Abstract
Our previous study showed that TNFR2 is preferentially expressed by CD4+FoxP3+ regulatory T cells (Tregs), and expression of this receptor identified maximally suppressive Tregs. TNFR2 is also expressed by a small fraction of CD4+FoxP3− conventional T cells (Tconvs) in normal mice, and its expression is upregulated by T cell activation. This raises questions about the role of TNFR2 signaling in the function of Tconv cells. In this study, by using FoxP3/gfp knock-in mice, we showed that TNFR2 signaling did not induce FoxP3− CD4 cells to become suppressive. Ki-67, a marker of proliferation, was concomitantly expressed with TNFR2 by CD4 cells, independent of forkhead box P3 expression, in normal mice and Lewis lung carcinoma-bearing mice. TNFR2 is associated with greater suppressive functions when expressed by Tregs and is associated with greater resistance to suppression when expressed by Tconv cells. In mice bearing 4T1 breast tumor or Lewis lung carcinoma, intratumoral Tconv cells expressing elevated levels of TNFR2 acquired the capacity to resist suppression by lymph node-derived Tregs. However, they remained susceptible to inhibition by more suppressive tumor-infiltrating Tregs,which expressed higher levels of TNFR2. Our data indicate that TNFR2 also costimulates Tconv cells. However, intratumoral Tregs expressing more TNFR2 are able to overcome the greater resistance to suppression of intratumoral Tconv cells, resulting in a dominant immunosuppressive tumor environment.
Naturally arising CD4+FoxP3+ regulatory T cells (Tregs), making up ~10% of peripheral CD4+ T cells, play an important role in preventing immunopathology by suppressing immune responses to autoantigens (1). However, they also attenuate natural and induced immune responses against tumor Ags and, therefore, represent a mechanism by which tumors evade immune destruction (2). It was reported that the intratumoral removal or inactivation of Tregs enhanced antitumor immunity and resulted in the eradication of advanced tumors (3, 4), suggesting that the suppression of antitumor immunity by Tregs occurs predominantly at the tumor site. Intratumoral CD4+ effector T cells are crucial helpers in the cytotoxic CD8 cell-mediated antitumor effect (5, 6). Therefore, the balance maintained by Treg and conventional T cell (Tconv cell) subsets of CD4 cells in the tumor microenvironment is likely to determine whether the host immune responses evoked by tumor Ag results in tolerance or antitumor responses.
TNFR2 has costimulatory function and enhances the responses of lymphocytes to TCR-mediated signaling (7–9). Our previous study revealed that TNFR2 identifies a subset of Tregs with maximally suppressive capacity in normal mouse lymphoid tissue. Furthermore, tumor-infiltrating Tregs were characterized by a high level of TNFR2 expression and markedly enhanced suppressive activity (10), presumably activated by TNF, a major proinflammatory mediator in the tumor microenvironment (11). We and other investigators reported that, in human and mouse resting CD4 cells, TNFR2 is predominantly expressed by Tregs (10, 12, 13). Although only a minor fraction of normal mouse CD4+CD25− Tconv cells express TNFR2, their expression of this receptor was also upregulated in the tumor environment (10) or upon activation by in vitro TCR stimulation (14). Thus, endogenous TNF present in the tumor environment may also costimulate Tconv cells through TNFR2. It is crucial to further define the effect of the intratumoral costimulatory TNF–TNFR2 pathway on Tconv cells and Tregs, because TNF and anti-TNF have been used to treat cancer (11).
We report our novel observations that intratumoral Tconv cells with enhanced TNFR2 expression exhibited an activated phenotype and acquired the capacity to completely resist the suppressive effects of lymph node (LN)-derived Tregs. However, intratumoral Tregs expressed much higher levels of TNFR2 and retained the capacity to markedly suppress intratumoral Tconv cells and, consequently, resulted in a dominant immunosuppressive tumor environment.
Materials and Methods
Mice, cells, and reagents
Female wild type 8–12-wk-old C57BL/6, BALB/c mice were provided by the Animal Production Area of the National Cancer Institute (NCI). FoxP3/gfp knock-in (KI) mice were kindly provided by Dr. Yasmine Belkaid, Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, and maintained in the Animal Production Area of the NCI. NCI-Frederick is accredited by the American Association for the Accreditation of Laboratory Animal Care International and follows the Public Health Service Policy for the Care and Use of Laboratory Animals. Animal care was provided in accordance with the procedures outlined in the Guide for Care and Use of Laboratory Animals (National Research Council; 1996; National Academy Press; Washington, D.C.). The C57BL/6-derived LLC cell line and BALB/c-derived 4T1 breast tumor cell line were obtained from American Type Culture Collection (Manassas, VA). Abs purchased from BD Biosciences (San Jose, CA) included anti-CD3 (145–2C11), CD4 (GK1.5), CD25 (PC61), CD45RB (16A), CD62L (MEL-14), CD44 (IM4), CD69 (H1.2F3), CD103 (M290), TNFR2 (TR75–89), CTLA4 (UC10–4F10–11), CD16/CD32 (2.4G2), and Ki-67 (B56). Functional grade purified anti-mouse CD3e Ab (eBio500A2), Foxp3 Staining Set (FJK-16s), and glucocorticoid-induced TNFR-related protein (GITR; DTA-1) Abs were purchased from eBioscience (San Diego, CA). Anti-mouse CD120b (TR75–89) Ab was from AbD SeroTec (Kidlington, Oxford, U.K.).
Mouse tumor inoculation and separation of tumor-infiltrating lymphocytes
LLC tumor cells were inoculated s.c. in the right flank of C57BL/6 mice in a single-cell suspension of 500,000 cells in 0.1 ml PBS per mouse. 4T1 tumor cells were injected into left and right mammary fat pads (thoracic #2 mammary glands) of recipient BALB/c mice in single-cell suspensions, with 100,000 cells in 0.1 ml PBS per mouse. After 2 wk, tumors were excised, minced, and digested in RPMI 1640 supplemented with 1 mg/ml collagenase IV and 0.1 mg/ml DNase I. The fragments were pushed through a 70-μm-pore size cell strainer to create a single-cell suspension. After density centrifugation of 40–80% Percoll, lymphocyte-enriched interphase was collected, washed, and stained with Ab for flow cytometry purification of tumor-infiltrating lymphocyte (TIL) Tregs. In some experiments, LLC tumor-bearing mice were sacrificed at day 1, 5, 10, or 15 after inoculation of tumor cells, and Tregs present in the lymphoid tissues and TILs were analyzed.
Cell purification,
CD4 subsets were purified and pooled from spleen and LNs (inguinal, axillary, and mesenteric regions) or TILs using a DakoCytomation MoFlo cytometer (Fort Collins, CO), yielding a purity of ~98% for both subsets. T-depleted spleen cells were used as APCs and were prepared by depletion of CD90+ cells with anti-mouse CD90 MicroBeads and LD column (Miltenyi Biotec, Auburn, CA). APCs were irradiated with 3000 Rad.
In vitro T cell-proliferation assay
CD4+CD25− T cells and CD4+CD25+ T cells from tumor-bearing mouse LNs or TILs or CD4+FoxP3/gfp+ and CD4+FoxP3/gfp− cells, with or without TNFR2 expression, were flow sorted. The sorted cells were cultured alone or cocultured at the desired ratio in a U-bottom 96-well plate in a medium with 2 × 3 105 APCs/well plus 0.5 μg/ml soluble anti-CD3 Ab. Cells were pulsed with 1 μCi [3H]thymidine (PerkinElmer, Wellesley, MA) per well for the last 6 h of the culture period. In some experiments, CD4+FoxP3/gfp+ Tregs were cocultured with freshly isolated CD4+FoxP3/gfp−TNFR2− Tconv cells or added to CD4+FoxP3/gfp−TNFR2− Tconv cells that were preactivated with APCs (2 × 105 APCs/well) and anti-CD3(0.5 μg/ml) for 24 h. In some experiments, flow-sorted CD4+CD25− TNFR2− Tconv cells from normal BALB/c mice were cultured with APCs (ratio of CD4+CD25−TNFR2− cells to APCs was 1:2) and anti-CD3 (0.5 μg/ml) for 72 h. The cells were flow sorted into TNFR2+ and TNFR2− fractions. The resorted cells (2.5 × 104 cells/well) were cultured alone or cocultured with freshly isolated Tregs at a desired ratio. The cells were stimulated with APCs (2 × 105 APCs/well) and anti-CD3 Ab (0.5 μg/ml). After incubation for 72 h, the proliferation was determined by [3H] thymidine incorporation assay. IFN-γ levels in the cell-culture medium were determined by SearchLight Mouse Cytokine Array (Pierce Biotechnology, Woburn, MA).
Flow cytometry
After blocking FcR, cells were incubated with appropriately diluted Abs. Acquisition was performed using a FACSort or an SLRII (BD Biosciences), and data analysis was conducted using FlowJo software (Tree Star, Ashland, OR).
Statistical analysis
Comparison of data was analyzed by the two-tailed Student t test using GraphPad Prism 4.0 (GraphPad, San Diego, CA).
Results
TNFR2-expressing Tregs and Tconv cells in normal mice are more proliferative
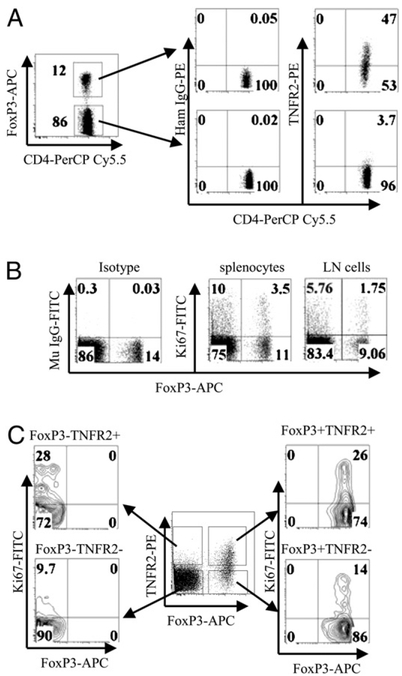
Forkhead box P3 (FoxP3) remains the best marker to identify mouse Tregs (15). Some of the FoxP3+ Tregs are CD25−, even in normal mice (10). Therefore, we further defined the relationship between the expression of TNFR2 and FoxP3 by CD4 cells in normal C57BL/6 mice and FoxP3/gfp KI mice. Our current data corroborated our previous observations showing greater TNFR2 expression on CD4+CD25+ Tregs (10, 14). As shown in Fig. 1A, TNFR2 was preferentially expressed by 47% of unstimulated FoxP3+ splenic CD4 cells, whereas ~4% of resting FoxP3− CD4 cells expressed only low levels of TNFR2. Tregs, which can be unequivocally identified by GFP expression in FoxP3/gfp KI mice, showed a similar pattern of TNFR2 expression on CD4 subsets from wild type mice: 45.9% of FoxP3/gfp+ cells were TNFR2-expressing cells, and only 6.4% of FoxP3/gfp− CD4 cells expressed TNFR2 (data not shown).
FIGURE 1.
Concomitant expression of TNFR2 and Ki-67 by CD4 subsets from normal mice. A, Spleen cells from normal C57BL/6 mice were stained with anti-CD4, TNFR2, and FoxP3 Abs. The expression of TNFR2 by CD4+FoxP3+ and CD4+FoxP3− cells was analyzed by FACS. B, Spleen cells and axillary and inguinal LN cells from normal C57BL/6 mice were stained with anti-CD4, Ki-67, and FoxP3 Abs. The expression of Ki-67 and FoxP3 was analyzed with FACS by gating on CD4. C, Spleen cells from normal C57BL/6 mice were stained with anti-CD4, TNFR2, FoxP3, and Ki-67 Abs. The expression of Ki-67 by FoxP3−TNFR2+, FoxP3− TNFR2−, FoxP3+TNFR2−, and FoxP3+TNFR2+ subsets was analyzed with FACS by gating on CD4 cells. Numbers indicate the percentage of cells in the respective quadrant. Data shown are representative of at least three separate experiments with similar results.
TNFR2 has costimulatory functions that markedly enhance the activation of lymphocytes by TCR signals (7–9). This led us to hypothesize that TNFR2-expressing Tregs and Tconv cells are likely to be more proliferative. Therefore, we assayed for Ki-67 expression, because it is exclusively expressed by proliferating cells(16). Furthermore, Ki-67hi CD4 cells were reported to express an increased level of TNFR2 (17). Consistent with this observation, normal C57BL/6 mouse splenic CD4+FoxP3+ cells with higher expression of TNFR2 contained twice as many Ki-67+ cells (24%) as CD4+FoxP3− cells (12%). In LNs from inguinal and axillary regions, 16.2% of CD4+FoxP3+ cells were Ki-67+ cells, compared with 6.5% of CD4+FoxP3− cells, although the overall Ki-67 levels were lower than in splenocytes (Fig. 1B). The Ki-67+ cells in the FoxP3+ splenic CD4 subset were largely present in the TNFR2+ subset (26%). Although a low level of Ki-67 was expressed in the population of FoxP3− CD4 cells, 28% of the TNFR2-expressing cells in this subset also were Ki-67+ cells. In contrast, only 14% and9.7% of Ki-67+ cells were present in TNFR2− FoxP3+ and FoxP3− CD4 cells, respectively (Fig. 1C). Thus, Tregs and Tconv cells in normal mouse lymphoid tissues exhibited concomitant expression of TNFR2 and Ki-67.
TNFR2+ Tregs potently inhibited proliferation of more resistant TNFR2+ Tconv cells
We next sought to determine whether TNFR2+ Tconv cells are more resistant to suppression by Tregs, by taking advantage of FoxP3/gfp KI mice, which allows purification of viable FoxP3+ Treg subsets based on GFP expression. First, we confirmed our previous observation that the suppressive capacity of TNFR2+ Tregs was considerably greater than that of TNFR2− Tregs over various Tconv/Treg ratios (p < 0.001–0.05; Fig. 2A, 2C) or of unfractionated Tregs (CD4+FoxP3/gfp+ cells), which usually consist ~40% TNFR2+ cells (p < 0.01; Fig. 2C). In fact, unfractionated Tregs were more suppressive than TNFR2− Tregs (p < 0.01; Fig. 2C). In contrast, TNFR2-expressing FoxP3/gfp− CD4 cells did not have any suppressive activity (Fig. 2B), indicating that TNFR2 by itself was not a specific indicator of CD4-suppressive cells. Therefore, the suppressive activity of CD4+CD25−TNFR2+ T cells present in normal C57BL/6 mice (10) was solely attributable to the more highly suppressive subset of TNFR2+FoxP3+ cells present in this CD4+CD25− subpopulation.
FIGURE 2.

Suppressive activity of CD4 subsets from FoxP3/gfp KI mice. Flow-sorted CD4+FoxP3/gfp−TNFR2− cells (Tconv cells), 5 × 104 cells/well, were cultured alone or cocultured with flow-sorted CD4+FoxP3/gfp+ TNFR2+ or CD4+FoxP3/gfp+TNFR2− cells (A) or CD4+FoxP3/gfp+TNFR2+ or CD4+FoxP3/gfp−TNFR2+ cells (B) from FoxP3/gfp KI mice at a ratio of 10:2, 10:5, and 10:10 (Tconv cells/Tregs). C, In other experiments, 2.5 × 104 cells/well of CD4+FoxP3/gfp−TNFR2− cells (Tconv) were cultured alone or cocultured at ratio of 1:1 with CD4+FoxP3/gfp+TNFR2+ (TNFR2+ Tregs), CD4+FoxP3/gfp+TNFR2− cells (TNFR2− Tregs), or unfractionated CD4+ FoxP3/gfp+ cells (total Tregs). The cells were stimulated with APCs (2 × 105 cells/well) and anti-CD3 (0.5 μg/ml) for 72 h. Proliferation was measured by [3H]thymidine incorporation. Data (mean ± SD, n = 3) shown are representative of at least three separate experiments with similar results. *p < 0.05; **p < 0.01; ***p < 0.001.
Furthermore, TNFR2+ Tregs potently inhibited proliferation of TNFR2− and TNFR2+ Tconv cells. In contrast, although TNFR2− Tregs exerted a weaker but detectable inhibitory effect on TNFR2− Tconv cells, this subset of Tregs had no suppressive activity when cocultured with TNFR2+ Tconv cells (Fig. 3A). Upon stimulation with APCs and anti-CD3, these more resistant TNFR2+ Tconv cells produced ~5-fold more INF-γ than did TNFR2− Tconv cells (p < 0.001; Fig. 3B), indicating that TNFR2+ Tconv cells were more activated. Thus, TNFR2 expression on Tconv cells enhances their capacity to resist Treg-mediated suppression; however, they were still susceptible to suppression by TNFR2-expressing Tregs.
FIGURE 3.
TNFR2+ Tconv cells are resistant to Treg-mediated inhibition. A, TNFR2− Tconv cells (CD4+FoxP3/gfp−TNFR2−) (left panel) or TNFR2+ Tconv cells (CD4+FoxP3/gfp−TNFR2+) (right panel), at 5 × 104 cells/well, were cultured alone or cocultured with TNFR2+ Tregs (CD4+FoxP3/gfp+ TNFR2+) or TNFR2− Tregs (CD4+FoxP3/gfp+TNFR2−) at a ratio of 1:1. The cells were stimulated with APCs (2 × 105 cells/well) and anti-CD3 (0.5 μg/ml) for 72 h. Proliferation was measured by [3H]thymidine incorporation. B, IFN-γ levels in TNFR2− and TNFR2+ Tconv cells that were cultured alone with the same conditions as in A. C, Flow-sorted CD4+FoxP3/gfp+TNFR2− Tconv cells, freshly isolated (left panel) or cultured with APCs (2 × 105 cells/well) and anti-CD3 (0.5 μg/ml) for 24 h (right panel) were stained with anti-CD4 and TNFR2 Abs. Expression of TNFR2 was analyzed by gating on CD4 cells. D, Flow-sorted CD4+FoxP3/gfp−TNFR2− Tconv cells, freshly isolated (left panel) or cultured with APCs (2 × 105 cells/well) and anti-CD3 (0.5 μg/ml) for 24 h (right panel), were cultured alone or cocultured at a 1:1 ratio with flow-sorted Tregs. The cells were stimulated with APCs and anti-CD3 Ab for 72 h. Proliferation was measured by [3H]thymidine incorporation. E, Flow-sorted CD4+CD25−TNFR2− Tconv cells from normal BALB/c mice were cultured with 2-fold greater number of APCs and anti-CD3 Ab (0.5 μg/ml). After 72 h of incubation, the cells were flow sorted into TNFR2+ and TNFR2− fractions. The resorted cells (2.5 × 104 cells/well) were cultured alone or cocultured with freshly isolated CD4+CD25+ Tregs at a ratio of 2:1 (Tconv cells Tregs). Freshly flow-sorted CD4+CD25−TNFR2− Tconv cells were used as a comparison. The cells were stimulated with APCs (2 × 105 cells/well) and anti-CD3 Ab (0.5 μg/ml) for 72 h, and proliferation was determined by [3H]thymidine incorporation assay. Percentage inhibition is shown. Data in A, B, D, and E are mean ± SD (n = 3). Data are representative of at least three separate experiments with similar results. *p < 0.05; ***p < 0.001.
This led us to hypothesize that induced expression of TNFR2 on Tconv cells would render them refractory to suppression by Tregs. To test this, we activated flow-sorted CD4+FoxP3/gfp−TNFR2− cells with APCs and anti-CD3 for 24 h and found their expression of TNFR2 was markedly upregulated (Fig. 3C). Unfractionated Tregs (CD4+FoxP3/gfp+) lost their capacity to inhibit the proliferation of these preactivated Tconv cells (Fig. 3D, right panel). In contrast, unfractionated Tregs potently inhibited the proliferation of unactivated TNFR2− Tconv cells (p < 0.05; Fig. 3D, left panel).
We further investigated whether only TNFR2-expressing Tconv cells acquired the Treg-resistant capacity or whether all preactivated Tconv cells developed this property. To provide a definite answer to this question, we resorted preactivated Tconv cells based on their TNFR2 expression and studied their responses to Tregs. Because our previous study showed that Tconv cells from BALB/c mice were more susceptible to inhibition by Tregs compared with Tconv cells from C57BL/6 mice (18), we used Tconv cells from this mouse strain to examine the induction of Treg resistance. First, we isolated CD4+CD25−TNFR2− cells from LNs and spleens of normal BALB/c mice by FACS cell sorting, and the cells were stimulated with APCs and anti-CD3 Ab. After 3 d of culture, ~30% of cells became TNFR2-expressing cells, which confirmed our previous report (14). The cells were resorted into TNFR2+ and TNFR2− fractions, and their responses to Tregs were assessed by coculturing them with freshly isolated CD4+CD25+ Tregs from normal BALB/c mice. As shown in Fig. 3E, resorted TNFR2+ Tconv cells were markedly more resistant than resorted TNFR2− Tconv cells to the suppression of proliferation by Tregs (p < 0.05). Interestingly, resorted TNFR2− Tconv cells were as susceptible as freshly isolated TNFR2− Tconv cells to suppression by freshly isolated Tregs (p < 0.05), indicating that the enhanced Treg-resistant capacity of activated Tconv cells was exclusively restricted to Tconv cells that were induced to express TNFR2. Thus, induced expression of TNFR2 on Tconv cells by TCR stimulation also enhanced their resistance to Tregs.
TNFR2+ Tregs and Tconv cells in draining LNs are more proliferative than in nondraining LNs in tumor-bearing mice
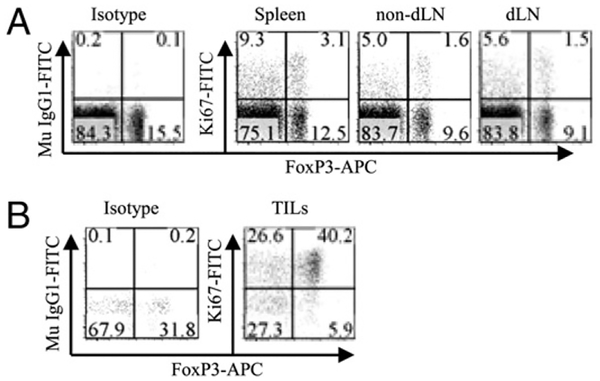
Our previous data showed that tumor-infiltrating Tregs and Tconv cells expressed high levels of TNFR2 (10), indicating that they were highly proliferating cells. Therefore, we further compared Ki-67 expressed by FoxP3+ and FoxP3− subsets of CD4 cells present in the spleen, draining LNs (dLNs), and nondraining LNs (non-dLNs) of mice inoculated with LLC tumor cells for 15 d, when the average tumor volume reached 301.6 mm3 (n = 6). As shown in Fig. 4A, the proportion of Ki-67+ cells in FoxP3+ and FoxP3− CD4 subsets was not increased in the spleen and non-dLNs of tumor-bearing mice. As expected, FoxP3+ cells in the dLNs were more proliferative than those in the non-dLNs, as evidenced by their higher level of Ki-67+ cells (p < 0.01; Fig. 4A, 4C). In contrast, the increase in Ki-67+ cells in FoxP3− CD4 subsets present in the dLNs did not differ from non-dLNs (p > 0.05; Fig. 4A, 4C). Thus, the proliferation of Tregs in the tumor-dLNs was more robust than that of Tconv cells. Furthermore, 50–60% of TNFR2+FoxP3+ cells in dLNs were Ki-67+, which was greater (p < 0.01) than Ki-67+ cells (20–30%) in non-dLNs (Fig. 4B, 4C). Similarly, the percentage of Ki-67+ cells in FoxP3−TNFR2+ population was greater (p < 0.05) in the dLNs (40–50%) than in non-dLNs (20–30%; Fig. 4B, 4C). In contrast, the proportion of Ki-67+ cells in FoxP3+ and FoxP3− subsets lacking TNFR2 expression remained at an equally low level (<10%) in the dLNs and non-dLNs (p > 0.05; Fig. 4B, 4C). We also examined the cellular numbers in the spleen, non-dLNs, and dLNs. As shown in Table I, the mean total cellularity in the dLNs (34.8 × 106/mouse) was 4-fold greater than in the non-dLNs (8.5 × 106/mouse; p < 0.001). The proportion of FoxP3+ Tregs in the dLNs was actually less (p < 0.05) than in non-dLNs, presumably due to the increase in other cell types in the dLNs, because the proportion of FoxP3+ cells in the CD4 subset of dLN cells was not decreased (data not shown). It is relevant that the mean number of FoxP3+ Tregs in the dLNs(1.22 × 106/mouse) was almost 3-fold greater than in the non-dLNs (0.41 × 106/mouse; p < 0.001). Consistent with the increase in Ki-67+ cells in the dLNs (Fig. 4), the mean number of Ki-67+ cells (0.37 × 106/mouse) was 4.6-fold greater than in the non-dLNs (0.08 × 106/mouse; p < 0.001). Thus, the percentage and number of Ki-67+ Tregs were increased in the dLNs of tumor-bearing mice.
FIGURE 4.
Expression of Ki-67 by CD4 subsets in the lymphoid organs of tumor-bearing mice. Spleen cells, nondLNs (left side inguinal and axillary LNs), or dLNs (right side inguinal and axillary LNs) from mice inoculated with LLC tumor cells for 15 d were stained with anti-CD45, CD4, FoxP3, Ki-67, and TNFR2 Abs. A, Expression of Ki-67 by FoxP3−and FoxP3+ cells were analyzed by gating on CD45+CD4+ cells. B, Expression of Ki-67 by FoxP3− TNFR2+, FoxP3+TNFR2+, FoxP3+TNFR2−, and FoxP3− TNFR2− CD4 subsets present in spleen, non-dLNs, and dLNs were analyzed by gating on respective populations. Data shown in A and B are representatives of at least three separate experiments with similar results. Numbers in the dot plots indicate the percentage of cells in the respective quadrant. C, Comparison of Ki-67 expression by CD4 subset present in non-dLNs and dLNs of tumor-bearing mice. Data (mean ± SD) shown are summarized from three separate experiments with similar results (n = 9). D, Kinetic expression of Ki-67 by FoxP3+ Tregs and TNFR2+FoxP3+ Tregs present in dLNs of mice inoculated with LLC tumor cells for 0, 1, 5, 10, or 15 d. Compared with tumor-free mice (day 0). Data (mean ± SD) shown are summarized from two separate experiments with similar results (n = 6). *p < 0.05;**p < 0.01.
Table I. Ki67+FoxP3+ Tregs in the lymphoid organs of LLC tumor-bearing mice (mean ± SD).
| Spleen | Non-dLN | dLN | |
|---|---|---|---|
| Total cellularity (×106) (n) | 192.3 ± 54.7 | 8.5 ± 1.61 | 34.8 ± 6.65* |
| FoxP3+ cells in total cells (%) | 2.37 ± 0.45 | 4.76 ± 0.62 | 3.55 ± 0.45** |
| FoxP3+ cells (×106) (n) | 4.4 ± 0.78 | 0.41 ± 0.03 | 1.22 ± 0.15* |
| Ki67+ cells in FoxP3+ cells (%) | 29.5 ± 4.48 | 20.1 ± 1.52 | 30.37 ± 1.55* |
| Ki67+FoxP3+ cells (×106) (n) | 1.31 ± 0.36 | 0.08 ± 0.002 | 0.37 ± 0.05* |
C57BL/6 mice were inoculated with LLC as described in Materials and Methods. Lymphoid tissues were harvested on day 15 after inoculation of tumor cells. Data were summarized from two separate experiments with similar results (n = 6).
*p < 0.001;
**p < 0.05, compared with non-dLN.
After s.c. inoculation of LLC tumor cells into syngeneic C57BL/6 mice, the tumor developed over time; the average tumor weight was 25.8 ± 7.3 mg, 88.1 ± 38.0 mg, and 267.4 ± 92.3 mg on days 5, 10, and 15 after tumor inoculation, respectively (n =6). We further examined the kinetics of Ki-67+ Tregs in the dLNs over this period of tumor development. The proportion of Ki-67+ cells present in the total FoxP3+ Tregs increased markedly beginning at day 15 and at day 10 in the TNFR2+FoxP3+ subset of Tregs (p < 0.01; Fig. 4D). Nevertheless, at the earlier stage of tumor development (e.g., day 5 of tumor inoculation), the proportion of Ki-67+ Tregs present in the dLNs was not different from that in the LNs in the same region of tumor-free mice (day 0; p > 0.05).
TNFR2+ Treg cell and Tconv cell subsets of TILs are more proliferative
To determine the relationship between TNFR2 expression and proliferation by Tconv cells and Tregs in TILs, we assessed Ki-67 and TNFR2 expression by FoxP3+ and FoxP3− CD4 TILs in C57BL/6 mice inoculated with LLC tumor cells at day 15, at a stage of full tumor development. As shown in Fig. 5A, 88.1% of FoxP3+ cells present in CD4 TILs were TNFR2+ cells, whereas only 52.8% of splenic FoxP3+ cells expressed TNFR2. Only 7% of splenic FoxP3− Tconv cells were TNFR2+ compared with 40.3% of FoxP3− CD4 TILs. Similarly, 84.7 and 48.8% of FoxP3+ and FoxP3− TIL CD4 cells expressed Ki-67, respectively. In comparison, 22.4 and 12% of FoxP3+ and FoxP3− splenic CD4 cells expressed Ki-67 (Fig. 5B). Similar data were observed in LLC- bearing FoxP3/gfp KI mice and 4T1 mammary tumor-bearing BALB/c mice (data not shown). These data clearly show that TNFR2 expression by FoxP3+ and FoxP3− CD4 cells in tumors was markedly increased; however, more of the FoxP3+ Tregs expressed TNFR2, and TNFR2 expression levels on Tregs were much higher on a per-cell basis than on FoxP3− Tconv cells, consistent with their Ki-67 expression.
FIGURE 5.
Tumor-infiltrating FoxP3+ and FoxP3− subsets of CD4 cells upregulated their TNFR2 expression and were actively replicating. Spleen cells and TILs from C57BL/6 mice inoculated with LLC tumor cells for 15 d were stained with anti-CD45, CD4, FoxP3, and Ki-67 Abs or isotype control Abs. A, The expression of TNFR2 by FoxP3− cells and FoxP3+ cells were analyzed by gating on CD45+CD4+ cells. B, The expression of Ki-67 by FoxP3− cells and FoxP3+ cells were analyzed by gating on CD45+CD4+ cells. Numbers indicate the percentage of cells in the respective quadrant. Data shown are representative of at least three separate experiments with similar results.
We also determined the Ki-67 expression by TIL Tregs and Tconv cells at an earlier stage (day 5) after tumor cell inoculation. As shown in Fig. 6A, at this stage, the proportion of Ki-67+ cells in FoxP3+ and FoxP3− CD4 subsets present in the spleen, non-dLNs, and dLNs was not increased compared with that in tumor-free mice (Fig. 6A). Nevertheless, the proportion of FoxP3+ cells in CD4 TILs was >40%. Furthermore, 49.4% of FoxP3− and 87.2% of FoxP3+ CD4 TILs expressed Ki-67 (Fig. 6B). Thus, there was an ~2-fold greater number of Ki-67+ cells in the FoxP3+ subset of TILs than in the FoxP3− subset of TILs at days 5 and 15 of tumor cell injection, suggesting that Tregs in the tumor are more replicative and have a higher turnover than Tconv cells.
FIGURE 6.
The expression of Ki-67 by FoxP3+ and FoxP3− subsets of CD4 cells in the lymphoid tissues and TILs from mice with early tumor development. Spleen cells, non-dLN cells, dLN cells, and TILs from C57BL/6 mice inoculated with LLC tumor cells for 5 d were stained with anti-CD45, CD4, FoxP3, and Ki-67 Abs. The expression of Ki-67 by FoxP3− and FoxP3+ cells was analyzed by gating on CD45+CD4+ cells. A, Lymphoid tissues. B, TILs. Numbers indicate the percentage of cells in the respective quadrant. Data shown are representative of two separate experiments with similar results.
Tumor-infiltrating Tregs and Tconv cells have an activated phenotype
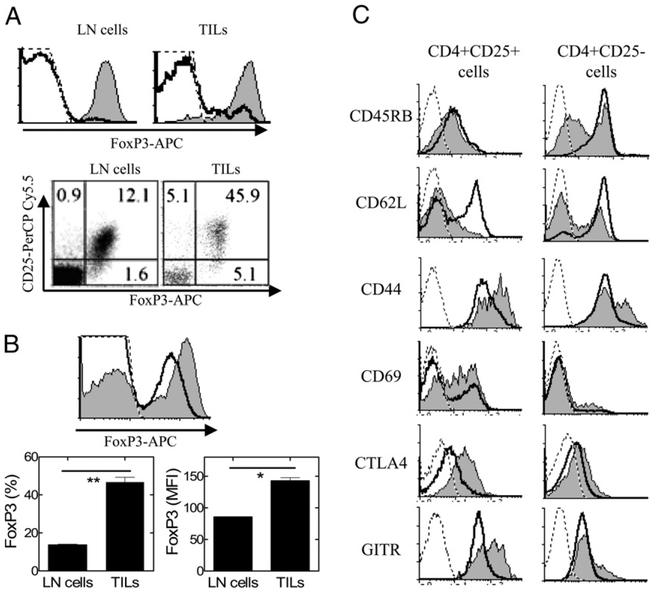
The enhanced expression of TNFR2 and Ki-67 by tumor-infiltrating Tregs and Tconv cells suggests they might have an activated phenotype. Isolation of Tregs in wild type mice is usually based on CD25 expression. Therefore, we compared the phenotype of 4T1 tumor-infiltrating CD4+CD25+ cells and CD4+CD25− cells from mice inoculated with tumor cells for 15 d. CD4+CD25+ cells in tumor-free BALB/c mouse LNs consisted of 93% FoxP3+ cells, whereas only ~2% of CD4+CD25− cells expressed FoxP3 (Fig.7A). Ninety percent of CD4+CD25+ TILs expressed FoxP3. In comparison, 10% of CD4+CD25− TILs were FoxP3+ (Fig. 7A). Thus, in 4T1 tumor, the proportion of FoxP3+ cells in CD4+ CD25− population was increased, as compared with cells from LNs. The proportion of FoxP3+ cells in CD4 TILs and the intensity of FoxP3 expression by TIL CD4 cells were greater than those of LN CD4 cells from tumor-free mice (p < 0.01 and p < 0.05, respectively; Fig. 7B); presumably, this resulted from stimulation by TNF, which has the capacity to increase the expression level of FoxP3 by Tregs on a per-cell basis (14). Our results differ from those in a previous report that showed that Tregs in 4T1 tumor at a later stage (by day 28) after tumor inoculation expressed a lower level of FoxP3 (5); the reason for this discrepancy will be addressed in a future study.
FIGURE 7.
Phenotype of CD25+ and CD25− subsets of tumor-infiltrating CD4 cells from 4T1 tumor-bearing mice. A, LN cells from normal BALB/c mice or 4T1 tumor TILs were stained with anti-CD45, CD4, CD25, and FoxP3 Abs. The expression of FoxP3 was analyzed by FACS, gating on CD4+CD25+ cells (shaded graph) or gating on CD4+CD25− cells (open graph, upper panel). The relationship of CD25 and FoxP3 expression on CD4 cells present in the control LN cells and in the TILs is shown in the dot plot (lower panel). B, Expression of FoxP3 by total CD4 cells from tumor-free BALB/c mouse LN cells (open graph) or by tumor-infiltrating CD4 cells from 4T1 tumor-bearing mice (shaded graph) was analyzed by FACS, gating on CD4+ cells (upper panel). The percentage and mean fluorescence intensity (MFI) of FoxP3+ cells in the CD4 population present in control LNs and in the TILs are shown in the lower panel. Data shown are mean 6 SD (n = 3). *p < 0.05; **p < 0.01. C, Splenocytes from tumor-free BALB/c mice (black line) and TILs from 4T1 tumor-bearing mice (shaded graph) were stained with anti-CD45, CD4, CD25 and phenotypic markers (CD45RB, CD62L, CD44, CD69, CTLA-4, and GITR). Expression of phenotypic marker was analyzed by FACS, gating on CD4+CD25+ cells (left panel) or CD4+CD25− cells (right panel). Isotype controls are represented by dashed line. Data shown are representative of three separate experiments with similar results.
Consistent with our previous results that TNFR2+ Tregs in the normal mouse had an activated phenotype, CD4+CD25+ TILs also exhibited a lower level of CD62L and higher levels of CD44, CD69, CTLA-4, and GITR compared with LN CD4+CD25+ cells from tumor-free mice. Moreover, TIL CD4+CD25− T cells also exhibited an activated phenotype, as evidenced by lower levels of CD45RB and CD62L and increased levels of CD44, CTLA-4, and GITR compared with CD4+CD25− T cells from tumor-free mouse LNs (Fig. 7C). Therefore, tumor-infiltrating Tconv cells are also activated at the tumor site.
Tumor-infiltrating Tconv cells are refractory to suppression by LN Tregs but remain susceptible to inhibition by TIL Tregs
Next we compared the TCR-dependent proliferative responses of tumor-infiltrating Tregs and Tconv cells. CD4+CD25− TILs from 4T1 tumor or LLC tumor exhibited lower proliferative responses upon APC/anti-CD3 stimulation than did CD4+CD25− T cells from LNs (p < 0.01–0.05; Fig. 8A, 8B). In contrast, CD4+CD25+ TILs from 4T1 and LLC tumors exhibited a greater proliferative response to APC/anti-CD3 stimulation than did the hyporesponsive CD4+CD25+ cells from tumor-free mouse LNs (Fig. 8C, 8D). Interestingly, although CD4+CD25− TILs from 4T1 and LLC tumors exhibited attenuated proliferative responses to TCR stimulation, unlike CD4+CD25− responder cells from tumor-free BALB/c and C57BL/6 mice (18), they resisted suppression by CD4+CD25+ cells from tumor-free mouse LNs at a ratio of 1:1 (Fig. 8E, 8F). Thus, consistent with their elevated levels of TNFR2 and Ki-67, TIL Tconv cells were more resistant to suppression by LN-derived Tregs. Nevertheless, when cocultured at a 1:1 ratio, CD4+CD25+ TILs from 4T1 and LLC tumors exhibited a potent suppressive effect on the proliferation of tumor-infiltrating CD4+CD25− cells (Fig. 8E, 8F). Therefore, in the tumor environment, the functional capabilities of Tregs and Tconv cells were altered, but Treg-mediated suppression still remained dominant over the more resistant Tconv TIL cells.
FIGURE 8.
Tumor-infiltrating Tregs potently inhibit tumor-infiltrating Tconv cells. Proliferative responses of Tconv cells: FACS-sorted CD4+ CD25− cells from LNs of tumor-free or from TILs of 4T1 tumor-bearing BALB/c mice (A) or from LNs of tumor-free or from TILs of LLC-bearing C57BL/6 mice (B) were cultured at 2.5 × 104 cells/well. The cells were stimulated with APCs (2 × 105 cells/well) and anti-CD3 for 72 h. Proliferation was measured by [3H]thymidine incorporation. Proliferative responses of Tregs: FACS-sorted CD4+CD25+ cells from LNs of tumor-free or from TILs of 4T1 tumor-bearing BALB/c mice (C) or from LNs of tumor-free or LLC-bearing C57BL/6 mice (D) were cultured at2.5 × 104 cells/well. The cells were stimulated with APCs (2 × 105 cells/well) and anti-CD3 for 72 h. Proliferation was measured by [3H]thymi-dine incorporation. Suppressive activity of Tregs: flow-sorted CD4+ CD25− cells (2.5 × 104 cells/well) from TILs of 4T1 tumor-bearing BALB/c mice were cultured alone or cocultured with the same number of CD4+CD25+ cells from LNs of tumor-free or TILs of 4T1 tumor-bearing BALB/c mice (E) or CD4+CD25− cells (2.5 × 104 cells/well) from TILs of LLC tumor-bearing C57BL/6 mice were cultured alone or cocultured with the same number of CD4+CD25+ cells from LNs of tumor-free or TILs of LLC tumor-bearing C57BL/6 mice (F). The cells were stimulated with APCs (2 × 105 cells/well) and anti-CD3 for 72 h. Proliferation was measured by [3H]thymidine incorporation. Data (mean ± SD, n = 3) shown are representative of at least three separate experiments with similar results.*p < 0.05;**p < 0.01.
Discussion
The failure of Tregs to proliferate after TCR stimulation in vitro initially led to their classification as naturally anergic cells. Our data show that FoxP3+ CD4 cells actually expressed a higher frequency of Ki-67+ cells than did FoxP3− CD4 cells from normal mice, suggesting that even in the steady-state, FoxP3+ CD4 cells are undergoing more robust replication and have a higher turnover than FoxP3− CD4 cells. Therefore, our data are consistent with a previous report that a subset of Tregs in normal mice proliferated even in the steady state, based on CFSE-dilution and BrdU-incorporation assays (19). Furthermore, the expression of Ki-67 correlated directly with TNFR2 expression, which is in agreement with our current understanding of TNFR2 as a costimulator of the proliferative response of lymphocytes to TCR stimulation (20–22). The cell source of TNFR2 ligands, which potentially contribute to the homeostasis of the Treg cell pool in the steady-state, remains to be defined.
The basis for the expansion of Tregs at the tumor site remains elusive. Three mechanisms have been proposed to account for the accumulation of Tregs in the tumor mass: the tumor microenvironment actively recruits natural Tregs by producing chemokine ligands that interact with chemokine receptors expressed by Tregs (23); naive CD4 cells can be converted into tumor-specific FoxP3+ Tregs by TGF-β produced by tumor cells (24) or alternatively by programed death ligand-1 expressed by dendritic cells in the tumor (25); and the tumor microenvironment stimulates the proliferative expansion of infiltrated Tregs (26). It was proposed that TGF-β produced by dendritic cells exhibiting an immature phenotype could stimulate the proliferation of Tregs in tumor (27). However, although known to induce de novo differentiation of naive CD4 cells into Tregs (28), TGF-β is actually a potent suppressor of proliferation of T cells (29). We observed that TGF-β also inhibits TNF-induced proliferation of Tregs in vitro (X. Chen, unpublished data). At day 5 after tumor inoculation, <20% of the FoxP3+ Tregs in the dLNs were proliferating (Figs. 4D, 6), whereas >80% of TIL FoxP3+ Tregs were Ki-67+ (Fig. 6). These data indicate that in situ proliferative expansion contributes substantially to the accumulation of Tregs in the tumor. TNF is a key mediator of cancer-associated inflammation produced by tumor cells and/or by infiltrating leukocytes (11), and it has the capacity to activate Tregs (14). IL-2 is known to be critical to the survival and proliferation of Tregs (30). However, the expression of IL-2 in the tumor tissue is low or absent (31, 32), presumably because of the hypoxic tumor environment, and IL-2 transcription is exquisitely sensitive to changes in oxygen tension (33). These facts argue against the likelihood of a major role for IL-2in the expansion of Tregs in the tumor. Although low levels of IL-2 are needed for the survival of Tregs, the TNF-enriched tumor microenvironment likely induces the proliferative expansion of TNFR2+ Tregs through TNF–TNFR2 interaction.
A small fraction of Tconv cells constitutively expresses TNFR2, even in the steady state (Fig. 1), and TNFR2-expressing Tconv cells are increased in the tumor (Figs. 5, 6). It was shown that memory Tconv cells are refractory to Treg-mediated inhibition (34) and that interaction of TNFR2 with its ligands plays a role in the maintenance of T cell memory (35). Whether TNFR2+ Tconv cells in normal or tumor-bearing mice actually represent memory cells merits future study. Furthermore, activated Tconv cells express more TNFR2 (14) (Fig. 3C). Such activated TNFR2+ Tconv cells were refractory to Treg-mediated suppression. Indeed, CD4+FoxP3/gfp−TNFR2− cells preactivated in vitro with APCs/anti-CD3 also acquired the capacity to resist suppression by Tregs, accompanied by the upregulation of TNFR2 (Fig. 3C, 3D), indicating that proliferating Tconv cells become more resistant than resting Tconv cells to Tregs. Importantly, induction of Treg cell resistance by TCR stimulation was restricted to the Tconv cells that were induced to express TNFR2 (Fig. 3E), underscoring the role of TNFR2 in the Treg cell resistance. TNFR2 was shown to promote TCR-mediated cellular activation (7–9). A recent study reported that the activation of TNFR2, but not TNFR1, on human T cells resulted in p100 processing and induced the activation of an alternative NF-kB pathway (36). Thus, expression of TNFR2 empowers Tconv cells to respond to the stimulation of its ligands and, consequently, enhances their resistance to the inhibition by Tregs.
It was reported that Tconv cells present in the 4T1 tumor with an activated phenotype were hyporesponsive to ex vivo TCR stimulation (5). Similarly, we found that tumor-infiltrating CD4+CD25− cells exhibited an activated phenotype (Fig. 7) and contained a higher proportion of TNFR2+ and Ki-67+ cells (Figs. 5, 6). Thus, TNF–TNFR2 costimulation also activates FoxP3− Tconv cells in the tumor, although they have attenuated proliferative responses to TCR stimulation in vitro, which may be due to TCR signaling defects of Tconv cells in the tumor (37). Presumably, the activated TIL Tconv cells become resistant to inhibition by LN-derived Tregs from tumor-free mice (Fig. 8), but they are still susceptible to inhibition by more highly activated tumor-infiltrating Tregs. Thus, TIL Tregs that express higher levels of TNFR2 on a greater number of cells may outcompete TIL Tconv cells for the use of stimulatory factors, including costimulatory TNFR2 signaling, provided by the tumor environment. It was reported that human and mouse Tregs expressed high levels of surface TNFR2 and had the capacity to shed TNFR2, which contributes to the suppressive action of Tregs (12). However, we did not detect elevated levels of soluble TNFR2 in human or mouse TNFR2+ Treg culture medium under our in vitro experimental condition (data not shown). Furthermore, TNFR2+FoxP3/gfp− T cells did not exhibit suppressive activity at all (Fig. 2). Thus, our data suggest that shed soluble TNFR2 may not represent a molecular basis for the immunosuppressive action of Tregs. Instead, we hypothesize that Tregs may outcompete Tconv cells in using TNF as costimulatory molecules for cellular activation and expansion; consequently, this further deprives Tconv cells of available TNFR2 ligand.
TIL Tregs override the activation of Tconv cells and produce a dominant immunosuppressive environment in tumors. Further defining the mechanisms by which the tumor environment enhances the resistance of Tconv cells to Treg-mediated inhibition may be instrumental in devising effective immunotherapy to circumvent Treg activity and may yield antitumor immune responses. In contrast to tumor-bearing states, the pathogenic activated effector T cells present in autoimmune conditions are more likely to outcompete Tregs in exploiting the TNF–TNFR2 costimulatory pathway. The possibility that this contributes to the therapeutic efficacy of anti-TNF therapy in autoimmune diseases (38) merits future study.
Collectively, our study demonstrated that TNFR2 was predominantly expressed by activated Tregs and, to a markedly lesser extent, by activated Tconv cells and enables Tregs and Tconv cells to respond to costimulatory signaling by TNFR2. Upregulation of TNFR2 was much greater in tumor-infiltrating Tregs than in Tconv cells, and both subsets were activated to proliferate, presumably in response to tumor-associated Ags and stimulation by the TNF-enriched cytokine milieu in the tumor microenvironment. However, the activated intratumoral Tregs still overcame the activated Tconv cells, which resulted in the predominance of the immunosuppressive environment in the tumor. Selectively amplifying TNF–TNFR2 costimulation of Tconv cells and/or blocking its effect on Tregs may improve the outcome of immunotherapy; therefore, it merits future study.
Acknowledgments
This work was supported in whole or in part by federal funds from the National Cancer Institute, National Institutes of Health, Contract HHSN26120080001E. This work was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. R.H. was supported by the International Training Program of the Japan Society for the Promotion of Science.
Abbreviations used in this paper:
- dLN
draining lymph node
- FoxP3
forkhead box P3
- GITR
glucocorticoid-induced
- TNFR
related protein
- KI
knock-in
- LN
lymph node
- MFI
mean fluorescence intensity
- NCI
National Cancer Institute
- non-dLN
nondraining lymph node
- Tconv cell
conventional T cell
- Treg
regulatory T cell
- TIL
tumor-infiltrating lymphocyte
Footnotes
Publisher's Disclaimer: The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Sakaguchi S, Yamaguchi T, Nomura T, and Ono M. 2008. Regulatory T cells and immune tolerance. Cell 133: 775–787. [DOI] [PubMed] [Google Scholar]
- 2.Zou W 2006. Regulatory T cells, tumour immunity and immunotherapy. Nat. Rev. Immunol 6: 295–307. [DOI] [PubMed] [Google Scholar]
- 3.Yu P, Lee Y, Liu W, Krausz T, Chong A, Schreiber H, and Fu YX. 2005. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J. Exp. Med 201: 779–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piconese S, Valzasina B, and Colombo MP. 2008. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J. Exp. Med 205: 825–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaput N, Darrasse-Jèze G, Bergot AS, Cordier C, Ngo-Abdalla S,Klatzmann D, and Azogui O. 2007. Regulatory T cells prevent CD8 T cell maturation by inhibiting CD4 Th cells at tumor sites. J. Immunol 179: 4969–4978. [DOI] [PubMed] [Google Scholar]
- 6.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, and Levitsky H. 1998. The central role of CD4(+) T cells in the antitumor immune response. J. Exp. Med 188: 2357–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim EY, Priatel JJ, Teh SJ, and Teh HS. 2006. TNF receptor type 2 (p75) functions as a costimulator for antigen-driven T cell responses in vivo. J. Immunol 176: 1026–1035. [DOI] [PubMed] [Google Scholar]
- 8.Kim EY, and Teh HS. 2001. TNF type 2 receptor (p75) lowers the threshold of T cell activation. J. Immunol 167: 6812–6820. [DOI] [PubMed] [Google Scholar]
- 9.Kim EY, and Teh HS. 2004. Critical role of TNF receptor type-2 (p75) as a costimulator for IL-2 induction and T cell survival: a functional link to CD28.J. Immunol 173: 4500–4509. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Subleski JJ, Kopf H, Howard OM, Ma¨nnel DN, and Oppenheim JJ. 2008. Cutting edge: expression of TNFR2 defines a maximally suppressive subset of mouse CD4+CD25+FoxP3+ T regulatory cells: applicability to tumor-infiltrating T regulatory cells. J. Immunol 180: 6467–6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balkwill F 2009. Tumour necrosis factor and cancer. Nat. Rev. Cancer 9: 361–371. [DOI] [PubMed] [Google Scholar]
- 12.van Mierlo GJ, Scherer HU, Hameetman M, Morgan ME, Flierman R,Huizinga TW, and Toes RE. 2008. Cutting edge: TNFR-shedding by CD4+ CD25+ regulatory T cells inhibits the induction of inflammatory mediators.J. Immunol 180: 2747–2751. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Subleski JJ, Hamano R, Howard OM, Wiltrout RH, and Oppenheim JJ. 2010. Co-expression of TNFR2 and CD25 identifies more of the functional CD4+FOXP3+ regulatory T cells in human peripheral blood. Eur.J. Immunol 40: 1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Bäumel M, Ma¨nnel DN, Howard OM, and Oppenheim JJ. 2007. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. J. Immunol 179: 154–161. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Y, and Rudensky AY. 2007. Foxp3 in control of the regulatory T cell lineage. Nat. Immunol 8: 457–462. [DOI] [PubMed] [Google Scholar]
- 16.Scholzen T, and Gerdes J. 2000. The Ki-67 protein: from the known and the unknown. J. Cell. Physiol 182: 311–322. [DOI] [PubMed] [Google Scholar]
- 17.van der Most RG, Currie AJ, Mahendran S, Prosser A, Darabi A, Robinson BW, Nowak AK, and Lake RA. 2008. Tumor eradication after cyclophosphamide depends on concurrent depletion of regulatory T cells: a role for cycling TNFR2-expressing effector-suppressor T cells in limiting effective chemotherapy. Cancer Immunol. Immunother 58: 1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X, Oppenheim JJ, and Howard OM. 2005. BALB/c mice have more CD4+CD25+ T regulatory cells and show greater susceptibility to suppression of their CD4+CD252 responder T cells than C57BL/6 mice. J. Leukoc. Biol 78: 114–121. [DOI] [PubMed] [Google Scholar]
- 19.Fisson S, Darrasse-Jèze G, Litvinova E, Septier F, Klatzmann D, Liblau R, and Salomon BL. 2003. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J. Exp. Med 198: 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carpentier I, Coornaert B, and Beyaert R. 2004. Function and regulation of tumor necrosis factor type 2. Curr. Med. Chem 11: 2205–2212. [DOI] [PubMed] [Google Scholar]
- 21.Aggarwal BB 2003. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol 3: 745–756. [DOI] [PubMed] [Google Scholar]
- 22.Tartaglia LA, Goeddel DV, Reynolds C, Figari IS, Weber RF,Fendly BM, and Palladino MA Jr. 1993. Stimulation of human T-cell proliferation by specific activation of the 75-kDa tumor necrosis factor receptor.J. Immunol 151: 4637–4641. [PubMed] [Google Scholar]
- 23.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. 2004. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med 10: 942–949. [DOI] [PubMed] [Google Scholar]
- 24.Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, Zhang Q,Lonning S, Teicher BA, and Lee C. 2007. Tumor evasion of the immune system by converting CD4+CD252 T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-beta. J. Immunol 178: 2883–2892. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, andNoelle RJ. 2008. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc. Natl. Acad. Sci. USA 105: 9331–9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou G, and Levitsky HI. 2007. Natural regulatory T cells and de novo-induced regulatory T cells contribute independently to tumor-specific tolerance.J. Immunol 178: 2155–2162. [DOI] [PubMed] [Google Scholar]
- 27.Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E,Kroemer G, Martin F, Chauffert B, and Zitvogel L. 2005. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+ CD25+ regulatory T cell proliferation. J. Exp. Med 202: 919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, and Wahl SM. 2003. Conversion of peripheral CD4+CD25- naive T cells to CD4+ CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3.J. Exp. Med 198: 1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li MO, Wan YY, Sanjabi S, Robertson AK, and Flavell RA. 2006. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol 24: 99–146. [DOI] [PubMed] [Google Scholar]
- 30.Oppenheim JJ 2007. IL-2: more than a T cell growth factor. J. Immunol 179: 1413–1414. [DOI] [PubMed] [Google Scholar]
- 31.Piancatelli D, Romano P, Sebastiani P, Adorno D, and Casciani CU. 1999. Local expression of cytokines in human colorectal carcinoma: evidence of specific interleukin-6 gene expression. J. Immunother 22: 25–32. [DOI] [PubMed] [Google Scholar]
- 32.Knerer B, Hulla W, Martinek H, Formanek M, Temmel A, and Kornfehl J. 1996. IL-1 and TNF-alpha but no IL-2 expression is found in squamous cell carcinomas of the head and neck by RT-PCR. Acta Otolaryngol. 116: 132–136. [DOI] [PubMed] [Google Scholar]
- 33.Zuckerberg AL, Goldberg LI, and Lederman HM. 1994. Effects of hypoxia on interleukin-2 mRNA expression by T lymphocytes. Crit. Care Med. 22: 197–203. [DOI] [PubMed] [Google Scholar]
- 34.Yang J, Brook MO, Carvalho-Gaspar M, Zhang J, Ramon HE,Sayegh MH, Wood KJ, Turka LA, and Jones ND. 2007. Allograft rejection mediated by memory T cells is resistant to regulation. Proc. Natl. Acad. Sci. USA 104: 19954–19959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabbagh L, Snell LM, and Watts TH. 2007. TNF family ligands define niches for T cell memory. Trends Immunol. 28: 333–339. [DOI] [PubMed] [Google Scholar]
- 36.Rauert H, Wicovsky A, Müller N, Siegmund D, Spindler V, Waschke J,Kneitz C, and Wajant H. 2010. Membrane tumor necrosis factor (TNF) induces p100 processing via TNF receptor-2 (TNFR2). J. Biol. Chem 285: 7394–7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whiteside TL 1999. Signaling defects in T lymphocytes of patients with malignancy. Cancer Immunol. Immunother 48: 346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feldmann M 2002. Development of anti-TNF therapy for rheumatoid arthritis. Nat. Rev. Immunol 2: 364–371. [DOI] [PubMed] [Google Scholar]