Abstract
Background
The high prevalence of cervical cancer at safety-net health systems requires careful analysis to best inform prevention and quality improvement efforts. We characterized cervical cancer burden and identified opportunities for prevention in a U.S. safety-net system.
Methods
We reviewed tumor registry and electronic health record (EHR) data of women with invasive cervical cancer ages 18+ diagnosed 2010–2015 in a large, integrated urban safety-net. We developed an algorithm to: (a) classify whether women had been engaged in care (≥1 clinical encounter between 6 months and 5 years before cancer diagnosis); and (b) identify missed opportunities (no screening, no follow-up, failure of a test to detect cancer, treatment failure) and associated factors among engaged patients.
Results
Of 419 women with cervical cancer, more than half (58%) were stage 2B or higher at diagnosis and 40% were uninsured. Most (69%) had no prior healthcare system contact; 47% were diagnosed elsewhere. Among 122 engaged in care prior to diagnosis, failure to screen was most common (63%), followed by lack of follow-up (21%), and failure of test to detect cancer (16%). Tumor stage, patient characteristics, and healthcare utilization differed across groups.
Conclusions
Safety-net healthcare systems face a high cervical cancer burden, mainly from women with no prior contact with the system. To prevent or detect cancer early, community-based efforts should encourage uninsured women to use safety-nets for primary care and preventive services.
Impact
Among engaged patients, strategies to increase screening and follow-up of abnormal screening tests could prevent over 80% of cervical cancer cases.
Keywords: Missed opportunities, prevention, cervical cancer, Pap smear, case review
INTRODUCTION
In the U.S., cervical cancer is preventable because precursor lesions can be identified through screening, additional diagnostic testing, and treated or monitored as indicated.[1] Screening programs have made cervical cancer an uncommon disease with diminishing incidence and mortality.[2, 3] However, significant disparities in screening exist for women who are racial/ethnic minorities, live in poverty, and have limited education and access to care.[4, 5]. Understanding whether these groups are also less likely to have diagnostic follow up after an abnormal screen result and receive treatment is limited by a lack of data. Most observational studies of follow-up are from privately insured populations receiving care from integrated healthcare systems.[6, 7] Less is known about follow-up among racial/ethnic minorities and within safety-net health systems that disproportionately care for disadvantaged women.[8, 9]
Studies from developed countries characterizing screening programs and contributors to cervical cancer occurrence are inadequate to inform quality improvement (QI) in safety-net systems for four key reasons: (1) Most were published before introduction of high-risk human papillomavirus (hrHPV) testing, which gave rise to multiple changes in screening and management guidelines since 2001;[10] (2) Many studies either predate adoption of electronic health records (EHRs) or rely on registry or claims data;[7, 11–17] (3) Only a few studies reviewed opportunities for improvement beyond initial Pap screening;[12, 18–20] most ignore subsequent steps in the screening process (diagnostic follow-up, treatment, and surveillance); and (4) The majority of studies are conducted among White women and those with commercial insurance, particularly those in health maintenance organizations (HMOs) with organized screening programs.[16, 17, 21] To our knowledge, like the majority of all U.S. settings[22, 23], most safety-net systems and clinics still deliver screening opportunistically. Thus, case reviews have significant potential to inform QI and improve delivery of the screening process in safety-net settings. We conducted this retrospective case series to:
-
(1)
Characterize women diagnosed with cervical cancer and their processes of care including varied pathways into a large, urban, safety-net healthcare system–e.g., whether they had been previously engaged in care in the system at the time of cancer diagnosis; and
-
(2)
(2) Identify healthcare system opportunities to improve the screening process through a correlates analysis of lack of screening and follow-up among women engaged in care.
MATERIALS AND METHODS
Study Setting and Screening Policies
This study was conducted at the Parkland Health and Hospital System (Parkland), a publicly funded safety-net healthcare system in Dallas, TX. Parkland includes a 862-bed hospital, 12 community-based adult primary care clinics, 11 women’s health clinics, and other specialty clinics, all connected by a single comprehensive EHR. Each year, Parkland provides care and offers cervical cancer screening to approximately 84,000 women yearly through its primary care and women’s health clinics. Parkland accepts commercial insurance plans, participates in federal-state programs (e.g., National Breast and Cervical Cancer Early Detection Program [NBCCEDP]) that pay for screening and diagnostic services, and administers a county-funded sliding fee scale medical assistance program for uninsured and underinsured Dallas County residents.
The delivery of the cervical screening process in all health systems is shaped by organizational factors and systems, funding sources, and resource constraints. Parkland is disproportionately dependent on government payers, which impacts delivery of care in multiple ways. For example, at the time this study was conducted, the NBCCEDP in Texas did not reimburse for genotyping or for cotests. Parkland, like all health systems, implements changes to the screening process in response to evolving guidelines. From 2010 to 2015 (study period), Pap alone every 2 to 3 years was the predominant screening strategy. However, providers were free to choose between Pap or co-testing (Pap plus hrHPV). Abnormal results were sent to performing providers’ EHR in-baskets; providers electronically referred those needing follow-up to a gynecology dysplasia clinic. The EHR health maintenance section alerted providers when cervical cancer screening was due for patients. In addition, cervical cancer screening is a clinical quality metric and providers were given monthly reports on screening rates for established patients seen that month.
Data Collection and Study Population
Data were obtained from the Parkland tumor registry and EHR. We used the registry to identify adult (ages 18 and older) women diagnosed with primary cervical cancer from 2010 to 2015. A board-certified gynecologist (CW) reviewed patients’ EHRs to verify primary cervical (i.e., not endometrial) origin. The EHR was then searched for outcome variables and covariates occurring between 6 months and 5 years prior to cervical cancer diagnosis date (hereafter the study window; Figure 1). Data collection focused on the screening process; thus, we excluded tests and procedures during the diagnostic workup period, defined as the 6 months prior to diagnosis.[12, 13, 19, 21, 24] Diagnostic procedures and results occurring prior to this time were abstracted.
Figure 1.
Timeline of retrospective data collection. Figure 1 includes 6-month diagnostic work-up period and overall 5-year study window in relation to date of cancer diagnosis.
Outcome: Identifying Prevention Opportunities
Using an iterative approach, we developed an algorithm applied to EHR data to classify two outcomes reflecting opportunity to prevent cervical cancer (Figure S1 Online Supplement). First, we classified engagement in care. A woman was defined as engaged in care if she had ≥1 inpatient, outpatient, or emergency department [ED] encounter between 6 months and 5 years before cancer diagnosis. We defined women as engaged in care, regardless of the type or location (e.g., ED) of care they had previously received because for all these women, Parkland had some opportunity either to provide screening or to refer the patient to primary care, the primary setting in which screening is delivered. In contrast, new patients presented to Parkland <6 months before diagnosis with a pre-existent cervical cancer. Second, among engaged patients who were screening-age eligible (<70 years at diagnosis, thus <65 years at some time during the study period), we categorized efforts to deliver the screening process and results of those tests/procedures into one of four mutually-exclusive categories in this order (see Table 1 for definitions):
Table 1.
Definitions of opportunities to prevent cervical cancer.
| Missed Opportunity | Definition |
|---|---|
| Did not receive screening | (1) Did not have a screening Pap or co-test within the study window or (2) did not have a screening Pap or co- test 3.5 years after a NILM Pap or ASC-US/HPV- result within the 5-year study window (if time permitted) given current recommendations for 3-year screening intervals. |
| Did not receive follow-up after an abnormal screening or diagnostic test | Had 1 or more Pap or co-test with an abnormal result (ASC-US/HPV+ or worse) and (1) did not have a colposcopy within 1 year after the first abnormal screening test in the study window, or (2) did not have a treatment procedure within 6 months after a colposcopy result of CIN 2/3 or greater, or (3) did not have a colposcopy within 1 to 1.5 years after a treatment during the study window (if time permitted). |
| Test(s) failure to detect a cervical abnormality (CIN 2/3 or cancer) | Developed cervical cancer subsequent to: (1) NILM Pap or negative co-tests during the study window, or (2) received a colposcopy/biopsy result less than CIN 2/3 within 1 year after first abnormal screening test in the study window. |
| Treatment failure | Developed cervical cancer within 1.5 years after receiving excisional or ablative treatment and surveillance as indicated. |
NILM= negative for intraepithelial lesion and malignancy; CIN=cervical intraepithelial neoplasia; ASC-US=atypical squamous cells of undetermined significance; HPV=human papillomavirus
-
1)
did not receive screening;
-
2)
did not receive follow-up after an abnormal screening test;
-
3)
developed cervical cancer subsequent to a negative screening or diagnostic test (failure to detect); or
4) developed cervical cancer after excisional or ablative treatment (treatment failure).
Covariates
We measured patient, tumor, and process of care characteristics. Patient characteristics included age, race/ethnicity, county of residence (Dallas or elsewhere), and year of diagnosis. Charlson comorbidity index[25] (excluding tumors) was assessed at cancer diagnosis for patients engaged in care. Insurance was classified as uninsured (including self-pay and charity care), Medicaid, Medicare, other government payer, or commercial. Tumor characteristics were gathered from the tumor registry and included stage, histology and tumor differentiation. Using International Federation of Gynecology and Obstetrics (FIGO) staging, we categorized clinical stage as local (≤2A) or advanced (≥2B); if missing, we used pathologic stage. If both were missing, stage was determined via manual review (CW). We categorized histology as adenocarcinoma, adenosquamous, squamous, or other/unknown. For process of care characteristics, we used the registry to determine if the diagnosis occurred elsewhere prior to presentation at Parkland and where women received subsequent cancer treatments. Using EHR data, we defined location of the women’s first Parkland visit (within the 6-month diagnostic window) as: ED, gynecology dysplasia clinic, or a primary care/women’s health/specialty clinic. The study was approved by the Institutional Review Board at UT Southwestern Medical Center (STU 042014–045).
Analysis
We compared new versus engaged patients across covariates using descriptive statistics and univariate logistic regression models. We compared characteristics of unscreened women to those lacking follow-up using chi-square and Fisher’s exact tests due to small cell sizes in order to identify statistically significant differences between women in these groups. For women whose screening or diagnostic tests did not detect cancer, we did not compare their demographics to other groups as such factors are unlikely to drive test failure (prior data indicate test failures are related to tumor characteristics such as histology).[26] Statistical significance was defined as p ≤ 0.05. Data analysis was conducted using SAS 9.4 (Cary, NC). Study authors selected case studies that illustrated and exemplified why engaged patients failed to receive screening or follow-up. Case descriptions were drafted by ACB and reviewed for accuracy by CW.
RESULTS
Characteristics of women diagnosed with cervical cancer and their processes of care
After review of 430 women in the registry, eleven (2.6%) were excluded because their cancer was not invasive or of cervical origin. Table 2 shows characteristics of the 419 eligible women. The majority were Dallas County residents at diagnosis (85.7%). Median age was 46 (range 20–89) with only 36 women (8.6%) over age 65. Nearly half were Hispanic (48.7%); the distribution of non-Hispanic White (23.9%) and Black (23.2%) women was about equal. Forty percent were uninsured/receiving charity care. The majority of tumors (58.2%) were advanced stage (≥2B). Histology was mostly squamous cell carcinoma (76.6%). More than a third (35.6%) had cancer diagnosed at another facility before coming to Parkland. Due to small cell sizes, we did not include pregnancy (n=5) or HIV status (n=4) in the tables. Figure 2 illustrates the distribution across the two primary outcomes.
Table 2.
Characteristics of women with cervical cancer and comparison of new patients versus engaged patients in an urban safety-net system, N = 419.
| Total N=419 (%) |
New Patients n=289 (69.1%) |
Engaged Patients n=130 (30.9%) |
Unadjusted Odds Ratioa (95% CI) |
|
|---|---|---|---|---|
| Age at Cancer Diagnosis | ||||
| 18–29 | 25 (6.0) | 20 (6.9) | 5 (3.8) | 1.89 (0.68, 5.25) |
| 30–49 | 221 (52.7) | 150 (51.9) | 71 (54.6) | Reference |
| 50–64 | 137 (32.7) | 94 (32.5) | 43 (33.1) | 1.04 (0.65, 1.64) |
| 65-Older | 36 (8.6) | 25 (8.7) | 11 (8.5) | 1.08 (0.50, 2.31) |
| Race-Ethnicity | ||||
| Hispanic | 204 (48.7) | 138 (47.8) | 66 (50.8) | Reference |
| Black | 97 (23.2) | 55 (19.0) | 42 (32.3) | 0.63 (0.38, 1.03) |
| White | 100 (23.9) | 84 (29.1) | 16 (12.3) | 2.51 (1.37, 4.62) |
| Other | 18 (4.3) | 12 (4.2) | 6 (4.6) | 0.96 (0.34, 2.66) |
| Payer at Cancer Diagnosis | ||||
| Uninsured (Charity) | 241 (57.5) | 156 (54.0) | 85 (65.4) | Reference |
| Commercial/Otherb | 83 (19.8) | 79 (27.3) | 4 (3.1) | 10.76 (3.81, 30.38) |
| Medicaid | 47 (11.2) | 29 (10.0) | 18 (13.8) | 0.88 (0.46, 1.67) |
| Other Government | 34 (8.1) | 15 (5.2) | 19 (14.6) | 0.43 (0.21, 0.89) |
| Medicare | 14 (3.3) | 10 (3.5) | 4 (3.1) | 1.36 (0.42, 4.47) |
| Charlson Comorbidity Score (No Tumor) | ||||
| 0 | 17 (13.1) | |||
| ≥ 1 | 32 (24.6) | |||
| Unknown | 81 (62.3) | |||
| Dallas County Resident at Cancer Diagnosis | ||||
| Yes | 359 (85.7) | 236 (81.7) | 123 (94.6) | Reference |
| No/unknown c | 60 (14.3) | 53 (18.3) | 7 (5.4) | 3.94 (1.74, 8.94) |
| Cancer Diagnosis Year | ||||
| 2010–2011 | 148 (35.3) | 105 (36.3) | 43 (33.1) | Reference |
| 2012–2013 | 150 (35.8) | 103 (35.6) | 47 (36.2) | 0.90 (0.55, 1.47) |
| 2014–2015 | 121 (28.9) | 81 (28.0) | 40 (30.8) | 0.83 (0.49, 1.39) |
| Cancer Stage | ||||
| Local | 167 (39.9) | 101 (34.9) | 66 (50.8) | Reference |
| Advanced | 244 (58.2) | 183 (63.3) | 61 (46.9) | 1.96 (1.28, 3.00) |
| Not assigned | 8 (1.9) | 5 (1.7) | 3 (2.3) | 1.09 (0.25, 4.71) |
| Histology | ||||
| Squamous | 321 (76.6) | 220 (76.1) | 101 (77.7) | Reference |
| Adenocarcinoma | 63 (15.0) | 39 (13.5) | 24 (18.5) | 0.75 (0.43, 1.31) |
| Adeno squamous | 20 (4.8) | 17 (5.9) | 3 (2.3) | 2.60 (0.75, 9.08) |
| Carcinoma NOS/Other | 15 (3.6) | 13 (4.5) | 2 (1.5) | 2.98 (0.66, 13.47) |
| Tumor Differentiation | ||||
| Low | 210 (50.1) | 141 (48.8) | 69 (53.1) | Reference |
| High | 143 (34.1) | 97 (33.6) | 46 (35.4) | 1.03 (0.66, 1.63) |
| Unknown | 66 (15.8) | 51 (17.6) | 15 (11.5) | 1.66 (0.87, 3.17) |
|
Location of First Visit during 6 Month Diagnostic Work-up Period |
||||
| Primary Care/Women’s/ Specialty | 95 (22.7) | 29 (10.0) | 66 (50.8) | Reference |
| Gynecology Dysplasia | 78 (18.6) | 66 (22.8) | 12 (9.2) | 12.5 (5.89, 26.61) |
| Emergency Department | 246 (58.7) | 194 (67.1) | 52 (40.0) | 8.49 (4.98, 14.47) |
| Initial Cancer Diagnosis Location | ||||
| Parkland/Affiliated | 270 (64.4) | 153 (52.9) | 117 (90.0) | Reference |
| Elsewhere | 149 (35.6) | 136 (47.1) | 13 (10.0) | 8.0 (4.31, 14.84) |
| Cancer Treatment Location | ||||
| All Parkland | 163 (38.9) | 103 (35.6) | 60 (46.2) | Reference |
| Some Parkland | 206 (49.2) | 146 (50.5) | 60 (46.2) | 1.42 (0.92, 2.20) |
| All Elsewhere | 50 (11.9) | 40 (13.8) | 10 (7.7) | 2.33 (1.09, 5.00) |
Univariate logistic regression models in which engaged patients are the referent group.
Other types of payers included veterans’ benefits and workman’s compensation.
46 patients had an unknown county of residence (N=42 new patients and N=4 engaged patients).
Figure 2.
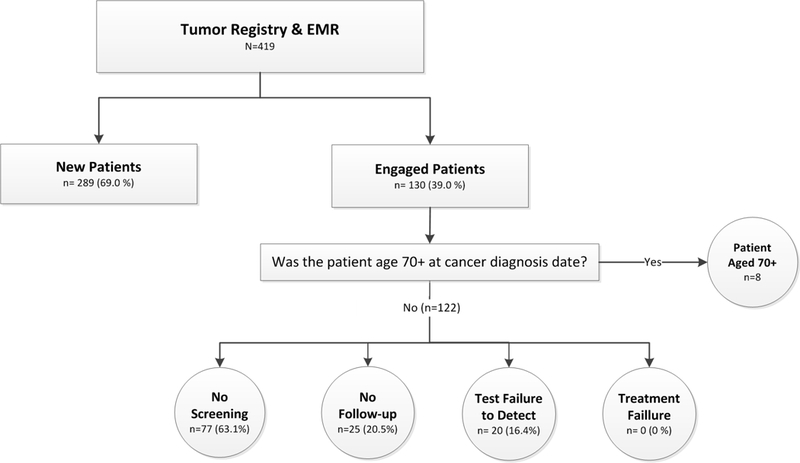
Study schema. Women with cervical cancer were classified using the tumor registry and electronic health record (EHR) as new patients or engaged patients; engaged patients aged <70 years were further classified by opportunity to prevent cervical cancer: no screening, no follow-up of abnormal screening, failure of a test to detect cancer, or a treatment failure.
Contact with the safety-net system: New versus engaged patients
The majority of cases (69.1%, n=289) were new patients with no history of care at Parkland prior to the 6-month diagnostic workup period. Compared to engaged patients, new patients were more likely to be non-Hispanic White (vs. Hispanic), to have commercial/other insurance (vs. uninsured), and to live outside of Dallas County (Table 2). New patients were more likely to be diagnosed with advanced tumors (63.3%) compared to engaged patients (46.9%).
As expected, new patients were more likely to have had their cancer diagnosed elsewhere; they were more likely to visit the ED or the gynecology dysplasia clinic (usually by referral), than primary care/women’s health/ specialty clinics. After diagnosis, new patients were more likely to receive cancer treatment outside of Parkland.
Among engaged patients, the extent of prior contact with Parkland varied: 25% had one primary care or women’s health visit during the study window; 38% had 2+ visits; 36% had one visit to the ED while 27% had 2+ ED visits. About twenty-five percent of engaged patients had a Charlson comorbidity score of ≥1 at cancer diagnosis (Table 2). Ten percent of engaged patients received their cancer diagnosis outside of Parkland and, for 40%, first visit in the diagnostic work-up period was at the ED.
Opportunities for prevention among engaged patients
Among engaged patients, 8 (6.2%) were age ≥70 years at diagnosis and were excluded. Of the remaining 122 (Figure 2), 63.1% were not screened, 20.5% did not receive follow-up, and 16.4% had a screening or diagnostic test that did not detect their cancer. There were no excisional or ablative treatment failures. Table 3 presents characteristics of engaged patients by opportunity to prevent cervical cancer. Table 4 provides case summaries that illustrate some of the challenges to delivering the screening process to safety-net populations.
Table 3.
Characteristics of engaged patients by opportunity to prevent cervical cancer among women aged <70 years at diagnosis in an urban safety-net system, N = 122.
| Total N=122 (%) |
Test did not Detect Cancer n=20 (16.4%) |
No Screening n=77 (63.1%) |
No Follow-up for Abnormal Test n=25 (20.5%) |
p-valuea | ||
|---|---|---|---|---|---|---|
| Age at Cancer Diagnosis | 0.180 | |||||
| 18–29 | 5 (4.1) | 0 (0.0) | 3 (3.9) | 2 (8.0) | ||
| 30–49 | 71 (58.2) | 16 (80.0) | 38 (49.4) | 17 (68.0) | ||
| 50–64 | 43 (35.2) | 4 (20.0) | 33 (42.9) | 6 (24.0) | ||
| 65-Older | 3 (2.5) | 0 (0.0) | 3 (3.9) | 0 (0.0) | ||
| Race-Ethnicity | 0.421 | |||||
| Hispanic | 63 (51.6) | 14 (70.0) | 37 (48.1) | 12 (48.0) | ||
| Black | 37 (30.3) | 6 (30.0) | 21 (27.3) | 10 (40.0) | ||
| White | 16 (13.1) | 0 (0.0) | 13 (16.9) | 3 (12.0) | ||
| Other | 6 (4.9) | 0 (0.0) | 6 (7.8) | 0 (0.0) | ||
| Payer at Cancer Diagnosis | 0.152 | |||||
| Uninsured (Charity) | 79 (64.8) | 7 (35.0) | 58 (75.3) | 14 (56.0) | ||
| Commercial | 4 (3.3) | 1 (5.0) | 2 (2.6) | 1 (4.0) | ||
| Medicaid | 17 (13.9) | 3 (15.0) | 10 (13.0) | 4 (16.0) | ||
| Other Government | 19 (15.6) | 7 (35.0) | 6 (7.8) | 6 (24.0) | ||
| Medicare | 3 (2.5) | 2 (10.0) | 1 (1.3) | 0 (0.0) | ||
| Charlson Comorbidity Score (No Tumor) | 0.822 | |||||
| 0 | 17 (13.9) | 8 (40.0) | 6 (7.8) | 3 (12.0) | ||
| ≥ 1 | 32 (26.2) | 4 (20.0) | 21 (27.3) | 7 (28.0) | ||
| Unknown | 73 (59.8) | 8 (40.0) | 50 (64.9) | 15 (60.0) | ||
| Dallas County at Cancer Diagnosis | 0.549 | |||||
| Yes | 115 (94.3) | 20 (100) | 72 (93.5) | 23 (92.0) | ||
| No/Unknownb | 7 (5.7) | 0 (0.0) | 5 (6.5) | 2 (8.0) | ||
| Cancer Diagnosis Year | 0.032 | |||||
| 2010–2011 | 42 (34.4) | 10 (50.0) | 23 (29.9) | 9 (36.0) | ||
| 2012–2013 | 44 (36.1) | 5 (25.0) | 31 (40.3) | 8 (32.0) | ||
| 2014–2015 | 36 (29.5) | 5 (25.0) | 23 (29.9) | 8 (32.0) | ||
| Cancer Stage | 0.002 | |||||
| Local | 62 (50.8) | 16 (80.0) | 28 (36.4) | 18 (72.0) | ||
| Advanced | 58 (47.5) | 4 (20.0) | 48 (62.3) | 6 (24.0) | ||
| Not assigned | 2 (1.6) | 0 (0.0) | 1 (1.3) | 1 (4.0) | ||
| Histology | 0.096 | |||||
| Squamous | 96 (78.7) | 10 (50.0) | 65 (84.4) | 21 (84.0) | ||
| Adenocarcinoma | 21 (17.2) | 8 (40.0) | 9 (11.7) | 4 (16.0) | ||
| Adeno squamous | 3 (2.5) | 2 (10.0) | 1 (1.3) | 0 (0.0) | ||
| Carcinoma NOS/Other | 2 (1.6) | 0 (0.0) | 2 (2.6) | 0 (0.0) | ||
| Tumor Differentiation | 0.010 | |||||
| Low | 64 (52.5) | 14 (70.0) | 34 (44.2) | 16 (64.0) | ||
| High | 44 (36.1) | 4 (20.0) | 34 (44.2) | 6 (24.0) | ||
| Unknown | 14 (11.5) | 2 (10.0) | 9 (11.7) | 3 (12.0) | ||
| Initial Cancer Diagnosis Location | 0.30 | |||||
| Parkland | 110 (90.2) | 17 (85.0) | 69 (89.6) | 24 (96.0) | ||
| Elsewhere | 12 (9.8) | 3 (15.0) | 8 (10.4) | 1 (4.0) | ||
| Cancer Treatment Location | 0.045 | |||||
| All Parkland | 57 (46.7) | 12 (60.0) | 32 (41.6) | 13 (52.0) | ||
| Some Parkland | 57 (46.7) | 7 (35.0) | 39 (50.6) | 11 (44.0) | ||
| All Elsewhere | 8 (6.6) | 1 (5.0) | 6 (7.8) | 1 (4.0) | ||
| Location of First Visit during 6 Month Diagnostic Work-up Period | 0.066 | |||||
| Primary Care/ Women’s/ Specialty | 60 (49.2) | 13 (65.0) | 37 (48.1) | 10 (40.0) | ||
| Gynecology Dysplasia | 12 (9.8) | 1 (5.0) | 5 (6.5) | 6 (24.0) | ||
| Emergency Department | 50 (41.0) | 6 (30.0) | 35 (45.5) | 9 (36.0) | ||
| Number of Missed Appointments during Study Window | 0.26 | |||||
| 0 | 66 (54.1) | 13 (65.0) | 43 (55.9) | 10 (40.0) | ||
| 1 | 15 (12.3) | 2 (10.0) | 8 (10.4) | 5 (20.0) | ||
| ≥2 | 41 (33.6) | 5 (25.0) | 26 (33.8) | 10 (40.0) | ||
p for comparison of screen and follow-up columns
Four patients had unknown county of residence (N=2 no screening and N=2 no follow-up).
Table 4.
Illustrative case summaries of engaged women with cervical cancer
| Failure to Screen |
|---|
|
Case 1: Age mid-thirties • Did not attend 16 scheduled Pap appointments in the HIV OB/GYN clinic in the 5 years prior to the abnormal Pap that led to cervical cancer diagnosis. • Routinely seen in the HIV clinics during the study period. • Poorly controlled HIV infection with increased viral load at multiple time points and self-reported non-adherence to medications. • During HIV visits, providers stressed the importance of medication adherence so that she would be well enough to care for her family, as well as the importance of keeping gynecology appointments. • Reported stress and competing demands, including caring for ill family members that involved travel. |
|
Case 2: Age late fifties • Post-menopausal with multiple comorbidities including tobacco use, hepatitis C, hypertension, dyslipidemia, multiple gastrointestinal conditions, and chronic abdominal pain. • Nine emergency department visits in first 2 years of study for severe abdominal pain and GI symptoms. Seen frequently in GI-Liver Clinic and received multiple GI diagnostic evaluations. Seen at least annually in the same primary care clinic for 4 of 5 study years. Treatment through both primary care and specialty clinics focused on extreme hyperlipidemia and control of chronic GI symptoms. • No Pap until seen for the first time at a women’s clinic at which time the patient was symptomatic (abnormal vaginal bleeding) and had a Pap performed indicative of cervical cancer. |
|
Case 3: Age early sixties • Post-menopausal with poorly controlled type II diabetes, hypertension, hyperlipidemia, congestive heart failure, and depression/anxiety. • Seen regularly in primary care for 2.5 years before cancer diagnosis, with focus on control of diabetes and hypertension. Hospitalized for hypertensive crisis approximately 1 year prior to cervical cancer diagnosis; thereafter, attended cardiology clinic in addition to primary care clinic. • CT angiography incidentally showed adnexal masses, confirmed by pelvic sonogram. The patient was seen in Gynecology Clinic 4 months later for further evaluation, which included her initial Pap within the system. Cervical cancer was subsequently diagnosed due to this Pap being abnormal. |
| Failure to Follow-Up Abnormal Test |
|
Case 4: Age late thirties • Reproductive-aged patient with history of diabetes mellitus and new diagnosis of advanced invasive breast cancer during the study period. Underwent mastectomy followed by neo-adjuvant chemotherapy for over 1 year. • Screened for cervical cancer with co-tests one year apart in primary care clinic; both showed a negative Pap with positive high risk HPV testing. However, the patient was not referred for colposcopy as per management guidelines following the second such result. • Thereafter, patient attended oncology follow-ups and infrequent primary care encounters that focused on her diabetes. • Cholecystectomy performed after positive evaluation for gall bladder disease. • Received a third Pap test approximately 2 years after the last co-test, with a negative result. • Patient presented to gynecology clinic with symptoms of cervical cancer approximately 1 year after the 3rd negative Pap. A cervical mass was identified upon presentation to Gynecology Clinic. |
Unscreened women were more likely to be diagnosed with advanced stage disease (62.3%) than those screened but not followed up (24%). Many had opportunities for screening: 22% had one primary care or women’s health visit during the study period and 32% had 2+ visits, while 45% visited the ED or a specialty clinic. Unscreened women had similar Charlson comorbidity scores when compared to women who did not receive follow-up; over one quarter (27.3%) had a score of ≥ 1 at the time of cancer diagnosis (excluding tumors; Table 3). Case summaries (Table 4) revealed that women who did not receive screening despite significant Parkland inpatient and outpatient utilization often were undergoing management of one or multiple comorbid conditions, including diabetes, hypertension, and human immunodeficiency virus (HIV). Some had cancelled or did not show for scheduled Pap appointments. Across the study window, 10.4% of unscreened women had a missed clinic appointment and 33.8% missed 2+ appointments (Table 3).
Among the 25 women who did not receive follow-up, the reference test was either a Pap/co-test (n=20) or a colposcopy/biopsy (n=5). About half (52%) had completed at least one primary care or outpatient clinic visit (Median =1, range 0–33) after the abnormal result (i.e., there were opportunities to stress the importance of or arrange for follow-up; see Table 4 for example case summaries). Notably, 40% (median=1, range 0–17) missed 2+ appointments for follow-up during the study window (Table 3). Case reviews revealed a variety of reasons for lack of follow-up with reasons documented in various EHR locations, including progress notes of patient encounters, telephone encounters, and under the “letters” tab. Reasons included competing demands such as caring for family, managing chronic and acute diseases, having limited contact with primary or gynecology clinics (vs. other sub-specialty care), or problems with results communication among providers, clinic or laboratory staff, and patients. For example, while reviews demonstrated that women were informed of their abnormal results in several ways (telephone, regular mail, certified letter), for many cases we could not determine in the EHR whether women actually received or understood these communications. Reviews indicated multiple reasons for lack of follow-up and cannot simply be attributed to patient non-adherence or provider/system failure to inform or arrange further evaluation.
For 16.4% (n=20 cases), we identified a prior test that did not detect a presumably existent cervical cancer or precursor lesion. Tests occurred a median of 31 months (range 7 to 45 months) prior to cancer diagnosis. Three women had a negative or CIN I colposcopy/biopsy prior to cancer diagnosis. Of these, two had squamous cancer and one had adenocarcinoma. Seventeen women had a negative screening test <3.5 years of cancer diagnosis. One of these was a negative co-test; one was an ASC-US/HPV negative co-test; the remaining 15 received cytology alone with a NILM result. Most women were previously under-screened: for 71% (12/17), the negative screening test was the first and only screen on record prior to cancer diagnosis. Approximately half of the 20 cancers in this group were adenocarcinomas.
DISCUSSION
We described the cervical cancer burden in a large, urban safety-net system, characterized clinical care pathways of engaged patients prior to their cervical cancer diagnosis, and, in doing so, identified opportunities to improve delivery of the screening process. Most women (69%) had no healthcare system contact prior to diagnostic work-up. In order to deliver the screening process and prevent cancer among these women, outreach to the broader community would be needed. Further, of the 122 engaged in care, 63% were not screened. This suggests that, to prevent cervical cancer at Parkland, QI efforts highest priority should be screening outreach. Comparing our data to the population-based Texas Cancer Registry[27] reveals that Parkland cared for 49% of all women with cervical cancer diagnosed in Dallas County and 10% of all cervical cancer cases in Texas. Dallas represents the 9th most populous U.S. county and Texas is the 2nd most populous U.S. state, according to the U.S Census Bureau 2011–2015 American Community Survey. Thus, future interventions within our system could significantly impact the population-level cervical cancer burden. The large burden observed in our study underscores the fact that cervical cancer is a disease predominantly borne by low socioeconomic status, African American, and Hispanic women— populations served by safety-net systems.
We identified multiple opportunities to improve cervical cancer prevention efforts. Consistent with prior findings, lack of screening was the leading driver of cervical cancer [16, 17, 28] occurring in 63% of our engaged patients. For the 16% whose test failed to detect cancer, the over representation of non-squamous histologies is somewhat expected because Pap tests are more sensitive for detecting squamous cell carcinomas than adenocarcinomas.[26, 29, 30] The patterns of care before cervical cancer and potential opportunities for prevention identified in our system, differed somewhat from prior reviews. For example, studies conducted in non-safety-net settings demonstrated a higher rate of screening test failures and treatment failures; we found none of the latter.[16, 17] These differences highlight the importance of healthcare systems conducting their own reviews to select interventions and deploy resources that will have the greatest impact for their patients. Our findings suggest that increasing screening and follow-up may potentially decrease cancer incidence irrespective of a system’s policies on screening test modality or interval.
Consistent with our findings, prior research underscore importance of encouraging women to obtain primary care as a key strategy for cervical cancer prevention.[31] Most new patients (67%) and even many engaged patients (40%) started their cervical cancer diagnostic process in the ED presenting with symptoms such as vaginal bleeding or pain. We could not determine whether and how long these new patients had delayed seeking care or their patterns of primary care contact with other healthcare systems.[32, 33] Notably, new patients were diagnosed at a later stage than engaged patients. Among engaged patients, primary care contact varied, ranging from dozens of visits over several years to a single visit or sporadic visits over a shorter time period. Furthermore, case reviews uncovered multi-factorial reasons for lack of screening and follow-up in engaged patients. These included ongoing management of comorbidities and problems with results reporting and communication, in addition to limited primary care contact or coordination with specialists.
We identified several factors associated with being a new patient, which reflect Parkland’s role as a regional provider of heavily subsidized care. First, we observed that many new patients did not live within Dallas County; these non-county residents are ineligible for subsidized primary care, but they are eligible for subsidized cervical diagnostic services through Parkland’s participation in the NBCCEDP. We also observed that those with commercial insurance (vs. uninsured/charity care) were more likely to be new patients. While counterintuitive, this finding may reflect issues of being under-insured with high deductibles. Other health systems may refer these patients because Parkland will subsidize diagnosis and treatment via county and federally supported programs, minimizing or eliminating out-of-pocket costs. We cannot assess specifics of individual patients’ insurance products to test this assumption.
To put our study population in context, we compared race/ethnicity and insurance status of the women in the present study to our observational cohort of 191,185 screening-eligible women who are engaged in primary care at Parkland. Overall, White race was more common among women with cervical cancer and Hispanic ethnicity was more common in the overall cohort. While uninsured/charity and commercial insurance was more common among women with cervical cancer, Medicaid and other government payers were more common among the overall cohort. These differences may reflect differing age distributions of cervical cancer cases vs. all primary care patients, demographics of out-of-county residents referred to Parkland for cancer care, or other factors. Notably, insurance status can change over time and the insurance status of a cancer patient at time of diagnosis may not be directly comparable to insurance status of our comparison population, which was defined at the time of a primary care visit.
Our study faces several limitations. Our algorithm assigned women into mutually exclusive categories. However, case reviews demonstrated that women could experience more than one missed opportunity for cervical cancer prevention. Realistically, health systems will never be able to fully eradiate cervical cancer due to patient noncompliance. Among women classified as “failure to detect,” we cannot rule out interval cancers arising quickly within the guideline-recommended intervals. We identified no treatment failures, which could be a result of our limited timeframe for post-treatment surveillance.
Our study has several key advantages. First, we evaluated the entire screening process, going beyond studies only focused on Pap screening.[12, 18–20] Second, we analyzed an array of patient, tumor, and process-of-care covariates and presented case summaries to further contextualize findings. Third, while conducted in a single healthcare system, our study sample represents half of the overall cervical cancer burden in one of the most populous U.S. counties, and is one of the few and largest cervical cancer case reviews conducted in a U.S. safety-net system without an organized screening program. Thus, it fills a significant gap in the literature. Moreover, given the increasing trend toward formation of integrated safety-net systems[34, 35] and the fact that many patients are referred to Parkland from federally qualified health centers and other stand-alone, smaller safety-net clinics, our study has relevance in other safety-net settings.
Implications
Our findings provide an initial evidence base to shape QI initiatives in safety-net systems and to encourage community-based interventions outside of safety-net walls. QI efforts should focus first on initial screening but also attend to increasing guideline-concordant follow-up. In our system, such strategies could potentially prevent over 80% of cervical cancers among engaged patients.
Our algorithm-driven approach, applied to longitudinal EHR data from a large, urban safety-net system, provides a starting point for other systems to conduct their own system audits. Case reviews/audits of cervical cancer patients are needed across diverse settings to identify targets for QI initiatives. We believe our framework for identifying missed opportunities can be applied—or adapted—successfully in other settings with EHRs, even in non-integrated and primary care safety-net systems with fewer resources. Sasieni and Cuzik encourage routine audits as an ethical requirement of large-scale screening programs.[36, 37] Our algorithm can be adapted for other health systems to characterize how cervical cancer occurs among engaged patients and prioritize interventions to improve prevention efforts. These efforts should be used to guide development, implementation, and dissemination of multilevel strategies to prevent cervical cancer.
Supplementary Material
Acknowledgments
We thank Dr. Noel Santini and colleagues at Parkland Health and Hospital System for supporting our PROSPR research initiatives. We also want to acknowledge the contributions of Mark Burkart for his programming expertise.
Financial Support: This study was conducted as part of the NCI-funded consortium Population-Based Research Optimizing Screening through Personalized Regimens (PROSPR; U54CA163308-S1). Additional support provided by the UTSW Center for Translational Medicine, through the NIH/National Center for Advancing Translational Sciences (UL1TR001105) and the Harold C. Simmons Comprehensive Cancer Center (1P30 CA142543). Ms. Betts is supported by a pre-doctoral fellowship, University of Texas School of Public Health Cancer Education and Career Development Program – (National Cancer Institute/NIH Grant R25 CA57712).
Footnotes
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Conflicts of Interest: None of the authors have any conflicts of interest to declare
References
- 1.Meggiolaro A, et al. , The role of Pap test screening against cervical cancer: a systematic review and meta-analysis. Clin Ter, 2016. 167(4): p. 124–39. [DOI] [PubMed] [Google Scholar]
- 2.Yang DX, et al. , Impact of Widespread Cervical Cancer Screening: Number of Cancers Prevented and Changes in Race-specific Incidence. Am J Clin Oncol, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaccarella S, et al. , Worldwide trends in cervical cancer incidence: Impact of screening against changes in disease risk factors. European Journal of Cancer, 2013. 49(15): p. 3262–3273. [DOI] [PubMed] [Google Scholar]
- 4.Sabatino SA, et al. , Cancer screening test use- United States, 2013. Morbidity and Mortality Weekly Report. May 8, 2015, 2015. 64(17): p. 464–8. [PMC free article] [PubMed] [Google Scholar]
- 5.White A, et al. , Cancer Screening Test Use - United States, 2015., in MMWR Morb Mortal Wkly Rep 2017. 2017. p. 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owens CL, et al. , Follow-up and clinical significance of unsatisfactory liquid-based Papanicolaou tests. Cancer Cytopathol, 2015. 123(1): p. 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinmann S, et al. , Cervical cancer screening and follow-up in 4 geographically diverse US health care systems, 1998 through 2007. Cancer, 2015. 121(17): p. 2976–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tejeda S, et al. , Patient barriers to follow-up care for breast and cervical cancer abnormalities. J Womens Health (Larchmt), 2013. 22(6): p. 507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Percac-Lima S, et al. , Barriers to follow-up of an abnormal Pap smear in Latina women referred for colposcopy. J Gen Intern Med, 2010. 25(11): p. 1198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith RA, et al. , Cancer screening in the United States, 2017: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin, 2017. 67(2): p. 100–121. [DOI] [PubMed] [Google Scholar]
- 11.Chattopadhyay SK, et al. , Use of cervical cancer screening among insured women: the extent of missed opportunities. Health Policy, 2005. 73(2): p. 194–201. [DOI] [PubMed] [Google Scholar]
- 12.Mema SC, et al. , Screening History in 313 Cases of Invasive Cancer: A Retrospective Review of Cervical Cancer Screening in Alberta, Canada. Journal of Lower Genital Tract Disease, 2017. 21(1): p. 17–20. [DOI] [PubMed] [Google Scholar]
- 13.Priest P, et al. , Pathways to diagnosis of cervical cancer: screening history, delay in follow up, and smear reading. BJOG: An International Journal of Obstetrics & Gynaecology, 2007. 114(4): p. 398–407. [DOI] [PubMed] [Google Scholar]
- 14.Bos AB, et al. , Nonattendance is still the main limitation for the effectiveness of screening for cervical cancer in the Netherlands. International journal of cancer, 2006. 119(10): p. 2372–2375. [DOI] [PubMed] [Google Scholar]
- 15.Kinney W, et al. , Missed Opportunities for Cervical Cancer Screening of HMO Members Developing Invasive Cervical Cancer (ICC). Gynecologic Oncology, 1998. 71(3): p. 428–430. [DOI] [PubMed] [Google Scholar]
- 16.Leyden WA, et al. , Cervical Cancer in Women With Comprehensive Health Care Access: Attributable Factors in the Screening Process. JNCI: Journal of the National Cancer Institute, 2005. 97(9): p. 675–683. [DOI] [PubMed] [Google Scholar]
- 17.Spence AR, Goggin P, and Franco EL, Process of care failures in invasive cervical cancer: Systematic review and meta-analysis. Preventive Medicine, 2007. 45(2–3): p. 93–106. [DOI] [PubMed] [Google Scholar]
- 18.Nygard JF, et al. , Screening histories of women with CIN 2/3 compared with women diagnosed with invasive cervical cancer: a retrospective analysis of the Norwegian Coordinated Cervical Cancer Screening Program. Cancer Causes Control, 2005. 16(4): p. 463–74. [DOI] [PubMed] [Google Scholar]
- 19.Andrae B, et al. , Screening-preventable cervical cancer risks: evidence from a nationwide audit in Sweden. J Natl Cancer Inst, 2008. 100(9): p. 622–9. [DOI] [PubMed] [Google Scholar]
- 20.Lewis H, et al. , Monitoring the performance of New Zealand’s National Cervical Screening Programme through data linkage. New Zealand Medical Journal, 2009. 122(1305): p. 15–25. [PubMed] [Google Scholar]
- 21.Sung H-Y, et al. , Papanicolaou smear history and diagnosis of invasive cervical carcinoma among members of a large prepaid health plan. Cancer, 2000. 88(10): p. 2283–2289. [PubMed] [Google Scholar]
- 22.Smith RA and Brawley OW, The National Breast and Cervical Cancer Early Detection Program: toward a system of cancer screening in the United States. Cancer, 2014. 120 Suppl 16: p. 2617–9. [DOI] [PubMed] [Google Scholar]
- 23.Miles A, et al. , A perspective from countries using organized screening programs. Cancer, 2004. 101(5 Suppl): p. 1201–13. [DOI] [PubMed] [Google Scholar]
- 24.Herbert A, et al. , Invasive cervical cancer audit: why cancers developed in a high-risk population with an organised screening programme. BJOG: An International Journal of Obstetrics & Gynaecology, 2010. 117(6): p. 736–745. [DOI] [PubMed] [Google Scholar]
- 25.Quan H, et al. , Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care, 2005. 43(11): p. 1130–9. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell H, et al. , Cervical cytology reported as negative and risk of adenocarcinoma of the cervix: no strong evidence of benefit. Br J Cancer, 1995. 71(4): p. 894–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Report prepared by the Texas Department of State Health Services, C.E.a.S.B., Texas Cancer Registry. Data Request # 17029, 3/14/17,.
- 28.Sung HY, et al. , Papanicolaou smear history and diagnosis of invasive cervical carcinoma among members of a large prepaid health plan. Cancer, 2000. 88(10): p. 2283–9. [PubMed] [Google Scholar]
- 29.Zappa M, et al. , Lower protection of cytological screening for adenocarcinomas and shorter protection for younger women: the results of a case-control study in Florence. Br J Cancer, 2004. 90(9): p. 1784–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castanon A, Landy R, and Sasieni PD, Is cervical screening preventing adenocarcinoma and adenosquamous carcinoma of the cervix? Int J Cancer, 2016. 139(5): p. 1040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bindman AB, et al. , Primary care and receipt of preventive services. J Gen Intern Med, 1996. 11(5): p. 269–76. [DOI] [PubMed] [Google Scholar]
- 32.Ashing-Giwa KT, et al. , Diagnostic and therapeutic delays among a multiethnic sample of breast and cervical cancer survivors. Cancer, 2010. 116(13): p. 3195–204. [DOI] [PubMed] [Google Scholar]
- 33.Battaglia TA, et al. , Predictors of timely follow-up after abnormal cancer screening among women seeking care at urban community health centers. Cancer, 2010. 116(4): p. 913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy J, et al. , Safety net integration: a shared strategy for becoming providers of choice. J Health Polit Policy Law, 2015. 40(2): p. 403–19. [DOI] [PubMed] [Google Scholar]
- 35.Cunningham P, Felland L, and Stark L, Safety-net providers in some US communities have increasingly embraced coordinated care models. Health Aff (Millwood), 2012. 31(8): p. 1698–707. [DOI] [PubMed] [Google Scholar]
- 36.Sasieni P and Cuzick J, Routine audit is an ethical requirement of screening. BMJ, 2001. 322(7295): p. 1179. [PMC free article] [PubMed] [Google Scholar]
- 37. Cuzick J, Routine Audit of Large-Scale Cervical Cancer Screening Programs. JNCI: Journal of the National Cancer Institute, 2008. 100(9): p. 605–606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


