Abstract
The role of serum vitamin D (Vit D) in cardiometabolic and muscle health remains unclear. The study aimed to evaluate associations of Vit D and factors of healthy aging among community-living middle-aged and older people in Taiwan. Analytic data on 1839 community-living older adults were excerpted from I-Lan Longitudinal Aging Study. All participants were collected demographic characteristics, serum Vit D, functional assessment, and cardiometabolic risk factors. The prevalence of Vit D insufficiency and deficiency in this study was 50.5% and 33.6%, respectively. Among 617 participants with Vit D deficiency, 72.3% of them were women. In multivariate logistic regression, the independent risk factors of Vit D deficiency were male gender (odds ratio [OR]: 0.266; 95% confidence interval [CI]: 0.213–0.333; P < 0.001), higher BMI (OR: 1.036; 95% CI: 1.005–1.067; P = 0.022), high total cholesterol (OR: 1.437; 95% CI: 1.160–1.779; P = 0.001), and high triglyceride (OR: 1.865; 95% CI: 1.446–2.404; p < 0.001). In multinomial logistic regression for 3-level Vit D status analysis, similar trend was found among participants with Vit D insufficiency. Insulin resistance increased in 2.31 and 1.71-folds in Vit D deficiency and insufficiency groups. Besides, association between Vit D deficiency and osteopenia was found only in women. In conclusion, Vit D deficiency was more common in women, and associated with poorer musculoskeletal health and higher cardiovascular and metabolic risk, including higher BMI, DBP, insulin resistance, total cholesterol, and triglyceride.
Keywords: cardiometabolic risk, musculoskeletal health, vitamin D
1. Introduction
Vitamin D (Vit D) was composed by a group of secosteroids, which was responsible for intestinal absorption of calcium, magnesium, and phosphate .[1] The major physiological function of Vit D was to maintain calcium homeostasis and metabolism, hence, deficiency of Vit D may result in impaired bone mineralization, subsequent rickets in children, osteomalacia and osteoporosis in adults .[2] A number of risk factors for Vit D deficiency had been reported, including older age, malnutrition, obesity, insufficient sun exposure, living conditions, critical illnesses, and others.[3] In addition to bone metabolism, Vit D also played active roles in maintaining physiological function of other organ systems. Owing to the impact of Vit D on multiple organ systems, Vit D deficiency has been reported to be associated with various dysfunctions, including musculoskeletal system (such as osteoporosis[4] and sarcopenia, [5] physical function,[6] cognitive function,[7] and cardiovascular risk).[8,9] A study of 12,644 participants from the Third US National Health and Nutrition Examination Survey (NHANES III) showed inverse association between Vit D level and SBP.[9] However, a randomized controlled trial of supplementation with 2800 IU of Vit D3 per day for 8 weeks did not reveal any significant treatment effects on blood pressure, cholesterol, or insulin resistance. Evidences support that Vit D receptors expressed throughout the cardiovascular system, and activations of Vit D receptor in molecular level demonstrate antiatherosclerotic and protective effects on cardiovascular risk factors.[10] However, mechanism for low Vit D may improve cardiovascular risk remained unclear, and more well-designed randomized controlled trials are needed.[11] There are several potential hypotheses including negative downregulation of the renin–angiotensin–aldosterone system on blood pressure, directly improving vascular compliance or improvement of glycemic control.[12] Interestingly, though previous work found similar prevalence of Vit D deficiency with only minor differences by gender or age, the associated multimorbidities actually showed prevalence diversity between genders of diabetes, hypertension, peripheral vascular disease, coronary artery disease, myocardial infarction, and stroke.[13] Age, gender, and body size also affected seasonal variation of Vit D levels.[14]
Internationally, approximately 1 billion people may be Vit D insufficient or deficient, including healthy community-living people, and even patients receiving medical treatment for osteoporosis .[2] Although supplementation of Vit D was expected to reverse the adverse impact of Vit D deficiency, the evidence was inconsistent.[8,15] The ineffectiveness of Vit D supplementation to prevent adverse outcomes may be resulted from some possibilities: the existence of hidden confounding factors that biased the association between Vit D deficiency and pathology, the lack of comprehensive data collection of study participants in evaluating those associations, and longer period of Vit D supplementation may needed to reverse the adverse impact. Therefore, the main aim of this study was to clarify the associations between serum Vit D status, musculoskeletal health condition, and cardiometabolic risk factors by using a cohort of community-living middle-aged and older adults with comprehensive data collection. Further, gender and age were analyzed in relation to the aforementioned dependent variables.
2. Materials and methods
2.1. Study participants
I-Lan Longitudinal Aging Study (ILAS) recruited community-dwelling people aged 50 years and older living in Yilan County of Taiwan, and the study protocol has been published in several previous studies.[16–20] ILAS aimed to investigate the complex interrelationship between aging, frailty, sarcopenia and cognitive declines. The inclusion criteria were: inhabitants of Yilan County with no intentions of relocating in the near future; and aged 50 years or older. ILAS excluded subjects as follows: unable to communicate with the interviewer or to sign the informed consent; unable to complete a 6-m walk within a reasonable time; with major illness, such as cancer, with life expectancy of less than 6 months; or currently institutionalized at the time of screening. Overall, 1839 participants received face-to-face interviews by the research staff, and all of them received subsequent body composition tests and physical examinations. All participants signed the written informed consent before they were enrolled for study. The institutional review board of the National Yang Ming University approved the study protocol. The study was designed and conducted in accordance with the principles of the Declaration of Helsinki; the cross-section and observational design and reporting format follow the STROBE guidelines.[21] The datasets generated during and/or analyzed during the current study are available from the Institutional Data Access of the National Yang-Ming University/Ethics Committee for researchers who meet the criteria for access to confidential data.
2.2. Demography and functional assessment
For all participants, the research nurses completed the questionnaire of demographic characteristics, past medical history, and personal health behavior. In addition, comprehensive functional assessment was performed for all participants, including Center for Epidemiologic Studies Depression Scale for depressive symptoms,[22] Charlson's comorbidity index for disease severity,[23] short-form mini-nutritional assessment for nutritional status;[24] Mini-Mental State Examination for cognitive status,[25] and Functional Autonomy Measurement System for functional status.[26]
Participants were defined as weakness in those who had lowest quintile of handgrip strength (measured using the Smedley Dynamometer; TTM, Tokyo, Japan), and as slowness in those who had slow walking speed in 6-m walking test. Weakness and slowness referred to those performances lower than the gender-specific lowest 20% of study population. [27]
2.3. Bone quality and appendicular muscle measurement
A whole body dual-energy X-ray absorptiometry scan was performed on each participant to measure their appendicular skeletal muscle mass by using a Lunar Prodigy instrument (GE Healthcare, Madison, WI), which was calculated as the sum of the lean soft tissue mass of all four limbs. Appendicular skeletal muscle mass index was adjusted by height (m) square (ASM/height2, kg/m2). Bone marrow density at the lumbar spine and bilateral hip joints was measured for analysis.
2.4. Laboratory measurements
All participants received venipuncture for blood sampling after 10-h overnight fast. Serum concentrations of fasting glucose (mg/dL), total cholesterol (TC, mg/dL), triglyceride (TG, mg/dL), low-density lipoprotein cholesterol (LDL-C, mg/dL), high-density lipoprotein cholesterol (HDL-C, mg/dL) were measured by using an automatic analyzer (ADVIA 1800, Siemens, Malvern, PA). Whole-blood glycated hemoglobin A1c was measured by enzymatic method using the Tosoh G8 HPLC Analyzer (Tosoh Bioscience, Inc., San Francisco, CA). Serum insulin level (uIU/mL) was measured by the chemiluminescence immunoassay analyzer (ADVIA Centaur, Siemens). Homeostatic model assessment-insulin resistance (HOMA-IR) was calculated as (glucose (mg/dL) × insulin (uIU/mL))/405.[28]
2.5. Definition of Vit D status
Serum level less than 20 ng/mL was defined as “deficiency," [2] whereas serum level was between 20 and 30 ng/mL was defined as “insufficiency," and those serum level more than (or equal to) 30 ng/mL was classified as “normal."[2,29]
2.6. Definition of cardiometabolic risk
In this study, cardiometabolic risk factors were defined as follows: high blood pressure was defined as “SBP ≥130 mm Hg” or “DBP ≥85 mm Hg,” TC ≥200 mg/dL as “high TC,” serum HDL-C <40 mg/dL in men or <50 mg/dL in women as “low HDL-C,” serum TG ≥150 mg/dL as “high TG,” serum LDL-C ≥130 mg/dL as “high LDL-C,” and waist circumference ≥ 90 cm in men and ≥80 cm in women as “abnormal waist circumference.”.[30] We defined “high HOMA-IR” as highest quintile from the population in this study.
2.7. Statistical analysis
Continuous variables were expressed as mean (standard deviation), and categorical data were expressed as frequency and percentage. Regarding basic characteristics analysis, comparisons between categorical variables were performed by Chi-square or Fisher's exact test when appropriate; comparisons between continuous variables were performed by Student t test. While analyzing variables between different serum Vit D levels, one-way analysis of variance was used to compare continuous variables between multiple categories. Analysis of covariance was applied to evaluate the association between serum Vit D level and physical function, musculoskeletal health, and cardiometabolic risk factors adjusted for age and sex.
Binary logistic regressions were used to explore the independent associative factors for Vit D deficiency, and factors with potential association (P < 0.10) were used for multivariate logistic regression model with Backward Wald method. Further, building the modeling of Vit D insufficiency and deficiency (reference group: participants with normal Vit D levels), multinomial logistic regression model was used to evaluate the independent association between parameters of cardiovascular system and musculoskeletal system after adjustment of potential confounding factors. Confounding factors including age and gender were adjusted to evaluate the odds ratio in different models. All statistical analyses were performed by statistical software SPSS 22.0 (SPSS, Chicago, IL). For all P value less than 0.05 was considered as statistically significant.
3. Results
Overall, data of 1839 participants were obtained for study. The mean age and prevalence of chronic illness were similar compared to the Taiwan Longitudinal Study on Ageing survey. [31]Table 1 summarized the baseline characteristics and demographic characteristics of all participants. The prevalence of Vit D deficiency in this study was 33.6%, and 50.5% for Vit D insufficiency. A significant sex difference was identified in this study that 72.3% of subjects with Vit D deficiency was women, and only 23.9% subjects with normal Vit D level was women. Compared to men, women were significantly younger, having lower educational level, lower bone mineral density, higher score of center for epidemiological studies depression, higher score of mini-mental status examination, and higher prevalence of dyslipidemia (Table 1).
Table 1.
Baseline sex stratified characteristics of participants.
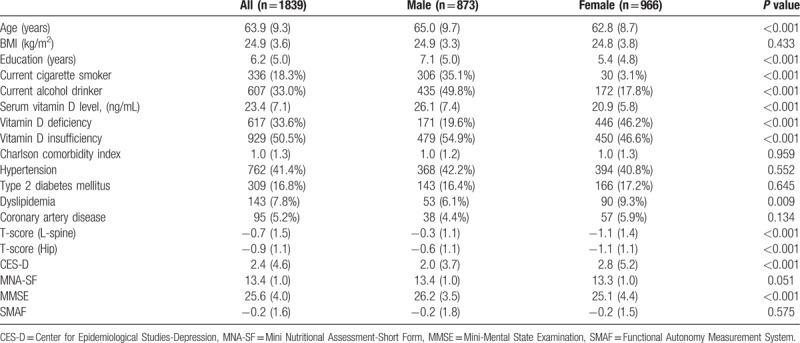
Table 2 compared subjects with different status of serum Vit D. Adjusted for age and sex, people with Vit D deficiency had significantly poorer physical function and nutritional status. Considering cardiometabolic parameters, subjects with Vit D deficiency had higher BMI, higher waist circumference, more abnormal blood pressure, higher insulin resistance, and higher serum levels of TC, LDL-C, and TG. However, no statistical difference was identified in depressive mood, comorbidity, mean carotid intima media thickness, or serum level of high sensitive C-reactive protein between groups.
Table 2.
Comparison between groups of different serum vitamin D status.
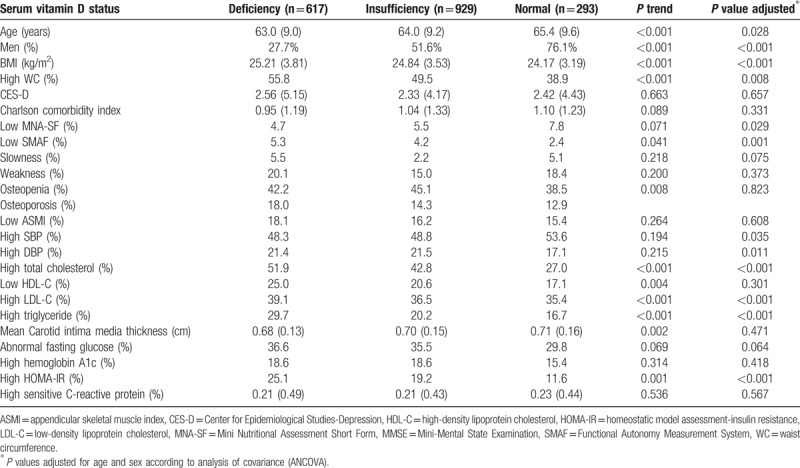
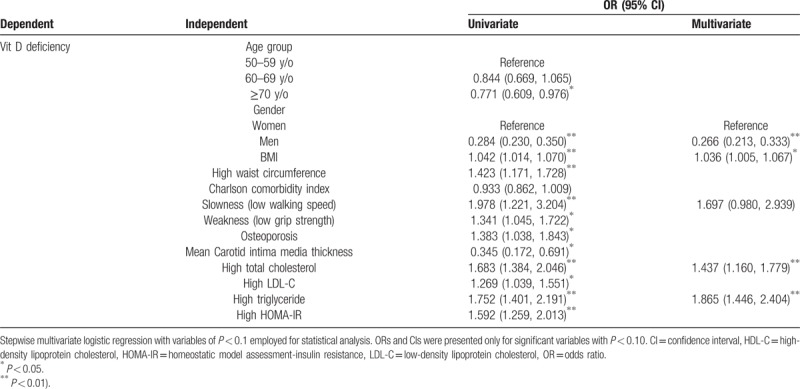
Table 3 showed the associative factors for Vit D deficiency in univariate analysis that age, gender, BMI, high waist circumference, slowness (low walking speed), weakness (low grip strength), osteoporosis, mean carotid intima media thickness, high TC, high LDL-C, high TG, and high HOMA-IR were all significantly associated with Vit D deficiency. In multivariate logistic regression, we found that male gender (odds ratio [OR]: 0.266; 95% confidence interval [CI]: 0.213–0.333; P < 0.001), higher BMI (OR: 1.036; 95% CI: 1.005–1.067; P = 0.022), high TC (OR: 1.437; 95% CI: 1.160–1.779; P = 0.001), and high TG (OR: 1.865; 95% CI: 1.446–2.404; P < 0.001) were associative factors for Vit D deficiency. Meanwhile, slowness (low walking speed) showed borderline association (OR: 1.697; 95% CI: 0.980–2.939; P = 0.059).
Table 3.
Independent associative factors of Vit D deficiency among community-dwelling older adults in Taiwan.
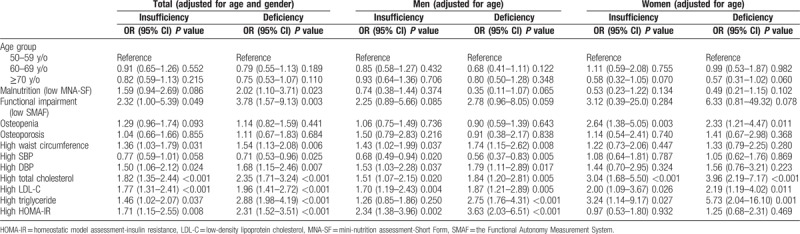
Further, adjusted for potential confounders and compare the 3 levels of vitamin status (deficiency, insufficiency, and normal), Vit D deficiency was significantly associated with higher malnutrition risk (OR: 2.02; 95% CI: 1.10–3.71; P = 0.023), poorer functional status (OR: 3.78; 95% CI: 1.57–9.13; P = 0.003), high waist circumference (OR: 1.54; 95% CI: 1.13–2.08; P = 0.006), high serum TC (OR: 2.35; 95% CI: 1.71–3.24; P < 0.001), high LDL-C (OR: 1.96; 95% CI: 1.41–2.72; P < 0.001), and high TG (OR: 2.88; 95% CI: 1.98–4.19; P < 0.001)(Table 4). Interestingly, higher SBP seemed to be a protective factor (OR: 0.71; 95% CI: 0.53–0.96; P = 0.025). Moreover, insulin resistance was significantly associated with Vit D status, and the lower the Vit D level was, the higher odds ratio and the stronger correlation was. Insulin resistance increased in 2.31 and 1.71-fold in Vit D deficiency and insufficiency group. Besides, DBP was also significantly higher among subjects of Vit D deficiency and insufficiency group (OR: 1.68; 95% CI: 1.15–2.46; P = 0.007 and OR: 1.50; 95% CI: 1.06–2.12; P = 0.024, respectively). Table 4 also showed the associations between Vit D status and other factors in men and women. In men, there were significant associations between Vit D deficiency and cardiometabolic risk including high waist circumference (OR: 1.74; 95% CI: 1.15–2.62; P = 0.008), high DBP (OR: 1.79; 95% CI: 1.11–2.88; P = 0.017), high TC (OR: 1.84; 95% CI: 1.20–2.81; P = 0.005), high LDL-C (OR: 1.87; 95% CI: 1.21–2.89; P = 0.005), and high TG (OR: 2.75; 95% CI: 1.76–4.31; P < 0.001). In women, however, the associations between Vit D deficiency and cardiometabolic risk were weaker. Besides, the association between serum Vit D status and bone health was only seen in osteopenia in women (OR: 2.33; 95% CI: 1.21–4.47; P = 0.011 in Vit D deficiency group and OR: 2.64; 95% CI: 1.38–5.05; P = 0.003 in Vit D insufficiency group).
Table 4.
Odds ratio adjusted for vitamin D status for parameters of function, musculoskeletal health, and cardiometabolic risks according to multinomial logistic regression.
4. Discussion
Findings from the study showed the inverse association between Vit D deficiency and cardiovascular risk factors including TC, TG, LDL-C, and insulin resistance. In addition, Vit D deficiency was associated with muscle strength and performance as handgrip strength and walking speed, respectively.
The prevalence of Vit D deficiency was 33.6%, which was significantly lower than that (66.2%) reported from the Nutrition And Health Survey in Taiwan.[32] The discrepancy may be resulted from the differences in lifestyle and living environment of study participants. Most participants in this study lived at rural communities and carried out heavy daily physical activities and sunlight exposure due to farming activities. Besides, we found significant sex differences in the associations between Vit D deficiency, musculoskeletal health, and cardiometabolic risk. Women were more commonly to be Vit D deficient than men, which was in line with results of the previous study.[33]
The reported associations between Vit D deficiency and cardiovascular risk were compatible with results of this study,[8,9] however, the sex-different associations were also noted in this study. Vit D had been shown to suppress pro-inflammatory cytokines, including tumor necrosis factor-α, and deferred the promotion of arterial stiffness,[34] which may play certain roles in the pathogenesis of cardiovascular disease. In kidney, Vit D may suppress renin production at the juxtaglomerular apparatus,[35] and further regulated the renin–angiotensin–aldosterone system to lower blood pressure. Thus, higher blood pressure may be a consequence to Vit D deficiency. Besides, Vit D also suppressed the expression of CD36 and peroxisome proliferator-activated receptor-r, and may slow the process of developing cardiovascular disease in patients with diabetes mellitus. Reduced Vit D receptor signaling may also increase foam cell formation and accelerated atherosclerosis that promoted the development of cardiovascular disease among subjects with Vit D deficiency.[36] Therefore, low serum Vit D may substantially increase the risk and development of cardiovascular diseases from multiple potential pathophysiological pathways.
The associations between Vit D deficiency and low bone mineral density had been reported in previous studies,[4] but the association was only seen in women with osteopenia in this study. Despite the association between Vit D deficiency and bone mineral density was noted in women, no association was found between skeletal muscle mass and muscle index in any age or sex-specific group. Serum levels of Vit D were not an appropriate biomarker or therapeutic targets for skeletal muscle mass when considering muscle health. [37]
Vit D deficiency may be resulted from multiple etiologies, and the negative impacts of Vit D deficiency were also associated with multiple mechanisms. Hence, the inconsistent results between Vit D supplementation in preventing adverse outcomes were not surprising. On one hand, simple Vit D supplementation may not sufficiently reverse all the cumulative negative impacts of Vit D deficiency, and on the other hand, the potential benefits of Vit D supplementation may need a longer period of time to show.
Despite all efforts went into this study by collecting comprehensive data to clarify the association between Vit D deficiency and various clinical characteristics, there were still some limitations. First, the cross-sectional study design did not provide sufficient information to clarify the causal relationship between Vit D deficiency and clinical characteristics. Second, the duration of Vit D deficiency for the study participants remained unknown, so estimating the overall effect of Vit D deficiency on these clinical characteristics may become inaccurate. Third, participants in ILAS residing in an agriculture county, hence the difference of lifestyle of study participants between this study and other previous ones may be another confounding factor for further comparisons, and might limit the generalizability to urban population.
5. Conclusions
In conclusion, Vit D deficiency or insufficiency was very common among community-living middle-aged and older adults in Taiwan. The associations between Vit D deficiency, musculoskeletal health, and cardiometabolic risk showed a strong age and sex-different relationship. In addition, the lower the Vit D level was, the higher odds ratio and the stronger correlation was. Further longitudinal study is needed to evaluate the clinical impact of Vit D deficiency status to health of older people.
Author contributions
1) Conceived and designed the experiments: LNP, WJL, LKC
2) Performed the experiments: N/A
3) Analyzed and interpreted the data: CHC, LKL, MJC
4) Contributed reagents, materials, analysis tools or data: LKC
5) Wrote the paper: CHC
6) Review and edit the paper: WJL, LKC
Conceptualization: Li-Kuo Liu, Mei-Ju Chen, Ming-Hsien Lin, Li-Ning Peng, Liang-Kung Chen.
Data curation: Chia-Hung Chen.
Formal analysis: Chia-Hung Chen.
Funding acquisition: Liang-Kung Chen.
Investigation: Chia-Hung Chen.
Methodology: Chia-Hung Chen, Li-Kuo Liu.
Project administration: Wei-Ju Lee, Liang-Kung Chen.
Software: Chia-Hung Chen.
Supervision: Li-Kuo Liu, Mei-Ju Chen, Ming-Hsien Lin, Li-Ning Peng, Liang-Kung Chen.
Validation: Chia-Hung Chen.
Visualization: Chia-Hung Chen, Wei-Ju Lee.
Writing – original draft: Chia-Hung Chen.
Writing – review & editing: Mei-Ju Chen, Wei-Ju Lee, Ming-Hsien Lin, Li-Ning Peng, Liang-Kung Chen.
Wei-Ju Lee orcid: 0000-0003-4326-333X.
Footnotes
Abbreviations: HDL-C = high-density lipoprotein cholesterol, HOMA-IR = Homeostatic model assessment-insulin resistance, ILAS = I-Lan Longitudinal Aging Study, LDL-C = low-density lipoprotein cholesterol, TC = total cholesterol, TG = triglyceride, Vit D = vitamin D.
This study was funded by the Ministry of Science and Technology, Executive Yuan of Taiwan (MOST107–2634-F-010–001).
The authors have no conflicts of interest to disclose.
References
- [1].Perez-Lopez FR. Vitamin D: the secosteroid hormone and human reproduction. Gynecol Endocrinol 2007;23:13–24. [DOI] [PubMed] [Google Scholar]
- [2].Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–81. [DOI] [PubMed] [Google Scholar]
- [3].Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc 2011;86:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev 2001;22:477–501. [DOI] [PubMed] [Google Scholar]
- [5].Visser M, Deeg DJ, Lips P, et al. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab 2003;88:5766–72. [DOI] [PubMed] [Google Scholar]
- [6].Halfon M, Phan O, Teta D. Vitamin D: a review on its effects on muscle strength, the risk of fall, and frailty. Biomed Res Int 2015;2015:953241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Miller JW, Harvey DJ, Beckett LA, et al. Vitamin D status and rates of cognitive decline in a multiethnic cohort of older adults. JAMA Neurol 2015;72:1295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pilz S, Gaksch M, Kienreich K, et al. Effects of vitamin D on blood pressure and cardiovascular risk factors: a randomized controlled trial. Hypertension 2015;65:1195–201. [DOI] [PubMed] [Google Scholar]
- [9].Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens 2007;20:713–9. [DOI] [PubMed] [Google Scholar]
- [10].Pilz S, Verheyen N, Grübler MR, et al. Vitamin D and cardiovascular disease prevention. Nat Rev Cardiol 2016;13:404–17. [DOI] [PubMed] [Google Scholar]
- [11].McGreevy C, Williams D. New insights about vitamin D and cardiovascular disease: a narrative review. Ann Intern Med 2011;155:820–6. [DOI] [PubMed] [Google Scholar]
- [12].Judd SE, Tangpricha V. Vitamin D deficiency and risk for cardiovascular disease. Am J Med Sci 2009;338:40–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Anderson JL, May HT, Horne BD, et al. Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol 2010;106:963–8. [DOI] [PubMed] [Google Scholar]
- [14].Lagunova Z, Porojnicu AC, Lindberg F, et al. The dependency of vitamin D status on body mass index, gender, age and season. Anticancer Res 2009;29:3713–20. [PubMed] [Google Scholar]
- [15].Witham MD, Nadir MA, Struthers AD. Effect of vitamin D on blood pressure: a systematic review and meta-analysis. J Hypertens 2009;27:1948–54. [DOI] [PubMed] [Google Scholar]
- [16].Hwang AC, Liu LK, Lee WJ, et al. Association of frailty and cardiometabolic risk among community-dwelling middle-aged and older people: results from the I-Lan longitudinal aging study. Rejuvenation Res 2015;18:564–72. [DOI] [PubMed] [Google Scholar]
- [17].Tang TC, Hwang AC, Liu LK, et al. FNIH-defined sarcopenia predicts adverse outcomes among community-dwelling older people in Taiwan: results from I-Lan longitudinal aging study. J Gerontol A Biol Sci Med Sci 2017;73:828–34. [DOI] [PubMed] [Google Scholar]
- [18].Liu LK, Guo CY, Lee WJ, et al. Subtypes of physical frailty: latent class analysis and associations with clinical characteristics and outcomes. Sci Rep 2017;7:46417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen LY, Wu YH, Liu LK, et al. Association among serum insulin-like growth factor-1, frailty, muscle mass, bone mineral density, and physical performance among community-dwelling middle-aged and older adults in Taiwan. Rejuvenation Res 2017;21:270–7. [DOI] [PubMed] [Google Scholar]
- [20].Wang CJ, Tang TC, Hung CH, et al. Less than one-fifth of people aged 75 years or older in the real world were suitable for SPRINT results. J Am Med Dir Assoc 2017;18:271–2. [DOI] [PubMed] [Google Scholar]
- [21].von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–7. [DOI] [PubMed] [Google Scholar]
- [22].Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- [23].Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- [24].Rubenstein LZ, Harker JO, Salva A, et al. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol A Biol Sci Med Sci 2001;56:M366–72. [DOI] [PubMed] [Google Scholar]
- [25].Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- [26].Hebert R, Carrier R, Bilodeau A. The functional autonomy measurement system (SMAF): description and validation of an instrument for the measurement of handicaps. Age Ageing 1988;17:293–302. [DOI] [PubMed] [Google Scholar]
- [27].Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- [28].Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- [29].Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 2006;81:353–73. [DOI] [PubMed] [Google Scholar]
- [30].National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;3143–421. [PubMed] [Google Scholar]
- [31].Yang YT, Iqbal U, Ko HL, et al. The relationship between accessibility of healthcare facilities and medical care utilization among the middle-aged and elderly population in Taiwan. Int J Qual Health Care 2015;27:222–31. [DOI] [PubMed] [Google Scholar]
- [32].Yeh CJ, Chang HY, Pan WH. Time trend of obesity, the metabolic syndrome and related dietary pattern in Taiwan: from NAHSIT 1993-1996 to NAHSIT 2005-2008. Asia Pac J Clin Nutr 2011;20:292–300. [PubMed] [Google Scholar]
- [33].Mithal A, Wahl DA, Bonjour JP, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int 2009;20:1807–20. [DOI] [PubMed] [Google Scholar]
- [34].Schleithoff SS, Zittermann A, Tenderich G, et al. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 2006;83:754–9. [DOI] [PubMed] [Google Scholar]
- [35].Yuan W, Pan W, Kong J, et al. 1,25-dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J Biol Chem 2007;282:29821–30. [DOI] [PubMed] [Google Scholar]
- [36].Oh J, Weng S, Felton SK, et al. 1,25 (OH)2 vitamin d inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation 2009;120:687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chen LK. Sarcopenia: Quality matters more than quantity. J Clin Gerontol Geriatr 2018;9:37–8. [Google Scholar]


