Abstract
Purpose:
Alarmins are constitutively present endogenous molecules that essentially act as early warning signals for the immune system. We provide a brief overview of major alarmins and highlight their roles in tumor immunity.
Methods:
We searched PubMed up to January 10, 2016, using alarmins and/or damage-associated molecular patterns (DAMPs), as key words. We selected and reviewed articles that focused on the discovery and functions of alarmin and their roles in tumor immunity.
Findings:
Alarmins are essentially endogenous immunostimulatory DAMP molecules that are exposed in response to danger (eg, infection or tissue injury) as a result of degranulation, cell death, or induction. They are sensed by chemotactic receptors and pattern recognition receptors to induce immune responses by promoting the recruitment and activation of leukocytes, particularly antigen-presenting cells.
Implications:
Accumulating data suggest that certain alarmins, High-mobility group nucleosome-binding protein 1 (HMGN1) in particular, contribute to the generation of antitumor immunity. Some alarmins can also be used as cancer biomarkers. Therefore, alarmins can potentially be applied for our fight against cancers.
Keywords: alarmin, biomarker, damage-associated molecular patterns, immune response, pattern recognition receptor, tumor immunity
INTRODUCTION
During the early 1990s, 2 competitive theories were proposed to explain the ability of the immune system to mount an effective immune response against infectious agents while maintaining tolerance toward self-antigens. The Infectious Non-Self theory put forward by Charles Janeway stated that antigen-presenting cells (APC) discriminate infectious nonself from noninfectious self by recognizing pathogen-associated molecular patterns (PAMPs) using pattern-recognition receptors (PRRs).1 The interaction between PRRs and corresponding PAMPs activates APC, leading to upregulation of costimulatory signals and subsequent activation of T cells. Although insightful and experimentally well supported, this theory could not sufficiently explain the generation of immune responses in the absence of an infectious nonself, such as graft rejection, unresponsiveness to normal microflora, and autoimmunity. The more general danger model proposed by Polly Matzinger2 suggested that the immune system is triggered to respond to dangerous signals known as damage-associated molecular patterns (DAMPs) as a result of cell death, tissue injury, or microbial attack. Research led to the identification of many exogenous PAMPs (eg, lipopolysaccharide, CpG DNA, and flagellin) and PRRs such as Toll-like receptors (TLRs) and RIG-I-like receptors in the late 1990s and early 2000s; however, the identification of endogenous DAMPs has lagged.1–3 Because a prominent DAMP, namely high-mobility group box B protein 1 (HMGB1), was discovered in the circulation of patients with septic shock, these DAMPs were considered dangerous mediators.4,5
In the late 1990s and early 2000s, extensive research into the role of several antimicrobial peptides and proteins (AMPs) and HMGB1 revealed that these structurally distinct multifunctional endogenous mediators shared certain common characteristics: they are present intracellularly in granules, the nucleus or cytosol, and are rapidly released as a result of degranulation, cell death, and/or induction; once they become extracellular, they are sensed by cellular receptors, resulting in recruitment and activation of immune cells, including the most potent APCs, which are known as dendritic cells (DCs); and they have the capacity to promote innate and adaptive immune responses. These constitutively present endogenous molecules essentially act as early warning signals for the immune system and therefore the term “alarmin” was coined by us to illustrate the unique function of these molecules.6 In essence, alarmins are immunostimulatory DAMPs that contribute to protective host defense. However, not all DAMPs are immunostimulatory. For example, phosphotidylserine exposed on the surface of dying cells can be considered a DAMP; however, it is anti-inflammatory and therefore certainly not an alarmin. DAMPs are considered dangerous. In contrast, alarmins function as intercellular signals and therefore behave as cytokines, and are dangerous only when they induce an excessive immune response known as a cytokine storm. Nevertheless, the terms “alarmins” and “DAMPs” are sometimes used interchangeably in the literature.
Alarmins identified so far can roughly be classified into several categories (Table), including certain AMPs (eg, defensin, cathelicidin, eosinophil-derived neurotoxin [EDN], and granulysin), nuclear binding proteins (eg, HMGB1 and interleukin [IL]-1α), heat shock proteins (HSPs) such as HSP60 and HSP96, ion-binders (eg, S100A8 and A9 and lactoferrin), nucleotides/metabolites (eg, adenosine triphosphate [ATP] and uric acid), and certain degradation products of the extracellular matrix.7–12 A property common to all alarmins is their capacity to promote inflammation and immunity; however, they are sensed by distinct receptors and have distinct roles.13–17 With progress in immunology, the functions of alarmins have been further elucidated and their potential clinical use has attracted more research enthusiasm.13,16,18–22 Based on their involvement in inflammation, antimicrobial defense, adaptive immunities, and wound healing, the potential clinical use of alarmins has become attractive.21,23–28 In this short review, we provide a general overview on the roles of well-characterized alarmins in immunity, followed by focusing on their roles in cancer.
Table.
Receptors and roles of well-characterized alarmins.
| Alarmins and their receptors | Biological activities and functions | |
|---|---|---|
| AMPs | Antimicrobial effects | |
| Defensins (α, β, and θ) | CCR2, CCR6, TLR4 | Leukocytes recruitment |
| Cathelicidins (eg, LL-37 and CRAMP) | FPRL1/FPR2,TLR7, 8, 9 | Cytokine induction |
| EDN and others | TLR2 | APC/DC activation Promotion of immune responses Inflammation |
| DNA binding proteins | Regulation of gene expression | |
| HMGN1 | TLR4 | Leukocytes recruitment |
| HMGB1 | CXCR4, RAGE, | Cytokine induction |
| IL-1α | TLR2, 4, 7, 8, 9 CD24 | APC/DC activation |
| IL-33 | IL-1R ST2 |
Promotion of immune responses Inflammation |
| HSP | Protein protection | |
| HSP70, 90, 96 | CD14, CD40, CD91, TLR2, 4 scavenger receptors (SRA1) | APC/DC activation Treg polarization Inflammation |
| Ion-binding proteins | Leukocytes recruitment | |
| Lactoferrin | RAGE, TLR2, 4 | Cytokine induction |
| S100 A8, A9 | RAGE, TLR4 | APC/DC activation Promotion of immune responses Inflammation |
| Others | Leukocytes recruitment | |
| ATP and others | P2Y2, 6, 12, P2X7 | Cytokine induction APC/DC activation Inflammation |
APC/DC = antigen-presenting cells/dendritic cells; AMP = antimicrobial peptides and proteins; ATP = adenosine triphosphate; CCR = CC chemokine receptor; CD = cluster of differentiation; CRAMP = cathelin-related antimicrobial peptide; CXCR = CXC chemokine receptor; EDN = eosinophil-derived neurotoxin; FPR = formyl peptide receptor; HMGB = High-mobility group protein with a ‘Box’ domain; HMGN = High-mobility group protein with a ‘nucleosome-binding’ domain; IL = interleukin; RAGE = receptor for advanced glycation end-products; TLR = Toll-like receptor; Treg = regulatory T cell.
OVERVIEW OF VARIOUS ALARMINS AMPs
Defensins consist of a family of small antimicrobial peptides with a characteristic β-sheet-rich fold and 6 cysteines forming 3 intrachain disulfide bonds that are classified into 3 (α, β, and θ) subfamilies.27 There are multiple α- and β-defensins in mice and humans29–32; however, only a few defensins have been studied at the protein level. Both α- and β-defensins are chemotactic for immature DCs, monocytes, and subsets of T cells.33–35 The receptors that β-defensins use to chemoattract DCs and macrophages are CC chemokine receptor 6 [CCR6] and CC chemokine receptor 2 [CCR2], respectively.36 A few β-defensins have been shown to induce DC maturation,37 with β-defensin 2 reported to use TLR4 as a receptor.38 Certain β-defensins can also form complexes with DNA and activate plasmacytoid DCs using TLR9.39 α-Defensins promote both Th1 and Th2 immune responses upon administration with antigen, whereas β-defensins predominantly stimulate a Th1 response.31,40,41
Cathelicidins have a conserved N-terminal prose-quence of approximately 100 residues known as the cathelin domain, and a C-terminal antimicrobial domain that is highly heterogeneous in terms of size and structure, which can be either an α-helix, β-hairpin with 1 or 2 intrachain disulfide bonds, or with extended polypro-line-type folding.42 Human cathelicidin (LL-37) and mouse cathelicidin (cathelin-related antimicrobial peptide [CRAMP]) can induce the migration of neutrophils and monocytes through human formyl peptide receptor like-1 [FPRL1] and mouse FPR2 (the ortholog of FPRL1).43–45 Cathelicidin can promote DC differentiation and maturation, and antigen-specific immune responses.46 By forming complexes with DNA and RNA, cathelicidin can induce the activation of plasmacytoid and myeloid DCs through the use of TLR7, 8, or 9, all of which have been shown to contribute to autoimmune diseases and wound healing.47–50
EDN present in the granules of eosinophils belongs to the ribonuclease family of proteins and shows a typical RNase V-shaped folding organized into 2 lobes, each consisting of 3 anti-parallel β-strands and 1 α-helix, and with 2 α-helices located between the 2 lobes.31 EDN is chemotactic for both immature and mature DCs, indicating it may regulate DC trafficking.51 EDN can activate myeloid DCs by triggering TLR2-MyD88 signaling pathway and promoting antigen-specific, preferentially Th2, immune responses.14
DNA Binding Proteins
High mobility group (HMG) proteins consist of a superfamily of nucleosome-binding proteins that are classified into HMGA, HMGB, and HMGN subfamilies, each of which contains several members, such as HMGB 1–3 and HMGN 1–4.52 HMGB1 is a nonhistone, chromatin-binding protein that is present in all mammalian nucleated cells. HMGB1 can be released by necrotic or apoptotic cells or secreted by activated macrophages, DCs, bone cells, or some tumor cells like melanoma cells.53–57 HMGB1 induces the migration of DCs, macrophages, and many nonleukocytes such as epithelial and neural/nerve cells.58–60 HMGB1 activates DC, monocytes, and macrophages, leading to upregulated expression of costimulatory molecules and inflammatory cytokines, including IL-6, IL-12, and tumor necrosis factor α.19,61–65 The effect of HMGB1 in partly complexing DNA and RNA is mediated by multiple receptors, including TLR2, 4,7, 8, and 9; receptor for advanced glycation end-products (RAGE); and CD24.60,66,67 Aside from promoting adaptive immune responses, HMGB1 has been shown to contribute to inflammation, such as during endotoxic shock,4,64,68 trauma-induced inflammation,69 and autoimmune disorders.70–73
HMGN1 is composed of 2 major domains, a C-terminal chromatin-unfolding domain and an N-terminal nucleosomal binding domain, and is highly expressed in the nucleoli of proliferative tissues that undergo constant turnover, such as epithelial and stem cells.52 Like HMGB1, HMGN1 has critical biological functions in development, host defense and tissue repair.52,63,74–76 In 2012, HMGN1 was identified as a novel alarmin demonstrating its contribution to the promotion of APC/DC maturation and induction of antigen-specific, preferentially Th1, immune responses.77 Despite the functional similarities of HMGB1 and HMGN1, the amino acid sequence of HMGN1 is distinct from that of HMGB1 and they are generated from distinct genes located on different chromosomes. HMGN1 lacks any cysteines in its sequence and is therefore not subject to oxidative inactivation as is HMGB1.78
IL-1α was initially identified and studied as a classical IL; however, recent studies have revealed that IL-1α and IL-33 each has the properties of alarmins. Both have the capacity to act as transcription factors that bind chromatin like HMGB1. They are released by producing cells passively via cell death or actively through a nonclassical “release” pathway. IL-1α transmits its signal by interacting with IL-1R, whereas IL-33 uses the ST2 receptor. IL-1α is a critical proinflammatory cytokine that participates in many inflammatory and immune responses.79,80 IL-33 favors Th2 polarization and can stimulate the production of IL-5 and IL-13 by type 2 innate lymphoid cells, and therefore plays important roles in allergic inflammation.81,82 More recently, IL-33 was reported to promote regulatory T cell [Treg] function in the intestine by enhancing Treg differentiation and accumulation.83
HSPs
HSPs act as intracellular chaperones to protect proteins against acute denaturation and aggregation. Extracellular HSPs induce maturation of DCs and promote the generation of innate and adaptive immune responses.84 HSP70, HSP90, and HSP96 can bind to TLRs, CD14, or SRA1 to trigger the activation of target cells.85 HSPs act as proinflammatory alarmins by promoting the production of proinflammatory cytokines.86,87 On the other hand, HSPs are also involved in the induction of Tregs in certain autoimmune situations.88
Ion-binding proteins
Lactoferrin is an 80-kilodalton iron-binding protein that belongs to the transferrin superfamily.89 It can be induced in neutrophils and epithelial cells and secreted into most exocrine fluids in an iron-free form.90,91 Lactoferrin has direct antimicrobial activity independent of its iron-binding property.92 Based on its iron-binding property, lactoferrin can also have antimicrobial effect against bacteria, viruses, fungi, and some parasites.93–97 Gonzalo et al98 reported in 2008 that lactoferrin acts as an alarmin capable in promoting the recruitment and activation of APCs and inducing antigen-specific immune responses.
S100 proteins or calgranulins are a family of more than 20 cytosolic proteins capable of binding calcium or zinc ion. They are predominantly expressed by neutrophils, macrophages, and cells of epithelial origin.99–101 S100A8 and A9 form heterodimers that can bind to TLR4 and RAGE to induce the production of proinflammatory cytokines via activation of nuclear factor kappa B.102–104 S100A7, 8, 12, and 15 are chemotactic for neutrophils, mast cells, and monocytes/macrophages likely via the use of RAGE and some G protein-coupled receptors.100,105 S100A8 and A9 heterodimers behave as alarmins that enhance many inflammatory and autoimmune conditions.106–108
Aside from the alarmins discussed above, ATP, uric acid, and certain degradative products of the extracellular matrix have also been classified as alarmins based on their functions in promoting maturation of APCs and induction of innate and adaptive immune responses.7,10–12 Ample amounts of ATP are released following cell death due to its high concentration inside cells. ATP can induce the migration of granulocytes, macrophages, and DC using P2Y2, P2Y6, and P2Y12 purinergic receptors.109 ATP activates macrophages and DCs by interacting with the P2X7 receptor and promoting the production of IL-1β by activating the NACHT, LRR and PYD domains-containing protein 3 [NALP3] inflammasome.110–112 Uric acid, a metabolite of purine nucleotides, forms urate microcrystals upon release from the cells.113 It induces inflammatory cytokines and activation of APCs, resulting in enhanced adaptive immune responses.114 Uric acid also activates the NALP3 inflammasome115 and is the major pathogenic factor of gout.116,117
ALARMINS AS POTENTIAL TUMOR BIOMARKERS
Certain alarmins may be used as biomarkers for cancer diagnosis or as indicators of prognosis. Clinical studies indicated that the expression profile of HMGB1 in patients with different cancers can provide diagnostic and prognostic insights as well as new HMGB1-targeted therapeutic agents. Elevated levels of HMGB1 in patients with malignant mesothelioma or colorectal carcinoma indicate poor prognosis.118,119 Combined positivity for microtubule-associated protein 1 light chain 3B and nuclear HMGB1 is a positive predictor for longer breast cancer survival.120 Metastatic breast cancer patients with high circulating HMGB1 level have a poor prognosis and a very poor response to cytotoxic therapies.121 The alarmin S100A9 was recently demonstrated to be a sensitive and specific marker for the activity of tumor-associated immune cells and represents a first in vivo imaging approach for prediction of local and systemic tumor development.122 Serum levels of HMGN1 have been shown to be closely associated with the clinical stages of non–small-cell lung cancer, indicating that HMGN1 may also be used as a biomarker.123
These preliminary clinical studies suggested that alarmins play a role in many kinds of cancers, including malignant mesothelioma and ovarian cancer.119,124
ALARMINS IN TUMOR IMMUNITY
The complicated interplay between the host immune system and cancer has been studied for decades. Since the early 20th century, many concepts concerning the development of cancer have been proposed. The most popular concepts that are now accepted widely are immunosurveillance,125 genetic changes,126 and immuno-editing.127 Immunoediting is a dynamic process that not only involves tumor prevention, but also shapes the immunogenicity of developing tumors. In the course of tumor development, many immune cells are provoked, such as T cells, natural killer cells, macrophages, and DCs. As professional APCs, DCs play a critical role in the induction of antitumor responses due to their ability to present antigens to T cells.128 An effective antitumor immune response by T cells requires efficient antigen presentation by activated mature DCs. The maturation of DCs depends on the local microenvironment and is influenced by various inflammatory stimulants. Unstimulated resting DCs are often in an immature/tolerant status induced by suppressive cells and their cytokines present in the tumor microenvironment.129,130 These immature DCs not only have poor ability to stimulate T cells, but also promote tumor growth by producing proangiogenic factors and enhancing endothelial cell migration that results in vasculogenesis.131 The development of large number of phenotypically and functionally matured DCs is important in the battle against tumors. Alarmins, based on their capacity to promote, recruit, activate, and mature DCs, can potentially play important roles in cancer progression, diagnosis, and potentially treatment.
ALARMINS AS ENHANCERS OF ANTITUMOR IMMUNITY
Numerous studies have shown that alarmins play an important role in the generation of antitumor immune responses.132,133 Immunization of mice with a DNA plasmid harboring a fusion of mouse β-defensin 2 or 3 with mouse B-cell lymphoma epitope sFv38 can stimulate the generation of antilymphoma immunity and protection.133,134 Inoculation of mice with acute lymphoid leukemia L1210 cells transfected to produce mouse β-defensin 2 results in the generation of L1210-specific protective immunity with enhanced activation of natural killer cells and CD8 T cells.132 Immunization of mice with a fusion protein of mouse β-defensin 2 and gp100 (a mouse melanoma antigen) promotes the induction of B16 melanomaspecific CD8 T cells and protective immunity.135 HSPs are also reported to be capable of promoting antitumor effector CD8 T cells and inhibiting tumor growth.136,137
IL-1α has been known for a long time to potentiate cell-mediated immunity and to play an essential role in the generation of antitumor immune defense.138,139 In 2014, the role of IL-33 in antitumor immunity was reported by Weiner et al.140 They demonstrated that both of the 2 isoforms of IL-33 can significantly expand the magnitude of antigen-specific CD8 T cells responses and elicit potent effector-memory CD8 T cells.140 Expression of IL-33 in the tumors is also reported to inhibit tumor growth by activating natural killer cells and CD8 T cells.141
HMGB1 is also an important alarmin for the generation of antitumor immunity in both mice and humans.142–144 Apetoh et al144 found that HMGB1 released by dying tumor cells and the TLR4-MyD88 signaling pathway are required for the antitumor responses in mice. In humans, TLR4 mutation has a negative prognostic influence on patients with breast cancer by affecting the binding of HMGB1 to its receptor.144 Blocking HMGB1 activity or knockdown of HMGB1 expression inhibited cancer growth and metastasis by inhibiting lymphangiogenesis.145,146 HMGB1 can be considered a central target in the diagnosis and treatment of a number of human cancers due to the important role it has in the promotion of cancer growth and metastasis.121,146,147 This suggests that alarmins capable of promoting Th1 immune response possess the capacity to enhance antitumor immunity.
HMGN1 was reported to have the capacity to promote antitumor immune responses as a vaccine adjuvant by Wei et al.21 They found that vaccination with a plasmid encoding the expression of a fusion protein consisting of gp100, a melanoma tumor-associated antigen, and HMGN1, could induce gp100-specific CD8 response and immune protection in mice against B16 melanoma.21
The importance of alarmins in tumor immunity has been demonstrated in another way. HMGN1 knockout mice develop more spontaneous tumors and are more susceptible to develop tumors in response to carcinogenic agents. Furthermore, in comparison with wild-type mice, malignant EG7 thymoma grows much faster in HMGN1 knockout mice.21 The increase in tumor growth in HMGN1 knockout mice is also accompanied by a reduction in the generation of EG7-specific CD8 T cells.21 Conversely, EG7 tumor cells transfected with HMGN1 that is secreted grow much less well in normal mice than untransfected EG7 cells. Therefore, intrinsic HMGN1 may be critical for the development of protective antitumor immunity.
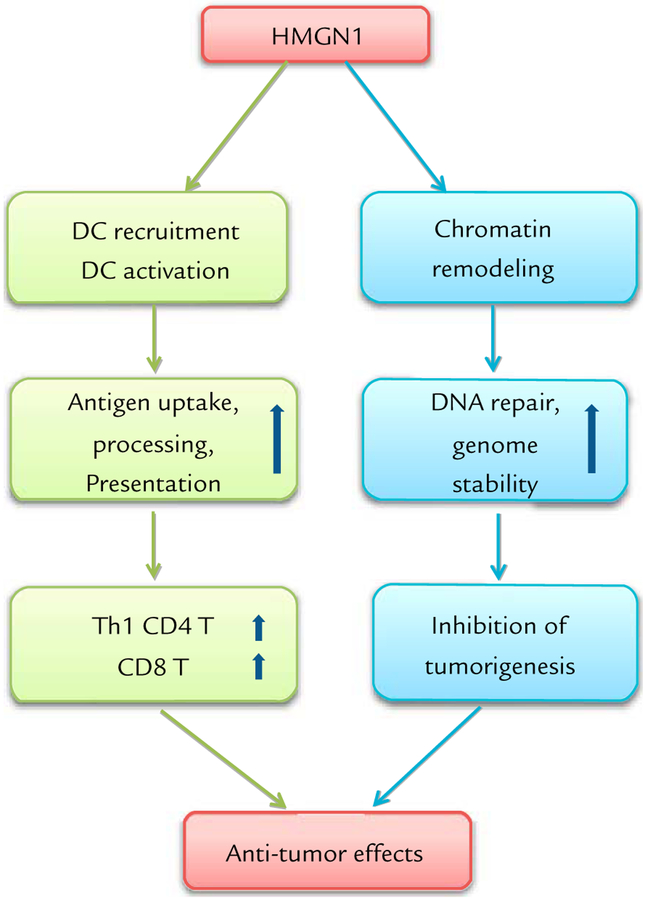
HMGN1 has so far been shown to preferentially promote Th1-polarized immune responses. Additionally, it is essential for the generation of protective immune defense against tumors. HMGN1 also inhibit tumorigenesis by promoting DNA repair and genome stability.148,149 Finally, we are obtaining data showing that HMGN1 can act as a therapeutic vaccine that enhances immunity to implanted tumors in mice (unpublished observations). Therefore, it appears that HMGN1 exerts only protective antitumor effects (Figure), and can potentially be developed as a therapeutic agent for the treatment of cancers.
Figure.
Potent antitumor effect of alarmin high-mobility groupnucleosome-binding protein1 [HMGN1]. HMGN1 manifests anti-tumor effects in at least 2 ways: promoting antitumor immunity by polarizing Th1 immune response and inhibiting tumorigenesis by promoting DNA repair and genome stability. DC = dendritic cell.
ALARMINS AS FACILITATORS OF TUMOR PROGRESSION
Besides their antitumor functions, certain alarmins have been shown to promote tumor progression. HMGB1 acts in an autocrine-regulated feedback loop to promote tumor angiogenesis through RAGE and TLR4 signaling in endothelial cells, which itself leads to increased HMGB1 secretion by endothelial cells themselves.150 HMGB1 can enhance cancer cell growth by upregulating expression of the micro-RNAs MiR-221 and MiR-222 in papillary thyroid cancer cells by controlling cell proliferation through inhibition of p27kipl, a cell-cycle regulator.151 HMGB1 can induce production of IL-10, which in turn promotes the generation of Treg and facilitates tumor progression.152 HMGB1 produced by cancer cells is also reported to promote tumor progression by hampering the activation of plasmacytoid DCs.153
β-Defensin 29 is reported to induce the recruitment of DC precursors to developing tumors and promote tumor progression by facilitating tumor angiogenesis.154 Other alarmins, due to their capacity to activate immune cells such as macrophages and DCs may also facilitate tumor progression by promoting inflammatory responses in the developing tumors. For a given alarmin molecule, whether it is beneficial for resistance to cancer is probably dependent on multiple factors, including concentration.
CLINICAL PERSPECTIVE
Alarmins or immunostimulatory DAMPS are endogenous mediators rapidly released in response to danger signals, and are sensed through interaction with receptors to initiate or promote a variety of responses (eg, inflammation, immunity, and wound healing) aimed at the elimination of danger and restoration of homeostasis. In the context of tumor immunity, it appears that alarmins exhibit both beneficial and harmful effects (Figure). Many alarmins can promote the generation of antitumor immunity through activation of APCs, including DC and macrophages.21,26,32,34,35,37–39 Yet, some alarmins can also promote tumor progression via enhancement of inflammation with production of growth factors and angiogenesis.121,145,146,150–154
CONCLUSIONS
Targeting alarmins or their sensing receptors has the potential to be used for the treatment of cancer patients. Alarmins, particularly those capable of enhancing Th1 responses, can be used as immunoadjuvants for the development of tumor vaccines or as therapeutic agents. Conversely, inhibition of alarmin-induced tumor-associated inflammation may slow down tumor progression, which would be beneficial for cancer patients. To achieve these approaches, the roles of individual alar-mins in the development of cancer and anticancer immunity in humans need to be further defined.
ACKNOWLEDGMENT
This project was funded in whole or in part with federal funds from the National Cancer Institute of the National Institutes of Health, under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. All authors contributed equally to this work.
Footnotes
CONFLICTS OF INTEREST
The authors have indicated that they have no conflicts of interest regarding the content of this article.
REFERENCES
- 1.Janeway CA Jr. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13:11–16. [DOI] [PubMed] [Google Scholar]
- 2.Tolerance Matzinger P., danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. [DOI] [PubMed] [Google Scholar]
- 3.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–119. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, et al. HMGB1 as a late mediator of lethal systemic inflammation. Am J Respir Crit Care Med. 2001; 164:1768–1773. [DOI] [PubMed] [Google Scholar]
- 5.Czura CJ, Wang H, Tracey KJ. Dual roles for HMGB1: DNA binding and cytokine. J Endotoxin Res. 2001;7:315–321. [DOI] [PubMed] [Google Scholar]
- 6.Yang D, Oppenheim JJ. Antimicrobial proteins act as “alarmins” in joint immune defense. Arthritis Rheum. 2004;50:3401–3403. [DOI] [PubMed] [Google Scholar]
- 7.Kol A, et al. Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J Immunol. 2000;164:13–17. [DOI] [PubMed] [Google Scholar]
- 8.Ogawa K, et al. Granulysin in human serum as a marker of cell-mediated immunity. Eur J Immunol. 2003;33: 1925–1933. [DOI] [PubMed] [Google Scholar]
- 9.Wargnier A, Sasportes M, Lagrange PH. [Granulysin: antimicrobial molecule of innate and acquired immunity in human tuberculosis]. Pathol Biol (Paris). 2005;53:516–521. [DOI] [PubMed] [Google Scholar]
- 10.Austermann J, et al. Alarmins MRP8 and MRP14 induce stress tolerance in phagocytes under sterile inflamma-tory conditions. Cell Rep. 2014;9:2112–2123. [DOI] [PubMed] [Google Scholar]
- 11.Wei F, et al. Alarmin HMGN1 Promotes Antitumor Immunity. J Immunother. 2012;35 747–747. [Google Scholar]
- 12.Yang D, et al. The alarmin functions of high-mobility group proteins. Biochimica Et Biophysica Acta-Gene Regulatory Mechanisms. 2010;1799:157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. [DOI] [PubMed] [Google Scholar]
- 14.Yang D, et al. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med. 2008; 205:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang D, et al. Alarmins link neutrophils and dendritic cells. Trends Immunol. 2009;30:531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang D, Oppenheim JJ. Alarmins and antimicrobial immunity. Med Mycol. 2009;47(Suppl 1):S146–S153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005; 17:359–365. [DOI] [PubMed] [Google Scholar]
- 18.Oppenheim JJ, et al. Alarmins initiate host defense. Adv Exp Med Biol. 2007;601:185–194. [DOI] [PubMed] [Google Scholar]
- 19.Yang D, et al. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol. 2007;81:59–66. [DOI] [PubMed] [Google Scholar]
- 20.Pugin J SIRS Dear. the concept of “alarmins” makes a lot of sense!. Intensive Care Med. 2008;34:218–221. [DOI] [PubMed] [Google Scholar]
- 21.Wei F, et al. The Alarmin HMGN1 Contributes to Antitumor Immunity and Is a Potent Immunoadjuvant. Cancer Res. 2014;74:5989–5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schelbergen RF, et al. Alarmins S100A8/S100A9 aggravate osteophyte formation in experimental osteoarthritis and predict osteophyte progression in early human symptomatic osteoarthritis. Ann Rheum Dis. 2016;75:218–225. [DOI] [PubMed] [Google Scholar]
- 23.Saito T, et al. Distinct expression patterns of alveolar “alarmins” in subtypes of chronic lung allograft dysfunction. Am J Transplant. 2014; 14:1425–1432. [DOI] [PubMed] [Google Scholar]
- 24.Vogl T, et al. Alarmin S100A8/S100A9 as a biomarker for molecular imaging of local inflammatory activity. Nature Communications. 2014:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alt JA, et al. Topical cathelicidin (LL-37) an innate immune peptide induces acute olfactory epithelium inflammation in a mouse model. Int Forum Allergy Rhinol. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Bosch MH, et al. Induction of Canonical Wnt Signaling by the Alarmins S100A8/A9 in Murine Knee Joints: Implications for Osteoarthritis. Arthritis Rheumatol. 2016;68:152–163. [DOI] [PubMed] [Google Scholar]
- 27.Ganz T Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–720. [DOI] [PubMed] [Google Scholar]
- 28.Chan JK, et al. Alarmins: awaiting a clinical response. J Clin Invest. 2012;122:2711–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tani K, et al. Defensins act as potent adjuvants that promote cellular and humoral immune responses in mice to a lymphoma idiotype and carrier antigens. Int Immunol. 2000;12:691–700. [DOI] [PubMed] [Google Scholar]
- 30.Com E, et al. Expression of antimicrobial defensins in the male reproductive tract of rats, mice, and humans. Biol Reprod. 2003; 68:95–104. [DOI] [PubMed] [Google Scholar]
- 31.Yang D, et al. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu Rev Immunol. 2004;22:181–215. [DOI] [PubMed] [Google Scholar]
- 32.Welkos S, et al. Humanized thetadefensins (retrocyclins) enhance macrophage performance and protect mice from experimental anthrax infections. Antimicrob Agents Chemother. 2011;55:4238–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang D, et al. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J Leukoc Biol. 2000;68:9–14. [PubMed] [Google Scholar]
- 34.Hubert P, et al. Defensins induce the recruitment of dendritic cells in cervical human papillomavirus-associated (pre)neoplastic lesions formed in vitro and transplanted in vivo. FASEB J. 2007;21:2765–2775. [DOI] [PubMed] [Google Scholar]
- 35.Cappelletti M, et al. Bright expression of CD91 identifies highly activated human dendritic cells that can be expanded by defensins. Immunol. 2015;144:661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang D, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. [DOI] [PubMed] [Google Scholar]
- 37.Presicce P, et al. Human defensins activate monocyte-derived dendritic cells, promote the production of proinflammatory cytokines, and up-regulate the surface expression of CD91. J Leukoc Biol. 2009; 86:941–948. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y, et al. Chicken intestine defensins activated murine peripheral blood mononuclear cells through the TLR4-NF-kappaB pathway. Vet Immunol Immunopathol. 2010;133:59–65. [DOI] [PubMed] [Google Scholar]
- 39.Lande R, et al. Cationic antimicrobial peptides in psoriatic skin cooperate to break innate tolerance to self-DNA. Eur J Immunol. 2015; 45:203–213. [DOI] [PubMed] [Google Scholar]
- 40.Bals R, et al. Mouse beta-defensin 3 is an inducible antimicrobial peptide expressed in the epithelia of multiple organs. Infect Immun. 1999;67:3542–3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schroder JM, Harder J. Human beta-defensin-2. Int J Biochem Cell Biol. 1999;31:645–651. [DOI] [PubMed] [Google Scholar]
- 42.Cathelicidins Zanetti M., multi-functional peptides of the innate immunity. J Leukoc Biol. 2004;75: 39–48. [DOI] [PubMed] [Google Scholar]
- 43.Wantha S, et al. Neutrophil-derived cathelicidin promotes adhesion of classical monocytes. Circ Res. 2013;112:792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Y, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen K, et al. The formylpeptide receptor 2 (Fpr2) and its endogenous ligand cathelin-related antimicrobial peptide (CRAMP) promote dendritic cell maturation. J Biol Chem. 2014;289:17553–17563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davidson DJ, et al. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J Immunol. 2004; 172:1146–1156. [DOI] [PubMed] [Google Scholar]
- 47.Chen K, et al. Signal relay by CC chemokine receptor 2 (CCR2) and formylpeptide receptor 2 (Fpr2) in the recruitment of monocyte-derived dendritic cells in allergic airway inflammation. J Biol Chem. 2013;288:16262–16273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kin NW, et al. Cathelin-related antimicrobial peptide differentially regulates T- and B-cell function. Eur J Immunol. 2011;41:3006–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gregorio J, et al. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med. 2010;207:2921–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ganguly D, et al. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med. 2009;206:1983–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang D, et al. Eosinophil-derived neurotoxin (EDN), an antimicrobial protein with chemotactic activities for dendritic cells. Blood. 2003;102:3396–3403. [DOI] [PubMed] [Google Scholar]
- 52.Hock R, et al. HMG chromosomal proteins in development and disease. Trends Cell Biol. 2007;17:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418: 191–195. [DOI] [PubMed] [Google Scholar]
- 54.Bell CW, et al. The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol. 2006;291:C1318–C1325. [DOI] [PubMed] [Google Scholar]
- 55.Charoonpatrapong K, et al. HMGB1 expression and release by bone cells. J Cell Physiol. 2006;207: 480–490. [DOI] [PubMed] [Google Scholar]
- 56.Ito N, et al. Cytolytic cells induce HMGB1 release from melanoma cell lines. J Leukoc Biol. 2007;81: 75–83. [DOI] [PubMed] [Google Scholar]
- 57.Vande Walle L, Kanneganti TD, Lamkanfi M. HMGB1 release by inflammasomes. Virulence. 2011;2: 162–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rouhiainen A, et al. Regulation of monocyte migration by amphoterin (HMGB1). Blood. 2004;104: 1174–1182. [DOI] [PubMed] [Google Scholar]
- 59.Dumitriu IE, et al. The secretion of HMGB1 is required for the migration of maturing dendritic cells. J Leukoc Biol. 2007;81:84–91. [DOI] [PubMed] [Google Scholar]
- 60.Dumitriu IE, et al. Requirement of HMGB1 and RAGE for the maturation of human plasmacytoid dendritic cells. Eur J Immunol. 2005; 35:2184–2190. [DOI] [PubMed] [Google Scholar]
- 61.Dumitriu IE, et al. HMGB1: guiding immunity from within. Trends Immunol. 2005;26:381–387. [DOI] [PubMed] [Google Scholar]
- 62.Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007;220:35–46. [DOI] [PubMed] [Google Scholar]
- 63.Campana L, Bosurgi L, Rovere-Querini P. HMGB1: a two-headed signal regulating tumor progression and immunity. Curr Opin Immunol. 2008;20:518–523. [DOI] [PubMed] [Google Scholar]
- 64.Andersson U, Rauvala H. Introduction: HMGB1 in inflammation and innate immunity. J Intern Med. 2011;270:296–300. [DOI] [PubMed] [Google Scholar]
- 65.Castiglioni A, et al. High-mobility group box 1 (HMGB1) as a master regulator of innate immunity. Cell Tissue Res. 2011;343:189–199. [DOI] [PubMed] [Google Scholar]
- 66.Rauvala H, Rouhiainen A. RAGE as a receptor of HMGB1 (Amphoterin): roles in health and disease. Curr Mol Med. 2007;7: 725–734. [DOI] [PubMed] [Google Scholar]
- 67.Kokkola R, et al. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand J Immunol. 2005;61:1–9. [DOI] [PubMed] [Google Scholar]
- 68.Yang H, Tracey KJ. Targeting HMGB1 in inflammation. Biochim Biophys Acta. 2010;1799:149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Venereau E, et al. HMGB1 and leukocyte migration during trauma and sterile inflammation. Mol Immunol. 2013;55:76–82. [DOI] [PubMed] [Google Scholar]
- 70.Chen Y, et al. The role of high mobility group box chromosomal protein 1 in rheumatoid arthritis. Rheumatology (Oxford). 2013;52: 1739–1747. [DOI] [PubMed] [Google Scholar]
- 71.He Z, et al. HMGB1 promotes the differentiation of Th17 via up-regulating TLR2 and IL-23 of CD14þ monocytes from patients with rheumatoid arthritis. Scand J Immunol. 2012;76:483–490. [DOI] [PubMed] [Google Scholar]
- 72.Zhang S, et al. HMGB1, an innate alarmin, in the pathogenesis of type 1 diabetes. Int J Clin Exp Pathol. 2009;3:24–38. [PMC free article] [PubMed] [Google Scholar]
- 73.Voll RE, et al. High mobility group box 1 in the pathogenesis of in-flammatory and autoimmune diseases. Isr Med Assoc J. 2008;10:26–28. [PubMed] [Google Scholar]
- 74.Birger Y, et al. Chromosomal protein HMGN1 enhances the rate of DNA repair in chromatin. EMBO J. 2003;22:1665–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rubinstein YR, et al. Chromosomal protein HMGN1 modulates the expression of N-cadherin. FEBS J. 2005;272:5853–5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hill DA, Imbalzano AN. HMGN1 is dispensable for myogenesis and adipogenesis. Gene. 2006;371:59–67. [DOI] [PubMed] [Google Scholar]
- 77.Yang D, et al. High-mobility group nucleosome-binding protein 1 acts as an alarmin and is critical for lipopolysaccharide-induced immune responses. J Exp Med. 2012;209: 157–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Venereau E, et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proin-flammatory cytokine release. J Exp Med. 2012;209:1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suwara MI, et al. IL-1alpha released from damaged epithelial cells is sufficient and essential to trigger inflammatory responses in human lung fibroblasts. Mucosal Immunol. 2014;7:684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tracy EC, et al. Interleukin-1alpha is the major alarmin of lung epithelial cells released during photo-dynamic therapy to induce inflammatory mediators in fibro-blasts. Br J Cancer. 2012;107:1534–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Halim TY, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oboki K, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci U S A. 2010;107:18581–18586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schiering C, et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bethke K, et al. Different efficiency of heat shock proteins (HSP) to activate human monocytes and dendritic cells: superiority of HSP60. J Immunol. 2002;169: 6141–6148. [DOI] [PubMed] [Google Scholar]
- 85.Yokota S, Minota S, Fujii N. Anti-HSP auto-antibodies enhance HSP-induced pro-inflammatory cytokine production in human monocytic cells via Toll-like receptors. Int Immunol. 2006;18:573–580. [DOI] [PubMed] [Google Scholar]
- 86.Lewthwaite JC, et al. Mycobacterium tuberculosis chaperonin 60.1 is a more potent cytokine stimulator than chaperonin 60.2 (Hsp 65) and contains a CD14-binding domain. Infect Immun. 2001;69:7349–7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chopra U, et al. TH1 pattern of cytokine secretion by splenic cells from pyelonephritic mice after invitro stimulation with hsp-65 of Escherichia coli. J Med Microbiol. 1997;46:139–144. [DOI] [PubMed] [Google Scholar]
- 88.Massa M, et al. Differential recognition of heat-shock protein dnaJ-derived epitopes by effector and Treg cells leads to modulation of inflammation in juvenile idiopathic arthritis. Arthritis Rheum. 2007;56: 1648–1657. [DOI] [PubMed] [Google Scholar]
- 89.Johansson BG. Isolation of crystalline lactoferrin from human milk. Acta Chem Scand. 1969;23:683–684. [DOI] [PubMed] [Google Scholar]
- 90.Fletcher J, Willars J. The role of lactoferrin released by phagocytosing neutrophils in the regulation of colony-stimulating activity production by human mononuclear cells. Blood Cells. 1986;11:447–457. [PubMed] [Google Scholar]
- 91.Chiyotani A, et al. Stimulation of Cl secretion by lactoferrin across canine airway epithelial cells in culture. Respiration. 1992;59:189–192. [DOI] [PubMed] [Google Scholar]
- 92.Valenti P, Antonini G. Lactoferrin: an important host defence against microbial and viral attack. Cell Mol Life Sci. 2005;62:2576–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lu L, et al. Protective influence of lactoferrin on mice infected with the polycythemia-inducing strain of Friend virus complex. Cancer Res. 1987;47:4184–4188. [PubMed] [Google Scholar]
- 94.Oram JD, Reiter B. Inhibition of bacteria by lactoferrin and other iron-chelating agents. Biochim Biophys Acta. 1968;170:351–365. [DOI] [PubMed] [Google Scholar]
- 95.Ellison RT 3rd. The effects of lactoferrin on gram-negative bacteria. Adv Exp Med Biol. 1994;357: 71–90. [DOI] [PubMed] [Google Scholar]
- 96.Lima MF, Kierszenbaum F. Lactoferrin effects on phagocytic cell function. I. Increased uptake and killing of an intracellular parasite by murine macrophages and human monocytes. J Immunol. 1985;134: 4176–4183. [PubMed] [Google Scholar]
- 97.Anand N, et al. Effect of lactoferrin protein on red blood cells and macrophages: mechanism of parasite-host interaction. Drug Des Devel Ther. 2015;9:3821–3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.De la Rosa G, et al. Lactoferrin acts as an alarmin to promote the recruitment and activation of APCs and antigen-specific immune responses. J Immunol. 2008;180: 6868–6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yano J, et al. Epithelial cell-derived S100 calcium-binding proteins as key mediators in the hallmark acute neutrophil response during Candida vaginitis. Infect Immun. 2010;78:5126–5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu K, Geczy CL. IFN-gamma and TNF regulate macrophage expression of the chemotactic S100 protein S100A8. J Immunol. 2000;164: 4916–4923. [DOI] [PubMed] [Google Scholar]
- 101.Guignard F, Mauel J, Markert M. The monoclonal antibody Mac 387 recognizes three S100 proteins in human neutrophils. Immunol Cell Biol. 1996;74:105–107. [DOI] [PubMed] [Google Scholar]
- 102.Schelbergen RF, et al. Alarmins S100A8 and S100A9 elicit a catabolic effect in human osteoarthritic chondrocytes that is dependent on Toll-like receptor 4. Arthritis Rheum. 2012;64:1477–1487. [DOI] [PubMed] [Google Scholar]
- 103.Villarreal A, et al. S100B alters neuronal survival and dendrite extension via RAGE-mediated NF-kappaB signaling. J Neurochem. 2011;117:321–332. [DOI] [PubMed] [Google Scholar]
- 104.Hermani A, et al. S100A8 and S100A9 activate MAP kinase and NF-kappaB signaling pathways and trigger translocation of RAGE in human prostate cancer cells. Exp Cell Res. 2006;312:184–197. [DOI] [PubMed] [Google Scholar]
- 105.Cornish CJ, et al. S100 protein CP-10 stimulates myeloid cell chemo-taxis without activation. J Cell Physiol. 1996;166:427–437. [DOI] [PubMed] [Google Scholar]
- 106.Xu W, et al. The expression and distribution of S-100 protein and CD 83 in thyroid tissues of auto-immune thyroid diseases. Cell Mol Immunol. 2004;1:378–382. [PubMed] [Google Scholar]
- 107.Foell D, Roth J. Proinflammatory S100 proteins in arthritis and auto-immune disease. Arthritis Rheum. 2004;50:3762–3771. [DOI] [PubMed] [Google Scholar]
- 108.Gloddek B, et al. Role of S-100beta as potential autoantigen in an autoimmune disease of the inner ear. J Neuroimmunol. 1999;101:39–46. [DOI] [PubMed] [Google Scholar]
- 109.Yang D, et al. Alarmin-induced cell migration. Eur J Immunol. 2013;43: 1412–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schnurr M, et al. Extracellular ATP and TNF-alpha synergize in the activation and maturation of human dendritic cells. J Immunol. 2000; 165:4704–4709. [DOI] [PubMed] [Google Scholar]
- 111.Sluyter R, Shemon AN, Wiley JS. Glu496 to Ala polymorphism in the P2X7 receptor impairs ATP-induced IL-1 beta release from human monocytes. J Immunol. 2004;172: 3399–3405. [DOI] [PubMed] [Google Scholar]
- 112.Ferrari D, et al. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- 113.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. [DOI] [PubMed] [Google Scholar]
- 114.Shi Y, Galusha SA, Rock KL. Cutting edge: elimination of an endogenous adjuvant reduces the activation of CD8 T lymphocytes to transplanted cells and in an autoimmune diabetes model. J Immunol. 2006;176:3905–3908. [DOI] [PubMed] [Google Scholar]
- 115.Martinon F, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. [DOI] [PubMed] [Google Scholar]
- 116.Roy A, et al. Mast cell chymase degrades the alarmins heat shock protein 70, biglycan, HMGB1, and interleukin-33 (IL-33) and limits danger-induced inflammation. J Biol Chem. 2014;289:237–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim B, et al. The Interleukin-1alpha Precursor is Biologically Active and is Likely a Key Alarmin in the IL-1 Family of Cytokines. Front Immunol. 2013;4:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee H, et al. Diagnostic significance of serum HMGB1 in colorectal carcinomas. PLoS One. 2012;7: e34318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jube S, et al. Cancer cell secretion of the DAMP protein HMGB1 supports progression in malignant mesothelioma. Cancer Res. 2012;72: 3290–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ladoire S, et al. Combined evaluation of LC3B puncta and HMGB1 expression predicts residual risk of relapse after adjuvant chemotherapy in breast cancer. Autophagy. 2015;11:1878–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stoetzer OJ, et al. Circulating immunogenic cell death biomarkers HMGB1 and RAGE in breast cancer patients during neoadjuvant chemotherapy. Tumour Biol. 2013; 34:81–90. [DOI] [PubMed] [Google Scholar]
- 122.Becker A, et al. Optical in vivo imaging of the alarmin S100A9 in tumor lesions allows for estimation of the individual malignant potential by evaluation of tumor-host cell interaction. J Nucl Med. 2015;56: 450–456. [DOI] [PubMed] [Google Scholar]
- 123.Wei F, et al. High-mobility group nucleosome-binding protein 1 is a novel clinical biomarker in non-small cell lung cancer. Tumour Biol. 2015;36:9405–9410. [DOI] [PubMed] [Google Scholar]
- 124.Ojalvo LS, et al. Emerging immunotherapies in ovarian cancer. Discov Med. 2015;20:97–109. [PubMed] [Google Scholar]
- 125.Burnet FM. The concept of immunological surveillance. Prog Exp Tumor Res. 1970;13:1–27. [DOI] [PubMed] [Google Scholar]
- 126.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 127.Dunn GP, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. [DOI] [PubMed] [Google Scholar]
- 128.da Cunha A, Michelin MA, Murta EF. Pattern response of dendritic cells in the tumor micro-environment and breast cancer. World J Clin Oncol. 2014;5:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jin JO, et al. Inhibition of breast cancer resistance protein (ABCG2) in human myeloid dendritic cells induces potent tolerogenic functions during LPS stimulation. PLoS One. 2014;9:e104753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Suciu-Foca N, Berloco P, CortesiniR. Tolerogenic dendritic cells in cancer, transplantation, and auto-immune diseases. Hum Immunol. 2009;70:277–280. [DOI] [PubMed] [Google Scholar]
- 131.Fainaru O, et al. Tumor growth and angiogenesis are dependent on the presence of immature dendritic cells. FASEB J. 2010;24:1411–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ma XT, et al. Vaccine with beta-defensin 2-transduced leukemic cells activates innate and adaptive immunity to elicit potent antileukemia responses. Cancer Res. 2006; 66:1169–1176. [DOI] [PubMed] [Google Scholar]
- 133.Biragyn A, et al. Mediators of innate immunity that target immature, but not mature, dendritic cells induce antitumor immunity when genetically fused with nonimmunogenic tumor antigens. J Immunol. 2001;167:6644–6653. [DOI] [PubMed] [Google Scholar]
- 134.Biragyn A, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002; 298:1025–1029. [DOI] [PubMed] [Google Scholar]
- 135.Park HJ, et al. Induction of TLR4-dependent CD8þ T cell immunity by murine beta-defensin2 fusion protein vaccines. Vaccine. 2011;29: 3476–3482. [DOI] [PubMed] [Google Scholar]
- 136.Wang L, et al. A Mage3/Heat Shock Protein70 DNA vaccine induces both innate and adaptive immune responses for the antitumor activity. Vaccine. 2009;28:561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kanegasaki S, Tsuchiya T. Alarmins released during local antitumor treatments play an essential role in enhancing tumor growth inhibition at treated and non-treated sites via a derivative of CCL3. Oncoimmunology. 2014;3:e958956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zeng XF, Li J, Li SB. A functional polymorphism in IL-1A gene is associated with a reduced risk of gastric cancer. Tumour Biol. 2014; 35:265–268. [DOI] [PubMed] [Google Scholar]
- 139.Xu H, Ding Q, Jiang HW. Genetic polymorphism of interleukin-1A (IL-1A), IL-1B, and IL-1 receptor antagonist (IL-1RN) and prostate cancer risk. Asian Pac J Cancer Prev. 2014;15:8741–8747. [DOI] [PubMed] [Google Scholar]
- 140.Villarreal DO, et al. Alarmin IL-33 acts as an immunoadjuvant to enhance antigen-specific tumor immunity. Cancer Res. 2014;74:1789–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gao X, et al. Tumoral expression of IL-33 inhibits tumor growth and modifies the tumor microenvironment through CD8þ T and NK cells. J Immunol. 2015;194:438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Saenz R, et al. TLR4-dependent activation of dendritic cells by an HMGB1-derived peptide adjuvant. J Transl Med. 2014;12:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rovere-Querini P, et al. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004;5:825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Apetoh L, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemo-therapy and radiotherapy. Nat Med. 2007;13:1050–1059. [DOI] [PubMed] [Google Scholar]
- 145.Chuangui C, Peng T, Zhentao Y. The expression of high mobility group box 1 is associated with lymph node metastasis and poor prognosis in esophageal squamous cell carcinoma. Pathol Oncol Res. 2012;18:1021–1027. [DOI] [PubMed] [Google Scholar]
- 146.Dong YD, et al. Expression and clinical significance of HMGB1 in human liver cancer: Knockdown inhibits tumor growth and meta-stasis in vitro and in vivo. Oncol Rep. 2013;29:87–94. [DOI] [PubMed] [Google Scholar]
- 147.Tang D, Lotze MT. Tumor immunity times out: TIM-3 and HMGB1. Nat Immunol. 2012;13:808–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Birger Y, et al. Increased tumor-igenicity and sensitivity to ionizing radiation upon loss of chromosomal protein HMGN1. Cancer Res. 2005;65:6711–6718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Postnikov YV, et al. Loss of the nucleosome-binding protein HMGN1 affects the rate of N-nitrosodiethylamine-induced hepatocarcinogenesis in mice. Mol Cancer Res. 2014;12:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.van Beijnum JR, Buurman WA, Griffioen AW. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis. 2008;11:91–99. [DOI] [PubMed] [Google Scholar]
- 151.Mardente S, et al. HMGB1 induces the overexpression of miR-222 and miR-221 and increases growth and motility in papillary thyroid cancer cells. Oncol Rep. 2012;28:2285–2289. [DOI] [PubMed] [Google Scholar]
- 152.Liu Z, Falo LD Jr, You Z. Knockdown of HMGB1 in tumor cells attenuates their ability to induce regulatory T cells and uncovers naturally acquired CD8 T cell-dependent antitumor immunity. J Immunol. 2011;187:118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Demoulin S, et al. HMGB1 secretion during cervical carcinogenesis promotes the acquisition of a tolerogenic functionality by plasmacytoid dendritic cells. Int J Cancer. 2015;137:345–358. [DOI] [PubMed] [Google Scholar]
- 154.Conejo-Garcia JR, et al. Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med. 2004; 10:950–958. [DOI] [PubMed] [Google Scholar]