Dr. McIntyre or an immediate family member serves as a paid consultant to Active Implants, Ceterix, Flexion, and Smith & Nephew and serves as a board member, owner, officer, or committee member of the American Academy of Orthopaedic Surgeons, Advocacy for Improvement in Mobility, the Arthroscopy Association of North America; the Medical Society of the State of New York, the Westchester County Medical Society, and Orthopaedic Practice Management Inc. Dr. Muschler or an immediate family member has received royalties from Fortus; serves as a paid consultant to the National Institutes of Health; serves as an unpaid consultant to Parker Hannifin; and has received research or institutional support from Fortus. Dr. Potter or an immediate family member serves as a paid consultant to Ortho RTI; has stock or stock options held in Imagen; has received research or institutional support from GE Healthcare and GE/NBA; and serves as a board member, owner, officer, or committee member of the International Society for Magnetic Resonance in Medicine. Dr. Spindler or an immediate family member has received royalties from NPhase; serves as a paid consultant to Cytori-Scientific Advisory Board, Mitek, and the National Football League; has received research or institutional support from the National Institutes of Health; and serves as a board member, owner, officer, or committee member of the American Orthopaedic Society for Sports Medicine and the Orthopaedic Research Society. Dr. Tokish or an immediate family member has received royalties from Arthrex; is a member of a speakers' bureau or has made paid presentations on behalf of Arthrex and Mitek; serves as a paid consultant to Arthrex, DePuy, and Mitek; and serves as a board member, owner, officer, or committee member of the Arthroscopy Association of North America. Dr. Tuan or an immediate family member serves as a paid consultant to Orthocell and serves as an unpaid consultant to AbbVie and Recellerate. Dr. Zaslav or an immediate family member is a member of a speakers' bureau or has made paid presentations on behalf of Lifenet and Vericel; serves as a paid consultant to Cartiheal and Lifenet; has stock or stock options held in Cartiheal and Orthospace; has received research or institutional support from Active Implants, Aesculap/B.Braun, Organogenesis, and Zimmer Biomet; and serves as a board member, owner, officer, or committee member of the International Cartilage Repair Society. Dr. Maloney or an immediate family member has received royalties from Stryker and Zimmer Biomet; has stock or stock options held in Bristol-Myers Squibb, Flexion Therapeutics, Medtronic, Novartis, Pfizer, and TJO; and serves as a board member, owner, officer, or committee member of the American Academy of Orthopaedic Surgeons. Dr. Mishra receives royalties from Zimmer-Biomet and DePuy. None of the following authors or any immediate family member has received anything of value from or has stock or stock options held in a commercial company or institution related directly or indirectly to the subject of this article: Dr. Chu, Dr. Bhutani, Dr. Lu, and Dr. Piuzzi.
February 15-17, 2018 at Stanford University, Stanford California.
Conference organizers
References
- Evidence-based Medicine: Levels of evidence are described in the table of contents. In this article, references 11, 15, 17, 21, 22, 28, 38, 39 are level I studies. References 23, 24, 25, 26, 34, 35, 36, 41, 44, 47, 51, 52 are level II studies. References 43, 48, 45 are level III studies. References 2, 4, 5, 12, 13, 16, 31, 40, 42, 50 are level IV studies. References 1, 3, 6, 7, 8, 9, 10, 14, 18, 19, 20, 27, 29, 30, 32, 33, 37, 46, 49 are level V expert opinion.
- References printed in bold type are those published within the past 5 years.
- 1.Chu C, Maloney W, Mao J, Rodeo S, Tuan R, Wang F: AAOS Optimizing Clinical Use of Biologics in Orthopaedic Surgery Planning Team Introductions/Overview of Symposium Goals General Session I: Determining Candidate Biologic Targets for Common Indications. 2018. https://www.aaos.org/uploadedFiles/PreProduction/Research/committee/research/symposia/Biologics Agenda_Web.pdf. Accessed July 17, 2018.
- 2.Yelin E, Weinstein S, King T: The burden of musculoskeletal diseases in the United States. Semin Arthritis Rheum 2016;46:259-260. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs JJ, King TR, Klippel JH, et al. : Beyond the decade: Strategic priorities to reduce the burden of musculoskeletal disease. J Bone Joint Surg Am 2013;95:e1251-e1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piuzzi N, Ng M, Chughtai M, et al. : The stem-cell market for the treatment of knee osteoarthritis: A patient perspective. J Knee Surg 2018;31:551-556. [DOI] [PubMed] [Google Scholar]
- 5.Turner L, Knoepfler P: Selling stem cells in the USA: Assessing the direct-to-consumer industry. Cell Stem Cell 2016;19:154-157. [DOI] [PubMed] [Google Scholar]
- 6.Schwarz A: New Procedure Uses Athletes' Own Blood to Treat Injuries. New York Times. 2009, A1 www.nytimes.com/2009/02/17/sports/17blood.html. Accessed July 17, 2018. [Google Scholar]
- 7.Dominici M, Nichols K, Srivastava A, et al. : Positioning a scientific community on unproven cellular therapies: The 2015 International Society for Cellular Therapy Perspective. Cytotherapy 2015;17:1663-1666. [DOI] [PubMed] [Google Scholar]
- 8.Sipp D, Caulfield T, Kaye J, et al. : Marketing of unproven stem cell–based interventions: A call to action. Sci Transl Med 2017;9:eaag0426. [DOI] [PubMed] [Google Scholar]
- 9.LaPrade RF, Dragoo JL, Koh JL, Murray IR, Geeslin AG, Chu CR: AAOS research symposium updates and consensus. J Am Acad Orthop Surg 2016;24:e62-e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stem Cell Basics I. https://stemcells.nih.gov/info/basics/1.htm. Accessed July 17, 2018.
- 11.Luangphakdy V, Boehm C, Pan H, Herrick J, Zaveri P, Muschler GF: Assessment of methods for rapid intraoperative concentration and selection of marrow-derived connective tissue progenitors for bone regeneration using the canine femoral multidefect model. Tissue Eng Part A 2016;22:17-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chahla J, Piuzzi NS, Mitchell JJ, et al. : Intra-articular cellular therapy for osteoarthritis and focal cartilage defects of the knee. J Bone Joint Surg 2016;98:1511-1521. [DOI] [PubMed] [Google Scholar]
- 13.Piuzzi NS, Chahla J, Jiandong H, et al. : Analysis of cell therapies used in clinical trials for the treatment of osteonecrosis of the femoral head: A systematic review of the literature. J Arthroplasty 2017;32:2612-2618. [DOI] [PubMed] [Google Scholar]
- 14.Muschler GF, Nakamoto C, Griffith LG: Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg Am 2004;86-A:1541-1558. [DOI] [PubMed] [Google Scholar]
- 15.Crisan M, Yap S, Casteilla L, et al. : A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008;3:301-313. [DOI] [PubMed] [Google Scholar]
- 16.Piuzzi NS, Hussain ZB, Chahla J, et al. : Variability in the preparation, reporting, and use of bone marrow aspirate concentrate in musculoskeletal disorders. J Bone Joint Surg 2018;100:517-525. [DOI] [PubMed] [Google Scholar]
- 17.Chu CR, Fortier LA, Williams A, et al. : Minimally manipulated bone marrow concentrate compared with microfracture treatment of full-thickness chondral defects. J Bone Joint Surg 2018;100:138-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muschler GF, Midura RJ: Connective tissue progenitors: Practical concepts for clinical applications. Clin Orthop Relat Res 2002;66-80. [DOI] [PubMed] [Google Scholar]
- 19.Caplan AI: Mesenchymal stem cells. J Orthop Res 1991;9:641-650. [DOI] [PubMed] [Google Scholar]
- 20.Bianco P, Cao X, Frenette PS, et al. : The meaning, the sense and the significance: Translating the science of mesenchymal stem cells into medicine. Nat Med 2013;19:35-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castillo TN, Pouliot MA, Kim HJ, Dragoo JL: Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med 2011;39:266-271. [DOI] [PubMed] [Google Scholar]
- 22.Mazzocca AD, McCarthy MBR, Chowaniec DM, et al. : Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am 2012;94:308-316. [DOI] [PubMed] [Google Scholar]
- 23.Xiong G, Lingampalli N, Koltsov JCB, et al. : Men and women differ in the biochemical composition of platelet-rich plasma. Am J Sports Med 2018;46:409-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weibrich G, Kleis WKG, Hafner G, Hitzler WE: Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg 2002;30:97-102. [DOI] [PubMed] [Google Scholar]
- 25.Payne KA, Didiano DM, Chu CR: Donor sex and age influence the chondrogenic potential of human femoral bone marrow stem cells. Osteoarthritis Cartilage 2010;18:705-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maletis GB, Chen J, Inacio MCS, Funahashi TT: Age-related risk factors for revision anterior cruciate ligament reconstruction. Am J Sports Med 2016;44:331-336. [DOI] [PubMed] [Google Scholar]
- 27.Baer PC, Geiger H: Adipose-derived mesenchymal stromal/stem cells: Tissue localization, characterization, and heterogeneity. Stem Cells Int 2012;2012:812693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trivanović D, Jauković A, Popović B, et al. : Mesenchymal stem cells of different origin: Comparative evaluation of proliferative capacity, telomere length and pluripotency marker expression. Life Sci 2015;141:61-73. [DOI] [PubMed] [Google Scholar]
- 29.Murray IR, Geeslin AG, Goudie EB, Petrigliano FA, LaPrade RF: Minimum information for studies evaluating biologics in orthopaedics (MIBO). J Bone Joint Surg 2017;99:809-819. [DOI] [PubMed] [Google Scholar]
- 30.Etkin CD, Springer BD: The American Joint Replacement Registry: The first 5 years. Arthroplast Today 2017;3:67-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chahla J, Cinque ME, Piuzzi NS, et al. : A call for standardization in platelet-rich plasma preparation protocols and composition reporting. J Bone Joint Surg 2017;99:1769-1779. [DOI] [PubMed] [Google Scholar]
- 32.Marks P, Gottlieb S: Balancing safety and innovation for cell-based regenerative medicine. N Engl J Med 2018;378:954-959. [DOI] [PubMed] [Google Scholar]
- 33.FDA, CDER: CBER: Guidance for Industry Expedited Programs for Serious Conditions—Drugs and Biologics. 2014. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm. Accessed July 17, 2018. [Google Scholar]
- 34.Hawker GA, Croxford R, Bierman AS, et al. : All-cause mortality and serious cardiovascular events in people with hip and knee osteoarthritis: A population based cohort study. PLoS One 2014;9:e91286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guccione AA, Felson DT, Anderson JJ, et al. : The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health 1994;84:351-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ravi B, Croxford R, Austin PC, et al. : The relation between total joint arthroplasty and risk for serious cardiovascular events in patients with moderate-severe osteoarthritis: Propensity score matched landmark analysis. BMJ 2013;347:f6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osteoarthritis: A serious disease, submitted to the U.S. Food and Drug Administration December 1, 2016. https://www.oarsi.org/sites/default/files/docs/2016/oarsi_white_paper_oa_serious_disease_121416_1.pdf. Accessed July 17, 2018.
- 38.Fitzpatrick J, Bulsara M, Zheng MH: The effectiveness of platelet-rich plasma in the treatment of tendinopathy: A meta-analysis of randomized controlled clinical trials. Am J Sports Med 2017;45:226-233. [DOI] [PubMed] [Google Scholar]
- 39.Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ: Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med 2016;44:792-800. [DOI] [PubMed] [Google Scholar]
- 40.White DK, Master H: Patient-reported measures of physical function in knee osteoarthritis. Rheum Dis Clin North Am 2016;42:239-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams AA, Titchenal MR, Andriacchi TP, Chu CR: MRI UTE-T2* profile characteristics correlate to walking mechanics and patient reported outcomes 2 years after ACL reconstruction. Osteoarthritis Cartilage 2018;26:569-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chughtai M, Piuzzi N, Yakubek G, et al. : Use of an app-controlled neuromuscular electrical stimulation system for improved self-management of knee conditions and reduced costs. Surg Technol Int 2017;31:221-226. [PubMed] [Google Scholar]
- 43.Kurtz S, Ong K, Lau E, Mowat F, Halpern M: Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg 2007;89:780-785. [DOI] [PubMed] [Google Scholar]
- 44.Crema MD, Nogueira-Barbosa MH, Roemer FW, et al. : Three-dimensional turbo spin-echo magnetic resonance imaging (MRI) and semiquantitative assessment of knee osteoarthritis: Comparison with two-dimensional routine MRI. Osteoarthritis Cartilage 2013;21:428-433. [DOI] [PubMed] [Google Scholar]
- 45.Peterfy CG, Guermazi A, Zaim S, et al. : Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 2004;12:177-190. [DOI] [PubMed] [Google Scholar]
- 46.Argentieri E, Burge A, Potter H: Magnetic resonance imaging of articular cartilage within the knee. J Knee Surg 2018;31:155-165. [DOI] [PubMed] [Google Scholar]
- 47.Chu CR, Williams AA, West RV: Quantitative magnetic resonance imaging UTE-T2* mapping of cartilage and meniscus healing after anatomic anterior cruciate ligament reconstruction. Am J Sports Med 2014;42:1847-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hunter DJ, Guermazi A, Lo GH, et al. : Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI osteoarthritis knee score). Osteoarthritis Cartilage 2011;19:990-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cyranoski D: Japan relaxes human stem-cell rules. Nature 2009;460:1068. [DOI] [PubMed] [Google Scholar]
- 50.Mardones R, Jofré CM, Tobar L, Minguell JJ: Mesenchymal stem cell therapy in the treatment of hip osteoarthritis. J Hip Preserv Surg 2017;4:159-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wheaton AJ, Casey FL, Gougoutas AJ, et al. : Correlation of T1rho with fixed charge density in cartilage. J Magn Reson Imaging 2004;20:519-525. [DOI] [PubMed] [Google Scholar]
- 52.Gallo MC, Wyatt C, Pedoia V, et al. : T1ρ and T2 relaxation times are associated with progression of hip osteoarthritis. Osteoarthritis Cartilage 2016;24:1399-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]