Abstract
Background:
The aim of this study was to compare transradial access (TRA) approach with transfemoral access (TFA) approach in patients undergoing hepatic interventions.
Methods:
We conducted a comprehensive search of the PubMed, Embase, and the Cochrane Library database to identify relevant available articles. Patients’ preference, success rate, intra- and postoperative outcomes were analyzed. The risk difference (RD), relative risk (RR), and weighted mean difference (WMD) values were reported with 95% confidence intervals (CIs). We used RevMan 5.3 to perform the pooled analyses.
Results:
Nine cohort studies were included. A total of 1096 procedures were performed in 877 patients. Of those, 545 procedures (49.7%) were performed by TRA, and 551 procedures (50.3%) were performed by TFA. Patients were significantly prefer the TRA (86.5%) to the TFA (13.5%) (RD = 0.88, P < .00001). The procedure time in TRA groups was longer (WMD = 3.36, 95% CI 1.24–5.47, P = .002). But there were no significant difference in terms of success rate, fluoroscopy time, radiation dosage, contrast volume, and postoperative vascular complications.
Conclusion:
For patients suffer from primary or secondary hepatic malignancy and undergoing hepatic interventions, the present meta-analysis demonstrated that patients prefer the TRA approach to the TFA approach. But the procedure time is longer in TRA group.
Keywords: hepatic interventions, transcatheter arterial chemoembolization, transcatheter arterial radioembolization, transfemoral, transradial
1. Introduction
Lucian Campeau[1] first reported the transradial access (TRA) approach for diagnostic coronary angiography in 1989. After 3 decades of development, the TRA has been shown a significant reduction in bleeding and vascular complications compared with the transfemoral access (TFA) in cardiology interventions.[2,3] With the development of TRA technology, it has been applied more widely for other visceral organs and peripheral intervention. And the feasibility and safety of noncoronary interventions via TRA, such as hepatic, splenic, uterine artery, pancreatic, and peripheral interventions, were shown in many large sample studies.[4–7]
For hepatic interventions in patients with hepatic malignancy, Shiozawa et al[8] first reported the use of TRA for transcatheter arterial chemoembolization (TACE) in patients with hepatocellular carcinoma (HCC) and compared with TFA in their retrospective study. With the development of interventional therapy for liver malignancy in recent years, the application of TRA approach for hepatic interventions was more and more extensive. Especially with the development of transcatheter arterial radioembolization (TARE) for hepatic malignancy with yttrium 90 microspheres,[9,10] there has been a growing number of studies on the use of TRA for TACE and hepatic arterial infusion chemotherapy (HAIC) and TARE of primary and metastatic liver malignancy in recent years.
Due to more and more clinicians are paying attention to and using and the lack of comprehensive comparison of TRA and TFA for hepatic interventions, we carried out this systematic review and meta-analysis, aiming to compare the patients’ preference for the choice of 2 operation approaches (TRA or TFA) and intraoperative and postoperative outcomes of TRA vs TFA for hepatic interventions in patients with primary or secondary liver malignancy.
2. Methods
2.1. Search strategy
We conducted a comprehensive electronic search in the PubMed, Embase, and Cochrane Library database to identify relevant available articles from their inception to April, 2018. The search terms included “hepatic,” “hepatocellular,” “liver,” “hepatoma,” “hepatomas” combined with the terms “chemoembolization,” “chemo-embolization,” “radioembolization,” “radio-embolization,” “Embolization,” “TACE,” “TARE,” “chemotherapy,” “radiotherapy,” “radiation” and “radial,” “transradial,” “trans-radial,” “TRA,” and “femoral,” “transfemoral,” “trans-femoral,” “TFA”. We also reviewed the reference lists of included studies and relevant reviews for identifying additional studies. And the searches were limited to articles published in the English language.
2.2. Inclusion and exclusion criteria
Two of the authors scrutinized the titles and abstracts of all identified articles for the 1st step of selection, and then we read the full text to further exclude the unqualified studies. All noncomparative trials were excluded from analysis. And the inclusion criteria were set as follows: research in adults; studies focused on TRA vs TFA in hepatic interventions, which include bland embolization, HAIC, TACE and TARE; only studies presented at least one of the outcomes of interest in TRA vs TFA in hepatic interventions were considered, including both randomized controlled trials (RCTs) and nonrandomized controlled trials (non-RCTs) matching. If we could not reach a consensus, it would be resolved by consulting with a 3rd author.
2.3. Data extraction and quality assessment
Following information was independently extracted by 1 author and checked carefully by others: basic information about the researches (1st author, year of publication, study location, study period, study design, number of patients, operation options), demographics, and clinical characteristics of the included patients (diagnosis, number of patients, number of procedures, age, gender), intraoperative and postoperative outcomes (patients’ preference, success rate, duration of the procedure, fluoroscopy time, radiation dosage, contrast volume, complications). And we define “complications” as puncture related complications, namely access site and related vascular complications after puncture as almost all studies focus on.
The primary outcome of interest in this systematic review was the patients’ preference (only patients experienced both femoral and radial accesses were included). Other intraoperative and postoperative outcomes were considered as secondary outcomes.
The quality of the selected studies was assessed using the Newcastle–Ottawa scale, which examines patient selection methods, comparability of study groups, and assessment of outcome. A study with 7 or more stars was considered to be of high quality. Regardless of total NOS score, all studies were included in the review.
2.4. Statistical analysis
The meta-analysis was conducted using the RevMan software version 5.3 (The Nordic Cochrane Center, Cochrane Collaboration, Copenhagen, Denmark). Quantitative statistical analysis for dichotomous variables was carried out using the Manthele–Haenszel method with the relative risk (RR) as the summary statistic. Weighted mean differences (WMDs) were used as the summary statistic for quantitative analysis of continuous variables. Both the RR and WMD values were reported with 95% confidence intervals (CIs). For those studies comprising continuous data, the mean and standard deviation (SD) were calculated using the methods described by Hozo et al.[11] “Patients’ preference,” as the primary outcome, was treated as noncomparative binary data by using the method described by Chen et al.[12] The level of heterogeneity between studies was evaluated by I2 statistics. I2 < 30% was considered to be low heterogeneity, and a fixed effects model was applied. 30% ≤ I2 ≤ 50% was considered to be moderate heterogeneity, and I2 > 50% represented high heterogeneity. A random effects model was applied when I2 ≥ 30%. A P-value <.05 showed that there was significant heterogeneity across the studies. Sensitivity analysis was performed by removing 1 study at a time to assess whether the results could have been markedly affected by a single study.
All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
3. Results
3.1. Search results and study characteristics
The PRISMA flow diagram is presented in Fig. 1. The search strategy identified 766 articles of which 182 were removed after deduplication. Then we excluded studies that were irrelevant to the topic based on the title and abstract, 62 articles left. Of those, 52 articles were excluded by full-text read, 1 article was excluded when we did data extraction because it did not identify the sample size.[13] Finally 9 researches[8,14–21] (2 prospective cohort studies, and 7 retrospective cohort studies) were selected for this meta-analysis.
Figure 1.
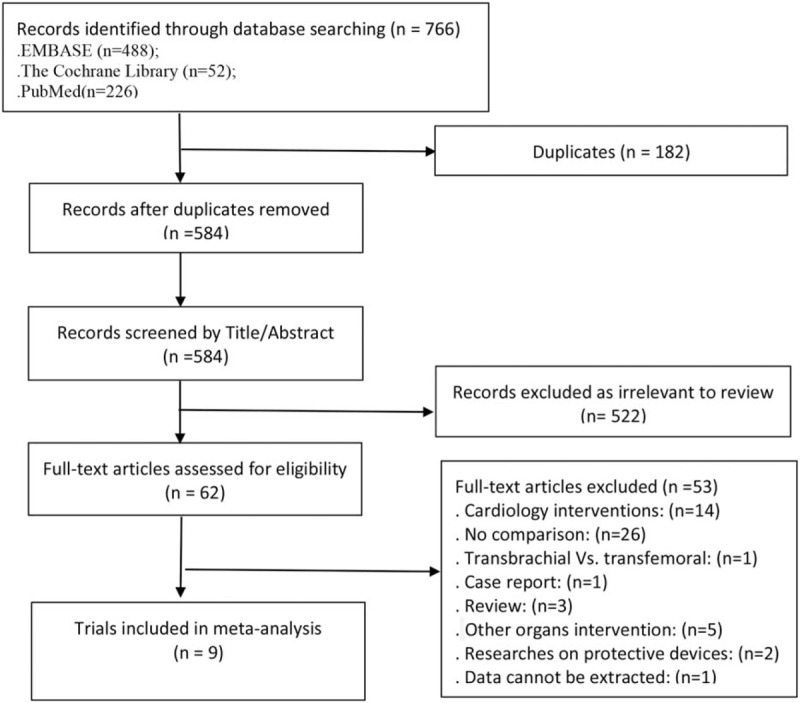
Study selection process.
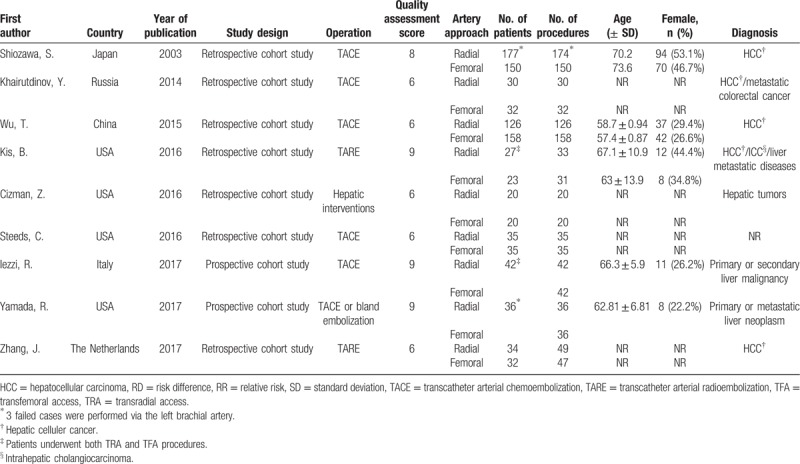
The characteristics and the results of the quality assessment of the 9 researches and the preoperative demographics and clinical characteristics of patients are shown in Table 1. The trials were conducted between 1997 and 2017 and published between 2003 and 2017. Nine studies included 877 patients, and 1096 procedures were performed. Of those, 545 procedures (49.7%) were performed with TRA, and 551 procedures (50.3%) were performed with TFA. In the 2 prospective studies which were performed by Iezzi et al[19] and Yamada et al,[20] patients were randomly assigned to undergo the initial procedure via TRA or TFA, the second procedure was performed via the alternate access by default, and only patients underwent at least 2 procedures were included. In the trial performed by Kis et al,[17] patients who underwent planning angiograms before the radioembolizations via femoral access. Therefore, patients in the TRA group experienced both TRA and TFA. And for the remaining 6 researches, patients in each access of groups were counted from different periods of time.
Table 1.
Characteristics of the 9 included studies and preoperative demographics and clinical characteristics of patients.
3.2. Patients’ preference
Three trials,[17,19,20] including 111 procedures, provided data for this outcome. High heterogeneity (I2 = 75%, P = .02) was found, therefore a random effects model was used. And 96 of 111 patients (86.5%) preferred TRA and 15 (13.5%) preferred TFA, the rate of patients preference for TRA was significant higher than TFA (risk difference [RD] = 0.88, 95% CI 0.77–0.99, P < .00001) (Table 2, Fig. 2A).
Table 2.
Intraoperative and postoperative outcomes.
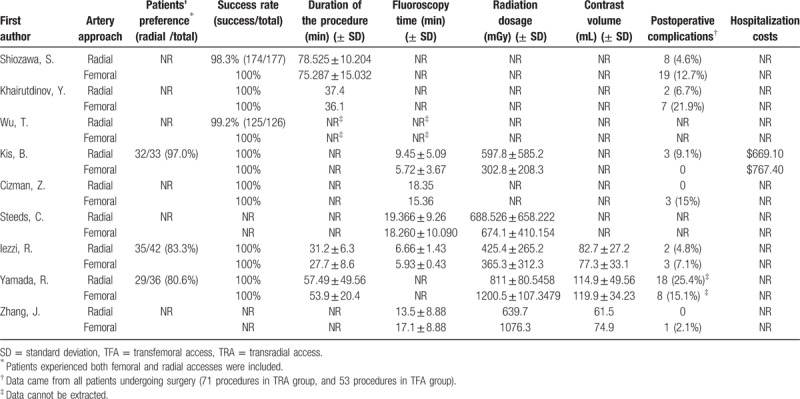
Figure 2.
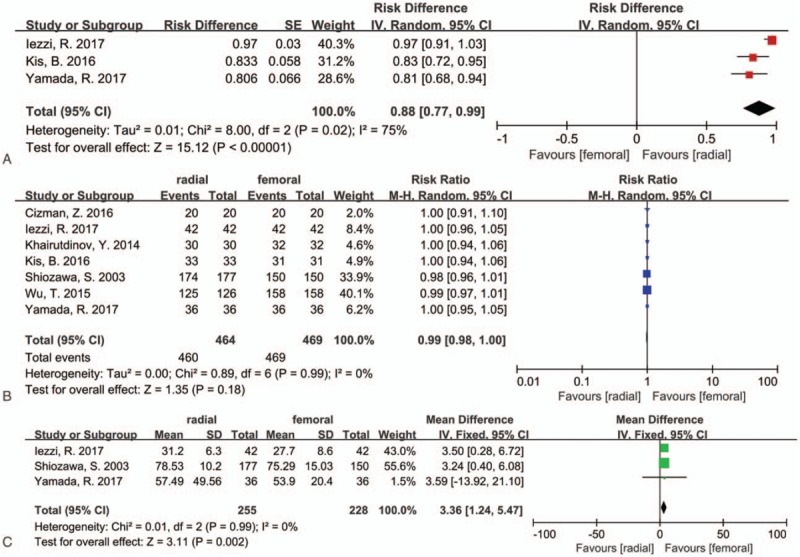
Forest plot of (A) patients’ preference, (B) success rate, and (C) procedure time.
3.3. Success rate
Seven studies,[8,14–17,19,20] including 461 procedures in the TRA group and 463 procedures in the TFA group, provided data for this outcome, and no heterogeneity (I2 = 0%, P = .99) was found. The operations were all successful in the TFA group. And only 4 cases failed in the TRA group due to the difficulty of insertion of the sheath from the radial artery. The success rate was lower in the TRA groups (457/461, 99.1%) than in the TFA groups (463/463, 100%), but the results were not statistically significant (RR = 0.99, 95% CI 0.98–1.00, P = .18) (Table 2, Fig. 2B).
3.4. Procedure time
Four trials[8,14,19,20] reported data for this outcome, but only 3 studies[8,19,20] provide effective data, and no heterogeneity (I2 = 0%, P = .99) was found. The procedure time was significantly shorter in TFA group than in TRA group (WMD = 3.36, 95% CI 1.24–5.47, P = .002) (Table 2, Fig. 2C).
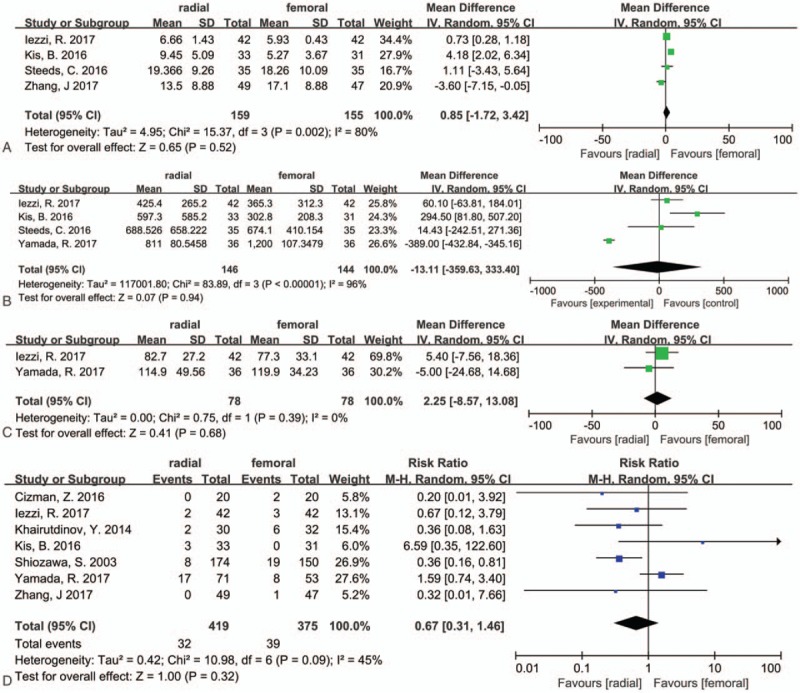
3.5. Fluoroscopy time
Five trials[16–19,21] analyzed this outcome, 4 trials[17–19,21] provide effective data for this outcome, and the SD were calculated using the methods described by Hozo et al[11] in Zhang's study.[21] There was high heterogeneity (I2 = 80, P = .002). The fluoroscopy time was longer in TRA groups, but the results were not statistically significant (WMD = 0.85, 95% CI −1.72 to 3.42, P = .52) (Table 2, Fig. 3A).
Figure 3.
Forest plot of (A) fluoroscopy time, (B) radiation dosage, (C) contrast volume, (D) postoperative complications.
3.6. Radiation dosage
Five trials[17–21] reported this outcome, and 4 trials[17–20] provide effective data. And high heterogeneity (I2 = 96%, P < .00001) was found, so a random effects model was chosen. The results were not statistically significant (WMD = −13.11, 95% CI −359.63 to 333.40, P = .94) (Table 2, Fig. 3B).
3.7. Contrast volume
Two trials[19,20] provide effective data for this outcome, and no heterogeneity (I2 = 0%, P = .39) was found. The results were not statistically significant. (WMD = 2.25, 95% CI −8.57 to 13.08, P = .68) (Table 2, Fig. 3C).
3.8. Postoperative complications
We defined “complications” as puncture related complications as almost all studies focused on. In addition, we did not make further efforts to extract the data from Wu and his colleagues’ study,[15] the data on the complications reported in their study were derived from the visceral reactions to chemotherapy, such as abdominal distension, vomiting, lumbago, and abdominal pain. And seven trials[8,14,16,17,19–21] provided data regarding this outcome, and moderate heterogeneity was found (I2 = 45%, P = .09). The overall complications were lower in TRA groups (32/419, 7.6%) than in TFA groups (39/375, 10.4%), but the results were not statistically significant (RR = 0.67, 95% CI 0.31–1.46, P = .32) (Table 2, Fig. 3D).
3.9. Sensitivity analysis
We performed a sensitivity analysis by removing 1 study at a time to assess whether the results could have been markedly affected by a single study in pooled data with moderate and high heterogeneity. Sensitivity analysis suggested that the data in this meta-analysis were relatively stable except the rate of complications. For the rate of complication, moderate heterogeneity was found, and when the Yamada's study[20] was removed, the I2 = 0 and the result was statistically significant (RR = 0.43, 95% CI 0.23–0.79, P = .007, Fig. 4).
Figure 4.
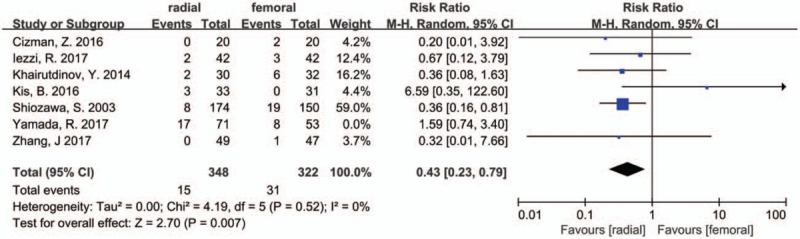
Sensitivity analysis of the postoperative complications.
4. Discussion
Hepatic interventions, which include HAIC, TACE, and TARE, is a safe and effective technology for the treatment of primary or secondary liver cancer, and most patients were successfully operated via femoral artery in the past years.[22] But the application of TRA for hepatic interventions was more and more extensive. The present meta-analysis demonstrated that compared to TFA, patients who received both via TRA and TFA interventional therapy preferred TRA (86.5% vs 13.5%, RD = 0.88, 95% CI 0.77–0.99, P < .00001). The success rate of TRA was lower, but only 4 cases of 461 procedures were failed via radial access due to the difficulty of insertion of the sheath from the radial artery, and there was no significant difference (99.1% vs 100%, RR = 0.99, 95% CI 0.98–1.00). The operative time in TRA groups was significantly higher than TFA groups (WMD = 3.36, 95% CI 1.24–5.47, P = .002). And the results about the fluoroscopy time, radiation dosage, contrast volume and postoperative vascular complications between the two groups were not statistically significant.
Interventional therapy for liver malignancy is different from interventional therapy for the cardiac and other organs, it usually needs to be performed more than once. Meanwhile, patients with liver malignancy usually have visceral reactions after the interventions. Therefore, the postoperative comfort and early mobility of the patients is a problem worthy of attention, especially for hepatic radioembolization, as it is an outpatient procedure in many medical centers. That may be the reason why researchers in recent trials comparing TRA with TFA all regarding the patient's preference as an important outcome,[19,20,23] and we treated it as the primary outcome. For patients undergoing hepatic interventions via TFA, bed rest in the supine position at least 6 hours are required, and it‘s the major impact on the postoperative experience. Due to the leg straightening, patients are unable to choose their comfortable position when they suffer from visceral reactions and extra nursing care may be required. And the above problems may be solved through TRA, and patients can suffer less pain and discomfort. That may be the reason why most patients prefer TRA. “Patients preference,” as a subjective assessment, was implemented in the form of questionnaire in patients who underwent both TRA and TFA for the trials included in the present meta-analysis.[17,19,20] Meanwhile, Iezzi et al[19] and Yamada et al[20] compared the degree of postoperative discomfort and patients’ dependence after procedure for TRA groups vs TFA groups by using a qualitative evaluation scale. Furthermore, other studies that were not included in this meta-analysis also reported the patients’ preference to TRA.[23–26]
The present meta-analysis demonstrated there was no significant difference in TRA vs TFA of the fluoroscopy time, radiation dosage, contrast volume, and postoperative complications, but the procedure time was significantly longer in the TRA groups. In cardiology intervention, procedure time is an important factor as it has been proven to be associated with stroke risk[27]; in addition, another meta-analysis demonstrated that TFA had a significantly higher risk for death and cardiac events than TRA.[28] But it is different from cardiac interventions, compared with TFA for hepatic interventions, longer procedure time in TRA groups did not accompanied by a higher incidence of postoperative mortality and complications of related vascular or viscera in the included trials. In addition, Wu et al reported that there were fewer viscera complications in the TRA group. Meanwhile, the procedure time may be influenced by the time for catheterization. For related vascular complications reported in the included trials, there was no major complication in the TRA groups, but 2[14,21] (2/551, 0.3%) cases of pseudoaneurysm were noted in TFA groups, which required further interventions. Other complications were limited to access site complications, except 1 partial radial artery thrombosis,[20] nearly of them were bruising, pain, or hematomas, which can spontaneously heal all, and the radial artery thrombosis was clinically silent as well.
For hospitalization cost, only Kis et al[17] reported this outcome, and the cost was lower in TRA group ($669.10 vs $767.40). There was no need for extra pressure device which may be the reason why the cost is less in TRA groups. Meanwhile, less extra nursing care may also decrease the overall cost in TRA groups.
Finally, there are certain limitations in the present meta-analysis. First, most of the included studies were retrospective cohort studies, which could limit the impact of the results in our study. Second, the primary outcome “patients’ preference” in our study was a subjective assessment, and patients could have an inherent bias before enrollment, as well as a lack of objective assessments in the included trials to evaluate this outcome. Third, we defined complications as vascular-related complications, and lack of data to confirm the influence of TRA and TFA to visceral reactions after the hepatic interventions.
5. Conclusion
For patients suffering from primary or secondary hepatic malignancy and undergoing hepatic interventions, the present meta-analysis demonstrated that patients are significantly prefer the TRA approach to the TFA approach. Meanwhile, the radial artery is a safe and feasible access for hepatic interventions. Although the technical success rate of the TRA approach is lower than TFA, it is high enough in the TRA groups. Although the procedure time is significantly longer in the TRA groups, there was no report on the adverse effects of the longer procedure time. In addition, more objective assessments are required to support the reasons for patients’ preference.
Author contributions
Conceptualization: Yuan-Yuan Chen.
Data curation: Yuan-Yuan Chen, Pan Liu.
Methodology: Pan Liu, Yu-Shen Wu, Huapeng Lin.
Writing – original draft: Yuan-Yuan Chen.
Writing – review & editing: Pan Liu, Yu-Shen Wu, Huapeng Lin, Xiaopin Chen.
Footnotes
Abbreviations: CIs = confidence intervals, HAIC = hepatic arterial infusion chemotherapy, HCC = hepatocellular carcinoma, non-RCTs = non-randomized controlled trials, RCTs = randomized controlled trials, RD = risk difference, RR = relative risk, SD = standard deviation, TACE = transcatheter arterial chemoembolization, TARE = transcatheter arterial radioembolization, TFA = transfemoral access, TRA = transradial access, WMDs = weighted mean differences.
YYC and PL contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Campeau L. Percutaneous radial artery approach for coronary angiography. Cathet Cardiovasc Diagn 1989;16:3–7. [DOI] [PubMed] [Google Scholar]
- [2].Jolly SS, Amlani S, Hamon M, et al. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am Heart J 2009;157:132–40. [DOI] [PubMed] [Google Scholar]
- [3].Piccolo R, Galasso G, Capuano E, et al. Transradial versus transfemoral approach in patients undergoing percutaneous coronary intervention for acute coronary syndrome. A meta-analysis and trial sequential analysis of randomized controlled trials. PLoS One 2014;9:e96127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Posham R, Biederman DM, Patel RS, et al. Transradial approach for noncoronary interventions: a single-center review of safety and feasibility in the first 1,500 cases. J Vasc Interv Radiol 2016;27:159–66. [DOI] [PubMed] [Google Scholar]
- [5].Resnick NJ, Kim E, Patel RS, et al. Uterine artery embolization using a transradial approach: initial experience and technique. J Vasc Interv Radiol 2014;25:443–7. [DOI] [PubMed] [Google Scholar]
- [6].Wu T, Sun R, Huang Y, et al. Partial splenic embolization of patients with hypersplenism by transradial or transfemoral approach: a prospective randomized controlled trial. Acta Radiol (Stockholm, Sweden: 1987) 2016;57:1201–4. [DOI] [PubMed] [Google Scholar]
- [7].Posham R, Patel RS, Kim E, et al. Transradial approach for peripheral and visceral interventions: a single-center review of safety and feasibility in the first 1000 cases. J Vasc Interv Radiol 2015;26:S101. [DOI] [PubMed] [Google Scholar]
- [8].Shiozawa S, Tsuchiya A, Endo S, et al. Transradial approach for transcatheter arterial chemoembolization in patients with hepatocellular carcinoma: comparison with conventional transfemoral approach. J Clin Gastroenterol 2003;37:412–7. [DOI] [PubMed] [Google Scholar]
- [9].Geschwind JF, Salem R, Carr BI, et al. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology 2004;127Suppl 1:S194–205. [DOI] [PubMed] [Google Scholar]
- [10].Salem R, Gabr A, et al. Institutional decision to adopt Y90 as primary treatment for HCC Informed by a 1,000-patient 15-year experience. Hepatology 2018;68:1429–40. [DOI] [PubMed] [Google Scholar]
- [11].Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chen YH, Du L, Geng XY, et al. Implement meta-analysis with non-comparative binary data in RevMan software. Chin J Evid Based Med 2014;14:889–96. [Google Scholar]
- [13].Khayrutdinov E, Arablinskiy A, Tsurkan V, et al. Radial versus femoral access for peripheral artery embolization. Cardiovasc Intervent Radiol 2016;39:S258. [Google Scholar]
- [14].Khairutdinov Y, Tsurkan V, Arablinskiy A. Radial versus femoral artery access for transarterial chemoembolization. Cardiovasc Intervent Radiol 2014;37:S318. [Google Scholar]
- [15].Wu T, Sun R, Huang Y, et al. Transradial arterial chemoembolization reduces complications and costs in patients with hepatocellular carcinoma. Indian J Cancer 2015;52Suppl 2:e107–111. [DOI] [PubMed] [Google Scholar]
- [16].Cizman Z, Ciszak T, Camacho J, et al. Comparison of radial and femoral artery access for interventional oncology. J Vasc Interv Radiol 2016;27:S250. [Google Scholar]
- [17].Kis B, Mills M, Hoffe SE. Hepatic radioembolization from transradial access: initial experience and comparison to transfemoral access. Diagn Interv Radiol 2016;22:444–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Steeds C, Baigorri B, Isaacson A, et al. Initial experience with the transradial approach for transarterial chemoembolization: Is there more radiation to the patient? J Vasc Interv Radiol 2016;27:S38. [Google Scholar]
- [19].Iezzi R, Pompili M, Posa A, et al. Transradial versus transfemoral access for hepatic chemoembolization: intrapatient prospective single-center study. J Vasc Interv Radiol 2017;28:1234–9. [DOI] [PubMed] [Google Scholar]
- [20].Yamada R, Bracewell S, Bassaco B, et al. Transradial versus transfemoral arterial access in liver cancer embolization: randomized trial to assess patient satisfaction. J Vasc Interv Radiol 2018;29:38–43. [DOI] [PubMed] [Google Scholar]
- [21].Zhang J, Stanley M, Richard H, et al. Transradial versus transfemoral access in radioembolization for hepatocellular carcinoma with yttrium-90 microspheres. Journal of Vascular and Interventional Radiology 2017;28:e21. [Google Scholar]
- [22].Rammohan A, Sathyanesan J, Ramaswami S, et al. Embolization of liver tumors: past, present and future. World J Radiol 2012;4:405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Thakor AS, Alshammari MT, Liu DM, et al. Transradial access for interventional radiology: single-centre procedural and clinical outcome analysis. Can Assoc Radiol J 2017;68:318–27. [DOI] [PubMed] [Google Scholar]
- [24].Aliberti C, Carandina R, Sarti D, et al. Transradial access (TRA) for TACE and hepatic arterial infusion chemotherapy (HAIC) of primary and metastatic liver cancer. Cardiovasc Interv Radiol 2017;40:S268. [Google Scholar]
- [25].Bishay V, Patel RS, Kim E, et al. Transradial approach for hepatic radioembolization: Initial results and technique. J Vasc Interv Radiol 2014;25:S90–1. [DOI] [PubMed] [Google Scholar]
- [26].Linville RM, Holayter RA, Dalvie P, et al. Transradial arterial access for intra-arterial liver directed therapy: a single center initial experience. J Vasc Interv Radiol 2015;26:e36–7. [Google Scholar]
- [27].Hamon M, Pristipino C, Di Mario C, et al. Consensus document on the radial approach in percutaneous cardiovascular interventions: position paper by the European Association of Percutaneous Cardiovascular Interventions and Working Groups on Acute Cardiac Care∗∗ and Thrombosis of the European Society of Cardiology. Euro Interv 2013;8:1242–51. [DOI] [PubMed] [Google Scholar]
- [28].Ferrante G, Rao SV, Juni P, et al. Radial versus femoral access for coronary interventions across the entire spectrum of patients with coronary artery disease: a meta-analysis of randomized trials. JACC Cardiovasc Interv 2016;9:1419–34. [DOI] [PubMed] [Google Scholar]


