Abstract
TNF-α (TNF) is a pleiotropic cytokine which can have proinflammatory or immunosuppressive effects, depending on the context, duration of exposure and disease state. The basis for the opposing actions of TNF remains elusive. The growing appreciation of CD4+FoxP3+ regulatory T cells (Tregs), which comprise ~10% of peripheral CD4 cells, as pivotal regulators of immune responses has provided a new framework to define the cellular and molecular basis underlying the contrasting action of TNF. TNF by itself can overcome the profound anergic state of T cell receptor-stimulated Tregs. Furthermore, in concert with IL-2, TNF selectively activates Tregs, resulting in proliferation, upregulation of FoxP3 expression and increases in their suppressive activity. Both human and mouse Tregs predominantly express TNFR2, making it possible for TNF to enhance Treg activity, which helps limit the collateral damage caused by excessive immune responses and eventually terminates immune response. TNFR2-expressing CD4+FoxP3+ Tregs comprise ~40% of peripheral Tregs in normal mice and present the maximally suppressive subset of Tregs. In this review, studies describing the action of TNF on Treg function will be discussed. The role of Tregs in the autoimmune disorders and cancer as well as the effect of anti-TNF therapy on Tregs, especially in rheumatoid arthritis, will also be considered.
Introduction
CD4+FoxP3+ Regulatory T Cells Are Pivotal Regulators of Immune Responses
The evidence that suppressive T cells downregulate antigen-specific response of effector T cells and maintain immune tolerance was reported as early as 1970s [1]. In the mid-1990s, Sakaguchi et al. [2] identified CD4 cells which constitutively coexpressed CD25, the IL-2 receptor α-chain, in normal rodents as potent suppressive regulatory T cells (Tregs), and showed that elimination of this population of cells elicited autoimmune responses. Subsequent studies extending over more than a decade have provided compelling evidence that CD4+FoxP3+ Tregs, comprising ~10% of peripheral CD4 cells, play an indispensable role in maintaining immune homeostasis and in suppressing deleterious excessive immune responses [3].
Two major sources of Tregs, namely naturally occurring Tregs (nTregs) and induced Tregs (iTregs), are engaged in normal tolerogenic surveillance of self-antigens and prevent potential autoimmune responses. nTregs develop in the thymus and are exported to the periphery [3], and iTregs are converted from naïve CD4 cells by TGF-β in conjunction with T cell receptor (TCR) stimulation in the periphery [4]. Tregs are preferentially self-reactive since their TCRs have higher affinity for self-antigens, and are similar to the TCR used by self-reactive pathogenic effector T cells (Teffs) [5]. Foxp3, a member of the forkhead/winged-helix family of transcription factors, is a master regulator of Treg development and function [6], as shown by deficiency of Tregs and lethal autoimmunity caused by the mutation of FoxP3 in human patients with IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) and its murine counterpart scurfy [reviewed in 7]. The characteristic phenotype of Tregs such as high expression of CD25, CTLA-4, GITR and low expression of CD127 was shown to be regulated by FoxP3 [reviewed in 8].
The activation, proliferation and effector functions of a large spectrum of immunocompetent cells, such as CD4 cells [9], CD8 cells [10], NK cells [11], NKT cells [12], dendritic cells [13], macrophages [14] and B cells [15] are susceptible to Treg-mediated suppression. The induction of Treg-suppressive activity is specific and requires antigenic stimulation through the TCR; however, the suppression exerted by Tregs is not antigen specific [16]. Therefore, a wide range of immune responses can be inhibited by Tregs through ‘bystander’ suppression [17]. In addition to suppressing immune responses to auto-antigens, Tregs also attenuate host defense responses against pathogens [reviewed in 18] and tumor antigens [reviewed in 19].
The exact mechanism(s) of Treg-mediated suppression remain incompletely understood. The in vitro suppressive activity of Tregs depends on cell-to-cell contact as over a short distance [9]. In addition, several molecules, such as IL-10, TGF-β, CTLA-4, indoleamine 2,3-dioxygenase and granzyme/perforin are reported to contribute to the suppressive activity of Tregs [reviewed in 20]. IL-35 is reportedly expressed by mouse FoxP3+ Tregs and contributes to Treg function [21]; however, this immunosuppressive cytokine is not expressed by human Tregs [22, 23]. Tregs express CD39/ENTPD1 and CD73/ecto-5′-nucleotidase, ectoenzymes which have the capacity to generate pericellular adenosine from extracellular nucleotides. The coordinated expression of CD39/CD73 on Tregs and the adenosine A2A receptor on activated Teffs therefore may generate an immunosuppressive loop [24]. Although Tregs are likely to use multiple mechanisms to suppress immune responses, CTLA-4 may have a dominant role. It has been recently shown that CTLA-4 was critically required for the function of Tregs in vivo by inhibiting activities of antigen-presenting cells (APCs) [25, 26].
Besides FoxP3+ Tregs, there are other types of Tregs that can be induced from naïve CD4 cells in the periphery, such as IL-10- and TGF-β-producing Tr1 cells and TGF-β-producing Th3 cells [3]. Thus, various Tregs by using distinct mechanism are likely to operate collaboratively to regulate the duration and magnitude of an immune response.
Contrasting Roles of TNF-α in Autoimmune Diseases
TNF-α (TNF) is a pleiotropic cytokine that is a major participant in the initiation and orchestration of complex events in inflammation and immunity [27]. TNF has well-documented proinflammatory effects. Nevertheless, increasing evidence reveals that TNF also has unexpected anti-inflammatory and immunosuppressive effects, especially after prolonged exposure [reviewed in 28–30]. For example, as expected, several transgenic mouse strains overproducing TNF consistently develop autoimmune disorders [reviewed in 31]. However, transgenic NOD mice overexpressing TNF in their pancreatic islets failed to develop autoimmune diabetes [32] and repeated injection of TNF suppressed both type I diabetes in NOD mice and lupus nephritis in susceptible mouse strains [33, 34]. Furthermore, NZB mice deficient in TNF exhibited acceleration of autoimmunity and lupus nephritis [35]. C67BL/6.129 mice deficient in TNF developed mild autoimmunity resembling the initial stages of lupus nephritis [36]. TNF knockout (KO) mice developed prolonged and exacerbated experimental auto-immune encephalomyelitis (EAE), although with a delayed onset, after EAE induction [37]. In multiple sclerosis patients, treatment with anti-TNF agents resulted almost uniformly in immune activation and exacerbation of disease [36]. Perhaps reflecting the strikingly contrasting activities of TNF, anti-TNF therapy in rheumatoid arthritis (RA) and inflammatory bowel disease, although impressively beneficial to the majority of patients, led to the development of lupus and neuroinflammatory diseases in some patients [36].
Regulatory T Cell Levels Are Increased at Autoimmune Inflamed Sites
It has been established for more than a decade that the breakdown of immune tolerance maintained by Tregs can cause organ-specific autoimmune responses in animal models [2]. The essential role of intact FoxP3 for Treg function in prevention of autoimmune responses has been convincingly confirmed by the development of autoimmunity in human patients with IPEX and in the homologous scurfy mouse [7]. It has therefore been proposed that the abnormality autoimmune patients generally have in common is either low Treg numbers with normal function or normal Treg numbers with compromised suppressive function [38]. However, the clinical and laboratory data lend very little support to this simplified notion and, instead, provide a much more complicated and often counterintuitive scenario. For example, the frequency and function of Tregs in the peripheral blood (PB) of RA patients is still controversial [reviewed in 38, 39]. The mounting evidence clearly indicates that the frequency of Tregs in RA patients with an activated phenotype and enhanced suppressive potential in the synovial fluid of inflamed joints was increased, as compared with those in the periphery [40–43]. Similarly, activated Tregs also accumulated in the inflamed joint of other arthropathies such as juvenile idiopathic arthritis and spondyloarthropathies [44–46]. Markedly elevated levels of Tregs were found in the synovial fluid in K/BxN mouse model of spontaneous inflammatory arthritis [47]. By crossing them with FoxP3gfp mice, the population of Tregs, which could be unequivocally identified by GFP expression, was also increased during development of arthritis in K/BxN mice, especially at sites of inflammation [48]. Consequently, functional Tregs are often increased at the site of autoimmune inflammation, presumably resulting from active recruitment and in situ proliferative expansion.
Elucidation of the effect of TNF on Treg activity is critically important in an era where the use of biological agents to block TNF in the autoimmune patients is becoming a routine therapy. This review focuses on the new developments that provide some insight into how TNF stimulates Treg activity and how this may explain the puzzling immunosuppressive property of TNF in chronic inflammation.
Inflammation in General Activates Regulatory T Cells
Inflammation is the hallmark of a wide variety of diseases, in addition to autoimmunity, including infection and cancer. Studies of both human patients and animal models show that the frequency and suppressive function of Tregs were increased in sepsis, which contributes to the postseptic immunosuppressive phase and its fatal consequence [reviewed in 49], in a TNFR2-dependent manner [50]. In chronic infections, Tregs are activated and accumulate at the site of infection, which limits the magnitude of effector responses to control infection, but also reduces collateral tissue damage caused by excessively vigorous antimicrobial immune responses [reviewed in 18]. Inflammation in the tumor microenvironment promotes tumor progression [reviewed in 51]. Tregs accumulate in the tumor microenvironment, which can dampen natural or induced immune responses against tumor antigens and is predictive of a poor prognosis [reviewed in 19]. Thus, activation of Tregs has been reported in various types of inflammatory responses, which may represent a negative feedback mechanism to curtail excessive inflammation and prevent self-tissue destruction.
TNF Activates Mouse Tregs
Administration of TNF Expands Tregs in Young Adult Autoimmune-Prone Mice
A study examining the effect of intraperitoneal administration of TNF on the number of Tregs in NOD mice shed some light on the in vivo effect of TNF on Treg activity. Injection of TNF into newborn NOD mice led to an accelerated development of diabetes, while TNF treatment of adult NOD mice inhibited the development of diabetes [34]. Wu et al. [52] found that administration of TNF into neonatal NOD as well as neonatal B6.NOD mice resulted in a reduction of CD4+CD25+ cells in the spleen. In contrast, administration of TNF into young adult NOD mice increased the number of splenic CD4+CD25+ cells. It is now known that self-reactive T cells are exported from the thymus prior to Tregs, and neonatal mice are virtually deficient in Treg in the periphery [3]. Thus, it is likely that the stimulatory action of TNF may preferentially activate Teffs in neonatal NOD mice. In adult NOD mice, when the peripheral Treg pool has reached its full size, TNF action is likely to expand Tregs and tip the immune balance maintained by Tregs and Teffs toward an immune-tolerant direction.
TNF Mediated the Capacity of Pertussis Toxin to Activate Tregs
Our studies showed that pertussis toxin in the immunizing cocktail to induce EAE was solely responsible for the resulting reduction in Treg activity [53, 54]. Furthermore, pertussis toxin inhibited TGF-β-induced FoxP3 expression by wild-type (WT) naïve CD4 cells cocultured with WT bone marrow-derived dendritic cells. However, although pertussis toxin markedly reduced Tregs number in WT mice, administration of pertussis toxin paradoxically expanded Tregs in IL-6 KO mice. Pertussis toxin also promoted FoxP3 induction when marrow-derived dendritic cells were from IL-6 KO mice [55]. Therefore, this led us to hypothesize that mediator(s) induced by pertussis toxin in the absence of IL-6 should be able to stimulate Tregs. We confirmed previous reports [56, 57] that proinflammatory cytokines (IL-1β, IL-6, TNF) and Th1 cytokine (IFN-γ) were produced by pertussis toxin-treated splenocytes. This led us to identify TNF as the sole mediator with the capacity to expand Tregs in this study [58].
TNF Stimulates Proliferative Responses of Both Regulatory T Cells and Effector T Cells
Our subsequent studies revealed that TNF actually stimulated proliferation of both CD25–as well as CD25+ subsets of mouse CD4 cells, and therefore enhanced proliferation of cocultures containing Tregs and Teffs. Thus, we showed for the first time that TNF had the ability to overcome the profound anergy of Tregs to TCR stimulation in vitro. TNF has the capacity to directly stimulate purified CD4 Treg cells free of APCs. Incubation of cocultures containing Tregs and Teffs over a shorter time of 48 h with TNF (0.5–10 ng/ml) partially reversed Treg-suppressive activity. However, after a more prolonged incubation of 72 h with TNF, Treg suppression prevailed and the degree of inhibition in co-cultures was restored to a normal level [50]. These data suggest that over the short-term, TNF, as seen in the early phases of inflammatory response, may enable Teffs to proliferate despite the presence of Tregs, whereas more prolonged exposure to TNF favors the expansion and activation of functional Tregs.
In Concert with Interleukin-2, TNF Selectively Activates Mouse Regulatory T Cells
TNF by itself was not sufficient to support the survival of Tregs in vitro [50]. To maintain the in vitro survival of Tregs, Treg cultures were supplemented with IL-2. In the presence of IL-2, TNF markedly increased level of the FoxP3 expression (MFI) by Tregs in a dose-dependent (0.1~10 ng/ml) and time-dependent (24~72 h) manner. IL-1β and IL-6 lacked this activity. Unlike TGF-β, TNF did not induce FoxP3 expression by anti-CD3-stimulated naïve CD4+CD25–T cells. In conjunction with IL-2, TNF was able to selectively expand the subset of CD4+FoxP3+ T cells and selectively increased the phosphorylation of Stat5 in Tregs. Importantly, Tregs pretreated with TNF and IL-2 were markedly more suppressive than Tregs pretreated with IL-2 alone [50]. Thus, activation of Tregs by TNF has proliferative and functional consequences.
IL-2 has been appreciated for its nonredundant role in maintaining Treg survival and function [reviewed in 59]. Our study showed that TNF selectively upregulated CD25 expression on Tregs [50], while IL-2 preferentially upregulated TNFR2 expression on Tregs (our unpubl. data). This suggests that TNF and IL-2 form a reciprocating receptor amplification circuit and synergistically upregulate Treg suppressive activity. Collectively, our data clearly demonstrate that TNF, in conjunction with IL-2, has the capacity to selectively activate Tregs, resulting in proliferation and upregulation of FoxP3 expression and Stat5 phosphorylation, and consequently to enhance the suppressive potential of Tregs.
TNFR2 Is Predominantly Expressed on Human and Mouse Regulatory T Cells
TNF mediates its biological functions through two structurally distinct receptors: TNFR1 (p55) and TNFR2 (p75); the latter is largely confined to cells of the immune system [60]. Unlike TNFR1, which contains a death domain in its cytoplasmic tail, the primary function of TNFR2 is to promote lymphocyte proliferation and survival [27]. Agonist antibodies against p75, like TNF, have the capacity to enhance T cell proliferative response, whereas the specific activation of p55 had no such effect [61]. Kim et al. [62–64] have convincingly demonstrated that TNFR2 has important costimulatory function and markedly enhanced the responses of lymphocyte to TCR-mediated signaling.
nTregs develop in the thymus as a distinct lineage of functionally mature suppressor T cell subset [3]. All human thymic CD4+CD25+ Tregs constitutively express TNFR2, while thymic CD4+CD25–cells do not express this receptor [65]. Interestingly, immunosuppressive human thymic CD4-CD8+CD25+ T cells also express TNFR2 mRNA and protein [66]. We found that the majority (~80%) of mouse thymic Tregs (CD8–CD4+CD25+) are also TNFR2-expressing cells [67]. The normal thymus is the only organ that constitutively expresses TNF [68]. Thus, TNF may regulate Treg development and differentiation in the thymus.
In human PB, all CD4+CD25hi T cells express the highest level of TNFR2, while 20~30% of CD4+CD25–T cells express a low level of this receptor [69 and our unpubl. data]. We confirmed that TNFR2 expression was highest on, but not exclusively confined to, human peripheral CD4+FoxP3+ T cells [69]. We demonstrated that 30–40% of CD4+CD25+ cells which comprised >90% FoxP3+ cells in peripheral lymphoid tissues of normal Balb/c mice and C57BL/6 (B6) mice expressed TNFR2, while only 8% of CD4+CD25–cells (which contain 25–40% of FoxP3+ cells) were TNFR2+cells. After TCR stimulation, surface expression of TNFR2 on both CD4+CD25+ and CD4+CD25–T cells was upregulated. However, the activated CD4+CD25+ T cells still expressed considerably higher levels of TNFR2 on 47% of cells as compared with 32% of activated CD4+CD25–T cells. TNFR1 was not detectable by FACS on either CD4+CD25+ or CD4+CD25–T cells [50, 67]. In contrast to human circulating CD4+CD25hi cells, virtually all of which express high levels of TNFR2 [69], less than 10% of mouse PB CD4+CD25+ cells are TNFR2+ cells [67], presumably because the laboratory mice live in a pathogen-free environment.
TNFR2-Expressing Mouse Tregs Are Maximally Suppressive Cells
The diverse biological activities of TNF may be caused as a distinct signaling consequence of a particular receptor [37, 70]. Several lines of evidence suggest that immunosuppressive action of TNF is mediated by TNFR2 [37, 71, 72]. For example, in the EAE mouse model, TNFR1-deficient mice were completely resistant to induction of disease, while TNFR2-deficient mice exhibited more severe EAE [37, 71]. The transmembrane form of TNF preferentially activates TNFR2 [73], and thus its role in selective stimulation of Tregs warrants future research. Although activation of Tregs can be a result of antigen-specific responses, proinflammatory cytokines such as TNF are also likely to enhance the activation of Tregs. Thus, TNFR2 is likely to costimulate Tregs in conjunction with TCR signaling and yields more activated functional Tregs.
To test this hypothesis, we have compared the phenotype and suppressive function of TNFR2+ Tregs with TNFR2–Tregs from normal C57BL/6 mice. The expression of TNFR2 actually defined a subset of activated/effector Tregs which were CD45RBloCD62LloCD44hi. Although TNFR2+ and TNFR2–Tregs expressed comparable levels of FoxP3 (91.0 and 92.8%, respectively), TNFR2+ Tregs expressed a much higher level of CTLA4 (83.6%, MFI: 101.1) than TNFR2–Tregs (41.0%, MFI: 50.9). More importantly, TNFR2+ Tregs were highly suppressive in vitro. In sharp contrast, CD4+CD25+TNFR2–T cells exhibited a naïve phenotype and only have minimal suppressive activity. CD103-expressing Tregs are generally known to be the most suppressive subset of mouse Tregs [74]. The suppressive activity of TNFR2+ Tregs was even superior to that of CD103-expressing Tregs. Further, the number of TNFR2+ Tregs was 5–7 times greater than that of CD103+ Tregs. Although not identified as Tregs previously, even CD4+CD25–TNFR2+ T cells had moderate suppressive activity [67], which correlated with expression of FoxP3 (our unpubl. data). Thus, TNFR2 expression identified maximally suppressive FoxP3+ Tregs.
Our studies showed that tumor-associated Tregs were characterized by highly suppressive activity and by high level of TNFR2 expression which may increase their capacity to respond to the elevated TNF production by tumors [67]. We characterized Tregs in several mouse tumor models. In both 4T1 breast tumor in Balb/c mice and Lewis lung carcinoma tumors in C57BL/6 mice, the majority of tumor-infiltrating Tregs were TNFR2+ Tregs with highly suppressive activity [67]. It has been reported that TGF-β, a cytokine crucial for de novo generation of Tregs [4], was also able to induce TNFR2 expression on CD4 cells [75]. Therefore, tumor-derived TGF-β may contribute to the increase in TNFR2+ Tregs in the tumor. Our study suggests that TNFR2+ Tregs may play a crucial role in immune evasion of the tumor by potently dampening host immune responses to tumor antigens. Thus, TNFR2+ Tregs may provide a therapeutic target. In support of this hypothesis, a recent study proposed that cyclophosphamide eradicated tumor by selectively eliminating TNFR2+ Tregs [76].
van Mierlo et al. [69] reported that both human and mouse Tregs not only strongly expressed TNFR2, but they also shed large amounts of soluble TNFR2 upon stimulation with anti-CD3/CD28 in conjunction with IL-2. This was paralleled by their ability to inhibit the action of TNF. In vivo, Tregs suppressed IL-6 production in response to LPS injection in mice. In contrast, Treg cells from TNFR2 KO mice were unable to do so despite their unhampered capacity to suppress T cell proliferation in a conventional in vitro suppression assay. This study suggests that shed TNFR2 represents a novel mechanism by which Tregs can inhibit the inflammatory action of TNF [69].
Taken together, TNFR2 expression on Tregs has either phenotypic or functional implications. Although we favor the idea the high expression of CTLA-4 account for the potent suppressive activity of TNFR2-expressing Tregs [67], other mechanism such as shedding TNFR2 molecules [69] may also contribute.
Effects of TNF on Human Treg Activity
Our understanding of the action of TNF on human Treg activity has been gained mainly from studies examining the effect of anti-TNF therapy in autoimmune patients. Due to the limitation in the study of human Tregs in inflammatory conditions, in vitro experiments have been used to clarify the direct action of TNF on human Treg activity. Using conventional in vitro Treg function assays, Ehrenstein et al. [77] first examined the effect of exogenous TNF (0.1–5 ng/ml) on cocultures containing CD4+CD25hi cells and CD4+CD25–cells from normal human healthy donors or from RA patients responsive to anti-TNF therapy. Their results showed that exogenous TNF neither increased nor decreased Treg activity, as evidenced by no alteration of percentage inhibition of proliferation in the cocultures. This was confirmed by another study [44]. Valencia et al. [78] examined the effect of higher concentrations of TNF (50 ng/ml) on in vitro normal human Treg activity. They reported that exogenous TNF downregulated FoxP3 expression and blocked Treg suppressive activity by signaling through TNFR2 and that an agonist monoclonal antibody to TNFR2 also reversed the suppressive activity of healthy donor PB CD4+CD25hi cells. In contrast, our own preliminary data suggest that TNF at the same concentration ranges has stimulatory activity on normal human Tregs. Thus, the current data regarding direct action of TNF on human Tregs is divergent, which is partially due to the lack of uniform criteria for the identification and isolation of functional human Tregs.
Anti-TNF Therapy Promotes Tr1 and Th3 Cells
TNF is a major inflammatory cytokine contributing to the pathogenesis of RA, which provides rationale for the development of anti-TNF biological agents in the treatment of RA [79]. The impressive clinical benefit of anti-TNF therapy in the majority of patients prompted investigators to examine the effect of anti-TNF treatment on the number and function of Tregs. Our findings that TNF promotes the proliferation and function of Tregs would predict that anti-TNF therapy should decrease the Treg levels in treated patients.
It was reported that 83.2% of PB CD4+CD25hi T cells from healthy donors expressed FoxP3 and exhibited suppressive activity, while only 37.1% of CD4+CD25hi T cells isolated from active RA patients were FoxP3+ and failed to exhibit suppressive function [78]. In contrast to the prediction, anti-TNF therapy reportedly increased FoxP3 expression as well as the suppressive activity of RA CD4+CD25hi cells [78]. Apparently, CD4+CD25hi population in active RA patients consist of a mixture of Tregs and activated effector cells. Anti-TNF therapy can attenuate inflammatory response and consequently reduce the number of ‘contaminating’ effector cells in CD4+CD25hi population, resulting in a relative increase in Treg activity. However, identification of Tregs in inflammatory states based only on CD25 may lead to incorrect enumeration and consequently inappropriate evaluation of Treg activity, simply because activated CD4 effector cells can also express high levels of CD25.
It was also reported that Tregs in RA patients exhibited a tendency to undergo apoptosis, which could be reversed by anti-TNF therapy [80]. However, as convincingly demonstrated in a study using the K/BxN-FoxPgfp mouse model of spontaneous arthritis, which is characterized by elevated level of TNF [79], Tregs exhibited both increased replication and more apoptosis, thereby maintaining equilibrium with Teff cells [48]. Thus, anti-TNF treatment may reduce Treg replication as well as Treg apoptosis.
Ehrenstein et al. [77] reported that PB CD4+CD25+ T cells of active RA patients were fully competent in inhibiting the proliferation of responder CD4+CD25–T cells, but they were unable to suppress the production of proinflammatory cytokines by activated T cells and monocytes. This failure of Tregs to inhibit cytokine production was reportedly restored by anti-TNF therapy. Furthermore, the number of ‘CD4+CD25hi Tregs’ was increased after anti-TNF therapy in responding patients. A subsequent study from the same group concluded that anti-TNF therapy did not expand or restore the function of preexisting nTregs in RA patients, but instead, resulted in the generation of a distinct population of suppressor cells lacking CD62L expression derived from naïve CD4 cells isolated from RA patients, but not from healthy donors. These induced suppressor cells are presumably Tr1 and/or Th3 cells since their suppressive activity was IL-10 and TGF-β dependent [81]. Similarly, Deepe and Gibbons [82] reported that neutralization of TNF gave rise to a population of antigen-specific CD4+CD25+ suppressor cells that inhibit protective immunity in murine histoplasmosis. These induced CD4+CD25+ suppressor cells did not express FoxP3, and their suppressive activity was IL-10 dependent. Thus, they were likely to be Tr1 cells rather than naturally occurring FoxP3+ Tregs. These studies clearly show that in RA patients anti-TNF therapy induces Tr1 and/or Th3 suppressor cells, but does not promote activity of nTregs which exert their suppressive function in a direct cell-to-cell contact manner. Taken together, current published evidence does not lend support to the notion that anti-TNF therapy can simply restore or promote Treg activity in RA patients.
Directly contradictory to its well-known anti-inflammatory action, anti-TNF therapy also has at times been shown to promote inflammation. A recent study showed that anti-TNF therapy in collagen-induced arthritis model expanded pathogenic Th1 and Th17 T cells in the LNs, mediated by the upregulation of IL-12/IL-23 p40 expression. However, anti-TNF therapy blocked the accumulation of Th1/Th17 cells in the synovium, providing an explanation for the paradox that anti-TNF therapy ameliorates experimental arthritis despite increasing numbers of pathogenic T cells in LNs [83]. This observation is consistent with previous studies showing that TNF selectively inhibits p40 expression in human and mouse myeloid cells [84, 85]. Furthermore, another study demonstrated that neutralization of TNF in the heart of healthy baboon resulted in myocarditis [86], which was hypothetically caused by eliminating Treg activity [87]. Together with studies showing that neutralization of TNF could reverse the attenuated TCR signaling resulting from chronic TNF exposure [reviewed in 29], these recent publications provide mechanistic insight into the puzzling autoimmune disorders caused by anti-TNF therapy in human patients, including induction of anti-dsDNA production and lupus, as well as neuroinflammatory diseases [reviewed in 30]. The basis for the proinflammatory effect of anti-TNF remains to be more precisely determined.
Nevertheless, anti-TNF therapy ameliorates clinical symptoms and laboratory parameters of inflammation in the majority of RA patients [79], which is probably achieved mainly by eliminating the TNF-TNFR costimulation pathway on activated pathogenic Teffs because they most likely expressed elevated level of TNFR2. In addition, immune complexes formed by cytokine and the antibody against it can markedly enhance the biological activity of the respective cytokine [recently reviewed in 88]. One such example is that IL-2:anti-IL2 Ab complexes expand Tregs more efficiently and consequently inhibit allergic inflammatory responses [89]. Whether complexes of endogenous TNF and therapeutic biological antagonists of TNF are able to stimulate Tregs through TNFR2 in autoimmune patients needs to be further investigated.
Conclusions
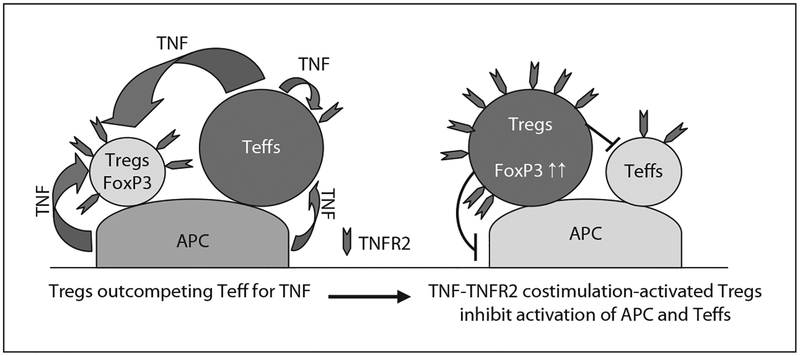
In normal Balb/c or C57BL/6 mice, TNFR2 is constitutively expressed by ~40% of Tregs, but TNFR2 is expressed by less than 10% of resting CD4 Teffs, suggesting that TNF may participate in the maintenance of Treg activity and immune homeostasis. Expression of TNFR2 by Teffs is also upregulated upon TCR stimulation [50], which may allow the TNF signal mediated by TNFR2 to effectively stimulate Teffs and render Teffs more resistant to Treg-mediated inhibition. The report showing that TNF downregulated Treg activity [38] is likely to reflect the stimulating effect of TNF on Teffs. Thus, liberating Teffs from Treg-mediated inhibition may account for proinflammatory effects of TNF. However, prolonged exposure to TNF favors the activation of Tregs [50]. Furthermore, in concert with IL-2 and perhaps with other common γ-chain cytokines, TNF selectively activates Tregs. Higher levels of TNFR2 expression by Tregs mediated by chronic inflammatory responses may enable them to outcompete Teffs for TNF. TNF-TNFR2-costimulated activation of Tregs may therefore account for the accumulation of activated Tregs found in inflammatory responses such as autoimmunity, sepsis, infection and tumors, and may also account for immunosuppression seen in the chronic exposure to TNF [28, 90]. The stimulatory effect of TNF on Tregs thus represents an important negative feedback mechanism that results in the attenuation and termination of prolonged or excessive immune responses, which otherwise may cause severe collateral damage (fig. 1). The polarizing action of TNF on Tregs and Teffs may be determined by timing, location, cytokine milieu, receptor usage, form (free vs. membrane bound) and cellular source of TNF. Further clarification of the pathways or cofactor(s) which polarize the stimulating activity of TNF will not only improve our understanding of cellular and molecular basis underlying the contrasting action of TNF, but may also allow us to identify better means of manipulating this powerful cytokine to the benefit of our patients.
Fig. 1.
Tregs predominately express TNFR2 and outcompete Teffs for TNF. TNFR2 is predominately expressed by both human and mouse Tregs. In the inflammatory responses, pathogenic Teffs and APCs are able to produce TNF. The chronic TNF exposure may favor the activation of Tregs by TNFR2 costimulation pathways. Accumulation of activated Tregs at the inflammatory site suppresses the activation of both innate immune cells as well as adaptive immune cells, and therefore may present an important negative feedback mechanism to limit the magnitude of the immune responses and avoid collateral damage.
Acknowledgements
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. This research was supported (in part) by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
The authors thank Drs. Arthur A. Hurwitz, O.M. Zack Howard and Jonathan M. Weiss and Ryoko Hamano for discussion and critical review of the manuscript, and are grateful to the members of the Laboratory of Molecular Immunoregulation, CIP, CCR, NCI-Frederick and to the collaborators for their contributions to the research discussed here. The authors apologize to those researchers whose work could not be cited due to space limitations.
Footnotes
The content of this chapter does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government.
References
- 1.Gershon RK, Kondo K: Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology 1970;18:723–737. [PMC free article] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M: Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995;155:1151–1164. [PubMed] [Google Scholar]
- 3.Sakaguchi S, Yamaguchi T, Nomura T, Ono M: Regulatory T cells and immune tolerance. Cell 2008; 133:775–787. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM: Conversion of peripheral CD4+CD25-naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 2003;198:1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY: An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol 2006;7:401–410. [DOI] [PubMed] [Google Scholar]
- 6.Zheng Y, Rudensky AY: Foxp3 in control of the regulatory T cell lineage. Nat Immunol 2007;8:457–462. [DOI] [PubMed] [Google Scholar]
- 7.Torgerson TR, Ochs HD: Immune dysregulation, polyendocrinopathy, enteropathy, X-linked: fork-head box protein 3 mutations and lack of regulatory T cells. J Allergy Clin Immunol 2007;120:744–750; quiz 751–742. [DOI] [PubMed] [Google Scholar]
- 8.Campbell DJ, Ziegler SF: FOXP3 modifies the phenotypic and functional properties of regulatory T cells. Nat Rev Immunol 2007;7:305–310. [DOI] [PubMed] [Google Scholar]
- 9.Thornton AM, Shevach EM: CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med 1998;188:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piccirillo CA, Shevach EM: Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol 2001;167:1137–1140. [DOI] [PubMed] [Google Scholar]
- 11.Trzonkowski P, Szmit E, Mysliwska J, Dobyszuk A, Mysliwski A: CD4+CD25+ T regulatory cells inhibit cytotoxic activity of T CD8+ and NK lymphocytes in the direct cell-to-cell interaction. Clin Immunol 2004;112:258–267. [DOI] [PubMed] [Google Scholar]
- 12.Azuma T, Takahashi T, Kunisato A, Kitamura T, Hirai H: Human CD4+ CD25+ regulatory T cells suppress NKT cell functions. Cancer Res 2003;63: 4516–4520. [PubMed] [Google Scholar]
- 13.Cederbom L, Hall H, Ivars F: CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol 2000;30: 1538–1543. [DOI] [PubMed] [Google Scholar]
- 14.Fu Y, Yi S, Wu J, Jimenez E, Simond D, Hawthorne WJ, O’Connell PJ: In vitro suppression of xenoimmune-mediated macrophage activation by human CD4+CD25+ regulatory T cells. Transplantation 2008;86:865–874. [DOI] [PubMed] [Google Scholar]
- 15.Seo SJ, Fields ML, Buckler JL, Reed AJ, Mandik-Nayak L, Nish SA, Noelle RJ, Turka LA, Finkelman FD, Caton AJ, Erikson J: The impact of T helper and T regulatory cells on the regulation of anti-double-stranded DNA B cells. Immunity 2002;16:535–546. [DOI] [PubMed] [Google Scholar]
- 16.Thornton AM, Shevach EM: Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol 2000;164:183–190. [DOI] [PubMed] [Google Scholar]
- 17.Karim M, Feng G, Wood KJ, Bushell AR: CD25+CD4+ regulatory T cells generated by exposure to a model protein antigen prevent allograft rejection: antigen-specific reactivation in vivo is critical for bystander regulation. Blood 2005;105: 4871–4877. [DOI] [PubMed] [Google Scholar]
- 18.Belkaid Y, Rouse BT: Natural regulatory T cells in infectious disease. Nat Immunol 2005;6:353–360. [DOI] [PubMed] [Google Scholar]
- 19.Zou W: Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 2006;6:295–307. [DOI] [PubMed] [Google Scholar]
- 20.Miyara M, Sakaguchi S: Natural regulatory T cells: mechanisms of suppression. Trends Mol Med 2007; 13:108–116. [DOI] [PubMed] [Google Scholar]
- 21.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA: The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 2007;450: 566–569. [DOI] [PubMed] [Google Scholar]
- 22.Allan SE, Song-Zhao GX, Abraham T, McMurchy AN, Levings MK: Inducible reprogramming of human T cells into Treg cells by a conditionally active form of FOXP3. Eur J Immunol 2008;38:3282–3289. [DOI] [PubMed] [Google Scholar]
- 23.Bardel E, Larousserie F, Charlot-Rabiega P, Coulomb-L’Hermine A, Devergne O: Human CD4+ CD25+ Foxp3+ regulatory T cells do not constitutively express IL-35. J Immunol 2008;181:6898–6905. [DOI] [PubMed] [Google Scholar]
- 24.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC: Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 2007;204:1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S: CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008;322:271–275. [DOI] [PubMed] [Google Scholar]
- 26.Friedline RH, Brown DS, Nguyen H, Kornfeld H, Lee J, Zhang Y, Appleby M, Der SD, Kang J, Chambers CA: CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. J Exp Med 2009;206:421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aggarwal BB: Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol 2003;3:745–756. [DOI] [PubMed] [Google Scholar]
- 28.Cope AP: Regulation of autoimmunity by proinflammatory cytokines. Curr Opin Immunol 1998;10: 669–676. [DOI] [PubMed] [Google Scholar]
- 29.Clark J, Vagenas P, Panesar M, Cope AP: What does tumour necrosis factor excess do to the immune system long term? Ann Rheum Dis 2005; 64(suppl 4):iv70–iv76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kollias G, Kontoyiannis D: Role of TNF/TNFR in autoimmunity: specific TNF receptor blockade may be advantageous to anti-TNF treatments. Cytokine Growth Factor Rev 2002;13:315–321. [DOI] [PubMed] [Google Scholar]
- 31.Kruglov AA, Kuchmiy A, Grivennikov SI, Tumanov AV, Kuprash DV, Nedospasov SA: Physiological functions of tumor necrosis factor and the consequences of its pathologic overexpression or blockade: mouse models. Cytokine Growth Factor Rev 2008;19:231–244. [DOI] [PubMed] [Google Scholar]
- 32.Grewal IS, Grewal KD, Wong FS, Picarella DE, Janeway CA Jr, Flavell RA: Local expression of transgene encoded TNF alpha in islets prevents autoimmune diabetes in nonobese diabetic (NOD) mice by preventing the development of auto-reactive islet-specific T cells. J Exp Med 1996;184:1963–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacob CO, McDevitt HO: Tumour necrosis factor-alpha in murine autoimmune ‘lupus’ nephritis. Nature 1988;331:356–358. [DOI] [PubMed] [Google Scholar]
- 34.Jacob CO, Aiso S, Michie SA, McDevitt HO, Acha-Orbea H: Prevention of diabetes in nonobese diabetic mice by tumor necrosis factor (TNF): similarities between TNF-alpha and interleukin 1. Proc Natl Acad Sci USA 1990;87:968–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kontoyiannis D, Kollias G: Accelerated autoimmunity and lupus nephritis in NZB mice with an engineered heterozygous deficiency in tumor necrosis factor. Eur J Immunol 2000;30:2038–2047. [DOI] [PubMed] [Google Scholar]
- 36.Kollias G, Kontoyiannis D, Douni E, Kassiotis G: The role of TNF/TNFR in organ-specific and systemic autoimmunity: implications for the design of optimized ‘anti-TNF’ therapies. Curr Dir Auto-immun 2002;5:30–50. [DOI] [PubMed] [Google Scholar]
- 37.Kassiotis G, Kollias G: Uncoupling the proinflammatory from the immunosuppressive properties of tumor necrosis factor (TNF) at the p55 TNF receptor level: implications for pathogenesis and therapy of autoimmune demyelination. J Exp Med 2001; 193:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valencia X, Lipsky PE: CD4+CD25+FoxP3+ regulatory T cells in autoimmune diseases. Nat Clin Pract Rheumatol 2007;3:619–626. [DOI] [PubMed] [Google Scholar]
- 39.Leipe J, Skapenko A, Lipsky PE, Schulze-Koops H: Regulatory T cells in rheumatoid arthritis. Arthritis Res Ther 2005;7:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao D, Malmstrom V, Baecher-Allan C, Hafler D, Klareskog L, Trollmo C: Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol 2003;33:215–223. [DOI] [PubMed] [Google Scholar]
- 41.van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS: CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum 2004;50:2775–2785. [DOI] [PubMed] [Google Scholar]
- 42.Mottonen M, Heikkinen J, Mustonen L, Isomaki P, Luukkainen R, Lassila O: CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol 2005;140:360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiao Z, Wang W, Jia R, Li J, You H, Chen L, Wang Y: Accumulation of FoxP3-expressing CD4+CD25+ T cells with distinct chemokine receptors in synovial fluid of patients with active rheumatoid arthritis. Scand J Rheumatol 2007;36:428–433. [DOI] [PubMed] [Google Scholar]
- 44.Ruprecht CR, Gattorno M, Ferlito F, Gregorio A, Martini A, Lanzavecchia A, Sallusto F: Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med 2005;201:1793–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Kleer IM, Wedderburn LR, Taams LS, Patel A, Varsani H, Klein M, de Jager W, Pugayung G, Giannoni F, Rijkers G, Albani S, Kuis W, Prakken B: CD4+CD25bright regulatory T cells actively regulate inflammation in the joints of patients with the remitting form of juvenile idiopathic arthritis. J Immunol 2004;172:6435–6443. [DOI] [PubMed] [Google Scholar]
- 46.Cao D, van Vollenhoven R, Klareskog L, Trollmo C, Malmstrom V: CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res Ther 2004; 6:R335–R346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen LT, Jacobs J, Mathis D, Benoist C: Where FoxP3-dependent regulatory T cells impinge on the development of inflammatory arthritis. Arthritis Rheum 2007;56:509–520. [DOI] [PubMed] [Google Scholar]
- 48.Monte K, Wilson C, Shih FF: Increased number and function of FoxP3 regulatory T cells during experimental arthritis. Arthritis Rheum 2008;58:3730–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Venet F, Chung CS, Monneret G, Huang X, Horner B, Garber M, Ayala A: Regulatory T cell populations in sepsis and trauma. J Leukoc Biol 2008;83:523–535. [DOI] [PubMed] [Google Scholar]
- 50.Chen X, Baumel M, Mannel DN, Howard OM, Oppenheim JJ: Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. J Immunol 2007;179:154–161. [DOI] [PubMed] [Google Scholar]
- 51.Coussens LM, Werb Z: Inflammation and cancer. Nature 2002;420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu AJ, Hua H, Munson SH, McDevitt HO: Tumor necrosis factor-alpha regulation of CD4+CD25+ T cell levels in NOD mice. Proc Natl Acad Sci USA 2002;99:12287–12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen X, Oppenheim JJ, Winkler-Pickett RT, Ortaldo JR, Howard OM: Glucocorticoid amplifies IL-2-dependent expansion of functional FoxP3+CD4+ CD25+ T regulatory cells in vivo and enhances their capacity to suppress EAE. Eur J Immunol 2006; 36:2139–2149. [DOI] [PubMed] [Google Scholar]
- 54.Chen X, Winkler-Pickett RT, Carbonetti NH, Ortaldo JR, Oppenheim JJ, Howard OM: Pertussis toxin as an adjuvant suppresses the number and function of CD4+CD25+ T regulatory cells. Eur J Immunol 2006;36:671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cassan C, Piaggio E, Zappulla JP, Mars LT, Couturier N, Bucciarelli F, Desbois S, Bauer J, Gonzalez-Dunia D, Liblau RS: Pertussis toxin reduces the number of splenic Foxp3+ regulatory T cells. J Immunol 2006; 177:1552–1560. [DOI] [PubMed] [Google Scholar]
- 56.Tonon S, Goriely S, Aksoy E, Pradier O, Del Giudice G, Trannoy E, Willems F, Goldman M, De Wit D: Bordetella pertussis toxin induces the release of inflammatory cytokines and dendritic cell activation in whole blood: impaired responses in human newborns. Eur J Immunol 2002;32:3118–3125. [DOI] [PubMed] [Google Scholar]
- 57.Ausiello CM, Fedele G, Urbani F, Lande R, Di Carlo B, Cassone A: Native and genetically inactivated pertussis toxins induce human dendritic cell maturation and synergize with lipopolysaccharide in promoting T helper type 1 responses. J Infect Dis 2002;186:351–360. [DOI] [PubMed] [Google Scholar]
- 58.Chen X, Howard OM, Oppenheim JJ: Pertussis toxin by inducing IL-6 promotes the generation of IL-17-producing CD4 cells. J Immunol 2007;178: 6123–6129. [DOI] [PubMed] [Google Scholar]
- 59.Malek TR, Bayer AL: Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol 2004;4: 665–674. [DOI] [PubMed] [Google Scholar]
- 60.Carpentier I, Coornaert B, Beyaert R: Function and regulation of tumor necrosis factor type 2. Curr Med Chem 2004;11:2205–2212. [DOI] [PubMed] [Google Scholar]
- 61.Tartaglia LA, Goeddel DV, Reynolds C, Figari IS, Weber RF, Fendly BM, Palladino MA Jr: Stimulation of human T-cell proliferation by specific activation of the 75-kDa tumor necrosis factor receptor. J Immunol 1993;151:4637–4641. [PubMed] [Google Scholar]
- 62.Kim EY, Priatel JJ, Teh SJ, Teh HS: TNF receptor type 2 (p75) functions as a costimulator for antigen-driven t cell responses in vivo. J Immunol 2006;176: 1026–1035. [DOI] [PubMed] [Google Scholar]
- 63.Kim EY, Teh HS: TNF type 2 receptor (p75) lowers the threshold of T cell activation. J Immunol 2001; 167:6812–6820. [DOI] [PubMed] [Google Scholar]
- 64.Kim EY, Teh HS: Critical role of TNF receptor type-2 (p75) as a costimulator for IL-2 induction and T cell survival: a functional link to CD28. J Immunol 2004;173:4500–4509. [DOI] [PubMed] [Google Scholar]
- 65.Annunziato F, Cosmi L, Liotta F, Lazzeri E, Manetti R, Vanini V, Romagnani P, Maggi E, Romagnani S: Phenotype, localization, and mechanism of suppression of CD4+CD25+ human thymocytes. J Exp Med 2002;196:379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cosmi L, Liotta F, Lazzeri E, Francalanci M, Angeli R, Mazzinghi B, Santarlasci V, Manetti R, Vanini V, Romagnani P, Maggi E, Romagnani S, Annunziato F: Human CD8+CD25+ thymocytes share phenotypic and functional features with CD4+CD25+ regulatory thymocytes. Blood 2003;102:4107–4114. [DOI] [PubMed] [Google Scholar]
- 67.Chen X, Subleski JJ, Kopf H, Howard OM, Mannel DN, Oppenheim JJ: Cutting edge: expression of TNFR2 defines a maximally suppressive subset of mouse CD4+CD25+FoxP3+ T regulatory cells: applicability to tumor-infiltrating T regulatory cells. J Immunol 2008;180:6467–6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baseta JG, Stutman O: TNF regulates thymocyte production by apoptosis and proliferation of the triple negative (CD3-CD4-CD8-) subset. J Immunol 2000;165:5621–5630. [DOI] [PubMed] [Google Scholar]
- 69.van Mierlo GJ, Scherer HU, Hameetman M, Morgan ME, Flierman R, Huizinga TW, Toes RE: Cutting edge: TNFR-shedding by CD4+CD25+ regulatory T cells inhibits the induction of inflammatory mediators. J Immunol 2008;180:2747–2751. [DOI] [PubMed] [Google Scholar]
- 70.Kollias G: TNF pathophysiology in murine models of chronic inflammation and autoimmunity. Semin Arthritis Rheum 2005;34:3–6. [DOI] [PubMed] [Google Scholar]
- 71.Suvannavejh GC, Lee HO, Padilla J, Dal Canto MC, Barrett TA, Miller SD: Divergent roles for p55 and p75 tumor necrosis factor receptors in the pathogenesis of MOG(35–55)-induced experimental autoimmune encephalomyelitis. Cell Immunol 2000;205:24–33. [DOI] [PubMed] [Google Scholar]
- 72.Ebach DR, Riehl TE, Stenson WF: Opposing effects of tumor necrosis factor receptor 1 and 2 in sepsis due to cecal ligation and puncture. Shock 2005;23: 311–318. [DOI] [PubMed] [Google Scholar]
- 73.Grell M, Douni E, Wajant H, Lohden M, Clauss M, Maxeiner B, Georgopoulos S, Lesslauer W, Kollias G, Pfizenmaier K, Scheurich P: The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell 1995;83:793–802. [DOI] [PubMed] [Google Scholar]
- 74.Lehmann J, Huehn J, de la Rosa M, Maszyna F, Kretschmer U, Krenn V, Brunner M, Scheffold A, Hamann A: Expression of the integrin alpha Ebeta 7 identifies unique subsets of CD25+ as well as CD25-regulatory T cells. Proc Natl Acad Sci USA 2002; 99:13031–13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gray JD, Liu T, Huynh N, Horwitz DA: Transforming growth factor beta enhances the expression of CD154 (CD40L) and production of tumor necrosis factor alpha by human T lymphocytes. Immunol Lett 2001;78:83–88. [DOI] [PubMed] [Google Scholar]
- 76.van der Most RG, Currie AJ, Mahendran S, Prosser A, Darabi A, Robinson BW, Nowak AK, Lake RA: Tumor eradication after cyclophosphamide depends on concurrent depletion of regulatory T cells: a role for cycling TNFR2-expressing effector-suppressor T cells in limiting effective chemotherapy. Cancer Immunol Immunother 2009;58:1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C: Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med 2004;200:277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE: TNF down-modulates the function of human CD4+CD25hi T regulatory cells. Blood 2006;108:253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Feldmann M: Development of anti-TNF therapy for rheumatoid arthritis. Nat Rev Immunol 2002;2:364–371. [DOI] [PubMed] [Google Scholar]
- 80.Toubi E, Kessel A, Mahmudov Z, Hallas K, Rozenbaum M, Rosner I: Increased spontaneous apoptosis of CD4+CD25+ T cells in patients with active rheumatoid arthritis is reduced by infliximab. Ann N Y Acad Sci 2005;1051:506–514. [DOI] [PubMed] [Google Scholar]
- 81.Nadkarni S, Mauri C, Ehrenstein MR: Anti-TNF-alpha therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-beta. J Exp Med 2007;204:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deepe GS Jr, Gibbons RS: TNF-alpha antagonism generates a population of antigen-specific CD4+ CD25+ T cells that inhibit protective immunity in murine histoplasmosis. J Immunol 2008;180:1088–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Notley CA, Inglis JJ, Alzabin S, McCann FE, McNamee KE, Williams RO: Blockade of tumor necrosis factor in collagen-induced arthritis reveals a novel immunoregulatory pathway for Th1 and Th17 cells. J Exp Med 2008;205:2491–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zakharova M, Ziegler HK: Paradoxical anti-inflammatory actions of TNF-alpha: inhibition of IL-12 and IL-23 via TNF receptor 1 in macrophages and dendritic cells. J Immunol 2005;175:5024–5033. [DOI] [PubMed] [Google Scholar]
- 85.Ma X, Sun J, Papasavvas E, Riemann H, Robertson S, Marshall J, Bailer RT, Moore A, Donnelly RP, Trinchieri G, Montaner LJ: Inhibition of IL-12 production in human monocyte-derived macrophages by TNF. J Immunol 2000;164:1722–1729. [DOI] [PubMed] [Google Scholar]
- 86.McTiernan CF, Mathier MA, Zhu X, Xiao X, Klein E, Swan CH, Mehdi H, Gibson G, Trichel AM, Glorioso JC, Feldman AM, McCurry KR, London B: Myocarditis following adeno-associated viral gene expression of human soluble TNF receptor (TNFRII-Fc) in baboon hearts. Gene Ther 2007;14: 1613–1622. [DOI] [PubMed] [Google Scholar]
- 87.Hajjar RJ, Zsebo K: AAV vectors and cardiovascular disease: targeting TNF receptor in the heart: clue to way forward with AAV? Gene Ther 2007;14:1611–1612. [DOI] [PubMed] [Google Scholar]
- 88.Mostbock S: Cytokine/antibody complexes: an emerging class of immunostimulants. Curr Pharm Des 2009;15:809–825. [DOI] [PubMed] [Google Scholar]
- 89.Wilson MS, Pesce JT, Ramalingam TR, Thompson RW, Cheever A, Wynn TA: Suppression of murine allergic airway disease by IL-2:anti-IL-2 monoclonal antibody-induced regulatory T cells. J Immunol 2008;181:6942–6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cope AP, Liblau RS, Yang XD, Congia M, Laudanna C, Schreiber RD, Probert L, Kollias G, McDevitt HO: Chronic tumor necrosis factor alters T cell responses by attenuating T cell receptor signaling. J Exp Med 1997;185:1573–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]