Abstract
Background:
p95HER2 is a truncated form of human epidermal growth factor receptor 2 (HER2) that confers resistance to trastuzumab in vitro, but clinical results have been conflicting to date. Given that p95HER2 levels correlate with total HER2 expression levels, which confer better outcomes, we sought to evaluate the p95HER2/HER2 ratio in the North Central Cancer Treatment Group N0337 and N98–32-52 trials.
Methods:
The HERmark assay and VeraTag technology (Monogram Biosciences) were used to measure total HER2 and p95HER2 expression levels in 91 patient samples.
Results:
In the multivariate model, increasing total HER2 level was significantly associated with longer overall survival (OS) (hazard ratio [HR]=0.33; P=.002) and decreasing p95HER2 level was significantly associated with longer OS (HR=4.2; P=.01). Total HER2 expression level was significantly associated with longer progression-free survival (PFS) (HR=0.57; P=.04) whereas p95HER2 level was not (HR= 1.7; P= .25). However, there was a positive association between p95HER2 and total HER2 expression levels (R2= 0.48; P<.001). Consistent with our hypothesis, the ratio of p95HER2/HER2 was significantly associated with worsening PFS (HR= 1.7; P= .04) and OS (HR=2.8; P=.002). Patients with the highest tertile of p95HER2/HER2 values had significantly less favorable PFS (HR=1.8; P=.06) and OS (HR=2.3; P=.02).
Conclusions:
A high p95HER2/HER2 ratio identified patients with metastatic breast cancer with poor outcomes on trastuzumab-based therapies.
Impacts:
p95HER2/HER2 ratio may provide better biomarker to identify patients with poor outcome on trastuzumab. Further investigation of the p95HER2/HER2 ratio as a potential prognostic or predictive biomarker for HER2-targeted therapy is warranted.
Background
Human epidermal growth factor receptor 2 (HER2) is a transmembrane tyrosine kinase receptor in the epidermal growth factor receptor family that promotes cell proliferation and resistance to apoptosis. Overexpression of HER2 occurs in approximately 20% of patients with early-stage breast cancer and is associated with poor outcomes, high recurrence rates, and worse overall survival (OS) (1). With the advent of HER2-targeted therapy, the outcome of patients with HER2-positive breast cancer has dramatically improved over the past decade. Trastuzumab is a humanized monoclonal antibody (mAb) that targets HER2 and has revolutionized the treatment of patients with HER2-positive breast cancer. Existing evidence suggests that trastuzumab exerts activity via multiple mechanisms, including HER2 degradation, inhibition of downstream mitogen-activated protein kinase and PI3K/Akt signaling, and antibody-dependent cellular cytotoxicity (1, 2). While trastuzumab is an effective therapy, a considerable number of patients develop recurrence or relapse, despite adjuvant trastuzumab-based chemotherapy.
Located on chromosome 17q12, HER2 gene encodes a 185-kDa transmembrane tyrosine kinase glycoprotein, often referred to as p185. The HER2 receptor is comprised of 3 distinct domains: extracellular, transmembrane, and intracellular. p95HER2, also known as p95HER2/611 carboxy terminal fragment (CTF) (3) and p110 (4), is a truncated form of HER2 receptor that arises either by the proteolytic shedding of the extracellular domain of the full-length receptor or by translation of the HER2 mRNA from internal initiation codons. The lack of the extracellular domain in p95HER2 results in the constitutive activation of downstream signaling pathways via an intact intracellular kinase domain. Previous studies have demonstrated that p95HER2 expression is prognostic and that high expression of p95HER2 is associated with poor outcome (4–7). p95HER2 expression is also associated with more aggressive disease (5–7) and resistance to trastuzumab in vitro, due to the lack of a trastuzumab-binding domain in the extracellular portion of the receptor. p95HER2-expressing cells retain sensitivity to lapatinib, a dual tyrosine kinase inhibitor of epidermal growth factor receptor and HER2 (8). However, conflicting results have been observed regarding the relationship between p95HER2 expression levels and clinical outcomes in patients with HER2-positive breast cancer treated with trastuzumab-based therapy. Given that high p95HER2 expression often correlates with high HER2 expression, which confers favorable outcomes in the setting of trastuzumab-based therapy, we sought to evaluate the correlation between quantitative levels of HER2, p95HER2, as well as p95HER2/HER2 and outcome in two phase II trials of trastuzumab-based chemotherapy in metastatic HER2-positive breast cancer, the North Central Cancer Treatment Group (NCCTG) N0337 and N98–32-52 trials.
Materials and Methods
Patients and Samples
The formalin-fixed, paraffin-embedded (FFPE) samples were obtained from two trials: the North Central Cancer Oncology Group (NCCTG) N0337 (9) (25 cases) and N98–32-52 (10) (26 cases). NCCTG is now part of the Alliance for Clinical Trials in Oncology. Patients in the N0337 trial received vinorelbine in combination with capecitabine and trastuzumab as a first or second line therapy in the metastatic setting. Patients in the N98–32-52 trial received the combination of paclitaxel, carboplatin, and trastuzumab once a week or once every 3 weeks as first line therapy in the metastatic setting. To increase statistical power, an additional 40 cases were provided from Tenon Hospital (Paris, France) from patients with metastatic breast cancer treated with trastuzumab-based therapy. The Tenon, N0337 and N98–32-52 patients were similar in that they all had no prior trastuzumab treatment, were treated with trastuzumab until progression and were treated with trastuzumab plus chemotherapy as first or second line metastatic treatment. There were no statistically significant differences in estrogen receptor (ER) positivity, progesterone receptor (PR) positivity, number of metastatic sites or age between the Tenon, N0337 and N98–32-52 patients. Treatment following progression was selected by the treating physician in all cases.
HERmark Quantitative HER2 Assay
Quantitative HER2 protein expression was determined using the HERmark assay (Monogram Biosciences, South San Francisco, CA) (11). Briefly, HER2 was measured by the release of a fluorescent tag conjugated to a HER2 mAb via a cleavable linker that is sensitive to singlet oxygen. This was paired with a biotinylated second HER2 mAb attached to a photosensitizer via a biotin-streptavidin bridge. The photosensitizer produced singlet oxygen upon illumination, cleaving the linker. Due to the short half-life of singlet oxygen, the tag was only cleaved when the two antibodies were bound in close proximity. The released fluorescent tag was quantified by capillary electrophoresis and normalized to the area of invasive tumor on the FFPE tissue section. The final measurement was proportional to the amount of receptor per tumor area in units of relative fluorescence per mm2 of tumor (RF/mm2) (12). Reported values were normalized to cell line standards included in each batch. HER2 measurements were compared to prespecified cutoffs for HERmark-negative determinations (total HER2 expression [H2T] <10.5 RF/mm2), HERmark positive determinations (H2T >17.8 RF/mm2), or HERmark equivocal determinations (H2T 10.5–17.8 RF/mm2) derived from the overlap of the lower and upper fifth percentiles of HER2-positive and HER2-negative distributions, respectively, as established using a reference database of 1,090 breast cancer patient samples (11).
Quantitative p95HER2 Assay
p95HER2 expression was measured using the p95HER2 VeraTag assay (Monogram Biosciences) (13, 14). Briefly, a mouse p95HER2 mAb (13) specific for the active M611 carboxy-terminal fragment form of p95HER2 (15) was paired with an anti-mouse secondary antibody conjugated to a fluorescent tag using a disulfide-containing tether that enables release of the tag by treatment with dithiothreitol. The amount of released tag was quantified by capillary electrophoresis, normalized to tumor area, and aligned to cell line standards as described above for the HERmark assay. p95HER2 measurements greater than 2.8 RF/mm2 were considered positive based on previous training (13) and validation studies (14, 16).
Statistical Analysis
All Cox proportional hazards and Kaplan-Meier analyses were stratified by hormone receptor status, except where explicitly described. Hazard ratios (HRs) for continuous variables in Cox proportional hazards analyses were expressed as fold-change in hazard rate per 10-fold change in the variable. Correlations of HER2 or p95HER2 with the number of metastatic sites were assessed by Spearman rank correlation coefficient. All analyses were 2-sided.
Results
Patient Characteristics
A total of 91 FFPE samples were analyzed from the primary tumors of patients with HER2-positive metastatic breast cancer from the NCCTG N0337 study (9), the N98–32-52 study (10), and from Tenon Hospital in Paris, France. The median patient follow-up time for all samples was 60 months (range, 3.6–106 months). Patient characteristics are summarized in Table 1. The median age was 56 years (range, 31–86 years). Patient tumor biopsies were 54% ER-positive and 59% hormone receptor-positive, defined as either ER-positive or PR-positive ≥ 1% (17). Among the 62 patient samples with available data, 35 (56%) tumors were grade 3. The majority of patients had metastases at multiple sites while 33 (36%) had a single metastatic site.
Table 1:
Patient Characteristics, HERmark and p95HER2 Testing Results (91 samples).
| Characteristic | Status | No. (%) |
|---|---|---|
| HERmarka | Positive | 66 (73) |
| Equivocal | 9 (10) | |
| Negative | 16 (18) | |
| p95HER2a (3 missing) |
Positive | 43 (51) |
| Negative | 45 (49) | |
| Estrogen receptor | Positive | 49 (54) |
| Negative | 42 (46) | |
| Progesterone receptor | Positive | 29 (32) |
| Negative | 62 (68) | |
| Hormone receptor status | Positive | 54 (59) |
| Negative | 37 (41) | |
| Grade (29 missing) |
3 | 35 (56) |
| 2 | 24 (39) | |
| 1 | 3 (5) | |
| Number of metastases | >3 | 10 (11) |
| 2–3 | 48 (53) | |
| 1 | 33 (36) | |
| Age at diagnosis (years) | Median | 56 |
| Range | 31–86 |
Cutoffs of 10.5 and 17.8 were used for HERmark and a cutoff of 2.8 was used for p95HER2. See Materials and Methods.
Concordance Between Clinical HER2 Status and HERmark Determination
All of the patient samples in the current study were clinically HER2-positive by standard the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines (18) measured by immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH) tests by local laboratories. Comparing HERmark (excluding equivocal) and conventional local HER2-positive determinations, HERmark results were 91% concordant with HER2 FISH testing (Figure 1A) and 91% concordant with HER2 IHC testing from N0337 and N98–32-52. Overall, the HERmark assay resulted in 66 (73%) positive, 9 (10%) equivocal, and 16 (18%) negative cases (Table 1). Upon further investigation, it was determined that the unexpectedly high HERmark-negative rate was mostly due to block-to-block (8 cases) or primary-to-metastasis heterogeneity (3 cases) rather than discordance (5 cases). Eleven of the 16 cases that were HERmark negative yet previously determined to be HER2-positive were attributable to different blocks from the same patient. Eight patient’s HER2-positive blocks used for clinical management from N0337 and N98–32-52 were depleted, and alternate blocks were provided and used in the HERmark assay which were later discovered to be HER2 FISH-negative and HER2 IHC-unknown. Additionally, three Tenon cases were treated in the metastatic setting based on HER2-positivity in the metastasis which had been depleted. The primary tumor used in the current study was HER2 IHC-negative.
Figure 1:
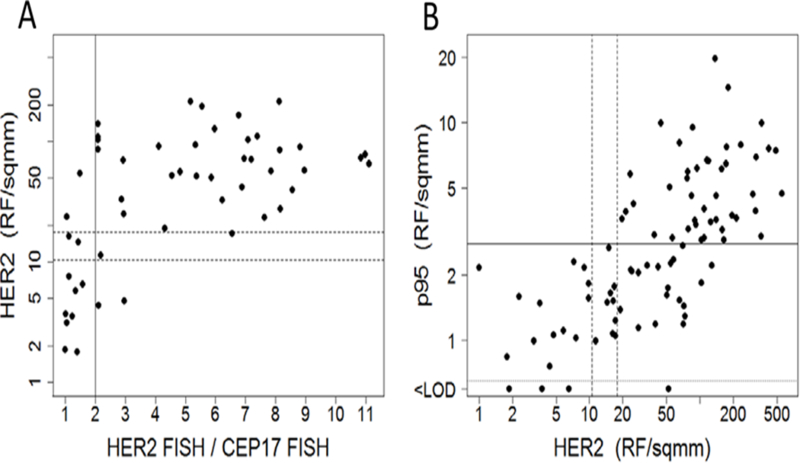
HER2 FISH, HER2 Protein, and p95HER2 Protein. A, HER2 protein expression vs. HER2/CEP17 FISH ratio; lines correspond to cutoffs for HER2 FISH-negative vs positive (solid) and HERmark-negative vs. equivocal vs. positive (dashed). B, p95HER2 protein expression vs. HER2 protein expression; lines correspond to HERmark cutoffs (dotted) and p95HER2-negative vs. positive (dashed). Abbreviations: FISH, fluorescence in situ hybridization; HER2, human epidermal growth factor receptor 2.
Association Between p95HER2 and Total HER2 Expression
Among the 88 samples with adequate specimens for p95HER2 testing, 45 (51%) were assigned as p95HER2-positive based on the previously established cutoff (13, 14). Overall, there was a positive association between the levels of p95HER2 expression and total HER2 expression (R2=0.48; P<.001). Among 66 HER2-positive cases by HERmark, 43 (65%) were considered p95HER2-positive, 20 (30%) were p95HER2-negative, and 3 (5%) had an unknown p95HER2 level (Figure 1B). In contrast, none of the HERmark-negative or equivocal tumors expressed p95HER2 protein above the clinical cutoff of 2.8 RF/mm2.
Association Between Total HER2, p95HER2, and p95HER2/HER2 Ratio and Clinical Outcomes
There was a significant improvement in OS with increasing HER2 expression (HR= 0.64; P=.047) and a trend toward improvement in progression-free survival (PFS) with increasing HER2 expression (HR=0.72; P=.10). However, p95HER2 expression was not significantly associated with either PFS (HR=1.5; P=.38) or OS (HR=1.4; P=.47) in univariate analyses. In the multivariate model, increasing HER2 levels were significantly associated with longer OS (HR=0.33; P=.002) and decreasing p95HER2 levels were significantly associated with longer OS (HR=4.2; P=.01; Table 2, Model A). Similar to the univariate analyses, total HER2 levels were significantly associated with longer PFS (HR=0.57; P=.04), while decreasing p95HER2 levels trended toward longer PFS (HR=1.7; P=.25; Table 2, Model A).
Table 2:
Total HER2, p95HER2, and p95HER2/HER2 Ratio and Clinical Outcome in Univariate and Multivariate Models.
| Variable | PFS (HRa) |
P Value | OS (HRa) |
P Value |
|---|---|---|---|---|
| Model Ab | ||||
| Log(HER2) | 0.57 | .04 | 0.33 | .002 |
| Log(p95HER2) | 1.7 | .25 | 4.2 | .01 |
| Model Bb | ||||
| Log(p95HER2/HER2) | 1.7 | .04 | 2.8 | .002 |
| Model C | ||||
| Log(p95HER2/HER2) | 1.9 | .02 | 2.7 | .003 |
| Hormone receptor status | 0.70 | .15 | 0.67 | .15 |
| Number of metastatic sites | 1.0 | .94 | 1.1 | .44 |
Abbreviations: HER2, human epidermal growth factor receptor 2; HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
Hazard ratios for log(HER2) and log(p95HER2) expressed as hazard ratio per 10-fold change.
Models A and B were stratified by hormone receptor status.
Given the fact that higher p95HER2 expression was associated with higher HER2 expression (Figure 1B), which was associated with better OS, we reasoned that the detrimental effect of p95HER2 could be masked by the beneficial effect of HER2 expression. Therefore, we evaluated the relationship between p95HER2/HER2 ratio and outcome in this study. In univariate analyses, the p95HER2/HER2 ratio was significantly associated with both shorter PFS (HR= 1.7; P= .04) and shorter OS (HR=2.8; P=.002), as hypothesized (Table 2, Model B). In a multivariate model that included hormone receptor status and number of metastatic sites, the ratio of p95HER2/HER2 remained significantly associated with worsening PFS (HR=1.9; P=.02) and OS (HR=2.7; P=.003; Table 2, Model C). We subsequently analyzed the p95HER2/HER2 ratio as a categorical variable by dividing p95HER2/HER2 ratios into tertiles. By comparing the ratios of the lowest and highest tertiles, the highest tertile trended toward shorter PFS (HR=1.8; P=.06; Figure 3A) and was significantly associated with shorter OS (HR=2.3; P=.02; Figure 3B).
Figure 3:
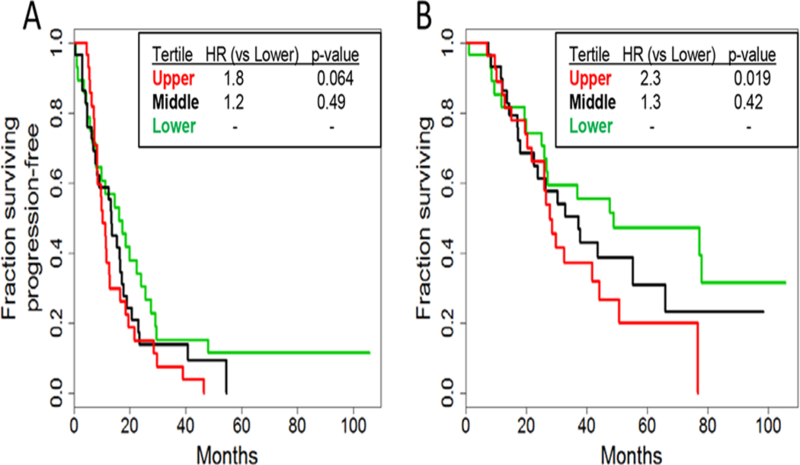
Kaplan-Meier Plots by Tertile for A, Progression-Free Survival and B, Overall Survival for Tertiles of p95/HER2 Ratio.
Discussion
There are at least 2 distinct forms of HER2 CTF that are historically referred to as p95, namely p95HER2/611CTF and 678CTF, which are generated by the utilization of alternative initiation codons at positions 611 and 678, respectively (5, 19). Previous studies have demonstrated that expression of HER2-CTFs confers a more aggressive tumor phenotype compared to the full-length HER2 receptor in both in vitro and in vivo studies (5–7). However, p95HER2/611CTF and 678CTF differentially activate the HER2 downstream signaling cascade. The ability to constitutively generate homodimers through intermolecular disulfide bonds has been proposed as a mechanism for the more aggressive tumor phenotype conferred by p95HER2/611CTF (15). More rapid activation of downstream signaling cascades has also been observed with p95HER2/611CTF compared to other HER2-CTFs (15). p95HER2/611CTF may also activate downstream target genes involved in metastatic progression that are distinct from those genes that are activated by the full-length HER2 (15). Furthermore, expression of p95HER2/611CTF in the mammary gland in transgenic mice results in more rapid tumor formation and metastatic potential compared to the full-length HER2 (15). In contrast, 678CTF lacks the cysteine required to generate a covalent bond and exhibits a low homotypic affinity to the transmembrane and intracellular domains (20). Given that the activity of 678CTF is insignificant compared to p95HER2/611CTF and that 678CTF is more abundant than p95HER2/611CTF in tumors (15), the ability to distinguish between p95HER2/611CTF and 678CTF is imperative. In this particular study, a specific monoclonal antibody against p95HER2/611CTF was used to quantify the biologically active form of p95HER2 (13).
Since p95HER2 lacks the extracellular epitope for trastuzumab binding, it has been postulated that HER2-positive breast cancers that express p95HER2 may be resistant to trastuzumab. In contrast, lapatinib, a small-molecule inhibitor of HER2 and epidermal growth factor receptor, inhibits the intracellular tyrosine kinase, and therefore, may retain activity against HER2-positive tumors that express high level of p95HER2. This hypothesis was substantiated by a report from Scaltriti et al. (8) that demonstrated that p95HER2 overexpression in MCF7 and T47D cell lines in vitro conferred resistance to trastuzumab, but retained sensitivity to lapatinib. Furthermore, the study reported that patients with p95HER2 expressing tumors had lower objective responses to trastuzumab-based therapy, although the sample size of the study was relatively small (46 patient samples) (8). Furthermore, the mAb used by Scaltriti et al. (8) to detect p95HER2 recognizes the intracellular domain of HER2 (clone CB11), which cannot discern between p95HER2/611CTF and 678CTF fragments.
Subsequently, several other studies have evaluated the level of p95HER2 expression and outcome in patients with HER2-positive metastatic breast cancer treated with anti-HER2 therapies. Previously, using the p95HER2/611CTF antibody in a VeraTag assay in FFPE tissue, we established the cutoff for p95HER2 detection used to identify patients with poor outcomes when treated with trastuzumab-based therapy (13). This cutoff was also validated in two separate cohorts of patients with HER2-positive breast cancer treated with trastuzumab-based therapy (14, 16). In contrast, another retrospective study has shown that lapatinib was equally effective in patients with high and low p95HER2 expression (21). These observations are consistent with the hypothesis that lapatinib inhibits the intracellular kinase domain of p95HER2 fragments and thus may represent an effective treatment option for patients with HER2-positive breast cancer with elevated levels of p95HER2 expression.
In the current study, p95HER2 was not found to significantly correlate with either PFS (HR=1.5; P=.38) or OS (HR=1.4; P=.47) in univariate analyses in contrast to other reports (13, 14). This may be due to inadequate power or a stronger association between p95HER2 and total HER2 in the current study. Consistent with previous studies (13, 14), the observations reported here have demonstrated positive associations between total HER2 and p95HER2 expression levels. Since high HER2 expression has been associated with improved outcome among patients treated with trastuzumab (22), it is conceivable that the protective effect of HER2 expression may mask a detrimental effect of p95HER2. Therefore, we evaluated the association between the ratio of p95HER2 and HER2 expression and the outcome in patients with HER2-positive breast cancer treated with trastuzumab-based therapy. As we hypothesized, the p95HER2/HER2 ratio was a stronger predictor of outcome among patients with HER2-positive breast cancer treated with trastuzumab compared to p95HER2 expression alone. Consistent with this observation, Montemurro et al. (23) also reported that p95HER2 expression alone was not associated with time to progression in a univariate analysis and hypothesized that the lack of significant association p95HER2 expression and disease progression may be due to the positive association between p95HER2 and total HER2 levels. In contrast to our current study, the inverse ratio of total HER2/p95HER2 was also evaluated by Montemurro et al. (23). As expected, a higher total HER2/p95HER2 was associated with improved outcome (HR=0.56 per 2-fold change in total HER2/p95HER2 ratio). When total HER2/p95HER2 ratio was dichotomized at its median value, patients with a higher ratio experienced a significantly longer time to progression than patients with a lower ratio (median, 2.0 months vs 9.6 months) (23).
In summary, the p95HER2/HER2 ratio may represent a useful parameter to identify patients with HER2-positive breast cancer with a poor prognosis when treated with trastuzumab-based therapies. Additional studies are needed to validate this finding and to define an optimal cutoff to differentiate patients with a poorer prognosis, who may benefit from the addition of a second HER2-targeted drug, particularly a tyrosine-kinase inhibitor such as lapatinib or neratinib. Given that several different methods and antibody reagents have been used to detect and quantitate p95HER2 expression, standardization and cross-validation studies are needed to advance the clinical validity and utility of p95HER2 testing.
Figure 2:
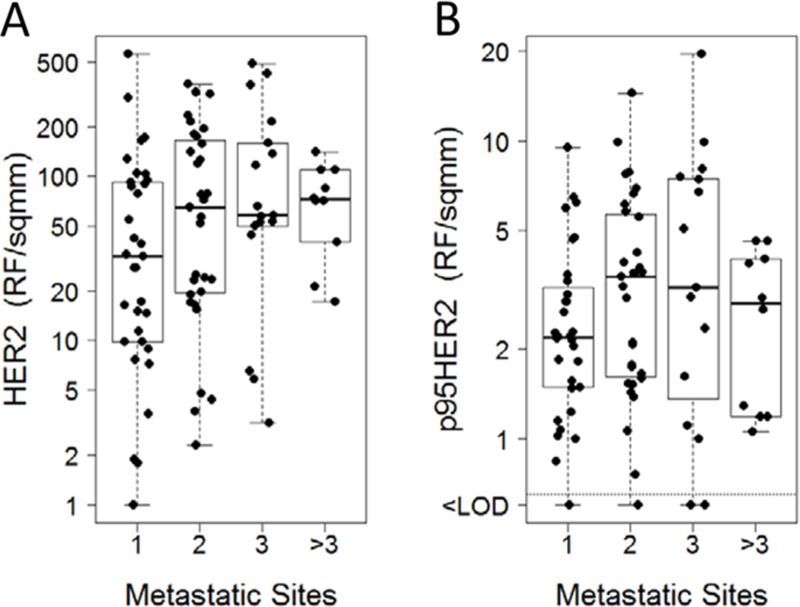
HER2 and p95HER2 vs. Number of Metastatic Sites. A, HER2 protein expression vs number of metastatic sites. B, p95HER2 protein expression versus number of metastatic sites. Measurements below the limit of detection are shown as < LOD. Abbreviations: HER2, human epidermal growth factor receptor 2; LOD, logarithm of odds.
Acknowledgments
ClinicalTrials.gov Identifiers: NCT00093808 (NCCTG-N0337); NCT00003612 (NCCTG-98–32-52)
Support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), U10CA025224, and U10CA180790. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest: Drs. Chumsri, Liu, Gligorov, Spano, Antoine, Moreno Aspitia, Tan, Bates and Weidler have no conflict of interest to report. Drs. Sperinde, Winslow, Petropoulos, Chenna, and Huang work for Monogram Biosciences. Dr. Perez works for Genentech.
Presented at American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, June 3–7, 2016
References
- 1.Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol 2012;9:16–32. [DOI] [PubMed] [Google Scholar]
- 2.Vu T, Claret FX. Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front Oncol 2012;2:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parra-Palau JL, Morancho B, Peg V, Escorihuela M, Scaltriti M, Vicario R, et al. Effect of p95HER2/611CTF on the response to trastuzumab and chemotherapy. J Natl Cancer Inst 2014;106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward TM, Iorns E, Liu X, Hoe N, Kim P, Singh S, et al. Truncated p110 ERBB2 induces mammary epithelial cell migration, invasion and orthotopic xenograft formation, and is associated with loss of phosphorylated STAT5. Oncogene 2013;32:2463–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christianson TA, Doherty JK, Lin YJ, Ramsey EE, Holmes R, Keenan EJ, et al. NH2-terminally truncated HER-2/neu protein: relationship with shedding of the extracellular domain and with prognostic factors in breast cancer. Cancer Res 1998;58:5123–9. [PubMed] [Google Scholar]
- 6.Molina MA, Saez R, Ramsey EE, Garcia-Barchino MJ, Rojo F, Evans AJ, et al. NH2-terminal truncated HER-2 protein but not full-length receptor is associated with nodal metastasis in human breast cancer. Clinical Cancer Research 2002;8:347–53. [PubMed] [Google Scholar]
- 7.Saez R, Molina MA, Ramsey EE, Rojo F, Keenan EJ, Albanell J, et al. p95HER-2 predicts worse outcome in patients with HER-2-positive breast cancer. Clin Cancer Res 2006;12:424–31. [DOI] [PubMed] [Google Scholar]
- 8.Scaltriti M, Rojo F, Ocana A, Anido J, Guzman M, Cortes J, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst 2007;99:628–38. [DOI] [PubMed] [Google Scholar]
- 9.Tan WW, Allred JB, Salim M, Flynn P, Fishkin PA, Stella PJ, et al. Phase II interventional study (N0337) of capecitabine in combination with vinorelbine and trastuzumab for first- or second-line treatment of HER2-positive metastatic breast cancer: a north central cancer treatment group trial. Clin Breast Cancer 2012;12:81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez E, Rowland K, Suman V. N98–32-52: efficacy and tolerability of two schedules of paclitaxel, carboplatin and trastuzumab in women with HER2 positive metastatic breast cancer: a North Central Cancer Treatment Group randomized phase II trial. Breast Cancer Res Treat 2003; 82 (suppl 1):S47; 2003; 2003. [Google Scholar]
- 11.Huang W, Reinholz M, Weidler J, Yolanda L, Paquet A, Whitcomb J, et al. Comparison of central HER2 testing with quantitative total HER2 expression and HER2 homodimer measurements using a novel proximity-based assay. Am J Clin Pathol 2010;134:303–11. [DOI] [PubMed] [Google Scholar]
- 12.Shi Y, Huang W, Tan Y, Jin X, Dua R, Penuel E, et al. A novel proximity assay for the detection of proteins and protein complexes: quantitation of HER1 and HER2 total protein expression and homodimerization in formalin-fixed, paraffin-embedded cell lines and breast cancer tissue. Diagn Mol Pathol 2009;18:11–21. [DOI] [PubMed] [Google Scholar]
- 13.Sperinde J, Jin X, Banerjee J, Penuel E, Saha A, Diedrich G, et al. Quantitation of p95HER2 in paraffin sections by using a p95-specific antibody and correlation with outcome in a cohort of trastuzumab-treated breast cancer patients. Clin Cancer Res 2010;16:4226–35. [DOI] [PubMed] [Google Scholar]
- 14.Duchnowska R, Sperinde J, Chenna A, Haddad M, Paquet A, Lie Y, et al. Quantitative measurements of tumoral p95HER2 protein expression in metastatic breast cancer patients treated with trastuzumab: independent validation of the p95HER2 clinical cutoff. Clin Cancer Res 2014;20:2805–13. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen K, Angelini PD, Laos S, Bach-Faig A, Cunningham MP, Ferrer-Ramon C, et al. A naturally occurring HER2 carboxy-terminal fragment promotes mammary tumor growth and metastasis. Mol Cell Biol 2009;29:3319–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duchnowska R, Sperinde J, Chenna A, Huang W, Weidler JM, Winslow J, et al. Quantitative HER2 and p95HER2 levels in primary breast cancers and matched brain metastases. Neuro Oncol 2015;17:1241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010;28:2784–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/college of American Pathologists clinical practice guideline update. Arch Pathol Lab Med 2014;138:241–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anido J, Scaltriti M, Bech Serra JJ, Santiago Josefat B, Todo FR, Baselga J, et al. Biosynthesis of tumorigenic HER2 C-terminal fragments by alternative initiation of translation. EMBO J 2006;25:3234–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell 2006;125:1137–49. [DOI] [PubMed] [Google Scholar]
- 21.Scaltriti M, Chandarlapaty S, Prudkin L, Aura C, Jimenez J, Angelini PD, et al. Clinical benefit of lapatinib-based therapy in patients with human epidermal growth factor receptor 2-positive breast tumors coexpressing the truncated p95HER2 receptor. Clin Cancer Res 2010;16:2688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipton A, Goodman L, Leitzel K, Cook J, Sperinde J, Haddad M, et al. HER3, p95HER2, and HER2 protein expression levels define multiple subtypes of HER2-positive metastatic breast cancer. Breast Cancer Res Treat 2013;141:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montemurro F, Prat A, Rossi V, Valabrega G, Sperinde J, Peraldo-Neia C, et al. Potential biomarkers of long-term benefit from single-agent trastuzumab or lapatinib in HER2-positive metastatic breast cancer. Mol Oncol 2014;8:20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


