Abstract
The presence of Alzheimer’s disease (AD)-related neuropathology among cognitively normal individuals has been well documented. It has been proposed that these individuals may represent a pre-clinical AD population. Previous studies have demonstrated a negative association between the presence of both amyloid-β (Aβ) plaques and neurofibrillary tangles with ante-mortem cognitive performance, a relationship which is likely influenced by a number of factors including age and APOE ε4 carrier status. The present study determined whether the presence of neuritic plaques (NPs) and diffuse plaques (DPs) are associated with performance in a number of cognitive domains after accounting for APOE ε4 carrier status and neurofibrillary tangle presence in a cohort of 123 older participants from the Rush Religious Order Study who died with a premortem clinical diagnosis of no cognitive impairment (NCI). After adjusting for age at death, education, gender, Braak stage, and APOE ε4 carrier status, the presence of NPs was associated with lower performance in the cognitive domains of Global Cognition (p = 0.002), Episodic Memory (p = 0.03), Semantic Memory (p = 0.009), and Visuospatial performance (p = 0.006), while DPs showed no association with any cognitive domain examined. These results suggest that decreases in cognition in elderly NCI individuals are associated with an increase in NPs and not DPs when age at death, education, gender, APOE ε4 status, and Braak stage are taken into consideration.
Keywords: Amyloid-β, Braak stage, cognition, neuropathology, pre-clinical Alzheimer’s disease
INTRODUCTION
The presence of Alzheimer’s disease (AD)-related neuropathology among cognitively normal individuals has been well documented [1–7]. These individuals may represent a pre-clinical disease stage based on the presence of AD neuropathology at autopsy despite a lack of clinically significant cognitive impairment premortem [8, 9]. However, studies have demonstrated a negative association between the presence of both amyloid-β (Aβ) plaques and neurofibrillary tangles (NFTs) with ante-mortem cognitive performance [7, 10–14], suggesting that individuals in the pre-clinical stages of AD are already demonstrating disease-related cognitive decline, despite the fact that their performance on cognitive tests is still within normal limits at the time of the assessment. The relationship between AD pathology and cognition is likely a multifactorial process. Factors such as APOE ε4 carrier status, age-related brain changes, gender, and education may have mediating or direct effects on AD pathology and cognition [4, 15, 16].
APOE ε4 carrier status has been reported to have differential effects on the presence of both tau and amyloid plaque pathology [17]. For example, the presence of tau pathology among ε4 carriers was associated with greater amyloid load, although ε4 and ε2 alleles were not related with tau pathology in the absence of Aβ pathology [17]. Others have reported that APOE genotype effects the distribution of cortical plaque and tangle pathology across brain regions [18] and that age and APOE-related tangle associations are mediated by the presence of neuritic plaques (NPs) [15].
Several studies suggest that cognition is impacted primarily by NPs and not diffuse plaques (DPs) [11, 19, 20]. Previously, our group found that increases in hippocampal NPs correlated with decreased episodic memory and global cognitive performance and that increases in the combined hippocampal/entorhinal cortex Aβ load was associated with greater NFT pathology [21]. However, this investigation did not find an association between cognition and NFT pathology [21]. Although research using amyloid imaging found that 21% of cognitively normal individuals displayed significant cortical amyloid deposition, there were no significant differences in cognitive performance between those classified as Aβ-positive and Aβ-negative [22]. By contrast, a recent meta-analysis concluded that there is a small, but significant negative association between increased Aβ deposition and cognitive performance among cognitively normal individuals [14]. Since Aβ imaging tracers are not specific to DPs or NPs [23], it is possible that the in vivo associations between Aβ deposition and cognition are confounded by the presence of DPs. NPs are thought to have a stronger association with cognition [11, 19, 20, 24].
The aim of the present study was to determine whether the presence of NPs and DPs are associated with performance in a number of cognitive domains after accounting for age at death, education, gender, APOE ε4 carrier status and NFTs in a cohort of older people who came to autopsy with an ante-mortem clinical diagnosis of no cognitive impairment (NCI).
METHODS
Data came from 123 older deceased and autopsied persons with a premortem clinical diagnosis of NCI, no coexisting clinical or neurological condition judged to contribute to cognitive impairment at their last clinical evaluation [25, 26], and who agreed to annual clinical evaluations and signed an informed consent and an Anatomic Gift Act donating their brains at time of death. Data from these subjects have been used in numerous clinical pathological studies supported by our ongoing NIA program project grant entitled the “Neurobiology of Mild Cognitive Impairment in the Elderly” (P01AG14449). At the time of these studies, individuals were chosen from all available Rush Religious Orders Study (RROS) cases that came to autopsy during a rolling admission [2]. Neuropathological procedures used to determine these conditions have been reported [2, 25, 26]. In addition, those taking anticholinesterases or medication for depression were also excluded. The Human Investigation Committee of Rush University Medical Center approved the study.
Clinical evaluation
Participants underwent a uniform, structured, clinical evaluation, self-reported medical history was obtained by a team led by a neurologist, and cognitive function was determined by a trained neuropsychological test technician [2, 25]. Medications used by the subjects within the previous fourteen days of the examination were reviewed and classified. After review of all clinical data and examination of the participant, a clinical diagnoses was made by a board certified neurologist or geriatrician with expertise in the evaluation of elderly persons with dementia. Clinical diagnostic classification of NCI was performed as described previously [2, 25]. A neurologist reviewed the medical history, medication use, neurologic examination, results of cognitive performance testing, and the neuropsychologist’s opinion of cognitive impairment and dementia. Each participant was evaluated in their home, emphasizing findings deemed clinically relevant.
Tissue preparation and neuropathological diagnosis
Brain accruement and processing was described previously [25, 27]. Briefly, each brain was cut into 1 cm thick coronal slabs using a brain slice apparatus and hemisected. One hemisphere was immersion fixed in 4% paraformaldehyde (24–72 h) and cryoprotected (10% glycerol and 2% dimethyl sulfoxide in phosphate buffer solution) until processing for immunohistochemistry.
Diagnostic blocks (mid-frontal, superior temporal, entorhinal cortex, hippocampus, inferior parietal cortex, basal ganglia, thalamus, and substantia nigra) from the opposite hemisphere were paraffin embedded and cut at 6 µm. Examination for cerebral infarctions was conducted as described previously [28]. Bielschowsky silver stain was used to visualize NPs, DPs, and NFTs. Sections were also immunostained for Aβ using antibody M0872 (1 : 100; Dako, CA) raised against Aβ1−40 and Aβ1−42. Paired helical filament tau (AT8; 1 : 800, Covance) immunohistochemistry [24] was also used to label NFTs. Neuropathological diagnoses were determined according to CERAD [29] and Braak staging [30] as recommended by the NIA-Reagan criteria [31]. Exclusion criteria included AD, Lewy body disease, mixed dementias, Parkinson’s disease, frontotemporal dementia, argyrophilic grain disease, vascular dementia, hippocampal sclerosis, and stroke.
Pathologic quantitation
A board-certified neuropathologist or trained technician, blinded to all clinical data, counted total number of NPs, DPs, and NFTs revealed by Bielschowsky silver stain in one square mm area (100× magnification) per cortical region as reported previously [27, 32]. Since the distribution of plaques and tangles was not normally distributed, standardized plaque and tangle counts from each area were converted to standard scores by dividing the standard deviation (SD) of mean raw counts per marker and region from the entire deceased cohort. Scaled scores for NPs and DPs derived from the mid-frontal, mid-temporal, inferior parietal, entorhinal, and hippocampal CA1 regions were summed to develop a summary count score of NPs and DPs for each subject.
Cognitive composite scores
Composite scores are based on the results of 17 individual cognitive tests divided into five domains of cognition [2, 27, 33, 34]. Mini-Mental State Examination (MMSE) described the cohort but not used in the composite scores. Briefly, episodic memory was evaluated with tests including immediate and delayed recall of story A from Logical Memory and of the East Boston Story, and Word List Memory, Recall, and Recognition from the Consortium to Establish a Registry for AD (CERAD). Semantic memory was assessed with three tests including a 15-item version of the Boston Naming Test, Verbal Fluency, which involves naming examples of semantic categories (i.e., animals, vegetables) in 1-min trials; and a reading test that involves reading single words aloud and a 10-item reading test. Scores on the three tests are converted to a standard scale and averaged to get the composite score. Working memory was assessed using Digit Span Forward and Backward and Digit Ordering. Two tests of perceptual speed included Symbol Digit Modalities Test, and Number Comparison. Finally, two tests of visuospatial ability included a 15-item version of Judgment of Line Orientation and a 9-item version of Standard Progressive Matrices [34]. For each test, raw scores were converted into z-scores based on the mean and standard deviation of the sample. The z-scores from the individual tests were averaged to create individual domain composite scores. The Global Composite Score (GCS) is an average of the 17 individual test z-scores.
Statistical analysis
The association between the presence of DPs and NPs with cognition was assessed using a series of linear regression models. The first series of models used the presence (>0) or absence (= 0) of DPs as the predictor with each of the cognitive domain scores as outcome variables while adjusting for Braak stage, APOE ε4 carrier status, age at death, education, and gender. The second series of models used the presence or absence of NPs as the predictor with each of the cognitive domain scores as outcome variables while adjusting for Braak stage, APOE ε4 carrier status, age at death, education, and gender. A third series of models used the presence or absence of both DPs and NPs as the predictor with each cognitive domain score as outcome variables while adjusting for Braak stage, APOE ε4 carrier status, age at death, education, and gender. Cohen’s d was used to estimate the effect size for group differences (Small = 0.00–0.49, Medium = 0.50–0.79, Large = ≥0.80). Spearman rank correlation was used to assess the relationship between Braak stage and total plaque scores. The Wilcoxon sign-rank test was used to determine whether DP and NP scores were significantly different within each Braak stage. Logistic regression was used to examine the association of Braak stage with the presence or absence of DPs and NPs. Separate analyses for DPs and NPs were run which adjusted for age, education, gender, APOE ε4 status, and the interaction of Braak stage and APOE ε4 status. When necessary, the false discovery rate was used to correct for multiple comparisons in order to maintain a significance level of ≤0.05.
RESULTS
Demographic and descriptive statistics
The cohort was comprised of 63 males and 60 females with an average age at death of 83.90 ± 6.12 years and a mean of 18.29 ± 3.59 years of education. The average duration between last clinical assessment and autopsy was 0.73 ± 0.79 years and average postmortem interval was 7.59 ± 7.40 h. The average MMSE score was 28.29 ± 1.37. Twenty-one individals were APOE ε4 carriers and 101 were non-ε4 carriers (APOE was not available for one individual). Among the non-carriers, 16 exhibited APOE ε2/3 and 85 were ε3/3. Of the 21 ε4 carriers, only one was APOE ε4/4 homozygous and none were APOE ε2/4. Of the cases examined meeting NIA-Reagan criteria, 56% (95% Confidence Interval (CI): 47%, 65%) were categorized with a low likelihood of AD and 40% (95% CI: 31%, 49%) with an intermediate likelihood of AD. CERAD criteria revealed 41% as no AD (95% CI: 32%, 50%), 13% as possible (95% CI: 8%, 20%), 36% as probable (95% CI: 28%, 45%), and 11% as definite AD: (95% CI: 6%, 18%).
Fifty-seven percent (n = 72) of the sample displayed both DPs and NPs while 32% (n = 39) had no plaque pathology. Five individuals had only NPs and seven only had DPs. Demographic, cognitive, and neuropathological results for those with and without DPs and NPs are shown in Table 1. For both DP and NP groups, individuals who had plaques were significantly older than those lacking plaques at autopsy (DPs and NPs, p < 0.001). No significant differences were noted for education, postmortem interval, brain weight, time between last clinical assessment and death, and MMSE for the presence or absence of either DPs or NPs. Females had a greater frequency of NP presence compared to males (p = 0.02), but not for the presence of DPs (p = 0.09). Further investigation of the differences in the presence or absence of NPs between gender found that females were more than twice as likely to display NPs compared to males [OR = 2.50, (1.17, 5.33), p = 0.02].
Table 1.
Demographic and neuropathological characteristics by presence or absence of diffuse and neuritic plaques
| DPs Present | DPs Absent | NPs Present | NPs Absent | NPs and DPs Present | NPs and DPs Absent | |
|---|---|---|---|---|---|---|
| N | 79 | 44 | 77 | 46 | 72 | 39 |
| Gender (M/F) | 36/43 | 27/17 | 33/44 | 30/16 | 31/41 | 25/14 |
| APOE ε4 (carrier/non-carrier) | 17/61 | 4/40 | 18/59 | 3/42 | 17/55 | 3/36 |
| Age at Death (y) | 85.74±5.09 | 80.58±6.46 | 85.51±5.23 | 81.19±6.58 | 85.71±5.24 | 80.21±6.50 |
| Education (y) | 18.29±3.27 | 18.30±4.13 | 18.23±3.40 | 18.39±3.92 | 18.33±3.31 | 18.36±4.04 |
| MMSE | 28.19±1.33 | 28.48±1.42 | 28.22±1.34 | 28.41±1.41 | 28.26±1.30 | 28.46±1.36 |
| PMI (h) | 7.61±8.37 | 7.55±5.31 | 7.35±8.44 | 7.97±5.29 | 7.58±8.80 | 7.81±5.45 |
| Brain Weight (g) | 1,230.71±145.69 | 1,251.14±156.03 | 1,227.37±152.04 | 1,255.87±143.94 | 1,224.55±148.58 | 1,254.97±153.10 |
| LCA and Death (y) | 0.77±0.93 | 0.66±0.44 | 0.76±0.95 | 0.68±0.43 | 0.81±0.98 | 0.68±0.45 |
| Braak Stage | ||||||
| 0 | 1 | 2 | 0 | 3 | 0 | 2 |
| I | 8 | 12 | 6 | 14 | 5 | 12 |
| II | 14 | 8 | 12 | 10 | 12 | 8 |
| III | 22 | 13 | 23 | 12 | 21 | 11 |
| IV | 29 | 9 | 31 | 7 | 27 | 6 |
| V | 5 | 0 | 5 | 0 | 5 | 0 |
DPs, diffuse plaques; LCA, Last clinical assessment; NPs, neuritic plaques; PMI, postmortem interval.
Diffuse and neuritic plaque association with cognition
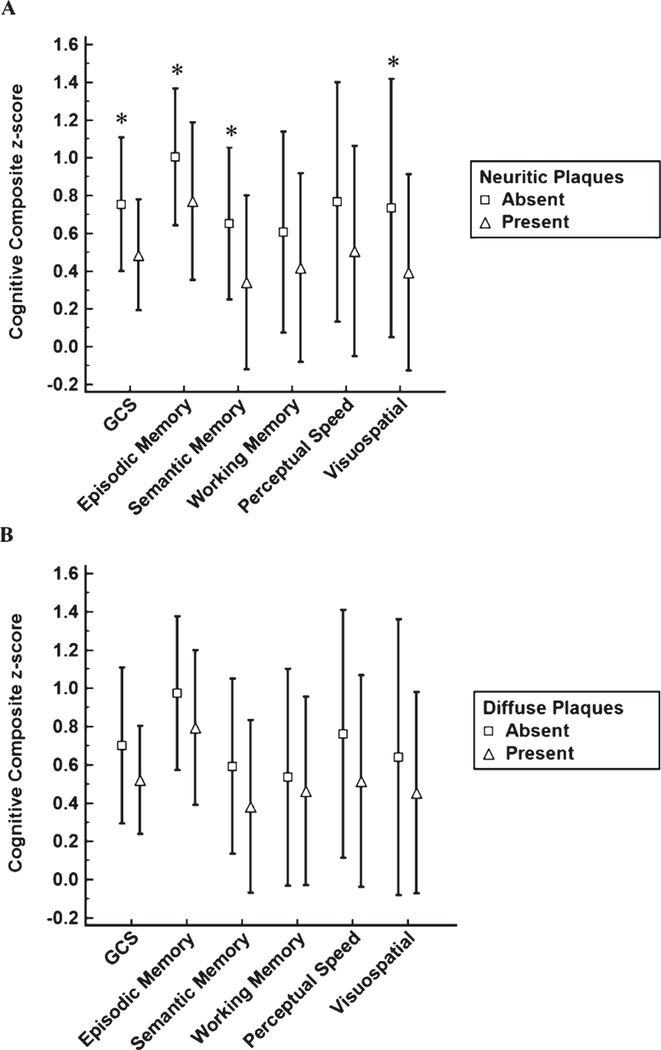
Associations between the cognitive domains and the presence or absence of DPs and NPs are shown in Table 2. GCS (p = 0.001), Episodic Memory (p = 0.03), Semantic Memory (p = 0.008), and Visuospatial (p = 0.004) domains were significantly lower among NCI individuals displaying NPs (Fig. 1A). These associations remained significant after adjusting for multiple comparisons. The effect size for the GCS was larger than that seen for the Episodic Memory, Semantic Memory, and Visuospatial effect sizes. None of the cognitive domains examined showed significant differences between individuals with and without DPs (Fig. 1B). Group differences for the combined presence of NPs and DPs for the cognitive domains examined were nearly identical to the analysis using only NPs (see Table 2).
Table 2.
Cognitive domain associations with diffuse and neuritic plaques
| Diffuse Plaques | ||||
|---|---|---|---|---|
| Cognitive Domain | Present (n = 79) | Absent (n = 44) | p-value | Cohen’s d |
| Global Cognitive Score | 0.52±0.28 | 0.70±0.41 | 0.10 | 0.51 |
| Episodic Memory | 0.80±0.41 | 0.97±0.40 | 0.16 | 0.42 |
| Semantic Memory | 0.38±0.45 | 0.59±0.46 | 0.14 | 0.46 |
| Working Memory | 0.46±0.49 | 0.54±0.57 | 0.72 | 0.15 |
| Perceptual Speed | 0.52±0.55 | 0.76±0.65 | 0.35 | 0.40 |
| Visuospatial | 0.45±0.53 | 0.64±0.72 | 0.24 | 0.30 |
|
Neuritic Plaques | ||||
| Cognitive Domain | Present (n = 77) | Absent (n = 46) | p-value | Cohen’s d |
| Global Cognitive Score | 0.49±0.29 | 0.75±0.35 | 0.001 | 0.81 |
| Episodic Memory | 0.77±0.42 | 1.01±0.36 | 0.03 | 0.62 |
| Semantic Memory | 0.34±0.46 | 0.65±0.40 | 0.008 | 0.72 |
| Working Memory | 0.42±0.50 | 0.61±0.53 | 0.14 | 0.37 |
| Perceptual Speed | 0.51±0.56 | 0.77±0.63 | 0.13 | 0.44 |
| Visuospatial | 0.39±0.52 | 0.73±0.68 | 0.004 | 0.56 |
|
Diffuse and Neuritic Plaques | ||||
| Cognitive Domain | Present (n = 72) | Absent (n = 39) | p-value | Cohen’s d |
| Global Cognitive Score | 0.50±0.29 | 0.77±0.36 | 0.001 | 0.83 |
| Episodic Memory | 0.78±0.42 | 1.02±0.36 | 0.04 | 0.61 |
| Semantic Memory | 0.36±0.45 | 0.65±0.41 | 0.04 | 0.67 |
| Working Memory | 0.44±0.51 | 0.61±0.56 | 0.25 | 0.32 |
| Perceptual Speed | 0.53±0.56 | 0.84±0.62 | 0.06 | 0.52 |
| Visuospatial | 0.41±0.53 | 0.74±0.70 | 0.006 | 0.53 |
Fig. 1.
Cognitive domain differences for neuritic (A) and diffuse (B) plaques. ∗p ≤ 0.05.
Diffuse and neuritic plaque association with Braak stage
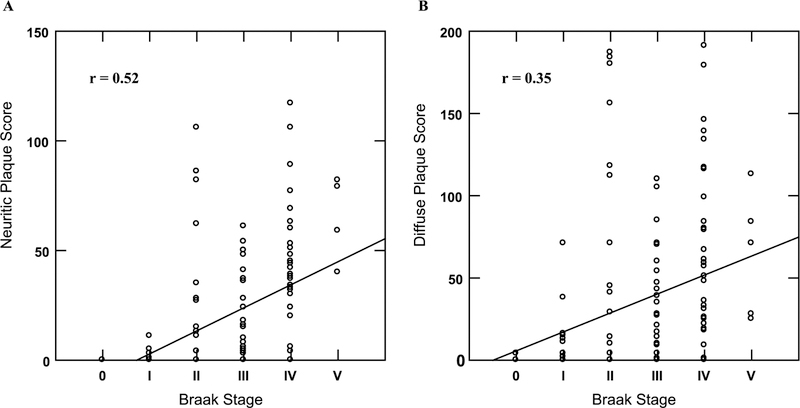
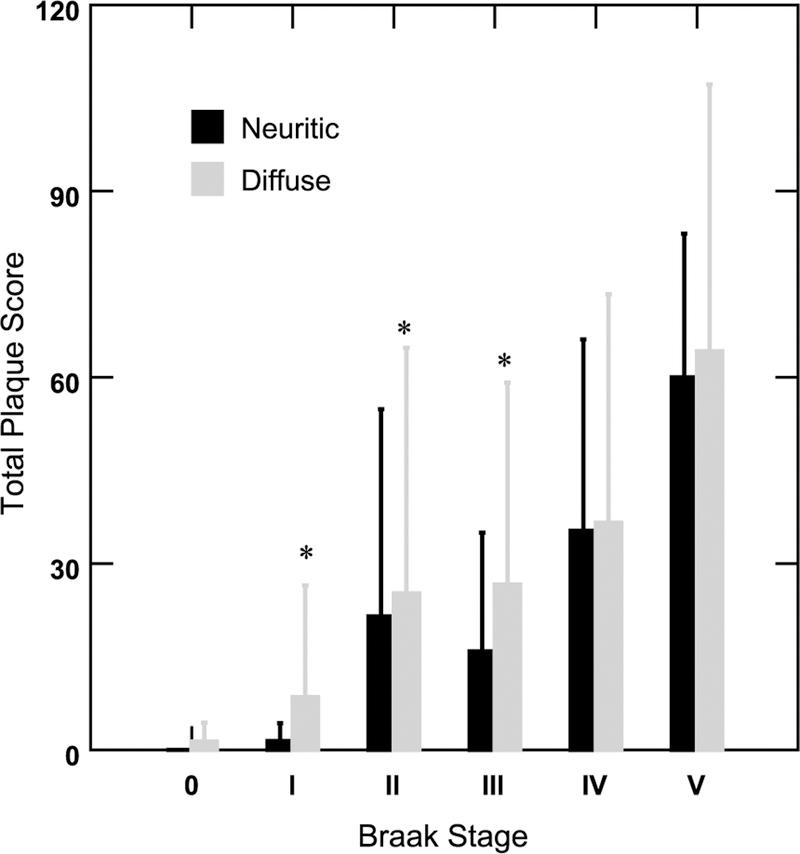
Total NP score was moderately correlated with Braak stage (rho = 0.52, p < 0.001; Fig. 2A) while total DP score showed a weak correlation (rho = 0.35, p < 0.001) with Braak stage (Fig. 2B. Total DP score was significantly greater than total NP score in Braak stages I (p = 0.02), II (p = 0.02), and III (p = 0.04) while there were no significant plaque score differences in the 0, IV, and V Braak stages (Fig. 3). Braak stage was not associated with the presence of DPs [OR = 1.22, (0.82, 1.83), p = 0.33] after adjusting for age at death, education, gender, APOE ε4 status, and the interaction of Braak stage and APOE ε4 status. However, higher Braak stage was significantly associated with the presence of NPs [OR = 1.84, (1.19, 2.83), p = 0.006] after adjusting for age at death, education, gender, APOE ε4 status, and the interaction of Braak stage and APOE ε4 genotype. In each of these models older age at death was significantly associated with an increased likelihood of plaque type (DP: [OR = 1.17, (1.07, 1.27), p < 0.001]; NP: [OR = 1.10, (1.01, 1.19), p = 0.03]).
Fig. 2.
Correlation of Braak stage with total neuritic (A) and total diffuse (B) plaque scores.
Fig. 3.
Total diffuse and neuritic plaque scores by Braak stage. ∗p ≤ 0.05 for neuritic versus diffuse in each Braak stage.
Follow-up analysis of Braak V NCI cases
For the five NCI cases classified in Braak stage V, we carried out an additional analysis to compare their NP and DP pathology with that of AD cases that were Braak stage V. The five NCI cases were matched on age, gender, education, and APOE genotype to AD Braak V cases that came from the same study cohort [2]. NP, DP, and NFT scores for each of the cortical areas examined are shown in Table 3. After adjusting for multiple comparisons no significant differences in NP, DP, or NFT pathology were noted between the Braak V NCI subjects and the Braak V AD cases.
Table 3.
NCI Braak V cases compared with age-, gender-, education-, and APOE genotype-matched AD Braak V cases
| NCI (n = 5) | AD (n = 5) | p-value | |
|---|---|---|---|
| Midfrontal Cortex NPs | 8 [5–18] | 32 [14–48] | 0.03 |
| Midtemporal Cortex NPs | 15 [5–29] | 28 [8–93] | 0.17 |
| Inferior Parietal Cortex NPs | 8 [4–19] | 23 [8–57] | 0.09 |
| Entorhinal Cortex NPs | 8 [1–20] | 15 [3–43] | 0.53 |
| Hippocampal CA1 NPs | 11 [4–23] | 12 [6–37] | 0.75 |
| Midfrontal Cortex DPs | 11 [5–35] | 22 [6–68] | 0.17 |
| Midtemporal Cortex DPs | 20 [6–21] | 31 [2–48] | 0.35 |
| Inferior Parietal Cortex DPs | 8 [4–16] | 21 [6–61] | 0.17 |
| Entorhinal Cortex DPs | 13 [4–24] | 3 [0–17] | 0.11 |
| Hippocampal CA1 DPs | 1 [0–50] | 6 [0–11] | 0.46 |
| Midfrontal Cortex NFTs | 0 [0–1] | 1 [0–10] | 0.42 |
| Midtemporal Cortex NFTs | 5 [4–43] | 9 [6–18] | 0.46 |
| Inferior Parietal Cortex NFTs | 1 [0–2] | 4 [0–20] | 0.16 |
| Entorhinal Cortex NFTs | 32 [3–66] | 36 [18–53] | 0.92 |
| Hippocampal CA1 NFTs | 20 [0–29] | 30 [7–47] | 0.14 |
NPs, neuritic plaques; DPs, diffuse plaques; NFTs, neurofibrillary tangles; median [range]; group differences were analyzed using the Mann-Whitney test; significance level is p ≤ 0.005 after correcting for multiple comparisons.
DISCUSSION
In a cohort of cognitively non-impaired aged individuals, we found that subjects who displayed NPs had significantly lower cognitive test performance across several domains of cognition while DPs were not associated with differences in cognition. This finding is supported by a recent study showing that cognitively normal individuals who progressed to MCI or AD had significantly lower cognitive test scores across several domains when compared to individuals who remained cognitively normal [35]. Others have also found strong associations between the presence of NPs and decreased performance in several cognitive domains [11]. We also found that NPs had a stronger correlation with Braak stage than DPs and that the latter plaque type was more prevalent in Braak stages I, II, and III even when adjusted for age, APOE ε4 carrier status, and Braak stage, all of which are factors related to Aβ deposition [15, 17, 18, 36]. The present significant association between NPs and cognition even after adjusting for APOE ε4 carrier status and Braak stage support previous findings [11, 19, 20, 24]. The significant differences between DPs and NPs within Braak stages is novel and shows that DPs are more prevalent than NPs in early Braak stages (I, II, III). In the present investigation, however, NPs tended to increase and were nearly equal to DPs in later Braak stages (IV, V). Others have reported that neuritic pathology but not diffuse amyloid deposits significantly affect cognition in AD supporting a difference between NP and DP deposition on cognitive ability [37]. It should be noted that DPs are a common, non-specific lesion among cognitively normal older adults that are not associated with APOE ε4 genotype [38]. Moreover, the observation of a significant increase in NPs, but not NFTs, in patients with mild AD compared with normal control cases further suggests that NPs play a key role in the earliest onset of AD symptoms [39]. Although others report that amyloid load was not related to cognitive status [7, 26], these studies did not differentiate plaque types making a direct comparison with other findings difficult.
The analyses using Braak stage as a predictor of the presence or absence of NPs and DPs provide additional evidence supporting the observation that NFT pathology is associated with NPs and not DPs. Although it has been suggested that NPs mediate the association between APOE status and NFT pathology, other age-related non-amyloid process may also play a role in NFT pathology [15]. In this regard, NPs display extracellular Aβ, tau [24], and cholinergic, noradrenergic, and other types of neurites [40–44] suggesting that the association between NPs and cognition is likely due to an interaction of multiple axonal defects [24, 36, 41]. In addition, complement receptor 1 (CR1) has been shown to be significantly associated with decline in several cognitive domains after controlling for several factors, including APOE genotype [45]. Although this association is thought to be mediated by both DPs and NPs, NFTs were not associated with CR1 and cognitive decline suggesting that immunological mechanisms may also play a significant role in the relationship between cognition and plaque pathology [45].
Several studies have noted that increased Aβ deposition is associated with APOE ε4 carrier status in pre-clinical AD [46–49], which supports the rationale for ongoing AD prevention trials using anti-Aβ agents as disease-modifiers [50, 51]. Although, the hypothesis that increased deposition of Aβ1‒42 precedes NFT accumulation [52] has also been used to support the rationale for anti-Aβ therapies, others have suggested that these therapies may not be effective once tau-related neurodegeneration has been initiated [53]. Although tau hyperphosphorylation is thought to be a downstream effect of Aβ deposition [54], it is currently being investigated as a therapeutic target [53, 55]. As the conduct of AD clinical trials has shifted to a paradigm of prevention in asymptomatic individuals at risk for developing AD [56], the relationship between pathological changes and subsequent cognitive decline has taken on greater importance. The observation in the present study of a significant inverse association between cognition and NPs suggests that the desired clinical benefits of anti-Aβ therapies may be achieved through increased binding specificity to NPs. However, this assumes that a therapy initiated early in the disease process would prevent the onset of tau-related degenerative processes induced by Aβ deposition. Bilousova et al. [57] found that oligomeric Aβ deposition within synaptic terminals within the parietal cortex drives the deposition of phosphorylated tau and is associated with the onset of AD, suggesting that therapeutic agents targeting synaptic Aβ oligomers early in the disease process may slow disease onset and trajectory. Moreover, this study also revealed high levels of Aβ oligomers in early AD compared to non-demented cases with histopathologic signs of AD-related pathology suggesting that dementia arises when Aβ oligomers within synaptic terminals reach a certain level [58]. In the present study, we found that NPs affected cognitive domain performance more than DPs and that NPs were related to higher Braak scores, suggesting that the development of NPs may coincide with higher levels of Aβ oligomers and tau phosphorylation at the synapse even before frank dementia occurs.
Recently, we reported that medial temporal lobe NFT pathology does not correlate with cognitive test performance in older persons without cognitive decline [21], suggesting that tangle pathology alone is not necessary or sufficient to induce impairments in cognition. In this regard, neuropathological observations have shown that amyloid pathology begins in the frontal cortex and spreads caudally within the cortex [30], whereas tau pathology is initiated in the medial temporal lobe [30], suggesting a discordance between the location of amyloid and its action as a putative initiator of NFT tau pathology. However, it is possible that oligomeric amyloid initiates tau signaling via either an antero- or retrograde transneuronal seeding process similar to the regional propagation of tau in human disease and animal models [58, 59] as Aβ first appears in neocortical regions that have extensive reciprocal connections with the medial temporal lobe [60, 61]. Levels of dimeric Aβ derived from AD cortex are sufficient for the induction of tau phosphorylation and dystrophic axonal profiles [38]. Together these findings lend support to the concept of a prion-mediated mechanism of AD disease pathogenesis [62]. It remains to be determined whether synaptic Aβ oligomers expression is increased and if this change would be associated with cognitive performance in the current cohort of cases.
Amyloid pathology is not always a pathogenic trigger for tau pathology. For example, primary age-related tauopathy (PART) is a neuropathological entity that can be distinguished from AD based on minimal, or complete absence of Aβ burden in individuals with significant NFT pathology [63]. However, others have suggested that PART represents a portion of the AD spectrum and is not distinguishable from AD [64]. Cases meeting neuropathologic criteria for PART display more advanced age at death and more severe forms of PART (higher Braak stage) display significantly lower cognitive scores [65]. PART is one example among many non-AD tauopathies without amyloid deposition suggesting that NFT pathology is not consistently a downstream effect of Aβ deposition in all tauopathies [66]. Tau pathology can be attributed to other factors not related to AD such as central nervous system infections, metabolic dysfunction, and physical injury (e.g., concussion) [67].
Another key factor that may play a role in the lack of frank cognitive impairment in the face of amyloid deposition in our population is the concept of brain reserve or cognitive reserve [18, 63]. Brain reserve suggests that some older individuals have a greater quantity of either neurons or synapses at disease onset allowing the continued performance of cognitive tasks [18, 63]. On the other hand, cognitive reserve involves the recruitment of other brain areas not severely affected by the disease process to aid in task performance [68]. Education level is a factor that may affect brain/cognitive reserve and the onset of dementia [69, 70]. In fact, Roe and colleagues [16] found a significant interaction between education level and NP densities for risk of dementia. When their sample was divided into three education levels (0–12 years, 13–16 years, ≥17 years), individuals with high levels of education and high levels of NP density (Frequent) were more likely to have dementia compared to those with the same education level at lower levels of NP density (Moderate and Sparse). From a clinical perspective, higher levels of education are also associated with better performance in a number of cognitive domains [71] suggesting that the protective effect that education confers upon the brain can be observed psychometrically. However, our sample had a relatively high education level regardless of the presence of DPs or NPs. Perhaps cases with a lower level of education and amyloid would have shown a greater degree of cognitive impairment. Our analysis of NCI cases but with high NFT pathology (Braak stage V) showed that these individuals displayed NPs, DPs, and NFT pathology at levels comparable to clinical AD subjects. This finding suggests that brain/cognitive reserve may play a role early in delaying the onset of AD up to a point but it is not able to offset cognitive impairment in patients with more advanced pathology suggesting the involvement of other factors.
The underlying mechanisms and pathways that drive the formation AD pathology are not completely understood and the likelihood of interactions adds a significant layer of complexity when trying to understand how these factors affect cognition. Whether Aβ deposition marks the onset of clinical AD or whether it is a by-product of other neurodegenerative processes has been debated in the field [72]. A number of proposed pathways that may play a role in the deposition of Aβ include inflammation [73], insulin resistance [74], and vascular dysfunction [75]. In fact, vascular disease strongly correlates with the onset of AD [76, 77]. The cases examined here were screened for vascular defects that would have effected cognition and not included in this study.
There are some limitations to this study. Subjects were from a community-based group of highly educated retired clergy who had excellent health care and nutrition and were used in multiple clinical pathological [78] and epidemiological investigations [33]. Individuals who volunteer may introduce bias by decreasing pathology but this is partially mitigated by high follow-up and autopsy rates [28]. None of the cases evaluated were Braak stage VI, which is highly associated with AD-dementia [30]. Although subtle changes in neuropsychological testing may not detect cognitive changes in non-demented people [79], our cognitive tests are standard in the dementia field [25]. Another limitation is the relatively small number of APOE ε4 carriers, particularly homozygous individuals and the impact this may have on the associations we reported. Future studies with a greater balance of APOE ε4 carriers and non-carriers will extend the results reported here. Strengths include uniform premortem clinical and postmortem pathological evaluation and that the final pathologic classification was performed without knowledge of clinical evaluation.
The results of this study found that alteration in cognitive performance seen in our NCI subjects is likely attributed to increases in NPs and not DPs when age, education, APOE ε4 status, and Braak stage are taken into consideration. With regard to NFT pathology, NPs show a stronger association with Braak stage than DPs. These results suggest that NPs confer a negative impact on both NFT pathology and cognition relative to DPs and may represent a more specific therapeutic target for therapies in preclinical AD.
ACKNOWLEDGMENTS
We thank the participants in the Rush Religious Order Study and members of the Rush ADC. Supported by National Institute on Aging grants PO1AG14449, P30AG10161, P30AG019610, RO1AG43775 and Barrow Neurological Institute Barrow and Beyond.
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/16-0365r2).
REFERENCES
- [1].Tomlinson BE, Blessed G, Roth M (1968) Observations on the brains of non-demented old people. J Neurol Sci 7, 331–356. [DOI] [PubMed] [Google Scholar]
- [2].Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS (2006) Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 66, 1837–1844. [DOI] [PubMed] [Google Scholar]
- [3].Dickson DW, Crystal HA, Mattiace LA, Masur DM, Blau AD, Davies P, Yen SH, Aronson MK (1992) Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol Aging 13, 179–189. [DOI] [PubMed] [Google Scholar]
- [4].Mormino EC (2014) The relevance of beta-amyloid on markers of Alzheimer’s disease in clinically normal individuals and factors that influence these associations. Neuropsychol Rev 24, 300–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Morris JC, Storandt M, McKeel DW Jr, Rubin EH, Price JL, Grant EA, Berg L (1996) Cerebral amyloid deposition and diffuse plaques in “normal” aging: Evidence for presympomatic and very mild Alzheimer’s disease. Neurology 46, 707–719. [DOI] [PubMed] [Google Scholar]
- [6].Green MS, Kaye JA, Ball MJ (2000) The Oregon brain aging study: Neuropathology accompanying healthy aging in the oldest old. Neurology 54, 105–113. [DOI] [PubMed] [Google Scholar]
- [7].Guillozet AL, Weintraub S, Mash DC, Mesulam MM (2003) Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch Neurol 60, 729–736. [DOI] [PubMed] [Google Scholar]
- [8].Jicha GA, Abner EL, Schmitt FA, Kryscio RJ, Riley KP, Cooper GE, Stiles N, Mendiondo MS, Smith CD, Van Eldik LJ, Nelson PT (2012) Preclinical AD Workgroup staging: Pathological correlates and potential challenges. Neurobiol Aging 33, 622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jack CR Jr, Knopman DS, Weigand SD, Wiste HJ, Vemuri P, Lowe V, Kantarci K, Gunter JL, Senjem ML, Ivnik RJ, Roberts RO, Rocca WA, Boeve BF, Petersen RC (2012) An operational approach to National Institute on Aging- Alzheimer’s Association criteria for preclinical Alzheimer disease. Ann Neurol 71, 765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rodrigue KM, Kennedy KM, Devous MD Sr, Rieck JR, Hebrank AC, Diaz-Arrastia R, Mathews D, Park DC (2012) beta-Amyloid burden in healthy aging: Regional distribution and cognitive consequences. Neurology 78, 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dowling NM, Tomaszewski FS, Reed BR, Sonnen JA, Strauss ME, Schneider JA, Bennett DA, Mungas D (2011) Neuropathological associates of multiple cognitive functions in two community-based cohorts of older adults. J Int Neuropsychol Soc 17, 602–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Braskie MN, Klunder AD, Hayashi KM, Protas H, Kepe V, Miller KJ, Huang SC, Barrio JR, Ercoli LM, Siddarth P, Satyamurthy N, Liu J, Toga AW, Bookheimer SY, Small GW, Thompson PM (2010) Plaque and tangle imaging and cognition in normal aging and Alzheimer’s disease. Neurobiol Aging 31, 1669–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del TK, Duyckaerts C, Frosch MP, Haroutunian V, Hof PR, Hulette CM, Hyman BT, Iwatsubo T, Jellinger KA, Jicha GA, Kovari E, Kukull WA, Leverenz JB, Love S, Mackenzie IR, Mann DM, Masliah E, McKee AC, Montine TJ, Morris JC, Schneider JA, Sonnen JA, Thal DR, Trojanowski JQ, Troncoso JC, Wisniewski T, Woltjer RL, Beach TG (2012) Correlation of Alzheimer disease neuropathologic changes with cognitive status: A review of the literature. J Neuropathol Exp Neurol 71, 362–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hedden T, Oh H, Younger AP, Patel TA (2013) Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology 80, 1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mungas D, Tractenberg R, Schneider JA, Crane PK, Bennett DA (2014) A 2-process model for neuropathology of Alzheimer’s disease. Neurobiol Aging 35, 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Roe CM, Xiong C, Miller JP, Cairns NJ, Morris JC (2008) Interaction of neuritic plaques and education predicts dementia. Alzheimer Dis Assoc Disord 22, 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Farfel JM, Yu L, De Jager PL, Schneider JA, Bennett DA (2016) Association of APOE with tau-tangle pathology with and without beta-amyloid. Neurobiol Aging 37, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sabbagh MN, Malek-Ahmadi M, Dugger BN, Lee K, Sue LI, Serrano G, Walker DG, Davis K, Jacobson SA, Beach TG (2013) The influence of Apolipoprotein E genotype on regional pathology in Alzheimer’s disease. BMC Neurol 13, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bancher C, Jellinger K, Lassmann H, Fischer P, Leblhuber F (1996) Correlations between mental state and quantitative neuropathology in the Vienna Longitudinal Study on Dementia. Eur Arch Psychiatry Clin Neurosci 246, 137–146. [DOI] [PubMed] [Google Scholar]
- [20].Nelson PT, Jicha GA, Schmitt FA, Liu H, Davis DG, Mendiondo MS, Abner EL, Markesbery WR (2007) Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: Neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol 66, 1136–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mufson EJ, Malek-Ahmadi M, Perez SE, Chen K (2016) Braak staging, plaque pathology, and APOE status in elderly persons without cognitive impairment. Neurobiol Aging 37, 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, Bi W, Cohen AD, Lopresti BJ, DeKosky ST, Halligan EM, Klunk WE (2008) Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol 65, 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lockhart A, Lamb JR, Osredkar T, Sue LI, Joyce JN, Ye L, Libri V, Leppert D, Beach TG (2007) PIB is a non-specific imaging marker of amyloid-beta (Abeta) peptide-related cerebral amyloidosis. Brain 130, 2607–2615. [DOI] [PubMed] [Google Scholar]
- [24].Nelson PT, Braak H, Markesbery WR (2009) Neuropathology and cognitive impairment in Alzheimer disease: A complex but coherent relationship. J Neuropathol Exp Neurol 68, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bennett DA, Schneider JA, Buchman AS, Mendes de LC, Bienias JL, Wilson RS (2005) The Rush Memory and Aging Project: Study design and baseline characteristics of the study cohort. Neuroepidemiology 25, 163–175. [DOI] [PubMed] [Google Scholar]
- [26].Mufson EJ, Chen EY, Cochran EJ, Beckett LA, Bennett DA, Kordower JH (1999) Entorhinal cortex beta-amyloid load in individuals with mild cognitive impairment. Exp Neurol 158, 469–490. [DOI] [PubMed] [Google Scholar]
- [27].Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE (2004) Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol 61, 378–384. [DOI] [PubMed] [Google Scholar]
- [28].Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA (2009) The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis 18, 691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mirra SS (1997) The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer’s disease: A commentary. Neurobiol Aging 18, S91–S94. [DOI] [PubMed] [Google Scholar]
- [30].Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82, 239–259. [DOI] [PubMed] [Google Scholar]
- [31].The National Institute on Aging and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease (1997) Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol Aging 18, S1–S2. [PubMed] [Google Scholar]
- [32].Mitchell TW, Nissanov J, Han LY, Mufson EJ, Schneider JA, Cochran EJ, Bennett DA, Lee VM, Trojanowski JQ, Arnold SE (2000) Novel method to quantify neuropil threads in brains from elders with or without cognitive impairment. J Histochem Cytochem 48, 1627–1638. [DOI] [PubMed] [Google Scholar]
- [33].Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J (2002) Natural history of mild cognitive impairment in older persons. Neurology 59, 198–205. [DOI] [PubMed] [Google Scholar]
- [34].Wilson RS, Leurgans SE, Boyle PA, Bennett DA (2011) Cognitive decline in prodromal Alzheimer disease and mild cognitive impairment. Arch Neurol 68, 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hassenstab J, Monsell SE, Mock C, Roe CM, Cairns NJ, Morris JC, Kukull W (2015) Neuropsychological markers of cognitive decline in persons With Alzheimer disease neuropathology. J Neuropathol Exp Neurol 74, 1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Oh H, Madison C, Villeneuve S, Markley C, Jagust WJ (2014) Association of gray matter atrophy with age, beta amyloid, and cognition in aging. Cereb Cortex 24, 1609–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Nagy Z, Esiri MM, Jobst KA, Morris JH, King EM, McDonald D, Litchfield S, Barnetson L (1996) Clustering of pathological features in Alzheimer’s disease: Clinical and neuroanatomical aspects. Dementia 7, 121–127. [DOI] [PubMed] [Google Scholar]
- [38].Jin M, Shepardson N, Yang T, Chen G, Walsh D, Selkoe DJ (2011) Soluble amyloid beta-protein dimers isolated from Alzheimer cortex directly induce tau hyperphosphorylation and neuritic degeneration. Proc Natl Acad Sci U S A 108, 5819–5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J (2004) The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology 62, 1984–1989. [DOI] [PubMed] [Google Scholar]
- [40].Besson FL, La JR, Doeuvre L, Gaubert M, Mezenge F, Egret S, Landeau B, Barre L, Abbas A, Ibazizene M, de LS V, Desgranges B, Eustache F, Chetelat G (2015) Cognitive and brain profiles associated with current neuroimaging biomarkers of preclinical Alzheimer’s disease. J Neurosci 35, 10402–10411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Masters CL, Selkoe DJ (2012) Biochemistry of amyloid beta-protein and amyloid deposits in Alzheimer disease. Cold Spring Harb Perspect Med 2, a006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Carvajal FJ, Inestrosa NC (2011) Interactions of AChE with Abeta aggregates in Alzheimer’s brain: Therapeutic relevance of IDN 5706. Front Mol Neurosci 4, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Geula C, Mesulam MM (1995) Cholinesterases and the pathology of Alzheimer disease. Alzheimer Dis Assoc Disord 9(Suppl 2), 23–28. [DOI] [PubMed] [Google Scholar]
- [44].Mesulam MM, Geula C (1994) Butyrylcholinesterase reactivity differentiates the amyloid plaques of aging from those of dementia. Ann Neurol 36, 722–727. [DOI] [PubMed] [Google Scholar]
- [45].Chibnik LB, Shulman JM, Leurgans SE, Schneider JA, Wilson RS, Tran D, Aubin C, Buchman AS, Heward CB, Myers AJ, Hardy JA, Huentelman MJ, Corneveaux JJ, Reiman EM, Evans DA, Bennett DA, De Jager PL (2011) CR1 is associated with amyloid plaque burden and age-related cognitive decline. Ann Neurol 69, 560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA (2010) APOE predicts amyloid beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol 67, 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Mormino EC, Betensky RA, Hedden T, Schultz AP, Ward A, Huijbers W, Rentz DM, Johnson KA, Sperling RA (2014) Amyloid and APOE epsilon4 interact to influence short- term decline in preclinical Alzheimer disease. Neurology 82, 1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lim YY, Villemagne VL, Pietrzak RH, Ames D, Ellis KA, Harrington K, Snyder PJ, Martins RN, Masters CL, Rowe CC, Maruff P (2015) APOE epsilon4 moderates amyloid- related memory decline in preclinical Alzheimer’s disease. Neurobiol Aging 36, 1239–1244. [DOI] [PubMed] [Google Scholar]
- [49].Thai C, Lim YY, Villemagne VL, Laws SM, Ames D, Ellis KA, Rainey-Smith SR, Martins RN, Masters CL, Rowe CC, Maruff P (2015) Amyloid-related memory decline in pre- clinical Alzheimer’s disease is dependent on APOE epsilon4 and is detectable over 18-Months. PLoS One 10, e0139082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Reiman EM, Langbaum JB, Tariot PN (2010) Alzheimer’s prevention initiative: A proposal to evaluate presymptomatic treatments as quickly as possible. Biomark Med 4, 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sperling RA, Rentz DM, Johnson KA, Karlawish J, Donohue M, Salmon DP, Aisen P (2014) The A4 study: Stopping AD before symptoms begin? Sci Transl Med 6, 228fs13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hu X, Li X, Zhao M, Gottesdiener A, Luo W, Paul S (2014) Tau pathogenesis is promoted by Abeta1–42 but not Abeta1–40. Mol Neurodegener 9, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yoshiyama Y, Lee VM, Trojanowski JQ (2013) Therapeutic strategies for tau mediated neurodegeneration. J Neurol Neurosurg Psychiatry 84, 784–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wolfe MS (2012) The role of tau in neurodegenerative diseases and its potential as a therapeutic target. Scientifica (Cairo) 2012, 796024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Himmelstein DS, Ward SM, Lancia JK, Patterson KR, Binder LI (2012) Tau as a therapeutic target in neurodegenerative disease. Pharmacol Ther 136, 8–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Langbaum JB, Fleisher AS, Chen K, Ayutyanont N, Lopera F, Quiroz YT, Caselli RJ, Tariot PN, Reiman EM (2013) Ushering in the study and treatment of preclinical Alzheimer disease. Nat Rev Neurol 9, 371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bilousova T, Miller CA, Poon WW, Vinters HV, Corrada M, Kawas C, Hayden EY, Teplow DB, Glabe C, Albay R III, Cole GM, Teng E, Gylys KH (2016) Synaptic amyloid-beta oligomers precede p-tau and differentiate high pathology control cases. Am J Pathol 186, 185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Jucker M, Walker LC (2011) Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann Neurol 70, 532–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].de Calignon A, Polydoro M, Sua´rez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, Pitstick R, Sahara N, Ashe KH, Carlson GA, Spires-Jones TL, Hyman BT (2012) Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron 73, 685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Thal DR, Ru¨b U, Orantes M, Braak H (2002) Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 58, 1791–1800. [DOI] [PubMed] [Google Scholar]
- [61].Na¨slund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, Buxbaum JD (2000) Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA 283, 1615–1617. [DOI] [PubMed] [Google Scholar]
- [62].Walker LC, Jucker M (2015) Neurodegenerative diseases: Expanding the prion concept. Annu Rev Neurosci 38, 87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Jellinger KA, Alafuzoff I, Attems J, Beach TG, Cairns NJ, Crary JF, Dickson DW, Hof PR, Hyman BT, Jack CR Jr, Jicha GA, Knopman DS, Kovacs GG, Mackenzie IR, Masliah E, Montine TJ, Nelson PT, Schmitt F, Schneider JA, Serrano-Pozo A, Thal DR, Toledo JB, Trojanowski JQ, Troncoso JC, Vonsattel JP, Wisniewski T (2015) PART, a distinct tauopathy, different from classical sporadic Alzheimer disease. Acta Neuropathol 129, 757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Duyckaerts C, Braak H, Brion JP, Buee L, Del TK, Goedert M, Halliday G, Neumann M, Spillantini MG, Tolnay M, Uchihara T (2015) PART is part of Alzheimer disease. Acta Neuropathol 129, 749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, Arnold SE, Attems J, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Gearing M, Grinberg LT, Hof PR, Hyman BT, Jellinger K, Jicha GA, Kovacs GG, Knopman DS, Kofler J, Kukull WA, Mackenzie IR, Masliah E, McKee A, Montine TJ, Murray ME, Neltner JH, Santa Maria I, Seeley WW, Serrano-Pozo A, Shelanski ML, Stein T, Takao M, Thal DR, Toledo JB, Troncoso JC, Vonsattel JP, White CL III, Wisniewski T, Woltjer RL, Yamada M, Nelson PT (2014) Primary age-related tauopathy (PART): A common pathology associated with human aging. Acta Neuropathol 128, 755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Dickson DW (2009) Neuropathology of non-Alzheimer degenerative disorders. Int J Clin Exp Pathol 3, 1–23. [PMC free article] [PubMed] [Google Scholar]
- [67].Giaccone G (2015) The existence of primary age-related tauopathy suggests that not all the cases with early Braak stages of neurofibrillary pathology are Alzheimer’s disease. J Alzheimers Dis 48, 919–921. [DOI] [PubMed] [Google Scholar]
- [68].Stern Y (2012) Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 11, 1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Roe CM, Xiong C, Miller JP, Morris JC (2007) Education and Alzheimer disease without dementia: Support for the cognitive reserve hypothesis. Neurology 68, 223–228. [DOI] [PubMed] [Google Scholar]
- [70].Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R (1994) Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA 271, 1004–1010. [PubMed] [Google Scholar]
- [71].Opdebeeck C, Martyr A, Clare L (2016) Cognitive reserve and cognitive function in healthy older people: A meta- analysis. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 23, 40–60. [DOI] [PubMed] [Google Scholar]
- [72].Morris GP, Clark IA, Vissel B (2014) Inconsistencies and controversies surrounding the amyloid hypothesis of Alzheimer’s disease. Acta Neuropathol Commun 2, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Martins IJ, Hone E, Foster JK, Sunram-Lea SI, Gnjec A, Fuller SJ, Nolan D, Gandy SE, Martins RN (2006) Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer’s disease and cardiovascular disease. Mol Psychiatry 11, 721–736. [DOI] [PubMed] [Google Scholar]
- [74].Kulstad JJ, Green PS, Cook DG, Watson GS, Reger MA, Baker LD, Plymate SR, Asthana S, Rhoads K, Mehta PD, Craft S (2006) Differential modulation of plasma beta- amyloid by insulin in patients with Alzheimer disease. Neurology 66, 1506–1510. [DOI] [PubMed] [Google Scholar]
- [75].Grammas P (2011) Neurovascular dysfunction, inflammation and endothelial activation: Implications for the pathogenesis of Alzheimer’s disease. J Neuroinflammation 8, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kling MA, Trojanowski JQ, Wolk DA, Lee VM, Arnold SE (2013) Vascular disease and dementias: Paradigm shifts to drive research in new directions. Alzheimers Dement 9, 76–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lorius N, Locascio JJ, Rentz DM, Johnson KA, Sperling RA, Viswanathan A, Marshall GA (2015) Vascular disease and risk factors are associated with cognitive decline in the alzheimer disease spectrum. Alzheimer Dis Assoc Disord 29, 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Mufson EJ, Binder L, Counts SE, DeKosky ST, de Toledo- Morrell L, Ginsberg SD, Ikonomovic MD, Perez SE, Scheff SW (2012) Mild cognitive impairment: Pathology and mechanisms. Acta Neuropathol 123, 13–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Smith GE, Pankratz VS, Negash S, Machulda MM, Petersen RC, Boeve BF, Knopman DS, Lucas JA, Ferman TJ, Graff- Radford N, Ivnik RJ (2007) A plateau in pre-Alzheimer memory decline: Evidence for compensatory mechanisms? Neurology 69, 133–139. [DOI] [PubMed] [Google Scholar]