Abstract
Gaucher disease is one of the common lysosomal storage diseases widespread all over the world. It is divided into three types such as type 1 (non-neuropathic), type 2 (acute infantile neuropathic) and type 3 (chronic neuropathic). This is caused by the deficiency of glucocerebrosidases from the midpoint nervous system. Recent years, computational tools are very important and play a vital role in identifying new leads for disease treatment. This study was performed to screen the effective bioactive molecules against glucocerebrosidases. In this study, Molecular docking and ADME profiles of bioactive molecules were found with the help of Schrödinger software. Results showed that, (−)-epicatechin are having best docking score and good binding affinity than other ligands. Hence, we concluded that the (−)-epicatechin may be a better drug candidate for gaucher disease which can be explored further.
Keywords: Acid β-glucosidase, Bioactive molecules, Molecular docking, ADME profile
Introduction
Gaucher disease (GD) is a most common metabolic storage disorders and is mainly caused due to the deficiency of acid β-glucosidase in human (Brady et al. 1965, 1966). It causes metabolic dysfunctions that include hepatosplenomegaly, cytopenias, bone disease, and central nervous system disorders (Mistry et al. 1992; Horowitz and Zimran 1994; Stone et al. 2000). Gaucher disease is displayed clinically in a heterogeneous disorder and also it is divided into three phenotypes: type 1-neuronopathic disease, which is the majority of widespread variant accounting for more than 90% of all the cases. Acute neuronopathic is a type 2 gaucher disease, and the chronic neuropathic disease is a type-3 gaucher disease (Beutler and Grabowski 2001; Schueler et al. 2003; Mankin et al. 2001). The gene mutations lead to the amino acid replacement in glucocerebrosidase which can reduce the protein stability reducing the essential catalytic activity (Mistry et al. 1992).
The pathological studies showed that the macrophages are infiltrated into the affected cells accounts for illnesses in the liver, spleen, bone marrow, skeleton, lungs, and occasionally in lymph nodes (Lu et al. 2010). The storage of the glucocerebroside damages the midpoint of nervous system at brain (Bhatia et al. 2002). Acid β-glucosidase (E.C.3.2.1.21) is widespread in plant kingdom (Ketudat et al. 2010) and it cleaves the β-1,4-glucosidic bonds in an assortment of naturally occurring glucosidases. This enzyme plays a vital role in the functions of cell wall catabolism and animal metabolism in certain organism. It also interacts with plant and microbes that are involved to stimulating the plant defence mechanisms against certain pathogens and releasing the plant hormones (Xue et al. 2009).
Acid β-glucosidase has been used to hydrolyze the flavanoids and isoflavonoid glucosides. These molecules are available naturally in plants and plant products such as fruits, red wines, soybeans, tea, and vegetables. There are two kinds of treatments recently available for treating the Gaucher disease namely enzyme replacement therapy (ERT) and substrate reduction therapy (SRT) (Barton et al. 1991; Grabowski et al. 1995). The enzyme replacement therapy is not available in everywhere in the world and not affordable to common people. Hence, this study seeks alternative therapy for Gaucher diseases. The ultimate aim of the study is to find an alternate drug molecule from naturally available bio active products using computational tools, mainly involving molecular docking and ADMET analysis.
Materials and methods
Software and hardware
The computational analysis was carried out in Maestro 10.2 version packages like ligprep, sitemap, grid generation and glide XP dock (Schrodinger, LLC, New York 2012). DELL PRECISION T1700 workstation machine running on Intel (R) Core (TM) i5-4590 CPU processor with 8 GB RAM and 240 GB hard disk on centos Linux as the operating system was used for this study.
Databases
In this study, 29 natural occurring bio-active compounds and 2 synthetic drugs were selected for this docking analysis. All these ligand molecules were retrieved from the chemical database (www.chemspider.com). The target was retrieved from the protein databank (PDB) (Berman et al. 2000).
Protein preparation
The target was taken and further prepared using Protein preparation wizard tool in Maestro 10.2 version. It is a vital step as it solves the problems present in the target molecule includes missing side chains, back chains are added and also updating molecule missing residues. This process was displayed the target heteromers and water molecules. We have removed the water molecules and it also there increasing the entropy of target molecule.
Validation of binding site and grid generation
Binding site validation is an important step in molecular docking. This process will cover entire protein molecule for validating active sites by using sitemap tool in maestro. And also it was showed that binding cavity active residues, volume of the site and site score. Based on this analysis, the selected site was taken further for grid generation. It was fixed that the ligand binding cavity is from target molecule. The selected ligand molecules are docked with acid-beta-glucosidase in order to find out the docking parameters with the help of Grid-based ligand docking (Glide, module 4.4 module 2012). Docking at the centroid of binding cavity grid box is generated with X 5.3; Y 3.71; Z − 20.86 coordination.
Ligand preparation
Ligand molecule of the phytocompounds was converted into 3D structure by using ligprep tool. The drawn ligand was geometry optimized via Optimized Potentials for Liquid Simulations 2005 (OPLS62005) force field (Ligprep, module 4.4 2012). Partial atomic charges are also computed by the OPLS62005 force field. Ligprep tool was used to generate 3D structures from 1D (Smiles) to 2D (SDF) representation, probing for tautomers and steric isomers and geometry minimization of the small molecules.
Molecular docking
Molecular docking predicts the ligand preferred orientation to a receptor when interacting with each other in order to form a higher stability complex. In this study, Maestro 10.2 version tool was used to perform extra precision (XP) docking for predicting the binding affinity, analyzing ligand efficiency, and inhibitory constant of ligand against target. Here, whole ligands are docked with the active site of target by using Glide Xtra precision (XP) tool which docks ligands flexibly to the target. Upon docking study, the suitable small molecules will have an available poses and also get better docking score with accurate hydrophobic contacts between target residues to ligand (Prabhu et al. 2017).
ADME profile
ADME profiles of the ligands are very important to know the druggability before it reaches to pharmaceuticals preparation. Recently, available gaucher drugs are highly expensive, that are only available in few developed countries. Therefore, to discover an anti-gaucher disease lead from bioactive molecules, it should possess good drug potentiality and free from toxicity. Present study has selected 29 bioactive molecules obtained from chemical databases for docking and their absorption, distribution, metabolism and excretion (ADME) properties using QikProp v4.4 module in Schrödinger suite (Quikprop, module 4.4 2012). Some of the major toxicity parameters included were liver toxicity, evaluation of Lipinski’s rule of five. Bio active molecules that have better docking poses and ADME parameters were classified to docking etiquette rationale study (Vijayakumar et al. 2017).
Results and discussion
Target site
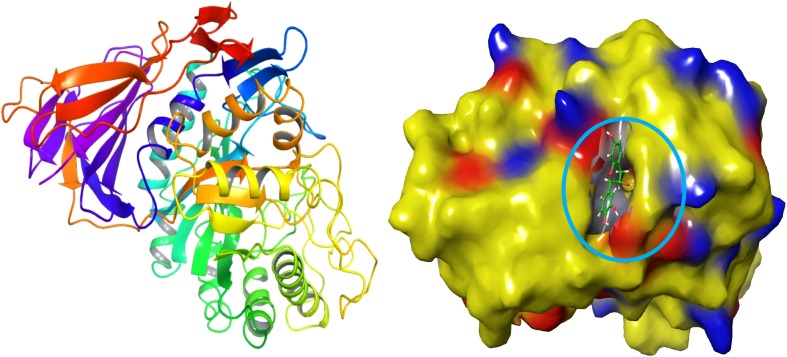
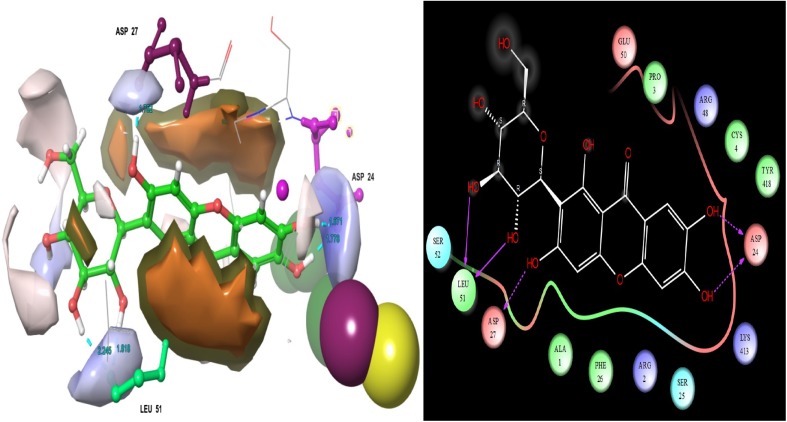
A binding site analysis was carried out by using Sitemap. Here, entire selected ligand molecules were binding with the cavity of enzyme acid-beta-glucosidase. Thus the active site was analyzed on the modelled target. The active site residues as follows ASP 27, ARG 2, PRO 3, ALA 1, GLU 50, ARG 48, CYS 4, LYS 413, TYR 418, ASP 24, SER 25, PHR 26, LEU 51, PRO 29, TYR 40, HIE 451, GLU 111, GLN 169, LYS 425 and PRO 30 (Fig. 1). The target site was showing much electrostatic interaction between ligands to target. Similar studies have been carried out for HCV NS5B target by using the tool of Molecular Operating Environment (MOE) software (Pandey et al. 2016).
Fig. 1.
Ligand catalytic and glucone-binding cavity of acid-β-glucosidase target molecule
Molecular docking
Molecular docking is one of the most widespread methods to explore the interactions between ligand and proteins. Docking reveals, the efficient ligand molecule that has better binding with that of target and it also shows the H-bond binding affinity (both side chain and backbone) and pi–pi interactions. During the docking analysis, there are participating so much of inter-atomic interactions essentially electrostatic energy and van der wall forces. These binding affinities are strongly relied on contribution from other factors such as entropy, de salvation and flexibility of receptor molecule to the ligand.
(−)-Epicatechin
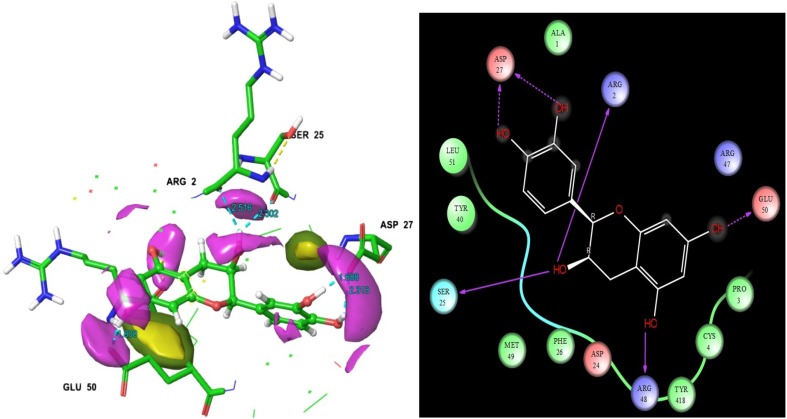
Among the 29 ligands, (−)-epicatechin was found to have a superior docking score than other ligands − 7.953 (Table 1). This ligand’s hydrogen, ammonia and functional groups were interacted with target residues. The interacting residues are as follows. They are ARG 2, ASP 27, SER 25, ARG 48 and GLU 50 (Fig. 2). The ligand to target residues contacts distances were measured and it is showed in Fig. 2. The interaction figure of (−)-epicatechin, as well as target shows the contacts of H-bonds namely HB side chain and HB back chain. Here, two lines of the contacts were displayed at the structure of interaction map. The initial one is HB side chain (blue dotted straight line). The second one is HB back chain (blue solid straight line). Through the two-dimensional interaction map, the ligand hydroxide, oxygen, and other groups interactions are shown. SER 25, ARG 42 and ASP 2 residues were displayed solid straight line contacts with that ligand hydroxide groups. ASP 27 and GLU 50 were represented as the contacts in hydroxide groups also. The molecule (−)-epicatechin is mainly found in fruits, vegetables, and teas. Catechin, (−)-epicatechin, epigallocatechin-3-gallate, epigallocatechin, and (−)-epicatechin gallate, are flavanols or polyphenols. (−)-Epicatechin is a sort of polyphenolic flavonoids which has identified and isolated from variety of medicinal plants such as Ricinus communis (Zahir et al. 2011), Theobroma cacao (Erlejman et al. 2008), Rubus coriifolius (Alanís et al. 2003), Camellia sinensis (Sannella et al. 2007), Crataegus laevigata (Kirakosyan et al. 2003) and Carapa guianensis (Qi et al. 2003). It is as essential phytoconstituents which is having wide spectrum of biological activities against certain globally challenged diseases and their related disorders as well. (−)-Epicatechin has tested on various diseases cell lines (SH-SY5Y-neuroblastoma), HepG2-hepatoma and MCF-7-breast cancer) for scrutinize the molecule biological efficiencies (Erlejman et al. 2008; Ramiro-Puig and Castell 2009; Granado-Serrano et al. 2010; Rodgers and Grant 1998). Previously, Paul et al. (2016) reported that the (−)-epicatechin posses good energy values with the viral proteins of DENV1, DENV2, DENV3 and DENV4. From their study, we found that one of the polyphenol flavonoids of catechin was also revealed superior glide energy values than other ligands with DENV 1-DENV 4. The previous report revealed that the potentiality of (−)-epicatechin against Paramphistomum cervi. It was firstly isolated from Ricinus communis which also recorded as better vivacious molecule for Paramphistomum cervi (Livestock disease causing parasites) in first time (Zahir et al. 2011). They consider that the molecule will be a better drug candidate for that parasite related diseases in animals. Monsalve et al. (2017) review stated that the efficacy of (−)-epicatechin containing medicinal plants and its diverse assortment of biological activities. It has also been reported as beneficial bioactive molecules, due to their antioxidant properties (Chobot et al. 2009). For the most part, it has been involved in oxidative stress protection and apoptosis (Thorpe et al. 2004).
Table 1.
Molecular docking score and the energy of phyto-ligands against the target glucocerebrosidases
| S. no. | Phyto-ligands | Glide docking score | Glide energy |
|---|---|---|---|
| 1. | Epicatechin | − 7.953 | − 46.864 |
| 2. | Apigenin | − 7.505 | − 39.963 |
| 3. | d-(+)-Catechin | − 7.353 | − 42.053 |
| 4. | Mangiferin | − 6.678 | − 47.79 |
| 5. | Syringic acid | − 6.551 | − 24.785 |
| 6. | Rac 8-prenylnaringenin | − 6.537 | − 41.438 |
| 7. | Carvacrol | − 6.503 | − 28.554 |
| 8. | l-(−)-Menthol | − 6.298 | − 22.213 |
| 9. | Curcumin | − 6.185 | − 42.906 |
| 10. | Berberine | − 6.082 | − 37.948 |
| 11. | Ellagic acid | − 6.046 | − 37.096 |
| 12. | Oseltamivir | − 6.008 | − 40.924 |
| 13. | Flavylium | − 5.985 | − 27.893 |
| 14. | g-Tokoferol | − 5.658 | − 36.835 |
| 15. | 6-Methyl-2-cyclohexen-1-ol | − 5.615 | − 19.753 |
| 16. | 2-cyclohexenol | − 5.607 | − 18.815 |
| 17. | Zanamivir | − 5.533 | − 34.138 |
| 18. | Methyl trifluoromethanesulfonate | − 5.474 | − 18.149 |
| 19. | Eugenol | − 5.332 | − 25.563 |
| 20. | 4,5-Epoxy-4,11,11-trimethyl-8-methylenebicyclo(7.2.0)undecane | − 5.31 | − 19.916 |
| 21. | 9-[(3-Methyl-2-buten-1-yl)oxy]-7H-furo[3,2-g] chromen-7-one | − 5.26 | − 34.033 |
| 22. | (−)-Menthone | − 5.231 | − 18.795 |
| 23. | Bilobalide | − 5.051 | − 29.533 |
| 24. | Biperiden | − 5.007 | − 31.98 |
| 25. | (−)-Andrographolide | − 4.674 | − 31.299 |
| 26. | Azadirachtin | − 4.411 | − 43.564 |
| 27. | Linalool | − 4.06 | − 22.073 |
| 28. | Oleanolic acid | − 3.92 | − 31.147 |
Fig. 2.
Epicatechin molecular structure was showed that ligand residues contacts, residues bonding distances and their contacts types
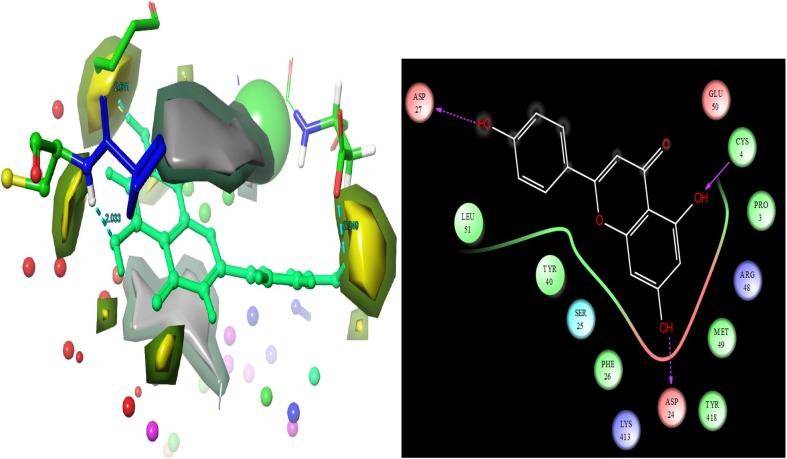
Apigenin
Apigenin is a second leading docking score − 7.505. Apigenin is having interactions with the target residues respectively ASP 27, ASP 24 and CYS 4 (Fig. 3). Molecular structure was represented as these residues contacts distances. ASP 27 and ASP 24 were represented in blue dotted aero line with contacts on ligand hydroxide groups. CYS 4 was displayed the contacts in the molecule of apigenin hydroxide group. Apigenin is a sort of flavonoids molecule which is well known active molecule compounds of flavonoids of medicinal plants. Flavonoids are the most important component of the secondary metabolites of medicinal plants (Kim et al. 2003). The flavonoids are recognized to functioned as an essentially in the management of disorders and diseases in human (Husain et al. 2017). The phytoconstituent of apigenin is protecting from the diverse range of diseases complications. Apigenin and isoflavones, which are known to reveals potent antibacterial activities against various pathogenic organisms. Their assessment of anti-microbial achievement is possibly associated to their efficiency to inactivate microbial adhesins, enzymes and cell transport proteins. Moreover, the apigenin has reported to the glucose intolerance through inhibition of microRNA maturation in transgenic mice (Ohno et al. 2013). Apigenin, which is present in numerous fruits and vegetables, also have different biological properties including enhancement of the malignant cells response to chemotherapy (Chan et al. 2012), tumorigenesis (Mafuvadze et al. 2012), altering immune cell functions (Nicholas et al. 2007), and anti-platelet action (Landolfi et al. 1984). Fajemiroye et al. (2016) review pointed out that the some medicinal plants including naming as Matricaria recutita, Mentha spicata, Ocimum basilicum, Origanum vulgare, Passiflora tripartite, Passiflora incarnata, Passiflora edulis, Onopordum illyricum, Scaevola sericea, Capsicum annuum, Medicago sativa, Asystasia gangetica, Petroselium crispum, Thymus vulgaris, Rosmarinus officinalis, Vernonia hymenolepis, Russelia equisetiformis, Vernonia amygdalina, Vernonia scorpioides and Passiflora foetida. These are listed due to the presence of apigenin. Apigenin has expressed wide spectrum of biological activities in various experimental approaches including anti-cancer (Zhao et al. 2011; Havsteen 2002), anti-microbial (Koo et al. 2002), anxiolytic (Kumar and Sharmal 2006), anti-depressant (Li et al. 2015; Hollman and Katan 1999), neuroprective capability (Zhang et al. 2009; Liu et al. 2013; Zhao et al. 2013a; Ha et al. 2008; Zhang et al. 2014; Patil et al. 2014; Zhao et al. 2013b), anti-chikungunya (Murali et al. 2015) and anti-inflammatory (Sithisarn et al. 2013; Drummond et al. 2013). Based on the computation analysis, we confirmed that the molecule will be represent good biological activities in gaucher diseases affected patients which was confirmed by this research outcomes (docking scores, hydrogen bond interactions and glide energy values).
Fig. 3.
Apigenin molecular structure was showed that ligand residues contacts, residues bonding distances and their contacts types
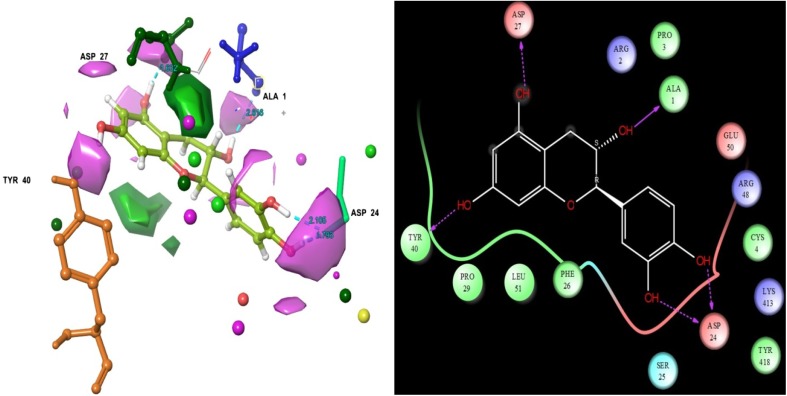
d-(+)-Catechin
The third docking scores were received the ligand d-(+)-Catechin − 7.353. d-(+)-Catechin interacts with the following target residues ASP 27, ASP 24, ALA 1 and TYR 40. Residues of ASP 27, ASP 24 and TYR 40 were formed h-bond side chain contacts with ligand hydroxide groups. ALA 1 was formed h-bond back chain with ligand hydroxide group (Fig. 4). Similar computational reported that the polyphenol flavonoids compound of catechin has exhibited better glide energy values with DENV 1-DENV 4. However, it’s also having acid-beta-glucocerebrosidase complications reducing capability (Paul et al. 2016). This was confirmed by present computational analysis.
Fig. 4.
Epicatechin molecular structure was showed that ligand residues contacts, residues bonding distances and their contacts types
Mangiferin
Mangiferin had lowest docking scores when compared with d-(+)-Catechin. ASP 27, ASP 24 and LEU 51 residues were interacting with the ligand. This interaction map was showed that type ligand binding affinities. From this interaction map examination ASP 24 and ASP 27 residues were formed h-bond chain contact with ligand hydroxide groups. LEU 51 residues was formed h-bond back chain with ligand hydroxide group (Fig. 5). Mangiferin is largely presence in the bark of Mangifera indica. It has precious pharmacological properties, which also having lots of health benefits including immunoregulation, cardio protective, memory enhancement, antiviral, luxitive, etc. The important and potentialities of Mangifera indica and their molecule mangiferin have been clearly reported by in silico, in vitro and in vivo approaches (Parvez 2016). Numerous studies documented that the molecule mangiferin is a vivacious promising bio-active molecule of mango (Mangifera indica L.). Particularly, the stem bark and leaf have accredited the significant biological actions to the human being (Sánchez et al. 2000). Previously, the different parts of Mangifera indica have been carried out in various pharmacological analyses, which are involved in extensive biological actions. These include antioxidant, anti-cancer, anti-microbial, anti-atherosclerotic, anti-allergenic, anti-inflammatory, analgesic, and immunomodulatory amongst numerous others. (Rouillard et al. 1998) research has examined the anti-oxidant probability of mangiferin by in vitro analysis. Additionally, the mangiferin has treated for stimulating immune cells and it was also used for inhibiting the viral replications (Zheng and Lu 1990), as well it was launch to defend from the hepatocytes, lymphocytes, neutrophils, and macrophages from oxidative stress; diminishes atherogenicity in streptozotocin injected diabetic rats; and to decrease the streptozotocin-induced damages like cardiac and renal tissues in rats (Muruganandan et al. 2002, 2005). Based on the computational outcome, we confirmed that this molecule may have potential drug properties to escape from the affection of gaucher disease.
Fig. 5.
Mangiferin molecular structure was showed that ligand residues contacts, residues bonding distances and their contacts types
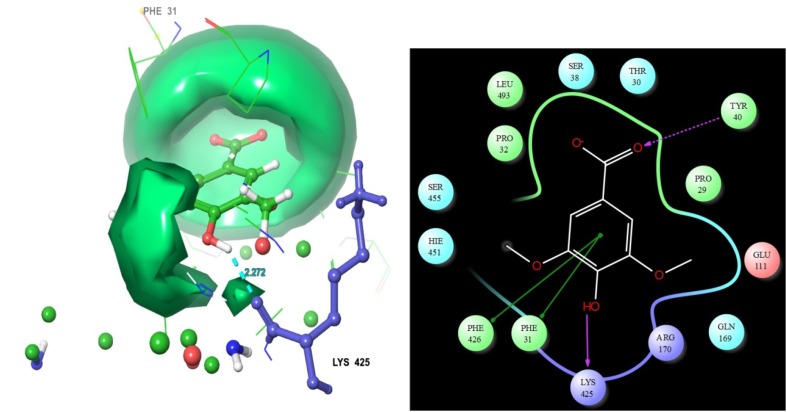
Syringic acid
Among the 29 molecules, syringic acid had an intermediate docking scores − 6.551. The molecular structure shows the ligand contacts distance values with the residues of acid-beta-glucosidase. Here, residue TYR 40 shows interaction with the ligand. This interaction map has displayed the kinds of contacts involved between ligand and target. From this interaction map examination TYR 40 residue is formed h-bond side chain contact with ligand oxygen group. And also this map displayed ligand main compound was formed two Pi–Pi stacking contacts with the target PHE 31 and PHE 426 (Fig. 6). In addition our study displayed the hydrophilic and hydrophobic area of each ligands. Hydrophobic collaborations include contacts between non-polar parts of the particle. In protein–ligand buildings non-polar parts at the communicating surfaces are covered upon ligand binding the target site. The hydrophobic associations are in this way entropy-driven and have been vital part in ligand binding cavity (Bissantz et al. 2010; Bohm 2003). The relationship between detention of non-polar surface region and tying liking is deep-rooted. This analysis suggests that upgrading non-polar contacts of ligand groups in hydrophobic protein takes results in more tightly tying to protein particle.
Fig. 6.
Syringic acid molecular structure was showed that ligand residues contacts, residues bonding distances and their contacts types
The electrostatic energy is a binding interface and important for complex formation from protein to the ligand. The major electrostatic interactions consist of hydrogen bonds (side and back chain), salt bridges, and Pi–Pi stacking. A hydrogen bond is one of the most important roles for biological macromolecules interaction, recognized for conferring stability to the protein molecule and to ligand (Hubbard and Haider 2010). Generally, hydrogen bonds occur in two kinds of electronegative atoms like donor and acceptor, (1) the donor has a covalent hydrogen atom, whereas the other (2) acceptor has a lone pair of electrons. Acceptor has a lone pair of electrons. The effective electrostatic attraction is arises from good-looking interaction flanked by the partial positive charge on the hydrogen atom and partial negative charge on the acceptor.
Synthetic drugs
Two synthetic drugs Eliglustat and Miglustat were showed minimal docking scores with fewer hydrogen bond contacts than phytoconstituents (Table 2). Miglustat has recommended treating mild to average problems of type I gaucher disease. Despite it can cross the blood–brain barrier, Despite it can cross the blood–brain barrier, which proven as to be not helpful in neuropathic lines of type III gaucher disease (Schiffmann et al. 2008). Hollak et al. (1995) reported that miglustat is having adverse effects, which includes diarrhoea, weight loss, tremors and parenthesis as well. In case of the overdose of miglustat pill is causing adverse effects during the treatment. The overdose of adverse effects symptoms such as blurred vision, chest pain or anxiety, perplexity, shortness of breath, sweating, vomiting, etc. (https://www.drugs.com/sfx/eliglustat-side-effects.html).
Table 2.
ADME profile of in this study used phyto-ligands
| S. no. | Phytoligands | HBD | HBA | MW | IP | EA | Meta | Human oral absorption | % of Human oral absorption |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Berberine | 5 | 5.45 | 290.272 | 8.752 | − 0.153 | 7 | 2 | 60.671 |
| 2. | d-(+)-Catechin | 0 | 4 | 164.099 | 12.172 | 1.809 | 0 | 3 | 83.767 |
| 3. | Methyl tri fluoro methane sulfonate | 2 | 4.25 | 198.175 | 9.216 | 0.573 | 3 | 2 | 72.119 |
| 4. | Syringic acid | 1 | 1.7 | 98.144 | 9.853 | − 0.939 | 2 | 3 | 95.85 |
| 5. | 2-cyclohexenol | 1 | 1.7 | 98.144 | 9.853 | − 0.939 | 2 | 3 | 95.85 |
| 6. | l-(-)-Menthol | 9 | 12.35 | 332.313 | 8.499 | 0.063 | 5 | 1 | 0 |
| 7. | Zanamivir | 3 | 7.2 | 312.408 | 9.595 | 0.356 | 3 | 3 | 68.923 |
| 8. | Oseltamivir | 5 | 5.45 | 290.272 | 8.753 | − 0.207 | 7 | 2 | 61.184 |
| 9. | Epicatechin | ||||||||
| 10. | Flavylium | 2 | 4 | 340.375 | 9.114 | 0.411 | 8 | 3 | 85.967 |
| 11. | Rac 8-prenylnaringenin | 2 | 4 | 340.375 | 9.113 | 0.412 | 8 | 3 | 85.364 |
| 12. | 6-Methyl-2-cyclohexen-1-ol | 1 | 1.7 | 112.171 | 9.855 | − 0.94 | 2 | 3 | 100 |
| 13. | Curcumin | 2 | 3.75 | 270.241 | 9.127 | 0.907 | 3 | 3 | 75.249 |
| 14. | Apigenin | 7 | 13 | 422.345 | 8.833 | 0.742 | 10 | 1 | 0 |
| 15. | Mangiferin | 5 | 7 | 360.32 | 8.857 | 0.874 | 6 | 1 | 38.245 |
| 16. | (R)-(+)-rosmarinic acid | 4 | 8 | 302.197 | 9.185 | 1.396 | 4 | 2 | 35.2 |
| 17. | Ellagic acid | 3 | 8.1 | 350.454 | 9.781 | 0.22 | 6 | 3 | 79.994 |
| 18. | (3E)-4-Hydroxy-3-{2-[(1R,8aS)-6 | 3 | 8.1 | 350.454 | 9.706 | 0.237 | 6 | 3 | 81.208 |
| 19. | Carvacrol | 5 | 5.45 | 290.272 | 8.752 | − 0.153 | 7 | 2 | 60.671 |
HBD hydrogen bond donor, HBA hydrogen bond acceptor, MW molecular weight, Meta metabolism
ADME analysis
In the earlier step of drug discovery physico-chemical properties were seen in order to find the vital properties to affecting the biological functions (ADME). In this analysis some important physic-chemical properties were listed such as permeability, solubility, lipophilicity, integrity and stability. But, the concept of absorption, distribution, metabolism and excretion (ADME) shows the toxicity level of small molecules (Pajouhesh and Lenz 2005; Mondal et al. 2009). At the initial stage of drug discovery not only the several end points related to potential hazardous effects that made an impact on drug efficiency and drug toxicity was not estimated but also carcinogenicity and mutagencity of a drug. Right from the beginning of drug discovery in silico method has been used to give an accurate prediction of pharmacokinetic properties for instant ADMET (Krafft et al. 2011).
Conclusions
The present study was focussed on gaucher disease, a lysosomal disorders that affects human beings due to the inadequacy of β-glycosidase. Currently, enzyme medication is the only treatment option available and it seems to be very expensive. These kinds of treatments are not available for rural people and they are unaware of the same due to lack of facilities in hospital. But, β-glycosidase balanced sources are available in naturally growing medicinal plants and their parts. Hence, present computational studies found few effective phyto-chemicals and their mode of interaction. It’s found that (−)-epicatechin have a better docking score and it was showed good binding affinities with the target. It is concluded that the phytocompound have a better drug candidate for gaucher disease. In vitro and in vivo evaluation will be essential and we hope this computational result will be helpful to proceed further with the effective drug development.
Acknowledgements
The authors are grateful to the DST-SERB (SB/YS/LS-109/2014) for providing financial assistance in this project. We specially express our thanks to the management of A.V.V.M. Sri Pushpam College (Autonomous), Poondi, for providing them necessary facilities and support to carry out this work.
References
- Alanís AD, Calzada F, Cedillo-Rivera R, Meckes M. Antiprotozoal activity of the constituents of Rubus coriifolius. Phytother Res. 2003;17:681–682. doi: 10.1002/ptr.1150. [DOI] [PubMed] [Google Scholar]
- Barton NW, Brady RO, Dambrosia JM, Di Bisceglie AM, Doppelt SH, Hill SC, Mankin HJ, Murray GJ, Parker RI, Argoff CE. Replacement therapy for inherited enzyme deficiency—macrophage-targeted glucocerebrosidase for Gaucher’s disease. N Engl J Med. 1991;19:1464–1470. doi: 10.1056/NEJM199105233242104. [DOI] [PubMed] [Google Scholar]
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) The Protein Data Bank. Nucleic Acids Res 28: 235–242. www.rcsb.com. Accessed 1971 [DOI] [PMC free article] [PubMed]
- Beutler E, Grabowski GA. Gaucher disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. 8. New York: McGraw-Hill; 2001. pp. 3635–3668. [Google Scholar]
- Bhatia Y, Mishra S, Bisaria VS. Microbial betaglucosidases: cloning, properties, and applications. Crit Rev Biotechnol. 2002;22:375–407. doi: 10.1080/07388550290789568. [DOI] [PubMed] [Google Scholar]
- Bissantz C, Kuhn B, Stahl MA. Medicinal chemist’s guide to molecular interactions. J Med Chem. 2010;53:5061–5084. doi: 10.1021/jm100112j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm HJ. Prediction of non-bonded interactions in drug design. In: Bohm HJ, Schneider G, editors. Protein-ligand interactions: from molecular recognition to drug design. Weinham: WILLEY-VCH Verlag GmbH & Co. KGaA; 2003. [Google Scholar]
- Brady RO, Kanfer JN, Shapiro D. Metabolism of glucocerebrosides. II. Evidence of an enzymatic deficiency in Gaucher’s disease. Biochem Biophys Res Commun. 1965;18:221–225. doi: 10.1016/0006-291X(65)90743-6. [DOI] [PubMed] [Google Scholar]
- Brady RO, Kanfer JN, Bradley RM, Shapiro D. Demonstration of a deficiency of glucocerebroside-cleaving enzyme in Gaucher’s disease. J Clin Investig. 1966;45:1112–1115. doi: 10.1172/JCI105417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns JRK, Esen A. β-Glucosidases. Cell Mol Life Sci. 2010;67:3389–3405. doi: 10.1007/s00018-010-0399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan LP, Chou TH, Ding HY, Chen PR, Chiang FY, Kuo PL, Liang CH. Apigenin induces apoptosis via tumor necrosis factor receptor- and Bcl-2-mediated pathway and enhances susceptibility of head and neck squamous cell carcinoma to 5-fluorouracil and cisplatin. Biochim Biophys Acta. 2012;1820:1081–1091. doi: 10.1016/j.bbagen.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Chobot V, Huber C, Trettenhahn G, Hadacek F. (±)-Catechin: chemical weapon, antioxidant, or stress regulator. J Chem Ecol. 2009;35:980–996. doi: 10.1007/s10886-009-9681-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond EM, Harbourne N, Marete E, Jacquier JC, O'Riordan D, Gibney ER. An in vivo study examining the antiinflammatory effects of chamomile, meadowsweet, and willow bark in a novel functional beverage. J Diet Suppl. 2013;10:370–380. doi: 10.3109/19390211.2013.830680. [DOI] [PubMed] [Google Scholar]
- Erlejman AG, Jaggers G, Fraga CG, Oteiza PI. TNF alpha-induced NFkappaB activation and cell oxidant production are modulated by hexameric procyanidins in Caco-2 cells. Arch Biochem Biophys. 2008;476:186–195. doi: 10.1016/j.abb.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Fajemiroye JO, Ferreira NL, de Oliveira LP, Elusiyan CA, Pedrino GR, da Cunha LC, da Conceição EC (2016) Matricaria recutita and its isolate-apigenin: economic value, ethnopharmacology and chemico-biological profiles in retrospect. J Pharma Phytochem 4:17–31
- Glide, module 4.4 module (2012) Schrodinger, LLC, New York
- Grabowski GA, Barton NW, Pastores G, Dambrosia JM, Banerjee TK, McKee MA, Parker C, Schiffmannn R, Hill SC, Brady RO. Enzyme therapy in type 1 Gaucher disease: comparative efficacy of mannose-terminated glucocerebrosidase from natural and recombinant sources. Ann Intern Med. 1995;122:33–39. doi: 10.7326/0003-4819-122-1-199501010-00005. [DOI] [PubMed] [Google Scholar]
- Granado-Serrano AB, Martín MA, Haegeman G, Goya L, Bravo L, Ramos S. Epicatechin induces NF-kappaB, activator protein-1 (AP-1) and nuclear transcription factor erythroid 2p45-related factor-2 (Nrf2) via phosphatidylinositol3-kinase/protein kinase B (PI3K/AKT) and extracellular regulated kinase (ERK) signalling in HepG2 ce. Br J Nutr. 2010;103:168–179. doi: 10.1017/S0007114509991747. [DOI] [PubMed] [Google Scholar]
- Ha SK, Lee P, Park JA, Oh HR, Lee SY, Park JH, Lee EH, Ryu JH, Lee KR, Kim SY. Apigenin inhibits the production of NO and PGE2 in microglia and inhibits neuronal cell death in a middle cerebral artery occlusion-induced focal ischemia mice model. Neurochem Int. 2008;52:878–886. doi: 10.1016/j.neuint.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Havsteen BH. The biochemistry and medicinal significance of the flavonoids. Pharmacol Ther. 2002;96:67–202. doi: 10.1016/S0163-7258(02)00298-X. [DOI] [PubMed] [Google Scholar]
- Hollak CE, Aerts JM, Goudsmit R, Phoa SS, Ek M, van Weely S. Individualised low-dose alglucerase therapy for type 1. Gaucher’s disease. Lancet. 1995;345:1474–1478. doi: 10.1016/S0140-6736(95)91037-9. [DOI] [PubMed] [Google Scholar]
- Hollman PC, Katan MB (1999) Health effects and bioavailability of dietary flavonols. Free Radical Res 31:S75–S80 [DOI] [PubMed]
- Horowitz M, Zimran A. Mutations causing Gaucher disease. Hum Mutat. 1994;3:1–11. doi: 10.1002/humu.1380030102. [DOI] [PubMed] [Google Scholar]
- Hubbard RE, Haider MK (2010) Hydrogen bonds in proteins: role and strength. In: Encyclopedia of life sciences (ELS), John Wiley & Sons, Ltd, Chichester. 10.1002/9780470015902.a0003011.pub2
- Husain FM, Ahmad I, Al-thubiani AS, Abulreesh HH, AlHazza IM, Aqil F (2017) Leaf Extracts of Mangifera indica L. Inhibit Quorum Sensing – Regulated Production of Virulence Factors and Biofilm in Test Bacteria. Front Microbiol. 10.3389/fmicb.2017.00727 [DOI] [PMC free article] [PubMed]
- Kim D, Jeong S, Weon L, Chang Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003;81:321–326. doi: 10.1016/S0308-8146(02)00423-5. [DOI] [Google Scholar]
- Kirakosyan A, Seymour E, Kaufman PB, Warber S, Bolling S, Chang SC. Antioxidant capacity of polyphenolic extracts from leaves of Crataegus laevigata and Crataegus monogyna (Hawthorn) subjected to drought and cold stress. J Agric Food Chem. 2003;51:3973–3976. doi: 10.1021/jf030096r. [DOI] [PubMed] [Google Scholar]
- Koo H, Pearson SK, Scott-Anne K, Abranches J, Cury JA, Rosalen PL, Park YK, Marquis RE, Bowen WH. Effects of apigenin and tt-farnesol on glucosyltransferase activity, biofilm viability and caries development in rats. Oral Microbiol Immunol. 2002;17:337–343. doi: 10.1034/j.1399-302X.2002.170602.x. [DOI] [PubMed] [Google Scholar]
- Krafft BA, Skaret G, Calise L. DocumentWG-EMM-11/23. Hobart: CCAMLR; 2011. [Google Scholar]
- Kumar S, Sharmal A. Apigenin: the anxiolytic constituent of Turnera afrodisíaca. Pharm Biol. 2006;44:84–90. doi: 10.1080/13880200600591758. [DOI] [Google Scholar]
- Landolfi R, Mower RL, Steiner M. Modification of platelet function and arachidonic acid metabolism by bioflavonoids. Structure–activity relations. Biochem Pharmacol. 1984;33:1525–1530. doi: 10.1016/0006-2952(84)90423-4. [DOI] [PubMed] [Google Scholar]
- Li R, Zhao D, Qu R, Fu Q, Ma S. The effects of apigenin on lipopolysaccharide- induced depressive-like behavior in mice. Neurosci Lett. 2015;594:17–22. doi: 10.1016/j.neulet.2015.03.040. [DOI] [PubMed] [Google Scholar]
- Ligprep, module 4.4 (2012) Schrodinger, LLC, New York
- Liu H, Ke W, Wei K, K, Hua Z (2013) The impact of IT capabilities on firm performance: the mediating roles of absorptive capacity and supply chain agility. Decis Support Syst 54:1452–1462
- Lu J, Chiang J, Iyer RR, Thompson E, Kaneski CR, David S, Yang C, Chen M, Richard J, Hodes B, Russell R. Decreased glucocerebrosidase activity in Gaucher disease parallels quantitative enzyme loss due to abnormal interaction with TCP1 and c-Cbl. Proc Nat Acad Sci USA. 2010;107:21665–21670. doi: 10.1073/pnas.1014376107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafuvadze B, Liang Y, Besch-Williford C, Zhang X, Hyder SM. Apigenin induces apoptosis and blocks growth of medroxyprogesterone acetate-dependent BT-474 xenograft tumors. Horm Cancer. 2012;3:160–171. doi: 10.1007/s12672-012-0114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin HJ, Rosenthal DI, Xavier R. Gaucher disease. New approaches to an ancient disease. J Bone Jt Surg Am. 2001;83:748–762. doi: 10.2106/00004623-200105000-00016. [DOI] [PubMed] [Google Scholar]
- Mistry PK, Smith SJ, Ali M, Cox TM, Hatton CSR, McIntyre N. Genetic diagnosis of Gaucher’s disease. Lancet. 1992;339:889–892. doi: 10.1016/0140-6736(92)90928-V. [DOI] [PubMed] [Google Scholar]
- Monsalve B, Meyer AC, Palomo I, Fuentes E (2017) Mechanisms of Endothelial Protection by Natural Bioactive Compounds from Fruit and Vegetables. Anais Academia Brasil Cienc 89:615–633 [DOI] [PubMed]
- Mondal SK, Mondal NB, Banerjee B, Mazumder UK. Determination of drug-like properties of a novel antileishmanial compound: in vitro absorption, distribution, metabolism, and excretion studies. Indian J Pharmacol. 2009;41:176–181. doi: 10.4103/0253-7613.56075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali KS, Sivasubramanian S, Vincent S, Murugan SB, Giridaran B, Dinesh S, Gunasekaran P, Krishnasamy K, Sathishkumar R. Anti-chikungunya activity of luteolin and apigenin rich fraction from Cynodon dactylon. Asian Pac J Trop Med. 2015;8:352–358. doi: 10.1016/S1995-7645(14)60343-6. [DOI] [PubMed] [Google Scholar]
- Muruganandan S, Gupta S, Kataria M, Lal J, Gupta PK. Mangiferin protects the streptozotocin-induced oxidative damage to cardiac and renal tissues in rats. Toxicology. 2002;176:165–173. doi: 10.1016/S0300-483X(02)00069-0. [DOI] [PubMed] [Google Scholar]
- Muruganandan S, Srinivasan K, Gupta S, Gupta PK, Lal J. Effect of mangiferin on hyperglycemia and atherogenicity in streptozotocin diabetic rats. J Ethnopharmacol. 2005;97:497–501. doi: 10.1016/j.jep.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Nicholas C, Batra S, Vargo MA, Voss OH, Gavrilin MA, Wewers MD, Guttridge DC, Grotewold E, Doseff AI. Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-kappaB through the suppression of p65 phosphorylation. J Immunol. 2007;179:7121–7127. doi: 10.4049/jimmunol.179.10.7121. [DOI] [PubMed] [Google Scholar]
- Ohno M, Shibata C, Kishikawa T, Yoshikawa T, Takata A, Kojima K, Akanuma M, Kang YJ, Yoshida H, Otsuka M, Koike K. The flavonoid apigenin improves glucose tolerance through inhibition of microRNA maturation in miRNA103 transgenic mice. Sci Rep. 2013;3:2553. doi: 10.1038/srep02553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajouhesh H, Lenz GR. Medicinal chemical properties of successful central nervous system drugs. NeuroRx. 2005;2:541–553. doi: 10.1602/neurorx.2.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey RK, Verma P, Sharma D, Bhatt TK, Sundar S, Prajapati V. High-throughput virtual screening and quantum mechanics approach to develop imipramine analogues as leads against trypanothione reductase of leishmania. Biomed Pharmacother. 2016;83:141–152. doi: 10.1016/j.biopha.2016.06.010. [DOI] [PubMed] [Google Scholar]
- Parvez GMM. Pharmacological activities of mango (Mangifera indica): a review. J Pharma Phytochem. 2016;3:01–07. [Google Scholar]
- Patil SP, Jain PD, Sancheti JS, Ghumatkar PJ, Tambe R, Sathaye S. Neuroprotective and neurotrophic effects of Apigenin and Luteolin in MPTP induced parkinsonism in mice. Neuropharmacol. 2014;86:192–202. doi: 10.1016/j.neuropharm.2014.07.012. [DOI] [PubMed] [Google Scholar]
- Paul A, Vibhuti A, Raj S (2016) Molecular docking NS4B of DENV 1-4 with known bioactive phyto-chemicals. Bioinformatics 3:140–148 [DOI] [PMC free article] [PubMed]
- Prabhu S, Vijayakumar S, Manogar P, Maniam GP, Natanamurugaraj G. Homology modeling and molecular docking studies on type II diabetes complications reduced PPARg receptor with various ligand molecules. Biomed Pharmacother. 2017;92:528–535. doi: 10.1016/j.biopha.2017.05.077. [DOI] [PubMed] [Google Scholar]
- Qi S-H, Wu D-G, Ma Y-B, Luo X-D. A Novel flavane from Carapa guianensis. Acta Bot Sin. 2003;45:1129–1133. [Google Scholar]
- Quikprop, module 4.4 (2012) Schrodinger suite, New York
- Ramiro-Puig E, Castell M. Cocoa: antioxidant and immunomodulator. Br J Nutr. 2009;101:931–940. doi: 10.1017/S0007114508169896. [DOI] [PubMed] [Google Scholar]
- Rodgers EH, Grant MH. The effect of the flavonoids, quercetin, myricetin and epicatechin on the growth and enzyme activities of MCF7 human breast cancer cells. Chem Biol Interact. 1998;116:213–228. doi: 10.1016/S0009-2797(98)00092-1. [DOI] [PubMed] [Google Scholar]
- Rouillard LF, Thiais AJ, Robin JR (1998) Cosmetic or pharmaceutical composition containing, as active ingredient, mangiferin or its derivatives, in pure or in plant extracts. US Patent 824,320
- Sánchez GM, Re L, Giuliani A, Núñez-Sellés AJ, Davison GP, León-Fernández OS. Protective effects of Mangifera indica L. extract, mangiferin and selected antioxidants against TPA-induced biomolecules oxidation and peritoneal macrophage activation in mice. Pharmacol Res. 2000;42:565–573. doi: 10.1006/phrs.2000.0727. [DOI] [PubMed] [Google Scholar]
- Sannella AR, Messori L, Casini A, Vincieri FF, Bilia AR, Majori G, Severini C. Antimalarial properties of green tea. Biochem Biophys Res Commun. 2007;353:177–181. doi: 10.1016/j.bbrc.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Schiffmann R, Fitzgibbon EJ, Harris C, DeVile C, Davies EH, Abel L, van Schaik IN, Benko W, Timmons M, Ries M, Vellodi A. Randomized, controlled trial of miglustat in Gaucher’s disease type 3. Ann Neurol. 2008;64:514–522. doi: 10.1002/ana.21491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrodinger, LLC, New York (2012)
- Schueler UH, Kolter T, Kaneski CR, Blusztajn JK, Herkenham M, Sandhoff V. Toxicity of glucosylsphingosine (glucopsychosine) to cultured neuronal cells: a model system for assessing neuronal damage in Gaucher disease type 2 and 3. Neurobiol Dis. 2003;14:595–601. doi: 10.1016/j.nbd.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Sithisarn P, Michaelis M, Schubert-Zsilavecz M, Cinatl J. Differential antiviral and anti-inflammatory mechanisms of the flavonoids biochanin A and baicalein in H5N1 influenza A virus-infected cells. Antivir Res. 2013;97:41–48. doi: 10.1016/j.antiviral.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Stone DL, Tayebi N, Orvisky E, Stubblefield B, Madike V, Sidransky E. Glucocerebrosidase gene mutations in patients with type 2 Gaucher disease. Hum Mutat. 2000;15:181–188. doi: 10.1002/(SICI)1098-1004(200002)15:2<181::AID-HUMU7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Thorpe GW, Fong CS, Alic N, Higgins VJ, Dawes LW. Cells have distinct mechanisms to maintain protection against different reactive oxygen species: oxidative-stress-response genes. PNAS. 2004;101:6564–6569. doi: 10.1073/pnas.0305888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar S, Manogar P, Prabhu S, Singh SK. Novel ligand-based docking; molecular dynamic simulations; and absorption, distribution, metabolism, and excretion approach to analyzing potential acetylcholinesterase inhibitors for Alzheimer’s disease. J Pharma Anal. 2017 doi: 10.1016/j.jpha.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- http://www.chemspider.com/Chemical-Structure.55081.html. Accessed 3 Aug 2015
- Xue Y, Song X, Yu J. Overexpression of β-glucosidase from Thermotoga maritima for the production of highly purified aglycone isoflavones from soy flour. World J Microbiol Biotechnol. 2009;25:2165–2172. doi: 10.1007/s11274-009-0121-4. [DOI] [Google Scholar]
- Zahir AA, Rahuman AA, Bagavan A, Geetha K, Kamaraj C, Elango G. Evaluation of medicinal plant extracts and isolated compound epicatechin from Ricinus communis against Paramphistomum cervi. Parasitol Res. 2011;111:1629–1635. doi: 10.1007/s00436-011-2589-8. [DOI] [PubMed] [Google Scholar]
- Zhang N, Yacoub E, Zhu XH, Ugurbil K, Chen W (2009) Linearity of Blood-Oxygenation-Level Dependent Signal at Microvasculature. Neuroimage 2:313–318 [DOI] [PMC free article] [PubMed]
- Zhang F, Li F, Chen G. Neuroprotective effect of apigenin in rats after contusive spinal cord injury. Neurol Sci. 2014;35:583–588. doi: 10.1007/s10072-013-1566-7. [DOI] [PubMed] [Google Scholar]
- Zhao M, Ma J, Zhu HY, Zhang XH, Du ZY, Xu YJ, Yu XD. Apigenin inhibits proliferation and induces apoptosis in human multiple myeloma cells through targeting the trinity of CK2, Cdc37 and Hsp90. Mol Cancer. 2011;29:104. doi: 10.1186/1476-4598-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Wang JL, Wang YR, Fa XZ. Apigenin attenuates copper-mediated β-amyloid neurotoxicity through antioxidation, mitochondrion protection and MAPK signal inactivation in an AD cell model. Brain Res. 2013;1492:33–45. doi: 10.1016/j.brainres.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Zhao H, Joo S, Xie W, Ji X (2013b) Using hormetic strategies to improve ischemic preconditioning and postconditioning against stroke. Int J Physiol Pathophysiol Pharmacol 2:61–72 [PMC free article] [PubMed]
- Zheng MS, Lu ZY. Antiviral effect of mangiferin and isomangiferin on herpes simplex virus. Chin Med J (Engl) 1990;103:160–165. [PubMed] [Google Scholar]