Abstract
The aim of the study was to explore the role of parity, maternal age, medical interventions, and birth weight with respect to labor duration and cervical dilation.
A total of 1601 pregnant women who had a singleton term gestation, spontaneous onset of labor, vertex presentation, vaginal delivery, and a normal perinatal outcome were reviewed. The retrospective study was conducted in patients from West China Second University Hospital of Sichuan University during June 2008 to June 2013.
There were 1367 nulliparous women and 234 multiparous women analyzed. The first stage (8.3 ± 3.8 vs 5.0 ± 2.6 hours), latent phase (5.1 ± 3.2 vs 3.5 ± 2.4 hours), active phase (3.2 ± 1.8 vs 1.5 ± 1.0 hours), second stage (44 ± 31 vs 18 ± 14 minutes), and total stage of labor (9.1 ± 3.9 vs 5.4 ± 2.6 hours) were all longer in nulliparous than in multipara women (all P < .05); but no significant difference in the third stage of labor (both 7 ± 4 minutes). In nulliparous women, the average time of first stage of labor increased by 58.257, 171.443, and 56.581 minutes due to artificial rupture of membranes, labor analgesia, and birth weight increased by 1 kg, respectively, but it decreased to 63.592 minutes by oxytocin usage, and the difference was significant. The average time of first stage of labor in nulliparous women aged from 26 to 30 years increased by 2.356 minutes compared to one in 20 to 26 years, but it increased by 1.802 minutes to the one in 30 to 39 years, compared to 20 to 26 years and the difference was not significant. The results were basically similar after multipara women were included.
Labor was significantly shorter in multiparous women than that in nulliparous women. Increased birth weight significantly increased in the length of the active phase and the second stage among nulliparous women. The increase of age, artificial rupture of membranes, labor analgesia, and the increase of birth weight tends to increase the time of first stage of labor and total labor duration, whereas oxytocin could shorten it.
Keywords: labor duration, labor stage, multipara women, nulliparous women
1. Introduction
The labor curve is a graphical design that records the progress of labor and salient features in the mother and fetus. In 1955, Friedman[1,2] published a landmark study, which depicted the relationship between duration of labor and cervical dilation as a Friedman curve.[3] However, Friedman's study was conducted more than half a century ago, and it was based on his observation of just 500 parturient women at term. Labor management has changed substantially during the last 60 years. Over the past decades, there have been many changes challenging the modern obstetrics practice, including but not limited to older maternal age, greater maternal body mass index and birth weights, more medical interventions (such as artificial rupture of membranes or oxytocin during labor or labor analgesia), and improved fetal monitoring during labor.[4]
A modern curve was, therefore, proposed in 2014. However, due to the subjectivity and inconsistency regarding the definition of the starting point of labor, it's difficult to evaluate the latent phase objectively. It has come to notice that, even with prolonged latent phase, mothers and children are rarely affected adversely. Some scholars have suggested that more attention should not be paid to the latent phase, but to the active phase and the second stage instead.[5] In addition, the continuous improvement and development of clinical research and statistical methods revealed the limitations of previous clinical research, such as the lack of comparability of baseline data and the bias of statistical methods.[6] For these reasons, some recent studies have suggested that the Friedman curve is no longer appropriate for labor management.[7,8]
In 2002, Zhang et al[9] demonstrated that the median time to progress from 4 to 10 cm cervical dilation was 5.5 hours for nulliparous women. Before it reaches 6 cm cervical dilation, the latent phase of labor was longer, and it progressed more slowly than the one described by Friedman.[9] A workshop was held in 2014 with experts from the Chinese Medical Association to redefine recommendations for labor management and diagnosis of labor arrest disorders.[4] They suggested that in a modern curve, there is no deceleration phase near 10 cm cervical dilation.[4] In 2018, Pitchaimuthu and Bhaskaran[10] conducted a prospective observational study, which included 156 primigravidas, and concluded that the mean rate of cervical dilatation in active phase in Indian women was approximately equivalent to the lowest acceptable rate of cervical dilatation in Friedman's study. It could lead to a huge number of C-sections which could have been spared. Such data are however lacking in China. Thus, it would be prudent to develop a customized labor curve based on the Chinese population with rich information on demographic and clinical characteristics.
Medical interventions (such as artificial rupture of membranes or oxytocin during labor or labor analgesia) are highly common now, as birth weights and maternal age have increased. So, updated labor definitions have focused on the influence of relative factors including medical interventions, maternal age, and birth weight.[4,11–14]
A recent review demonstrated that women with labor analgesia had a longer second stage than women without analgesia,[12] but other studies reported that there was no significant effect on the second stage, or even the first stage of labor by labor analgesia.[15–17] Therefore, the impact of analgesia on labor remains controversial. In addition, effects of artificial rupture of membranes or oxytocin during labor, maternal age, and birth weight on the duration of normal labor still remains unclear.[18,19] Although there are many reports about normal labor and its associating factors,[20–23] clinical data on it are still lacking in China. Hence, this study aimed to explore the association of parity, maternal age, medical interventions, and birth weight with labor duration and cervical dilation in Chinese women.
2. Materials and methods
2.1. Participants
This retrospective study reviewed vaginal labor, singleton, cephalic term deliveries at the West China Second University Hospital of Sichuan University between June 2008 and June 2013. Inclusion criteria include women with vaginal delivery, singleton, term pregnancy (>37 weeks and <42 weeks) and cephalic presentation, good neonatal outcomes, and complete clinical records. Exclusion criteria include premature rupture of membranes; previous uterine scar and cervical surgery; macrosomia and newborns with low birth weight (birth weight <2500 g), malformations, and Apgar score <7 at 1 and 5 minutes; women with induced labor, <2 vaginal examinations, or >3 cm cervical dilatation when admitted into hospital. The women were grouped by parity as nulliparous women and multiparous women groups. The retrospective study was approved by the Ethics Committee of the West China Second University Hospital of Sichuan University. Informed consent was waived.
2.2. Data collection
The maternal age, body weight index (BMI) before delivery (weight [kg]/height2 [m2]), medical interventions (amniotomy or oxytocin or labor analgesia [epidural or spinal-epidural anesthesia]), infant length (cm), and weight (kg) were collected. The duration of labor including latent phase, active phase, first stage of labor, second stage of labor, and third stage of labor, along with the total stage of labor (specific definitions follow) were reviewed.
2.3. Stages of labor
The stages of labor were defined based on reported standard gynecology methods.[24] The first stage of labor: begins with the onset of regular uterine contractions and ends only when the cervical is completely dilated. The latent phase of labor: from 0 to 3 cm cervical dilatation; the active phase: from 3 to 10 cm cervical dilatation; the second stage of labor: from the end of the first stage until the delivery of the infant is completed. The third stage of labor from the delivery of the child until the placenta and membrane are expelled. To shed light on the current practice, we also performed an additional analysis based on the present labor definition recommended by the Chinese Medical Council.[4] In this analysis, the latent phase was redefined as cervical dilation of 0 to 6 cm (referred as “N latent phase”), whereas the cervical dilation of 6 to 10 cm for the active phase (referred as “N active phase”).
2.4. Subgroup division
Medical interventions refer to artificial rupture of membranes or oxytocin or labor analgesia during labor. When the labor process was in the active phase (cervix 3 cm), if irregular contractions, delayed labor progress, and the imbalance of the first basin occurs, artificial rupture of membranes was implemented. If the artificial rupture of membranes within half an hour was optimal, then oxytocin was waived. Otherwise, if the artificial rupture of the anterior chamber after half an hour was not optimal, then 500 mL balanced solution containing 2.5 U oxytocin was injected, starting from 8 drops/min, adjusted the dose according to the contractions at a 20-minute interval, each adjustment was at 4 drops/min, the maximum dose is 40 drops/min, until the effective contraction contraindicated oxytocin. Labor analgesia methods include continuous epidural block and spinal anesthesia-epidural block. For pregnant women without contraindications who volunteered contraception, analgesia was performed before the cervix was <2 cm. Birth weight was divided at a 500 g interval: 2500 g ≤birth weight < 3000 g, 3000 g ≤birth weight < 3500 g and 3500 g ≤birth weight< 4000 g. Maternal age was divided at 5 years intervals: age <25, 25 ≤ age <30, 30 ≤ age <35, and 35 ≤ age.
2.5. Statistical analysis
All statistical analyses were performed using windows-based SPSS version 20.0 (IBM Corp, Armonk, NY). Data with normal distribution were presented as mean ± standard deviation, the comparison between the 2 groups was assessed by independent samples t test, multiple comparison was determined by analysis of variance, and pairwise comparison was detected by the Student-Newman-Keuls method. Non-normal distribution of data was expressed by the median (maximum) that the comparison between groups using nonparametric test. Count data between the 2 groups were analyzed using χ2 test. A general linear model was used to perform multivariate analysis of labor time. A 2 tail P < .05 was considered statistically significant.
3. Results
3.1. Demographic data
From June 2008 to June 2013, 37,072 women were reviewed. Among them, 10,539 women had a normal vaginal birth. Of these, 1601 women were suitable for analysis. The women were grouped by parity to the nulliparous women group (n = 1367) and the multiparous women group (n = 234) (Fig. 1). The maternal age, BMI, and neonatal weight of nulliparous women were significantly lower than that of multiparous women. The pregnancy complications (such as gestational diabetes mellitus, intrahepatic cholestasis of pregnancy), and neonatal birth weight were comparable between the groups (P > .05) (Table 1).
Figure 1.
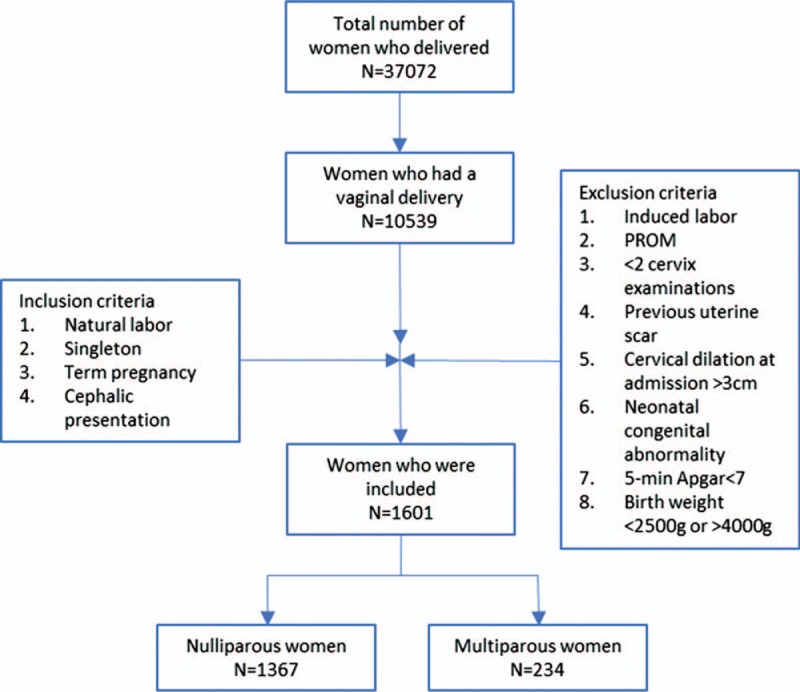
Flow chart of patient selection. PROM = premature rupture of membranes.
Table 1.
Demographic data.

Based on the possible factors affecting the birth process, women were further subdivided into medical intervention group (amniotomy or oxytocin or labor analgesia), and nonmedical intervention group to analyze the possible association of these factors with labor duration.
3.2. The characteristics of the labor stages
The mean and upper limit time of the first stage of labor in the nulliparous women was 8.3 and 15.9 hours; the latent phase was 5.1 and 11.5 hours; the active phase is 3.2 and 6.8 hours; the second stage was 44 to 106 minutes; the third stage was 7 to 15 minutes; and the total labor was 9.1 and 16.9 hours, respectively. Using the modern definition,[4] the mean and upper limit time of latent phase was 7.0 and 14.2 hours, and active phase was 1.3 and 3.1 hours, respectively (Table 2). The mean and upper limit of the first stage of labor in the multiparous women was 5.0 and 10.2 hours; the latent phase was 3.5 and 8.3 hours; the active phase was 1.5 and 3.5 hours, respectively; the second stage was 18 to 46 minutes; the third stage was 7 to 15 minutes, and the total labor was 5.4 to 10.6 hours. Furthermore, the mean and upper limit time of latent phase was 4.4 and 9.6 hours, active phase was 0.6 and 1.6 hours, respectively (Table 2). Nulliparous women's latent period, active period and second stage, and the total labor were all significantly longer, compared to multipara women (P < .05); and there was no significant difference between the 2 groups in the third stage of labor (P > .05).
Table 2.
Labor duration.

3.3. Medical interventions
As shown in Table 3, under the medical intervention, for a primipara who used artificial rupture of membranes and oxytocin at the same time, in the absence of labor analgesia, the average time of first stage of labor was the shortest, which was 347.33 ± 193.30 minutes, and the total labor duration was very short, which was 396.87 ± 210.06 minutes.
Table 3.
Description of birth process after medical intervention in primipara (mean ± standard deviation).

As shown in Table 4, under the medical intervention, for all parturients who used artificial rupture of membranes and oxytocin at the same time, under the condition of absence of labor analgesia, the average time of first stage of labor was the shortest, which was 326.47 ± 187.79 minutes, and the total labor duration was very short, which was 368.62 ± 205.09 minutes.
Table 4.
Description of birth process after medical intervention in all parturients (mean ± standard deviation).

3.4. Maternal age
There were no statistically significant differences in the duration of labor among the different age groups in nulliparous women. Except, a significantly prolonged second stage was seen in women who were 30 to 35 years old compared to women who were younger than 25 years. There was no significant difference in the dilatation rate of the uterine orifice among the delivery age groups (P > .05). There were no statistically significant differences between the median lengths of labor among the different age groups in multiparous women, except for a significantly longer second stage in women who were older than 35 years compared with women who were younger than 25 years (Table 5).
Table 5.
Effects of age on labor duration in nulliparous women and multiparous women (mean ± standard deviation).

3.5. Birth weight
There were significant differences in the first stage of labor, the active phase, the second stage of labor, and total labor among the different birth weight groups in nulliparous women (P < .05). Nevertheless, there was no significant difference in latent period time (P > .05) (Table 6). Differences in all the labor length were not significant among the different birth weight groups in multiparous women (P > .05) (Table 6).
Table 6.
Effects of birth weight on labor duration in nulliparous women and multiparous women (mean ± standard deviation).

As shown in Tables 7 and 8, under the circumstance in which other factors did not change, among primipara, the average time of first stage of labor and total labor duration increased by 58.257 and 62.431 minutes by using artificial rupture of membranes; the average time of first stage of labor and total labor duration increased by 171.443 and 173.379 minutes by using labor analgesia, the birth weight increased by 1 kg, the average time of first stage of labor and total labor duration increased by 56.581 and 66.001 minutes, the average time of first stage of labor and total labor duration decreased by 63.592 and 66.982 minutes by using oxytocin, respectively, and the difference was significant. The average time of first stage of labor and total labor duration in primipara aged from 26 to 30 years increased by 2.356 and 9.248 minutes, respectively, in comparison with primipara aged from 20 to 26 years; the average time of first stage of labor and total labor duration in primipara aged from 30 to 39 years increased by 1.802 and 14.134 minutes, respectively, in comparison with primipara aged from 20 to 26 years; the difference was not significant. As shown in Tables 9 and 10, among all the puerperants, there was no significant difference in the effect of various medical interventions on the time of first birth process and total labor duration in comparison with primipara.
Table 7.
Multiple factor analysis of primipara (time of first stage of labor was used as dependent variable).

Table 8.
Multiple factor analysis of primipara (total labor duration was used as dependent variable).

Table 9.
Multivariate analysis of all puerperants (time of first stage of labor was used as dependent variable).

Table 10.
Multivariate analysis of all puerperants (total labor duration was used as dependent variable).

4. Discussion
In this study we explored whether parity, maternal age, medical interventions, and birth weight were associated with labor duration in Chinese pregnant women. The results showed that the length of labor was significantly shorter in multiparous women than that in nulliparous women, which was similar to previous study.[25] Increased neonatal birth weight significantly increased the length of the active phase and the second stage among nulliparous women. However, the impact of these factors was not observed in multiparous women. We also found that the second stage of labor increased with increase in maternal age in both groups.
Since the first publications on partographs, the issues of the labor phase and dilatation of the cervix have been controversial. Friedman[1,2] defined the length of active phase as 4.9 hours in nulliparous women and 2.2 hours in multipara, respectively. More recently in United States, Zhang et al[4] published a study based on their observations from 62,415 parturient. They identified that nulliparas and multiparas appeared to progress at a similar pace before 6 cm. However, after 6 cm dilation, labor accelerated much faster in multiparas than in nulliparas. The phases of labor in this study were consistent with previous Chinese studies,[22] but were shorter than the data published from other countries.[26] Several factors may be attributable to the difference. The first, 45% of the parturients included in our study were given interactions for augmentation, which may have altered the natural labor progression. The second, measurement of cervical dilation and station was subjective. The third, the majority of women in our data set did not have a deceleration phase, but this difference seen between the Friedman curve and ours may be due to the frequency of vaginal examination in the late first stage of labor. Whether the deceleration phase is caused by delayed vaginal examination needs more research. The fourth, bias may arise due to the increased cesarean rates in China, and the last 1 was that our population study was from a single institution, differences observed in our local population may not exist in other populations.
It was believed that medical interventions with amniotomy and oxytocin, could accelerated labor, and also by labor analgesia.[27] However, a Cochrane review[28] assessed the use of amniotomy in spontaneously started labor. The evidence showed that the length of first stage of labor was shorter in the women having their membranes ruptured, but not significant different. The researchers also concluded that routine amniotomy might probably increase the likelihood of cesarean surgery and should not be done routinely. In the present study, 721 (45%) women had their membranes ruptured or were given oxytocin due to their prolonged labor. The length of active phase was longer in nulliparous women with labor medical intervention than the control group. In the multipara group, there was no significant difference (P > .05). The results showed that the use of oxytocin could shorten labor duration, whereas the use of artificial rupture of membrane and labor analgesia increases the first stage of labor and total labor duration. There was no obvious progress in the birth process of puerperant's with poor progress of labor after using artificial rupture of membrane, whereas labor duration could be decreased after using oxytocin. There was no correlation between oxytocin, labor analgesia, and artificial rupture of membrane.
Previous study had shown that the duration of the second stage of labor increases directly with maternal age.[29] The myometrial tissues may undergo a physiologic aging process, or the myometrium may become less effective or responsive to oxytocic agents with age. In addition, skeletal muscular strength declines with age. Other factors include increased complications and great psychological pressure. Greenberg et al[19] conducted a retrospective study of 2500 delivery cases. They reported that length of labor differed significantly by maternal age for both nulliparous and multiparous women. Older nulliparous women were more likely to experience a longer first stage than younger nulliparous women, and both nulliparous and multiparous women who were older were more likely to experience a prolonged second stage of labor.[19] Our study showed that the first stage of labor and total labor duration would be prolonged as maternal age increased.
Leftwich et al[30] have shown that in a large cohort of contemporary laboring women, as birth weight increases, progression in labor is, in fact slower. In the present study, in nulliparous women, there were significant differences in the length of first stage of labor, active phase, the second stage of labor, and total labor duration among the different neonatal birth weight groups. With the increase in birth weight, the first stage of labor and total labor duration were prolonged.
The limitations of the current study are worth mentioning. First, although this is a retrospective study, the information on the exposures and outcome was recorded to the medical charts at the time being. Thus the information bias is considered as minimal. Although we controlled for many identifiable confounders using multivariable analysis, there may be other confounders that we did not conceive. For example, women with medical interventions were more likely to have prolonged labor processes. Second, obstetric practices differ between hospitals. And the higher cesarean rate could cause an inherent selection bias from informative censoring.
Acknowledgment
Not applicable.
Author contributions
Conceptualization: Rong Zhou.
Data curation: Hongqin Chen, Liyuan Cao, Wen Cao.
Formal analysis: Hongqin Chen, Liyuan Cao, Wen Cao, Hui Wang.
Investigation: Liyuan Cao, Wen Cao, Hui Wang.
Methodology: Hongqin Chen, Liyuan Cao, Hui Wang, Rong Zhou.
Project administration: Hongqin Chen, Wen Cao, Hui Wang.
Resources: Cairong Zhu.
Software: Hongqin Chen, Hui Wang, Cairong Zhu.
Supervision: Hui Wang, Cairong Zhu.
Visualization: Cairong Zhu.
Writing – original draft: Hongqin Chen.
Writing – review and editing: Hongqin Chen, Liyuan Cao, Hui Wang, Cairong Zhu, Rong Zhou.
Footnotes
Abbreviation: BMI = body weight index.
The study was supported by the Key Research and Development Program of Sichuan Science and Technology Agency (No. 2017FZ0067).
The authors have no conflicts of interest to disclose.
References
- [1].Friedman E. The graphic analysis of labor. Am J Obstet Gynecol 1954;68:1568–75. [DOI] [PubMed] [Google Scholar]
- [2].Friedman EA. Primigravid labor; a graphicostatistical analysis. Obstet Gynecol 1955;6:567–89. [DOI] [PubMed] [Google Scholar]
- [3].Friedman EA. An objective approach to the diagnosis and management of abnormal labor. Bull N Y Acad Med 1972;48:842–58. [PMC free article] [PubMed] [Google Scholar]
- [4].Zhang J, Landy HJ, Branch DW, et al. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol 2010;116:1281–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yang L, Xu B, Hu Y. A meta-analysis of changes in duration of labor. Chin J Obstet Gynecol 2012;47:431–5. [PubMed] [Google Scholar]
- [6].Lavender T, Cuthbert A, Smyth RM. Effect of partograph use on outcomes for women in spontaneous labor at term and their babies. Cochrane Database Syst Rev 2018;8:CD005461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Laughon SK, Branch DW, Beaver J, et al. Changes in labor patterns over 50 years. Am J Obstet Gynecol 2012;206:419.e1-e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yang L, Xu BY, Hu YL. Change of labor duration: a systematic analysis. Zhonghua Fu Chan Ke Za Zhi 2012;47:431–5. [PubMed] [Google Scholar]
- [9].Zhang J, Troendle JF, Yancey MK. Reassessing the labor curve in nulliparous women. Am J Obstet Gynecol 2002;187:824–8. [DOI] [PubMed] [Google Scholar]
- [10].Pitchaimuthu N, Bhaskaran S. Labor pattern among primigravida in local population. J Obstet Gynaecol India 2018;68:482–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Worstell T, Ahsan AD, Cahill AG. Length of the second stage of labor what is the effect of an epidural. Obstet Gynecol 2014;123:84S. [Google Scholar]
- [12].Cheng YW, Shaffer BL, Nicholson JM, et al. Second stage of labor and epidural use: a larger effect than previously suggested. Obstet Gynecol 2014;123:527–35. [DOI] [PubMed] [Google Scholar]
- [13].Zondag DC, Gross MM, Grylka-Baeschlin S, et al. The dynamics of epidural and opioid analgesia during labour. Arch Gynecol Obstet 2016;294:967–77. [DOI] [PubMed] [Google Scholar]
- [14].Leighton BL, Halpern SH. The effects of epidural analgesia on labor, maternal, and neonatal outcomes: a systematic review. Am J Obstet Gynecol 2002;186:S69–77. [DOI] [PubMed] [Google Scholar]
- [15].Wang F, Shen X, Guo X, et al. Epidural analgesia in the latent phase of labor and the risk of cesarean delivery: a five-year randomized controlled trial. Anesthesiology 2009;111:871–80. [DOI] [PubMed] [Google Scholar]
- [16].Wong CA, McCarthy RJ, Sullivan JT, et al. Early compared with late neuraxial analgesia in nulliparous labor induction: a randomized controlled trial. Obstet Gynecol 2009;113:1066–74. [DOI] [PubMed] [Google Scholar]
- [17].Halpern SH, Abdallah FW. Effect of labor analgesia on labor outcome. Curr Opin Anaesthesiol 2010;23:317–22. [DOI] [PubMed] [Google Scholar]
- [18].Papadias K, Christopoulos P, Deligeoroglou E. Creatsas G, Mastorakos G, Chrousos GP. Maternal age and the duration of the second stage of labor. Women's Health and Disease: Gynecologic, Endocrine, and Reproductive Issues. New Jersey: Wiley-Blackwell; 2006. 414–7. [DOI] [PubMed] [Google Scholar]
- [19].Greenberg MB, Cheng YW, Sullivan M, et al. Does length of labor vary by maternal age? Am J Obstet Gynecol 2007;197:428.e1-7. [DOI] [PubMed] [Google Scholar]
- [20].Suzuki R, Horiuchi S, Ohtsu H. Evaluation of the labor curve in nulliparous Japanese women. Am J Obstet Gynecol 2010;203:226.e1-6. [DOI] [PubMed] [Google Scholar]
- [21].Incerti M, Locatelli A, Ghidini A, et al. Variability in rate of cervical dilation in nulliparous women at term. Birth 2011;38:30–5. [DOI] [PubMed] [Google Scholar]
- [22].Zhang J, Troendle J, Mikolajczyk R, et al. The natural history of the normal first stage of labor. Obstet Gynecol 2010;115:705–10. [DOI] [PubMed] [Google Scholar]
- [23].Albers LL, Schiff M, Gorwoda JG. The length of active labor in normal pregnancies. Obstet Gynecol 1996;87:355–9. [DOI] [PubMed] [Google Scholar]
- [24].Steven G. Obstetrics: Normal and Problem Pregnancies. 7th edPhiladelphia, PA: Elsevier; 2007. [Google Scholar]
- [25].Vahratian A, Hoffman MK, Troendle JF, et al. The impact of parity on course of labor in a contemporary population. Birth 2006;33:12–7. [DOI] [PubMed] [Google Scholar]
- [26].Neal JL, Lowe NK, Ahijevych KL, et al. Active labor” duration and dilation rates among low-risk, nulliparous women with spontaneous labor onset: a systematic review. J Midwifery Womens Health 2010;55:308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Vincent M. Amniotomy: to do or not to do? RCM Midwives 2005;8:228–9. [PubMed] [Google Scholar]
- [28].Smyth RM, Markham C, Dowswell T. Amniotomy for shortening spontaneous labour. Cochrane Database Syst Rev 2013. CD006167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rasmussen S, Bungum L, Hoie K. Maternal age and duration of labor. Acta Obstet Gynecol Scand 1994;73:231–4. [DOI] [PubMed] [Google Scholar]
- [30].Leftwich HK, Gao W, Wilkins I. Does increase in birth weight change the normal labor curve? Am J Perinatol 2015;32:87–92. [DOI] [PubMed] [Google Scholar]


