Abstract
Migraine is a relatively common disease that is associated with high disability and reduced quality-of-life. This study aimed to investigate the prevalence, epidemiological characteristics, and risk factors of migraine in Han Chinese from Fujian Province, China.
A cross-sectional epidemiological survey study was conducted to evaluate characteristics of migraine in Han Chinese. Demographic and clinical data were collected through a survey administered in face-to-face interviews by trained investigators, and a physical exam and symptom review were performed. Univariate and multivariate regression analyses were performed to assess independent risk factors for migraine.
A total of 7860 subjects aged 15 years and older were surveyed, of which 9.1% (n = 717) were diagnosed with migraine. Among these, a higher percentage was female (12.6%) than male (5.3%). Only 114 subjects (15.9%) were diagnosed as having migraine with aura, which was closely associated with family history of migraine. Multivariate regression analysis showed that the odds of migraine were significantly lower in subjects aged ≥50 years compared with those aged <30 years (odds ratio [OR] ranged from 0.40 to 0.64; P ≤.013) and was higher in females compared with males (OR = 2.89, P <.001). The odds of migraine was significantly greater in subjects with a history of alcohol consumption (OR = 1.81, P <.00) and insomnia (OR = 2.77, P <.001).
Han Chinese in Fujian province has a relatively high prevalence of migraine, and female gender, <50 years of age, insomnia, and use of alcohol are associated with increased odds of having migraine in this population.
Keywords: aura, epidemiology, Fujian, Han Chinese, headache, migraine
1. Introduction
Migraine and tension-type headache are among the most prevalent disorders of humans and migraine alone is responsible for 1.3% of all years of life lost to disability worldwide.[1] Consequently, the World Health Organization (WHO) considers migraine to be a high-priority public health concern.[2] Migraine affects a person's daily life, work, and ability to study in about 2-thirds of people with migraines, and about 50% are unable to work, study, or complete daily housework.[3,4] The prevalence of migraine in China shows an increasing trend; in 2011 it was reported to be 4.32%,[5] which was higher than the 0.69% reported in 1985.[6] However, the reported prevalence of migraine in China is still lower than that reported in France (7.9%),[7] the United States (15.3%),[8] Sweden (13.2%),[9] and Spain (8.4%).[10] A cross-sectional study in China found that the frequency of primary headache was 50.1% in a general neurological outpatient population, of which 23.8% were considered to be migraine.[11] Data suggest that migraine is under-recognized and undertreated in China, highlighting the importance of adopting public health policies to address the disease.[5]
The Chinese Fujian province contains multiple ethnic groups, including Han and She Chinese. Our prior cross-sectional study investigated the prevalence and risk factors of migraine in She Chinese residing in Fujian province, finding that being female and having a history of insomnia were significantly associated with migraine.[12] The She Chinese are an ethnic minority in Fujian province and have their own lifestyle, customs, and genetic traits distinct from Han Chinese and other Asian populations.[13] The Han Chinese represent about 97.8% of the population in Fujian province. Although a recent study investigated migraine susceptibility genes in Han Chinese of Fujian province,[14] little is known regarding the prevalence and epidemiology of migraine in the Han population in this province. Knowledge of prevalence data on migraine, risk factors, and comorbidities are considered crucial in helping to assess the public health burden of migraine.[15] Therefore, the aim of this epidemiological study was to characterize the prevalence, characteristics, and associated risk factors of migraine in the Han population in Fujian province, China.
2. Methods
2.1. Study design and ethical considerations
This study is a cross-sectional survey with multi-step stratification sampling based on gender and age and was conducted between June 2014 and June 2015 in the Han Chinese population of Fujian province. It follows the study design of our earlier epidemiological study of the She population in Fujian province, as described previously.[12] The study protocol was approved by the Ethics Committee of Ningde Municipal Hospital, Ningde City, Fujian, China. All included subjects provided signed informed consent.
The included subjects and their parents were confirmed to be Han Chinese and to be permanent residents based on the household registration of the local government. The sampling process was stratified according to the 5 geographic regions of Fujian province (north, south, east, west, and middle) from which a total of 30 villages or communities were randomly sampled. All subjects were Han Chinese with traditional lifestyle and diets.
2.2. Study subjects
Included subjects were Han Chinese ≥15 years of age who were permanent residents of Fujian province and had the ability to describe their symptoms. Subjects were excluded if they had been diagnosed with other diseases or disorders that could cause headache. Included subjects were registered by the leader or coordinator of the village and screened in the social activity center of the village. Administration of the questionnaire and the physical exam were also performed at the social activity center.
2.3. Study questionnaire and interview
The study questionnaire was designed according to the International Criteria for migraine developed by the Headache Classification Committee of the International Headache Society (see Supplemental Material for a sample of the questionnaire).[16] The first part of the questionnaire collected baseline information including name, gender, age, occupation, height, body weight, and education level. The second part of the questionnaire captured the clinical characteristics per the diagnostic criteria for migraine with aura and migraine without aura developed by the International Headache Classification Committee (third edition; ICHD-3).[16] This part of the questionnaire also collected family history, duration of symptoms, predisposing factors, and treatments. Symptoms sensed by the subjects and dysphasic speech were directly evaluated by the investigators during the face-to-face interview and were not included in the questionnaire.
The survey was conducted via face-to-face structured interviews by trained investigators and not by subject self-report. Investigators were from the Department of Neurology from the Ningde Municipal Hospital. Before the study, investigators participating in the survey received 1-week training on communication skills, questioning skills, use of questionnaire, explanation of the questions, data analysis, and data storage.
2.4. Physical examination and diagnosis of headache
The height, body weight, and sitting blood pressure were measured for each subject on the first day of the study. Fasting blood was collected on the second day and stored at -20oC until laboratory testing was performed. Glucose tolerance was assessed by having the subject drink glucose solution (75 g) and measuring blood glucose concentration 2 hours later. If the subjects had chronic diseases (such as diabetes and hypertension) or other diseases (common somatic and neurological diseases) the subject was reexamined with their previous medical records at the time of their physical examination to confirm any prior diagnosis.
For this study, hypertension was defined according to the 2017 Clinical Practice Guidelines of the Centers for Disease Prevention and Control (CDC),[17] as follows: systolic blood pressure greater than or equal to 140 mmHg (18.6 kPa) and/or diastolic blood pressure greater than or equal to 90 mmHg (12 kPa) in the absence of antihypertensive drugs. Insomnia was defined according to the following diagnostic criteria from the Diagnostic and Statistical Manual of Mental Disorders (DSM), 5th edition,[18] as follows:
Sleep disorder symptoms, including difficulty in falling asleep, easy or early awakening, dreaminess, difficulty in sleeping after waking, and feeling tired after waking, or sleepy during the day;
Sleep disorder symptoms occurring at least 3 times a week for more than 1 month;
Insomnia causing significant distress, or decreased efficiency of mental activity, or impedes social function; and
Insomnia occurring independent of any known physical or mental disorder.[18]
The diagnosis of migraine was made per the information collected in the interview and ICHD-3 criteria and diagnosed by ≥2 chief physicians according to the international diagnostic criteria for migraine developed by the Headache Classification Committee of International Headache Society.[16]
2.5. Statistical analysis
The sample size was validated using the power calculation:
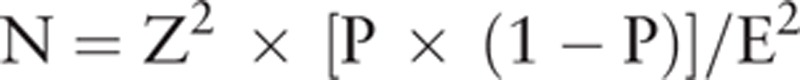 |
Continuous variables are reported as mean and standard deviation. For continuous variables, comparison between subjects with or without migraine was performed by independent t test. Categorical variables are presented as counts and percentages. For categorical variables, chi-square tests were used to compare the differences between subjects with and without migraine or migraine with and without aura. Univariate and multivariate logistic regression analyses were performed to investigate which factors were associated with headache. Factors significantly associated with headache in univariate analysis were included in the multivariate model. The parameters “Z”, “P”, “E” from the analytic equation 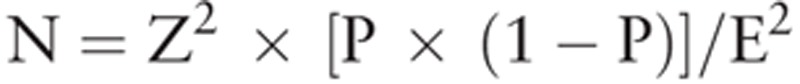 are defined as follows: N = sample size; Z = level of confidence according to the standard normal distribution (for 95% level of confidence, z = 1.96; for 99% level of confidence, z = 2.575); and P = estimated proportion of the population that presents the characteristic (when unknown we used P = .5); and E = tolerated margin of error (e.g., to reveal the real proportion within 5%). Statistical analyses were performed using the IBM SPSS statistical software version 22 for Windows (IBM Corp., Armonk, NY). Two-tailed P value of <.05 indicated statistical significance.
are defined as follows: N = sample size; Z = level of confidence according to the standard normal distribution (for 95% level of confidence, z = 1.96; for 99% level of confidence, z = 2.575); and P = estimated proportion of the population that presents the characteristic (when unknown we used P = .5); and E = tolerated margin of error (e.g., to reveal the real proportion within 5%). Statistical analyses were performed using the IBM SPSS statistical software version 22 for Windows (IBM Corp., Armonk, NY). Two-tailed P value of <.05 indicated statistical significance.
3. Results
3.1. Study population and ethical considerations
A total of 9134 subjects aged ≥15 years participated in the survey (Fig. 1). Among subjects meeting the inclusion criteria, 1274 (13.95%) were later excluded because they refused to receive the physical examination or to provide signed informed consent. Thus, the remaining 7860 subjects were included in the study, including 3749 males (47.7%) and 4111 females (52.3%).
Figure 1.
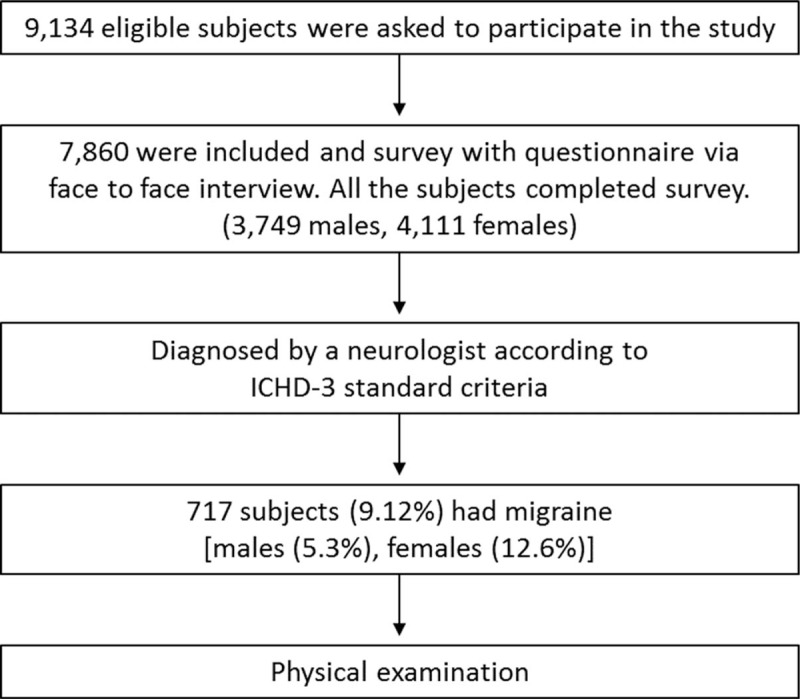
Flow of subjects’ enrollment.
3.2. Subjects’ demographic and clinical characteristics, migraine prevalence and features
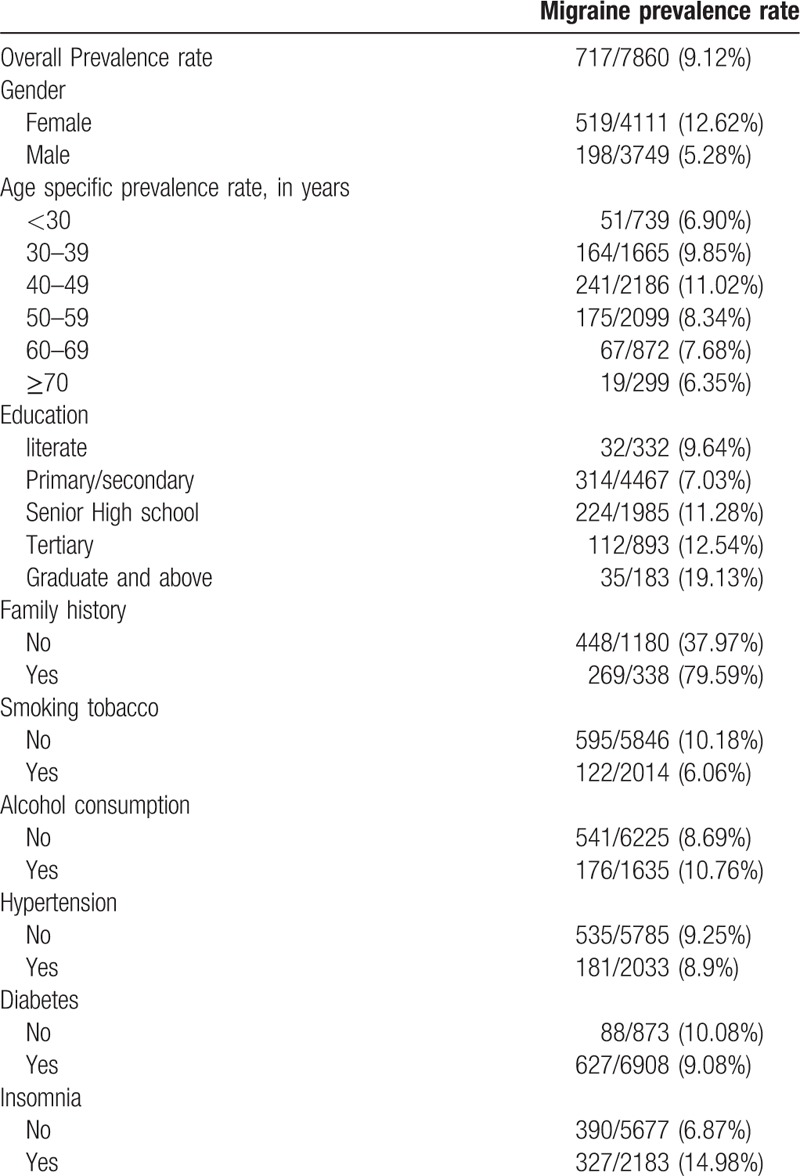
Within the study population, 717 subjects (9.12%) had migraine (Table 1). The prevalence of migraine was greater in females (12.6%) than in males (5.3%). A higher percentage of subjects with graduate or above education (19.1%) and between the ages of 40 to 49 years (11.0%) had migraine compared with other education groups (≤12.5%) or age groups (≤9.9%). Migraine was associated with a family history of migraine (79.6% with a family history vs 38.0% without), no history of smoking (10.2% vs 6.1% with smoking history), insomnia (15% vs 7% without insomnia), and history of drinking alcohol (10.8% vs 8.7% without drinking history). Within the overall population, similar percentages of patients with migraine had hypertension, diabetes, and a history of drinking alcohol.
Table 1.
Demographic and clinical characteristics of study subjects showing prevalence rates of migraine.
A similar percentage of males and females did not have migraine (50.3% vs 49.7%, respectively) (Table 2). In patients with migraine, a greater percentage was female (72.4%) than male (27.6%) (P <.001). A higher percentage of subjects without or with migraine were 40 to 59 years of age (54.2% and 58.0%, respectively), and a greater percentage had a primary/secondary education (58.1% and 43.8%) (P <.001). In subjects without or with migraine, the majority had no family history of migraine (91.4% and 62.5%, respectively), had not smoked (73.5% and 83%), did not consume alcohol (79.8% and 75.5%), and did not have hypertension (73.9% and 74.7%), diabetes (88.9% and 87.7%), or insomnia (74.0% and 54.4%). Between the without migraine and migraine groups, a significantly greater percentage without migraine were male, had no history of migraine, did not drink alcohol, did not smoke, and did not have insomnia (P ≤.010). The 2 groups differed in education levels and age distribution (P <.001). The incidence of hypertension and diabetes was similar between groups (P ≥.333).
Table 2.
Distribution of subjects with and without migraine by demographic and clinical characteristics.

The percentage of males and females with or without migraine with aura was similar (P =.207) (Table 3). A higher percentage of subjects with aura compared with those without aura had a family history of aura (67.5% vs 31.8%) (P <.001). The most common locations of migraine, regardless of aura, was the forehead (38.6% for with aura and 34.8% for without aura; P =.440) and temporal region (49.1% for with aura and 49.8% for without aura: P =.902). The most common method of relieving the headache for subjects with and without aura is sleep (84.2% and 78.6%, respectively) and medication (98.3% and 97.4%; P =.574). In both subjects with and without migraine with aura, the most frequently used method for treating migraine was hospital treatment (51.8% and 53.4%; P =.462). The majority of subjects (51.8% for with aura and 48.6% for without aura; P =.772) lost ≥7 days of work per year due to migraine.
Table 3.
Migraine with and without aura shown by demographic and clinical characteristics.

3.3. Univariate and multivariate analysis of factors associated with migraine
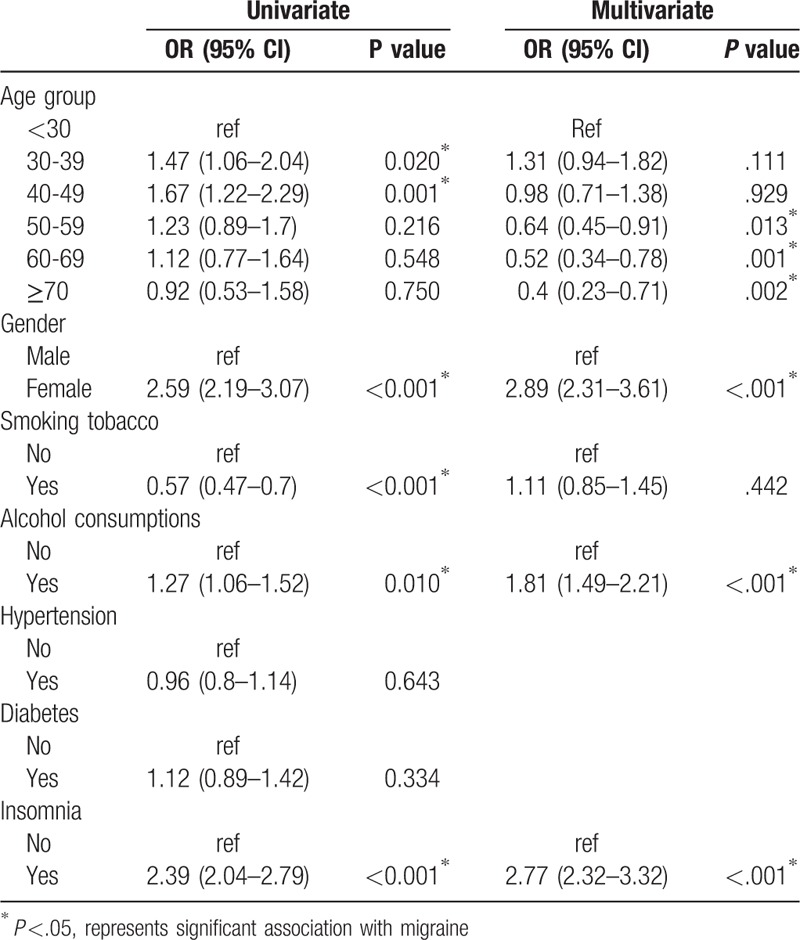
In univariate analyses, age group, gender, smoking, drinking alcohol, and insomnia were found to be significantly associated with migraine. Subjects aged 30 to39 and 40 to 49 years had significantly higher odds of migraine than those aged <30 years (30–39: odds ratio [OR] = 1.47, P =.02; 40–49: OR = 1.67, P =.001). The risk of migraine was significantly increased in females compared with males (OR = 2.59, P <.001), and was significantly lower in subjects who smoked compared with those that did not (OR = 0.57, P <.001). The chance of migraine was significantly increased in subjects with history of drinking alcohol and insomnia (drinking: OR = 1.27, P = .01; insomnia: OR = 2.39, P <.001).
Factors significantly associated with migraine in univariate analysis (i.e., age group, gender, smoking, drinking alcohol, and insomnia) were included in the multivariate analysis (Table 4). After adjusting for other factors, the odds of migraine were significantly lower in subjects aged ≥50 years compared with those <30 years of age (OR ranged from 0.40 to 0.64; P ≤.013) and were higher in females compared with males (OR = 2.89, P <.001). The odds of migraine was significantly greater in subjects with a history of drinking alcohol (OR = 1.81, P <.001) and insomnia (OR = 2.77, P <.001).
Table 4.
Factors associated with migraine.
4. Discussion
In the present study, investigation of the prevalence, epidemiological characteristics, and risk factors of migraine in Han Chinese from Fujian province found that overall prevalence of migraine was 9.6% and a higher percentage of those with migraine were female (12.6%) than male (5.3%). Only 114 subjects (15.9%) were diagnosed as having migraine with aura, which was closely related to the family history of migraine. The odds of migraine were significantly lower in subjects aged ≥50 years compared with those <30 years of age and also was higher in females compared with males. The odds of migraine were also increased in subjects <50 years of age, in females, and in subjects with a history of drinking alcohol or having insomnia.
The prevalence of migraine in the present study was higher than that observed in another survey study that assessed migraine in a neurology clinic of a tertiary care hospital in China, which found that prevalence was 4.3% (401/9282) among the subjects surveyed.[4] The percentages of females and males with migraine in the present study were also higher than those of the prior study (6.9% for males and 11.3% for females). Prevalence was also higher than that of a recent community-based, cross-sectional study that assessed migraine in the Songbei district of Harbin, China, which found that the prevalence of migraine was 8.9% and the frequency of migraine in males and females was 3.7% and 12.2%, respectively (P <.001).[15] Those authors also found that female sex, depression, coronary artery disease, chronic obstructive pulmonary disease, ischemic stroke, and hypertension were positively associated with migraine, while age and education level were negatively associated with migraine.[15] In contrast, the present study found no association between hypertension and the odds of having migraine. Interestingly, an Australian study reported that population studies of migraine reported prevalence rates from 2.6% to 21.7%, averaging about 12%; however, migraine prevalence reported by neurologists is significantly higher, ranging from 27.6% to 48.6%, which may suggest that non-neurologists tend to underestimate migraine.[19]
Female gender was positively associated with migraine in the present study and the frequency of migraine was about 2 times higher in females than in males. This gender difference is comparable to results of a recent US study reporting the prevalence of migraine as 20.7% in females and 9.7% in males.[8] The prevalence of migraine worldwide is 14% in females, which is also about 2-fold higher than in males (6%).[20] Migraine is shown to be associated with the female reproductive cycle, including menarche, pregnancy, and menopause. Female reproductive hormones may regulate several mediators and receptor systems to affect the pathophysiology of migraine.[21]
Results of the present study are consistent with prior data indicating that the frequency of migraine varies between different age groups. Around the world, the prevalence of migraine in adults aged 25 to 60 years is 11% and is higher than that reported in children/adolescents (7%) and older adults (≥60 years) (6%).[22] Childhood migraine is often associated with psychological problems and psychiatric comorbidities, particularly in children with migraine without aura.[23] Children with migraine without aura are also observed to be more anxious, fearful, vulnerable to frustration and to have less overall physical endurance.[24] Motor coordination impairment is another comorbidity associated with migraine in children.[25] Assessment of maternal personality, including maternal stress levels, is suggested as a way to understand migraine in children and to manage these children clinically.[26] Although the present study evaluated teens aged 15 to 19 along with adults of all ages, children and adolescents were not included in the analysis.
In the present study, the prevalence of migraine in Han Chinese in Fujian province was highest among subjects aged 40 to 49 years (11.28%). These results are comparable to those from a study conducted in a community in Northeast China, in which the prevalence of migraine was the highest (11.21%) in subjects aged 40 to 59 years.[15] However, our findings differ from those reported in studies from the United States and the United Kingdom. In 2018 in the United States, the highest burden of migraine was reported for subjects aged 18 to 44 (17.9%) and among elderly and disabled individuals (16.4%).[8] In the UK, the prevalence of migraine was found to vary among different age groups; young adults had relatively high prevalence but adults aged 40 to 50 years had a low prevalence of migraine.[27]
Differences across studies appear to reflect the impact of geography, cultural habits, and genetics on the frequency of migraine within a population. In the United States, for example, prevalence was highest among Native Americans and Alaskan Natives compared to white, black, and Hispanic populations, and the lowest prevalence was among Asian Americans.[8] Epidemiological studies have confirmed that migraine is significantly associated with genetic factors and has familial susceptibility, suggesting the important roles of pathogenic genes.[28] Linkage studies in family pedigrees have identified regions of the genome and potential genes that may influence migraine susceptibility.[29] In addition, better understanding of the underlying pathophysiology of migraines has resulted in identification of candidate genes in case-control populations.[29] Clinical expression of migraine is thought to be genetically influenced in up to 60% of cases and influenced by endogenous factors in the other 40%.[30] Recent genome-wide analysis of whole-blood expression probes led investigators to suggest that immune-inflammatory pathways may be involved in the pathogenesis and progression of migraine in some patients.[31] In support of the genetic link with migraine, our study found that family history of migraine was noted in 67.5% of subjects with migraine with aura, which was significantly higher than that in those with migraine without aura (37.5%).
Geography and weather are also thought to impact the incidence and prevalence of migraine. A previous study that investigated the epidemiology of migraine in 22 regions of China, found that the prevalence of migraine tended to be higher in Southern China compared with Northern China,[4] which might be related to differences in climate that are considered predisposing factors of migraine.[32] In addition, certain foods, food preparation, and supplements also have been found to impact the incidence of migraine within a given population.[33]
Migraine has also been shown to be associated with clinical factors such as overweight and obesity.[34] A 2017 study in China showed that patients with episodic and chronic migraine were more likely to be overweight, obese, or morbidly obese, and body mass index (BMI) was significantly associated with migraine attacks, but not with the severity or duration of the attacks.[35]
We also found that insomnia increased the risk of migraine in our study population. In a study from the United States, headache with and without aura was found to be closely associated with sleep disorders, and investigators proposed that headache and insomnia should be treated simultaneously.[36] Another study found that sleep quality was reduced in patients with migraine, but the prevalence of fatigue in these patients was comparable to that in healthy controls.[37] A relationship between sleep disorders and headache was also noted in children, including especially daytime somnolence in children without aura.[38] Nervousness, stress, emotion, strong light, and either lack of or excess sleep have also been associated with migraine.[39] Other factors found to predispose a person for migraine include hormone levels, light, and work stress.[40] Similar to our findings, drinking alcohol was found to be weakly associated with migraine.[40]
The present study has several limitations. Although all subjects received physical examinations and blood analysis, hyperlipidemia and hyperuricemia were not investigated in this study. Hence, we did not address whether migraine is associated with these diseases. This study investigated migraine in Han Chinese of Fujian province, but simple sampling was employed, which may confound the results. In addition, the questionnaire we used was employed to guide face-to-face interviews, but was not strictly verified for assessing migraine. Additional studies are needed that are specifically designed to assess the validity of the questionnaire for migraine. Although all investigators received training before the study started, bias due to the face-to-face interview cannot be excluded. Further epidemiological studies are required to further investigate factors that may influence development of migraine.
5. Conclusion
In conclusion, our findings indicate that Han Chinese in Fujian province has a relatively high prevalence of migraine compared to other ethnic groups in China. Female gender and insomnia are independent risk factors for migraine. Migraine is associated with a family history of migraine, especially in individuals having migraine with aura. Our findings may provide evidence for designing educational plans to help medical policy makers improve the knowledge and treatment of migraine.
Acknowledgments
We would like to extend our thanks to all participants of this study.
Author contributions
Conceptualization: Qi-fang Lin.
Data curation: Long-teng Yao, Yong-tong Xin.
Formal analysis: Qiao-qing Xia, Yu-li Zeng, Long-teng Yao.
Supervision: Qi-fang Lin, Genbin Huang.
Writing - original draft: Yu-li Zeng, Xiao-yang Wu, Lin-feng Ye.
Writing - review & editing: Genbin Huang.
Footnotes
Abbreviation: OR = odds ratio.
This work was supported by grant 2014-CXB-26 from the Fujian Medical Innovating Program, Research Foundations for Young Scholars, Health Department of Fujian Province (Grant No.2014-1-99) and The Ningde Planning Project of Science and Technology (Grant No.20140113).
The authors declare that there is no conflict of interest.
References
- [1].World Health Organization Lifting the Burden, Atlas of Headache Disorders and Resources in the World 2011,. 1st editionGeneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- [2].Leonardi M, Steiner TJ, Scher AT, et al. The global burden of migraine: measuring disability in headache disorders with WHO's classification of functioning, disability and health (ICF). J Headache Pain 2005;6:429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Millichap JG. Acute treatment regimens for migraine in the ED. Pediatr Neurol Briefs 2015;29:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Messali A, Sanderson JC, Blumenfeld AM, et al. Direct and indirect costs of chronic and episodic migraine in the United States: a web-based survey. Headache 2016;56:306–22. [DOI] [PubMed] [Google Scholar]
- [5].Li X, Zhou J, Tan G, et al. Diagnosis and treatment status of migraine: a clinic-based study in China. J Neurol Sci 2012;315:89–92. [DOI] [PubMed] [Google Scholar]
- [6].Zhao F, Tsay JY, Cheng XM, et al. Epidemiology of migraine: a survey in 21 provinces of the People's Republic of China, 1985. Headache 1988;28:558–65. [DOI] [PubMed] [Google Scholar]
- [7].Henry P, Auray JP, Gaudin AF, et al. Prevalence and clinical characteristics of migraine in France. Neurology 2002;59:232–7. [DOI] [PubMed] [Google Scholar]
- [8].Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine headache in the United States: figures and trends from government health studies. Headache 2018;58:496–505. [DOI] [PubMed] [Google Scholar]
- [9].Dahlof C, Linde M. One-year prevalence of migraine in Sweden: a population-based study in adults. Cephalalgia 2001;21:664–71. [DOI] [PubMed] [Google Scholar]
- [10].Matías-Guiu J, Porta-Etessam J, Mateos V, et al. One-year prevalence of migraine in Spain: a nationwide population-based survey. Cephalalgia 2011;31:463–70. [DOI] [PubMed] [Google Scholar]
- [11].Wang Y, Zhou J, Fan X, et al. Classification and clinical features of headache patients: an outpatient clinic study from China. J Headache Pain 2011;12:561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Huang GB, Yao LT, Hou JX, et al. Epidemiology of migraine in the She ethnic minority group in Fujian province, China. Neurol Res 2013;35:684–92. [DOI] [PubMed] [Google Scholar]
- [13].Lin Y, Lai X, Chen B, et al. Genetic variations in CYP17A1, CACNB2 and PLEKHA7 are associated with blood pressure and/or hypertension in She ethnic minority of China. Atherosclerosis 2011;219:709–14. [DOI] [PubMed] [Google Scholar]
- [14].Lin QF, Chen ZC, Fu XG, et al. Migraine susceptibility genes in Han Chinese of Fujian province. J Clin Neurol 2017;13:71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang X, Xing Y, Sun J, et al. Prevalence, associated factors, and impact on quality of life of migraine in a community in northeast China. J Oral Facial Pain Headache 2016;30:139–49. [DOI] [PubMed] [Google Scholar]
- [16].Headache Classification Committee, International Headache Society The international classification of headache disorders 3rd ed. Cephalagia 2018;38:1–60. [DOI] [PubMed] [Google Scholar]
- [17].Centers for Disease Prevention and Control. Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Atlanta, GA, USA: CDC, 2017. [Google Scholar]
- [18].American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 2013;Washington, D.C: APA, 5th edition. [Google Scholar]
- [19].Yeh WZ, Blizzard L, Taylor BV. What is the actual prevalence of migraine? Brain Behav 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stovner LJ, Hagen K, Jensen R, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia 2007;27:193–210. [DOI] [PubMed] [Google Scholar]
- [21].Vetvik KG, MacGregor EA. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol 2017;16:76–87. [DOI] [PubMed] [Google Scholar]
- [22].Bigal ME, Liberman JN, Lipton RB. Age-dependent prevalence and clinical features of migraine. Neurology 2006;67:246–51. [DOI] [PubMed] [Google Scholar]
- [23].Esposito M, Parisi L, Gallai B, et al. Attachment styles in children affected by migraine without aura. Neuropsychiatr Dis Treat 2013;9:1513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Esposito M, Marotta R, Gallai B, et al. Temperamental charcteristics of children with migraine without aura: a multicenter study. Neuropsychiatr Dis Treat 2013;9:1187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Esposito M, Verrotti A, Gimigliano F, et al. Motor coordination impairment and migraine in children: a new comorbidity? Eur J Pediatr 2012;171:1599–604. [DOI] [PubMed] [Google Scholar]
- [26].Esposito M, Roccella M, Gallai B, et al. Maternal personality profile of children affected by migraine. Neuropsychiatr Dis Treat 2013;9:1351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Steiner TJ, Scher AI, Stewart WF, et al. The prevalence and disability burden of adult migraine in England and their relationships to age, gender and ethnicity. Cephalalgia 2003;23:519–27. [DOI] [PubMed] [Google Scholar]
- [28].Lemos C, Castro MJ, Barros J, et al. Familial clustering of migraine: further evidence from a Portuguese study. Headache 2009;49:404–11. [DOI] [PubMed] [Google Scholar]
- [29].Gasparini CF, Sutherland HG, Griffiths LR. Studies on the pathophysiology and genetic basis of migraine. Curr Genomics 2013;14:300–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ferrari MD, Klever RR, Terwindt GM, et al. Migraine pathophysiology: lessons from mouse models and human genetics. Lancet 2015;14:65–80. [DOI] [PubMed] [Google Scholar]
- [31].Gerring ZF, Powell JE, Montgomery GW, et al. Genome-wide analysis of blood gene expression in migraine implicates immune-inflammatory pathways. Cephalagia 2018;38:292–303. [DOI] [PubMed] [Google Scholar]
- [32].Yang AC, Fuh JL, Huang NE, et al. Temporal associations between weather and headache: analysis by empirical mode decomposition. PLoS One 2011;6:e14612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sun-Edelstein C, Mauskop A. Foods and supplements in the management of migraine headaches. Clin J Pain 2009;25:446–52. [DOI] [PubMed] [Google Scholar]
- [34].Lillis J, Graham J, Thomas EK. Importance of pain acceptance in relation to headache disability and pain interference in women with migraine and overweight/obesity. Headache 2017;57:709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Huang QQ, Lian XP, Wang SQ, et al. Association between body mass index and migraine: a survey of adult population in China. Behav Neurol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lateef T, Swanson S, Cui L, et al. Headaches and sleep problems among adults in the United States: findings from the National Comorbidity Survey–Replication Study. Cephalalgia 2011;31:648–53. [DOI] [PubMed] [Google Scholar]
- [37].Seidel S, Hartl T, Weber M, et al. Quality of sleep, fatigue and daytime sleepiness in migraine—a controlled study. Cephalalgia 2009;29:662–9. [DOI] [PubMed] [Google Scholar]
- [38].Esposito M, Roccella M, Parisi L, et al. Hypersomnia in children with migraine without aura: a questionnaire-based case-control study. Neuropsychiatr Dis Treat 2013;9:289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hauge AW, Kirchmann M, Olesen J. Trigger factors in migraine with aura. Cephalalgia 2010;30:346–53. [DOI] [PubMed] [Google Scholar]
- [40].Hauge AW, Kirchmann M, Olesen J. Characterization of consistent triggers of migraine with aura. Cephalalgia 2011;31:416–38. [DOI] [PubMed] [Google Scholar]


