Abstract
Both DHPS (dihydropteroate synthase) and DHFR (dihydrofolate reductase) play important physiological roles in the survivability of Mycobacterium tuberculosis (MTB). Sulfonamides are the potent drugs to monitor growth and proliferation of MTBs by inhibiting the activity of DHPS and DHFR which could explain the mechanism of action of these molecules. In this work, 102 heterocyclic sulfonamides (HSF) have been screened by discovery studio molecular docking programme to search the best suitable molecule for the treatment of MTBs. Lipinski’s rule of five protocols is followed to screen drug likeness of these molecules and ADMET (absorption, distribution, metabolism, excretion and toxicity) filtration has been used to value their toxicity. Only fourteen molecules are found to obey the Lipinski’s rule and able to cross the ADMET filter. A small difference between HOMO and LUMO energy signifies the electronic excitation energy which is essential to calculate molecular reactivity and stability of the best docked compound and easy activation of drug in the protein environment. Both 4-amino-N-(6-hydroxypyridin-2-yl)benzenesulfonamide (M1) and 4-amino-N-(9H-carbazol-2-yl)benzenesulfonamide (M2) show the best theoretical efficiency with DHPS and DHFR, respectively. These compounds are also found to bind to the adenine–thymine region of tuberculosis DNA.
Electronic supplementary material
The online version of this article (10.1007/s40203-018-0041-9) contains supplementary material, which is available to authorized users.
Keywords: DHPS and DHFR inhibitors, Heterocyclic sulfonamide compounds, Structure based drug design, Molecular docking, ADMET
Introduction
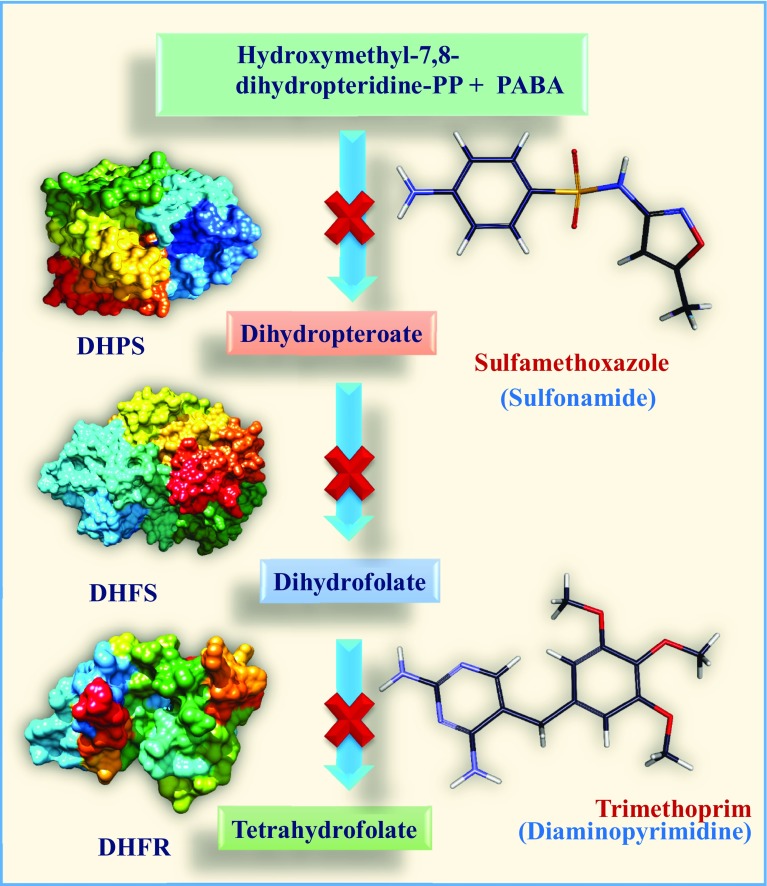
Dihydrofolate reductase (DHFR) is a significant drug target in anti-mycobacterial drugs design process. It integrates into the folate pathway by dihydropteroate synthase (DHPS) and dihydrofolate synthase (DHFS) to generate a hydroxyl dihydrofolate antimetabolite, which in turn inhibits DHFR enzymatic activity (Zheng et al. 2013; Minato et al. 2015). Introduction of new drug with novel therapeutic application is a time consuming and costly process. It has to be also pass through many legal restrictions in the animal experiments before to be used in living body. However, the use of computational drug designing method as a predictive guideline towards the successful synthesis of drug in the experimental laboratories, has drastically simplified and changed the scenario in the pharmaceutical research landscape by optimizing the drug development process (Myers and Baker 2001). Antibacterial drug discovery has been developed significantly during the last few years following the use of in silico models (Chung et al. 2013). Tuberculosis (TB) caused by pathogenic bacteria Mycobacterium tuberculosis (MTB), is an infectious and lethal disease. It is one of the top three infectious killing diseases in the world (Sandhu 2011). Despite discovery of several new drugs for curing TB, unfortunately, millions of patients are still suffering from this disease world-wide and lost their life. So, there is an urgent need for development of new drugs to treat tuberculosis in a better manner by avoiding the present lengthy and complex treatment process and resolving the problems of drug resistance and persistence (Sacchettini et al. 2008). Action of antibiotics to control the proliferation of microbes is a complex path. They interfere in the cell wall synthesis, cell permeability and also hinder the synthesis of DNA and protein (Fig. 1). Sulfonamides are class of antibiotics which inhibit the synthesis of folic acid by competing with p-aminobenzoic acid (PABA) (Winum et al. 2005). The hypersensitivity of sulfa drugs may be due to partial oxidation of aromatic-NH2 group of sulfonamides by cytochrome-P450 to hydroxylamine and/or nitroso derivative. These toxicity and drug resistance of sulfonamides have initiated world wide effort to search for new generation drugs (Summan and Cribb 2002). Sulfonamides show potent anti-microbial activity and recent literature reports that they are active against MTB (Freilich et al. 1939). Sulfamethoxazole (sulfonamide)–trimethoprim (diaminopyrimidine) (co-trimoxazole) has been used as a potential medicine to treat drug-resistant TB. Co-trimoxazole has been found to be surprisingly active against drug-resistant MTBs, not only in vitro but also in vivo (Palomino and Martin 2016). This report has motivated us to consider sulfonamides for their anti-mycobacterial activity.
Fig. 1.
Mode of action of sulfonamides on Tetrahydrofolate synthesis pathway
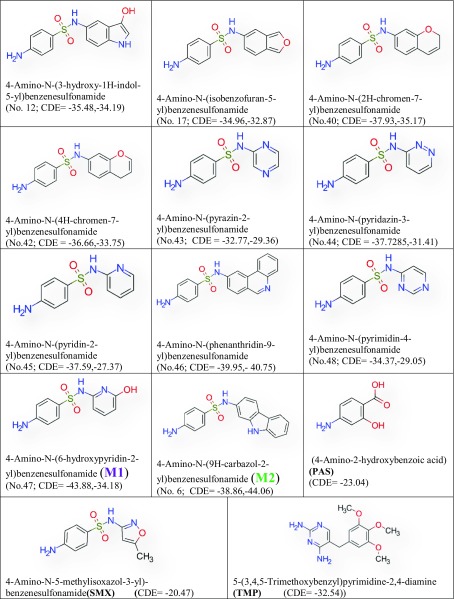
In this work, in silico techniques are used to compare the drug-like activity of some substituted sulfonamides containing five and six-member heterocyclic ring with O-/N-/S-hetero atoms (heterocyclic sulfonamides, HSF) to predict future drugs for TB treatment. Using in silico approach, HSFs have been screened to search the binding affinity of HSFs with DHPS and DHFR receptors, following Lipinski’s rule of five filtrations, for their drug-like efficiency with both DHPS and DHFR, taken from a large chemical library of small molecules (Lipinski et al. 2001). Out of 102 HSFs only fourteen compounds exhibit docking score (binding affinity) higher than approved tuberculosis drugs, p-amino salicylic acid (PAS), sulfamethoxazole (SMX) and trimethoprim (TMP). These drugs (PAS, SMX and TMP) also interfere with the folate biosynthetic pathway (Rengarajan et al. 2004; Masters et al. 2003).
In this work, the molecular orbitals are used to calculate the electronic configuration, molecular reactivity and stability of the compounds, which are obtained through the density functional theory (DFT) computational process (Rozhenko 2014). ADMET filtration has been applied to check the toxicity of the compounds (Hou and Wang 2008). Out of 102 HSFs only two compounds, 4-amino-N-(6-hydroxypyridin-2-yl) benzenesulfonamide (M1) and 4-amino-N-(9H-carbazol-2-yl) benzenesulfonamide (M2) exhibit docking score higher than approved tuberculosis drugs, p-amino salicylic acid (PAS) and sulfamethoxazole (SMX). They also cross the ADMET and Lipinski’s rule of five filter and has been found to bind to adenine–thymine region of tuberculosis DNA.
Methods and materials
Sequence alignment analysis
Sequence alignment method has been used to identify the functional similarity between two or more sequences of amino acids by simultaneously aligning the sequences. The process is called multiple-sequence alignment (MSA) and important for finding the functionality of proteins and evolution history of the species.
The DHFR enzymes found in human body have different functions from that of MTBs. Using target validation protocol HSFs have been validated as potential drug and not harmful to human health (Wyss et al. 2003). To identify the homology and relationship between human-DHFR and MTB-DHFR, sequence comparison was performed using multiple sequence alignment in Clustal omega (Sievers et al. 2011). DHPS does not exist in human body, so there is no risk of drug interaction.
High throughput virtual screening (HTVS)
Structure-based drug discovery is a useful rapid and economic procedure and has been recognized to be more effective than the conventional wet lab drug discovery scheme (Lionta et al. 2014; Pradhan et al. 2016). High throughput virtual screening (HTVS) is a methodology for drug discovery that has become widely used tool for structure-based drug discovery. It is basically a procedure of screening of large number of drug like molecules against selected and specific drug targets. HTVS are used for screening of various types of libraries, including combinatorial chemistry, genomics, protein and peptide. It involves docking of drug-like molecule (ligand) into a protein target followed by applying a scoring function to estimate the likelihood that the ligand will bind to the protein with high affinity (Szymański et al. 2012).
Discovery studio (DS) uses CDOCKER as a docking tool which is an application of a CHARMm based docking tool using a rigid receptor (Wu et al. 2003). It is used to calculate quantitative data to compare the relative performance and accuracy of various grid-based approximations using all-atom force field calculations. The protein is rigid while the ligands are treated as fully flexible and a final minimization step is used to refine the docked poses. The results are statistically indistinguishable from the detailed atomic dockings and provide up to a six-fold reduction in computation time (Wu et al. 2003).
In discovery studio, the output (.sd) file of docked ligand is used. In addition to the structural information, each record includes the CDOCKER score (negative value, i.e., -CDOCKER_ENERGY), where a higher value indicates a more favorable binding. This enables the energy to be used like a score. This score includes internal ligand strain energy, receptor (target enzyme)-ligand interaction energy, and is used to sort the poses of each input ligand. On comparing the CDOCKER energies, drug-like molecules are selected for further processing (Wu et al. 2003).
The crystallographic co-ordinates for DHPS (PDB ID: 1EYE) and DHFR (PDB ID: 4KM2) have been retrieved from the protein data bank (PDB). Before docking procedure, protein structures are prepared by using protein preparation wizard of DS 4. Following which loops are built up, protonation has been done by adding hydrogen atoms to the crystal structures and CHARMm force field has been applied. Finally, receptor cavity (active site of protein) has been obtained from the PDB files using DS 4.
Reference drugs approved by FDA (Food and Drug Administration), sulfamethoxazole (DB01015), trimethoprim (DB00440) and p-amino salicylic acid (DB00233) are obtained from drug bank database (Wishart et al. 2008). All HSFs are drawn using Accelrys draw software. All 3D coordinates and change of ionization are obtained from ligand preparation wizard of DS 4 software.
Drug likeness
‘Drug likeness’, a qualitative attributes of chemicals assigned, is widely used in the early stages of the selected lead molecule and drug discovery. Its theoretical progress is assumed to be Lipinski’s ‘rule of five’ (Lipinski et al. 2001).
ADMET properties
ADMET is a high throughput in silico screening method which has been widely accepted in the early stage of the drug design process (Cheng et al. 2012). It can predict any toxicity and undesired effect of drug in human body. High quality ADMET annotated data points for unique compounds have been carefully curated from a large number of diverse literatures and quantitative structure–activity relationship (QSAR) models (Cheng et al. 2012). In this work, a comparative study has been made between approved TB drugs and HSFs. QSAR model and the prediction accuracy were given by various models like, naive Bayes classifier, classification and regression tree, random forest, support vector machine and k nearest neighbor (Cheng et al. 2012). This relationship determination usually achieved by comparing the results given by two major components of the approach: objects clustering and activity prediction (Hou and Wang 2008).
Quantum chemistry calculation
Electronic structure (molecular orbital) and the effect of substituent of drug-like molecules have significant role in the drug efficiency (Rozhenko 2014). A molecular function can be used to calculate chemical and physical properties. The difference of the energies of the HOMO (highest occupied molecular orbital) and LUMO (lowest unoccupied molecular orbital) termed as the band gap, may use to compute the molecular reactivity, strength and stability of the complexes (Zhan et al. 2003).
In the present study, DFT computation has been carried out by Gaussian 09W software (windows platform with 8 GB RAM computer containing Intel I 5 processor) (Frisch et al. 2009). Gaussian calculation setup has been done in Gaussian 09 panel using Becke’s three-parameter exchange potential and Lee–Yang–Parr correlation functional (B3LYP) theory with basis set 6–31G (Becke 1993; Gill et al. 1992; Stephens et al. 1994). Afterward, surface (molecular orbital, density, potential) and electrostatic potential charges (EPS) are used to calculate the HOMO and LUMO of the compound.
Drug–DNA interaction
Understanding of interaction between drug molecules and DNA has become an active research area at the interface of chemistry, molecular biology and medicine (Sirajuddin et al. 2013). To find the binding pattern of HSFs with MTBs DNA (PDB ID: 3PVP), the docking method is used. Transcription and replication, vital to cell survival and proliferation are affected by the interaction of exogenous agents like drug and the DNA function could be artificially modulated. Drug–DNA docking has been done by Autodock vina (Trott and Olson 2010).
Results and discussion
Sequence alignment analysis
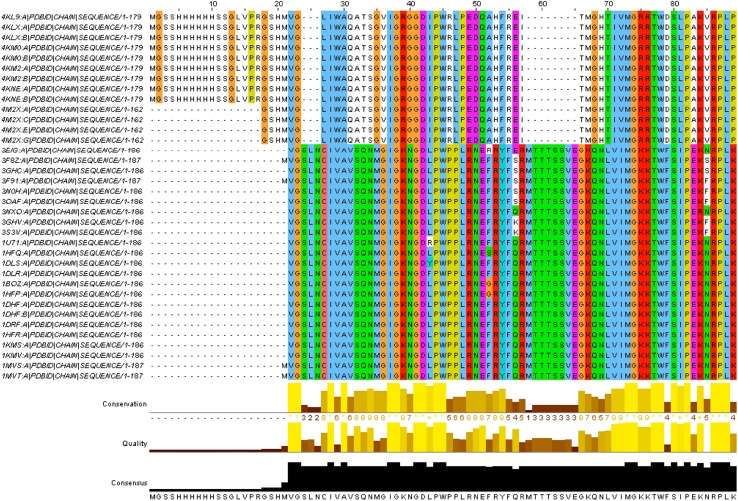
The sequence alignment analysis has been carried out by Clustal omega and the result is depicted in Table 1 and Fig. 2. It is evident that there is no significant match between human and MTBs DHFR (Table 1). The PDB ID 3NXX is a human DHFR and except PDB ID 2DHF all are MTBs DHFR. Clustal omega has shown 100% similarity between 3NXX and 2DHF, which is also a Homo sapiens DHFR. But, with other sequences which are of MTBs DHFR, the sequence similarities are less than 30%. Similarity between human and MTBs DHFR is less than 30%, there is no functional similarity between human and MTBs DHFR. Similarities between these two DHFRs are shown in Table 1. Correctly identifying and aligning of these sequences, has developed an idea of biological evolution. Sequence alignment analysis makes sure that the drugs do not harm biological activities of human health because there are no evolutionary and functional similarities between human and MTBs DHFR.
Table 1.
Sequence alignment results (percent identity matrix) as obtained from Clustal omega (partial view)
| Sequence no. | PDB ID and chain | Total amino acids | Sequence no. | PDB ID and chain | Total amino acids | Similarity (%) |
|---|---|---|---|---|---|---|
| 25 | 3NXX_A|PDBID|CHAIN|SEQUENCE | 186 | 75 | 2DHF_A|PDBID|CHAIN|SEQUENCE | 186 | 100.0 |
| 25 | 3NXX_A|PDBID|CHAIN|SEQUENCE | 186 | 76 | 2DHF_B|PDBID|CHAIN|SEQUENCE | 186 | 100.0 |
| 25 | 3NXX_A|PDBID|CHAIN|SEQUENCE | 186 | 77 | 4M2X_A|PDBID|CHAIN|SEQUENCE | 162 | 24.07 |
| 25 | 3NXX_A|PDBID|CHAIN|SEQUENCE | 186 | 78 | 4M2X_C|PDBID|CHAIN|SEQUENCE | 162 | 24.07 |
| 25 | 3NXX_A|PDBID|CHAIN|SEQUENCE | 186 | 79 | 4M2X_E|PDBID|CHAIN|SEQUENCE | 162 | 24.07 |
| 25 | 3NXX_A|PDBID|CHAIN|SEQUENCE | 186 | 80 | 4M2X_G|PDBID|CHAIN|SEQUENCE | 162 | 24.07 |
| 25 | 3NXX_A|PDBID|CHAIN|SEQUENCE | 186 | 81 | 4KNE_A|PDBID|CHAIN|SEQUENCE | 179 | 21.79 |
| 25 | 3NXX_A|PDBID|CHAIN|SEQUENCE | 186 | 82 | 4KNE_B|PDBID|CHAIN|SEQUENCE | 179 | 21.79 |
| 25 | 3NXX_A|PDBID|CHAIN|SEQUENCE | 186 | 83 | 4KM2_A|PDBID|CHAIN|SEQUENCE | 179 | 21.79 |
| 25 | 3NXX_A|PDBID|CHAIN|SEQUENCE | 186 | 84 | 4KM2_B|PDBID|CHAIN|SEQUENCE | 179 | 21.79 |
| 25 | 3NXX_A|PDBID|CHAIN|SEQUENCE | 186 | 85 | 4KM0_A|PDBID|CHAIN|SEQUENCE | 179 | 21.79 |
| 25 | 3NXX_A|PDBID|CHAIN|SEQUENCE | 186 | 86 | 4KM0_B|PDBID|CHAIN|SEQUENCE | 179 | 21.79 |
| 25 | 3NXX_A|PDBID|CHAIN|SEQUENCE | 186 | 87 | 4KLX_A|PDBID|CHAIN|SEQUENCE | 179 | 21.79 |
| 25 | 3NXX_A|PDBID|CHAIN|SEQUENCE | 186 | 88 | 4KLX_B|PDBID|CHAIN|SEQUENCE | 179 | 21.79 |
| 25 | 3NXX_A|PDBID|CHAIN|SEQUENCE | 186 | 89 | 4KL9_A|PDBID|CHAIN|SEQUENCE | 179 | 21.79 |
| 25 | 3NXX_A|PDBID|CHAIN|SEQUENCE | 186 | 90 | 1DG8_A|PDBID|CHAIN|SEQUENCE | 159 | 24.53 |
| 25 | 3NXX_A|PDBID|CHAIN|SEQUENCE | 186 | 91 | 1DG7_A|PDBID|CHAIN|SEQUENCE | 159 | 24.53 |
| 25 | 3NXX_A|PDBID|CHAIN|SEQUENCE | 186 | 92 | 1DG5_A|PDBID|CHAIN|SEQUENCE | 159 | 24.53 |
Both PDB ID 3NXX and 2DHF belong to human DHFR and the rest belong to M. tuberculosis DHFR. It is evident that the 3NXX sequences show 100% similarity with 2DHF and with other sequences similarities are less than 30%. All similarities are shown in the last column
Fig. 2.
Protein (DHFR) multiple sequence alignment (Jalview) generated by Clustal omega (partial view). The amino acid sequence of the human DHFR protein has been compared with various M. tuberculosis DHFR. Site specific similarities between amino acids are shown by the colored letters while the degree of conservation, quality and consensus of those amino acids among all organisms are shown in yellow and black
Docking and bond analysis
The specific binding of a ligand (drug) to a drug target molecule is the key to drug action. Each ligand binds favourably to a specific site on the surface of the target molecule. Identification of the ligand-binding site for each specific protein molecule is crucially important when trying to find a suitable drug molecule for the target, and it is also important to understand the function of the protein (Soga et al. 2007). Interactions within 4 Å have been taken into consideration for both protein and DNA docking (Walker et al. 1953). The docking analysis scores and bonds are discussed below (Table 2). Additional data are given in Online Resource 1 (Table S1).
Table 2.
Fourteen HSFs show higher CDOCKER energy (CDE) than PAS, SMX and trimethoprim with mycobacterial DHPS and DHFR
M1 and DHPS
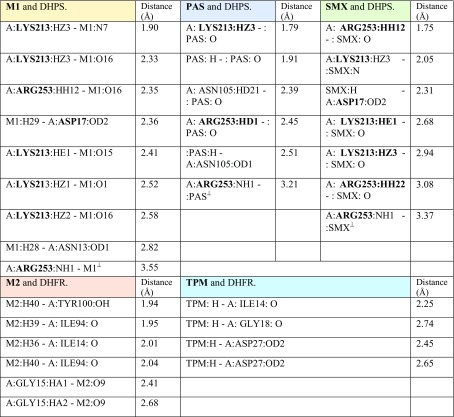
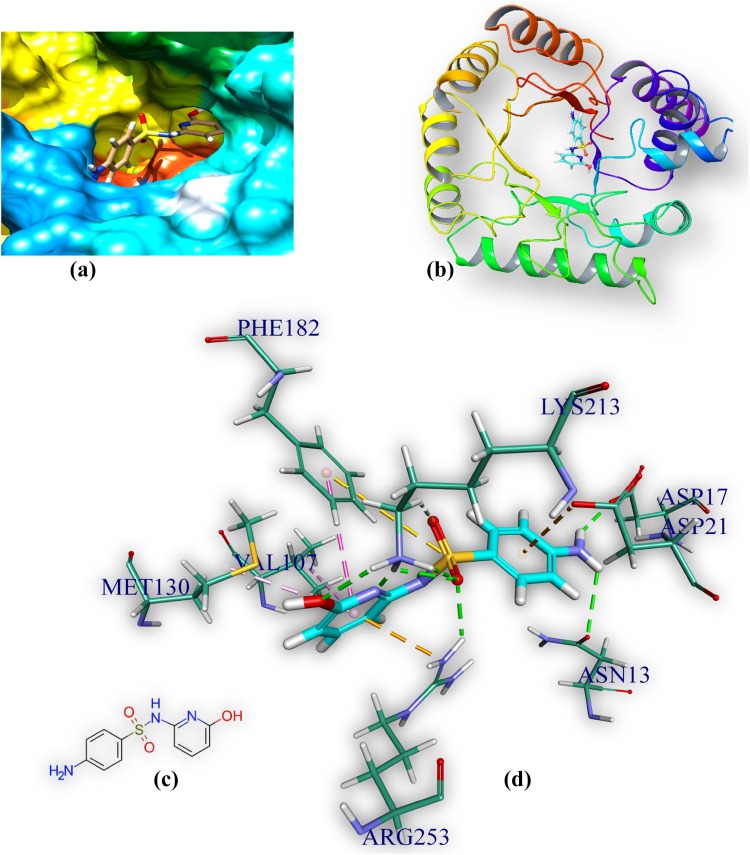
M1 is docked in the active site of DHPS of MTB. It forms eight hydrogen bonds with amino acids of the DHPS within range distance of 1.90–2.82 Å (Table 3, Fig. 3). The hydrogen bond forming amino acids are asparagine (ASN13), aspartic acid (ASP17), lysine (Lys213) and arginine (Arg 253). It also forms an electrostatic bond (3.55 Å) with arginine (Arg253). The CDOCKER energy is − 43.88 a.u.
Table 3.
Bond distances and categories of M1, PAS, SMX and DHPS and M2 and DHFR, trimethoprim (TPM)
⊥Electrostatic bond
Fig. 3.
Comprehensive perception of DHPS and M1 after docking, a secondary structure of MTB DHPS represented by hydrophobic surface and M1 represented is by stick model and coloured according to elements, b DHPS is represented by ribbon and M1 is represented by stick, c 2D structure of M, and d interactions of M1 with DHPS amino acids. Bonds are in dots. M1 surrounding amino acids are in three letters code, represented in blue. For brevity explicit H-atoms are deleted in 2D structure
PAS and DHPS
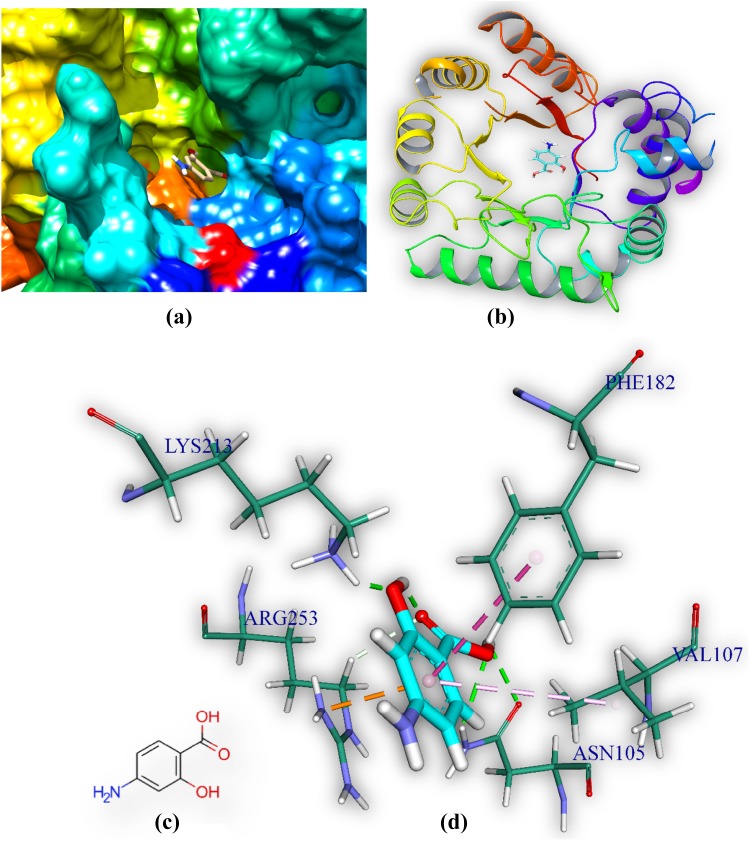
PAS is docked in the active site of DHPS MTB. It forms five hydrogen bonds with amino acids of the DHPS within range distance of 1.79–2.51 Å (Table 3, Fig. 4). The hydrogen bond forming amino acids are asparagine (Asn105), lysine (Lys213) and arginine (Arg253). It also forms an electrostatic bond (3.21 Å) with arginine (Arg253). The CDOCKER energy is − 23.04 a.u. The common surrounding amino acids of the PAS and M2 are marked in bold Table 3.
Fig. 4.
Comprehensive perception of DHPS and PAS after docking. Figure legends are same as Fig. 3
Sulfamethoxazole and DHPS
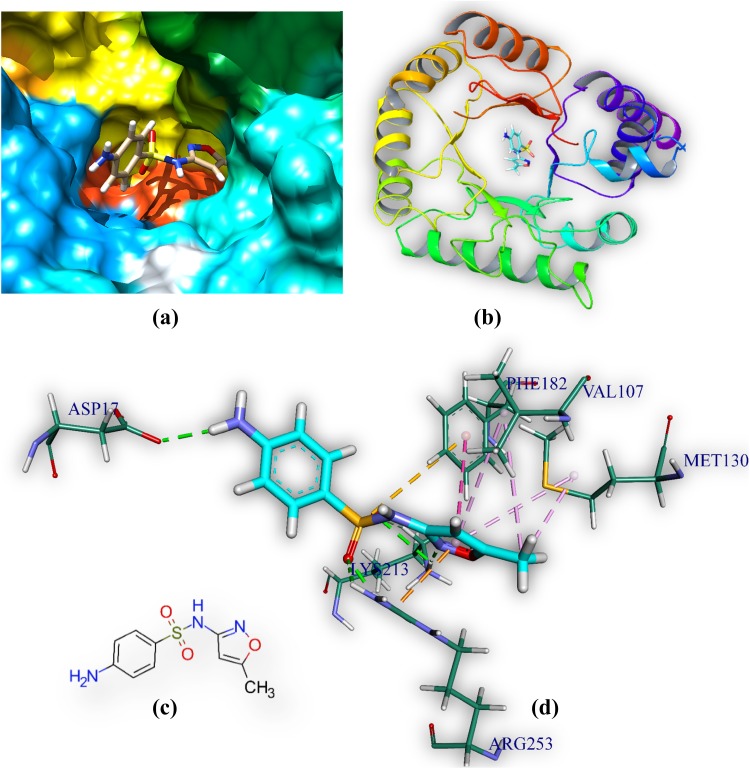
Sulfamethoxazole is docked in the active site of DHPS MTB. It forms six hydrogen bonds with amino acids of the DHPS within range distance of 1.75–3.08 Å (Table 3, Fig. 5). The hydrogen bond forming amino acids are aspartic acid (Asp17) lysine (Lys213) and arginine (Arg253). It also forms an electrostatic bond (3.37 Å) with Methionine (MET130). The CDOCKER energy is − 20.47 a.u.
Fig. 5.
Comprehensive perception of DHPS and sulfamethoxazole after docking. Figure legends are same as Fig. 3
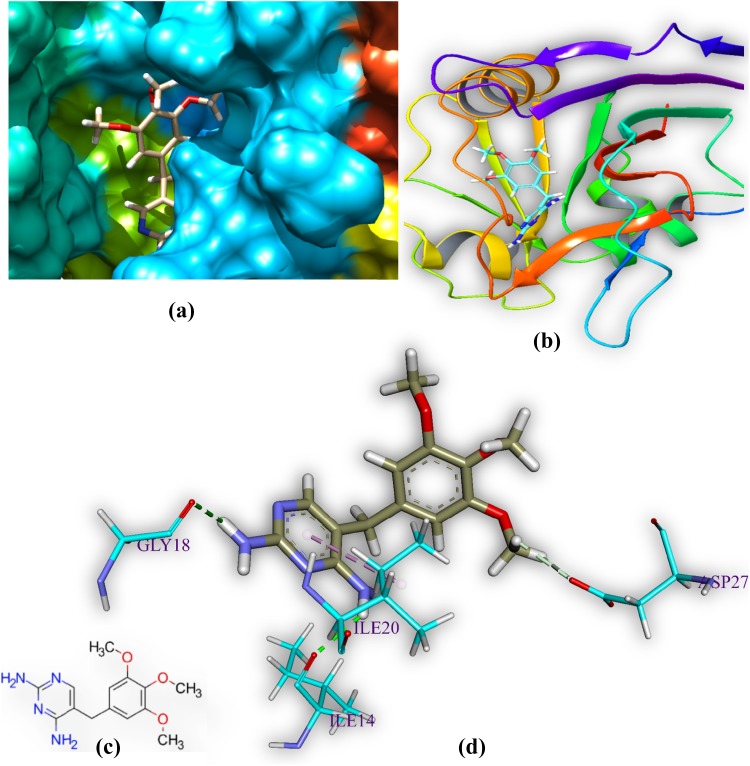
M2 and DHFR
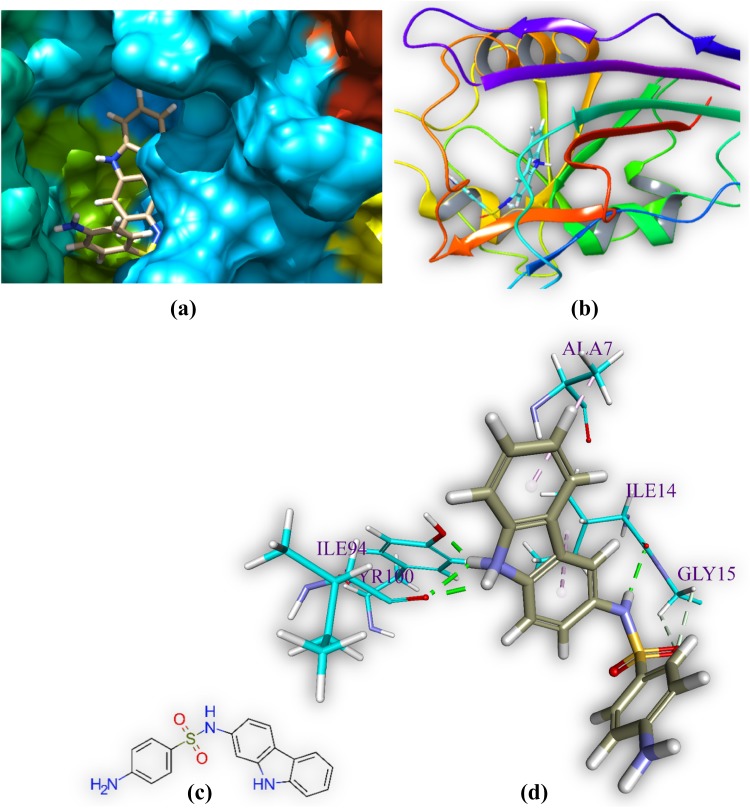
M2 is docked in the active site of DHFR MTB. It forms six hydrogen bonds with amino acids of the DHFR within range distance of 1.94–2.68 Å (Table 3 and Fig. 6). The hydrogen bond forming amino acids are glycine (GLY15), isoleucine (ILE14 and ILE94), and tyrosine (TYR100) and. The hydrophobic bond forming amino acid is alanine (ALA7). The CDOCKER energy is − 44.06 a.u.
Fig. 6.
Comprehensive perception of DHFR and M2 after docking. Figure legends are same as Fig. 3
Trimethoprim and DHFR
Trimethoprim is docked in the active site of DHFR MTB. It forms four hydrogen bonds with amino acids of the DHFR within range distance of 2.25–2.65 Å (Table 3, Fig. 7). The hydrogen bond forming amino acids are isoleucine (ILE14), glycine (GLY18) and aspartic acid (ASP27). The CDOCKER energy is − 32.54 a.u.
Fig. 7.
Comprehensive perception of DHFR and trimethoprim after docking. Figure legends are same as Fig. 3
ADMET property analysis
ADMET properties have been determined by in silico analysis by means of Caco-2 cell, BBB (blood–brain barrier), HIA (human intestinal absorption), p-glycoprotein substrate/inhibitor, renal organic cation transporter, AMES toxicity and other parameters. ADMET data have been summarized in Online Resource 1 (Table S2 and Table S3).
Most pharmaceutical drugs, and all oral ones, must necessarily cross at least one cell membrane to initiate their bio-medical activity (O’Hagan and Kell 2015). Drugs which can penetrate the BBB are assigned +ve. This is crucial in pharmaceutical sphere, because central nervous system (CNS)-active compounds must pass across it and CNS-inactive compounds must not pass across it in order to avoid the CNS side effects (Shen et al. 2010). Most of the drugs in data sets are BBB positive (Ajay et al. 1999). Human intestinal absorption data are the sum of bioavailability and absorption (Zhao et al. 2001) of drugs which can penetrate the HIA and is assigned to +ve (Ajay et al. 1999). Caucasian colon adenocarcinoma cell (Caco-2) lines are derived from a human colon carcinoma. They have been used as a model of the intestinal barrier because the cells have characteristics that resemble intestinal epithelial cells (van Breemen and Li 2005). Drugs with good intestinal absorption were set to be positive samples (Ajay et al. 1999). To predict the suitability drugs for oral dosing and to investigate drug efflux, Caco-2 permeability is used.
The p-glycoprotein is one of the drug transporters that determine the uptake, efflux and evacuation of drugs (Finch and Pillans 2014). Drugs which induce or inhibit p-glycoprotein can interact with other drugs (Finch and Pillans 2014). Non-substrate and non-inhibitor data for M2 and M1 signify that they will not interact with other drugs and will easily eliminate from cell. Organic cation transporters are responsible for drug absorption and disposition in the kidney, liver, intestine and indicate the renal excretion of drug (Zhang et al. 1998). ADMET results of M1 and M2 show non-inhibition of renal organic cation transporter.
The human cytochromes P450 (CYP), particularly isoforms CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1 and CYP3A4 have vital function in drug metabolism and clearance in the liver (Vasanthanathan et al. 2009). Inhibition of human CYP enzymes will lead to drug–drug interactions and failure of drug metabolism (Vasanthanathan et al. 2009). ADMET result shows M1 and M2 are non-inhibitor of the cytochromes P450 (CYPs).
The hERG (human Ether-à-go-go-Related Gene) is a gene, sensitive to drug binding and shows the risk of cardiotoxicity (heart arrhythmia) produced by drug (Mitcheson 2008). The hERG inhibition is a crucial regulation that must be avoided during drug development (Sanguinetti and Tristani-Firouzi 2006). ADMET result shows both these drugs are weak inhibitor and non-inhibitor of hERG inhibition (predictor I and II).
The Ames test is a widely employed method to test carcinogenicity and genotoxicity. More formally, it is a biological assay to evaluate the mutagenic potential of chemical compounds (Ames et al. 1972; Mortelmans and Zeiger 2000). ADMET result shows that M1 and M2 are non-Ames toxic and non-carcinogenic.
Fish toxicity, honey bee toxicity and Tetrahymena pyriformis are the environmental risk assessments of the drug based on fish and Tetrahymena pyriformis as environmental indicators (Cheah et al. 2014). In Tetrahymena pyriformis (a protozoa), evaluation of the cytotoxic effects of xenobiotics may be predicted by observing various parameters like growth rate, cell density, etc. (Sauvant et al. 1999).
From the results of in silico ADMET, it can be concluded that M1 and M2 may be absorbed by BBB, Caco-2 and human intestines. M1 and M2 will excrete safely without harming liver heart and kidney. Moreover, M1 and M2 do not display any acute toxicity and mutagenic effect in regard to the cellular and environmental toxicity. Through in silico approach we have proposed a less toxic drug than existing, which is a boosting to synthetic chemists. PAS, sulfamethoxazole and trimethoprim are not positive in all absorption section like M1 and M2.
Density functional theory analysis
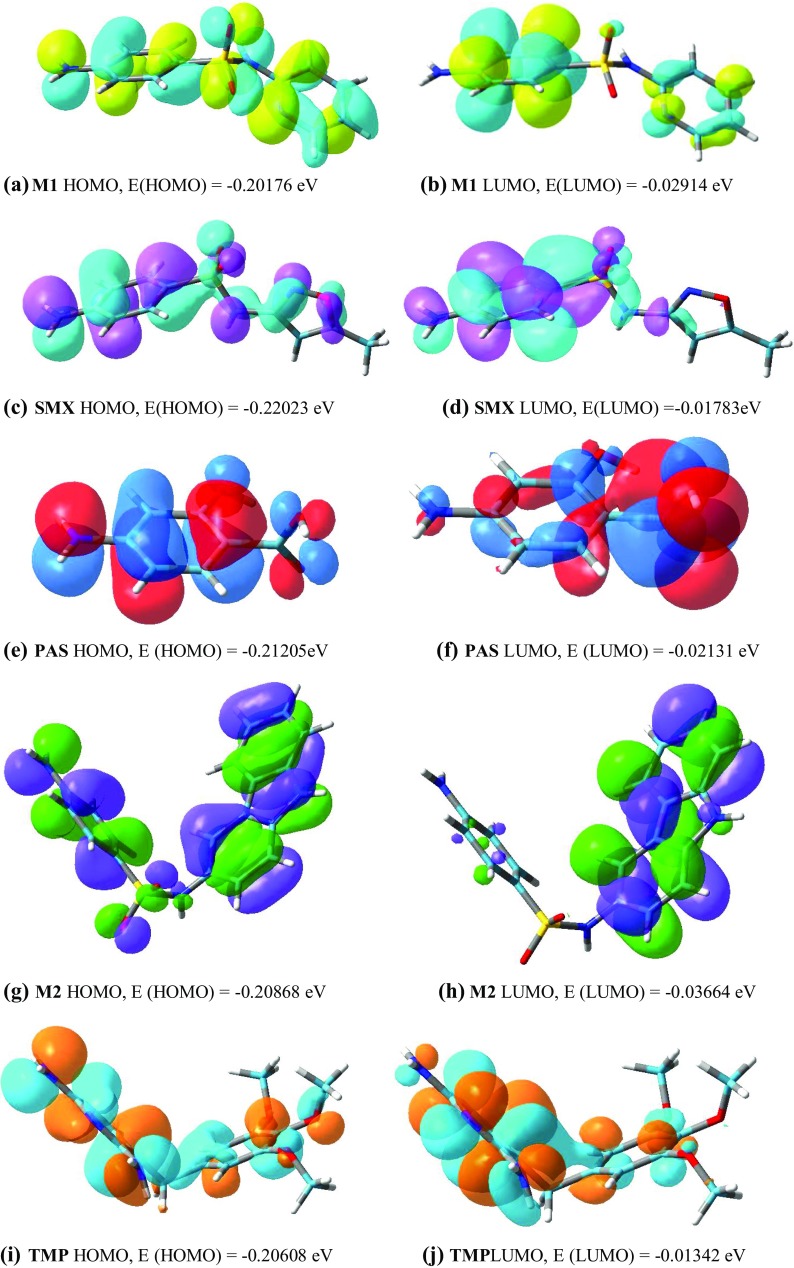
All HSFs from the best binding pose have been transferred to Gauss view 5 and the difference in HOMO and LUMO energy is obtained. It indicates (Fig. 8) the electronic excitation energy necessary to compute the molecular reactivity and stability of the compounds (Rozhenko 2014; Zhan et al. 2003). Details of DFT analysis are given in Table 4.
Fig. 8.
HOMO and LUMO of M1, SMX, PAS, M2 and TPM
Table 4.
HOMO, LUMO and band gap
| Molecules | HOMO | LUMO | Band gap | Positive electron density colour | Negative electron density colour |
|---|---|---|---|---|---|
| M1 | − 0.20176 | − 0.02914 | − 0.17262 | Yellow | Light blue |
| Sulfamethoxazole (SMX) | − 0.22023 | − 0.01783 | − 0.2024 | Violet | Light blue |
| para-Amino salicylic acid (PAS) | − 0.21205 | − 0.02131 | − 0.23336 | Red | Dark blue |
| M2 | − 0.20868 | − 0.03664 | − 0.17204 | Green | Violet |
| Trimethoprim (TPM) | − 0.20608 | − 0.01342 | − 0.19266 | Orange | Light blue |
Drug–DNA interaction
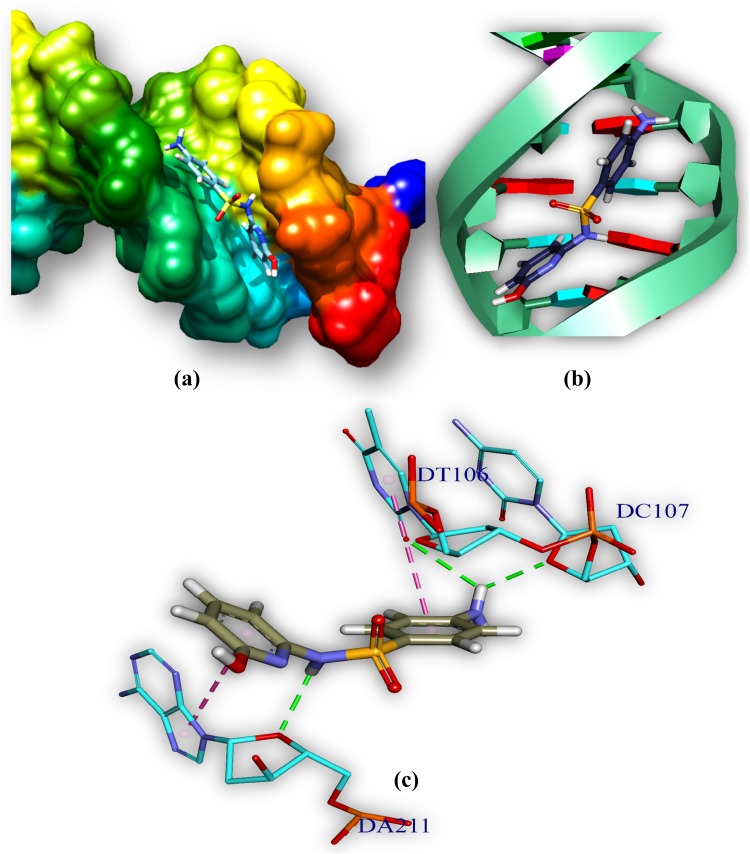
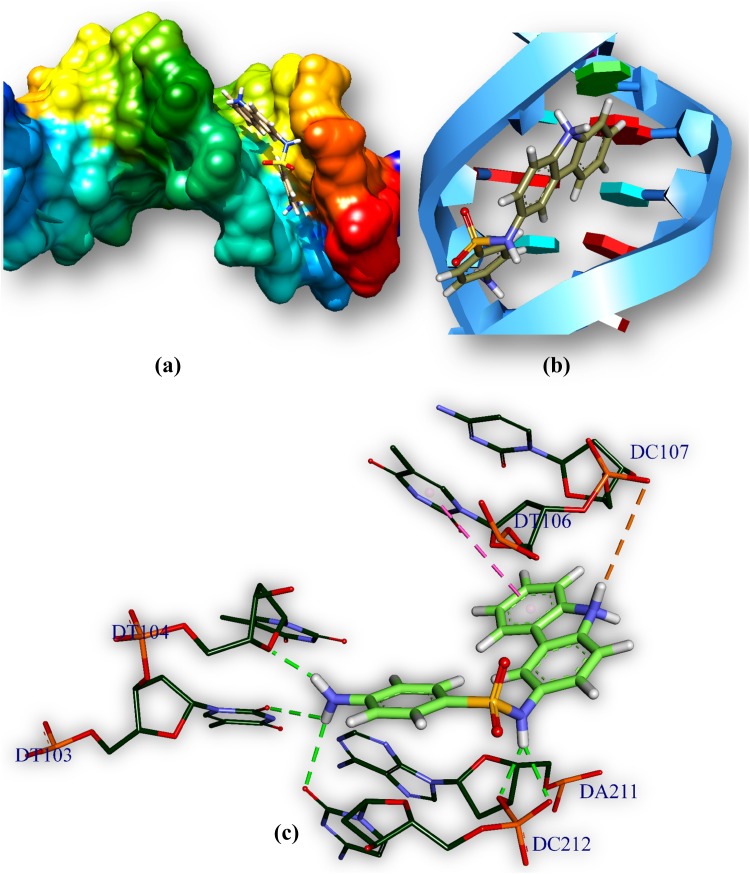
The drug (HSFs)–DNA interaction has been computationally examined by docking techniques and has been used to study the interactions between DNA and small molecules those are of potentially pharmaceutical interest. Drug–DNA interaction gives an idea about binding pattern of drug with DNA. HSFs (drug) docked with MTB DNA and has been used to understand the drug–DNA interaction. HSFs interact with AT-rich regions (represented by red and turquoise coloured rings, respectively) of DNA in the minor groove by forming hydrogen bonding and hydrophobic interactions. The amino group (–NH2) of guanine prevents M1 (Fig. 9; Table 5) and M2 (Fig. 10; Table 5) from binding to the G–C base pairs by steric hindrance, thus conferring AT-selectivity on the drug molecule. Minor groove binding molecules are usually constructed a series of heterocyclic or aromatic hydrocarbon rings that possess rotational freedom. This allows that the molecules to fit into the minor groove, with displacement of water. These drugs can form hydrogen bonds to bases (Jin and Cowan 2014).
Fig. 9.
Comprehensive perception of MTB DNA and M1 after docking. M1 bound to MTB DNA in minor groove, a double helical structure of DNA represented by hydrophobic surface and M1 is represented by stick model and are coloured according to elements, b M1 is represented by stick and double helical DNA structures is represented by ladder and rings, c interactions of ligand with DNA base pairs (A, T, G, C), the interaction type. Bonds are in dots, ligand surrounding base pairs are in two letter code represented in blue
Table 5.
Bond distances and categories between M1, M2 and DNA
| Bond name | Distance (Å) | Category | Bond name | Distance (Å) | Category |
|---|---|---|---|---|---|
| M1:H—D:DA211:O3′ | 2.30 | Hydrogen bond | M2:H—C:DT104:O2 | 2.59 | Hydrogen bond |
| M1:H—D:DC212:OP1 | 2.38 | Hydrogen bond | M2:H—D:DA211:O4′ | 2.29 | Hydrogen bond |
| M1:H—C:DT103:O2 | 3.07 | Hydrogen bond | C: DT106:C5′—:M2:O | 3.21 | Hydrogen bond |
| M1:H—D:DC212:O2 | 2.80 | Hydrogen bond | D: DA211:C4′—:M2:O | 3.72 | Hydrogen bond |
| M1:H—C:DT104:O4′ | 2.10 | Hydrogen bond |
Fig. 10.
Comprehensive perception of MTB DNA and M2 after docking. Figure legends are same as Fig. 9
Out of 102 HSFs fourteen derivatives have successfully passed Lipinski’s and ADMET filters. They are (4-amino-N-(5-methyl-1H-pyrrol-2yl) benzenesulfonamide), (4-amino-N-(9H-carbazolyl) benzenesulfonamide), (4-amino-N-(1H-indol6yl)benzenesulfonamide), (4-amino-N-(2H-isoindol5yl)benzenesulfonamide), (4-amino-N-(3-hydroxy1Hindol5yl)benzenesulfonamide), (4-amino-N-(isobenzofuran-5-yl) benzenesulfonamide), (4-amino-N-(2H-chromen-7-yl)benzenesulfonamide), (4-amino-N-(4H-chromen-7-yl)benzenesulfonamide), (4-amino-N-(pyrazin-2-yl)benzenesulfonamide), (4-amino-N-(pyridazin-3-yl)benzenesulfonamide), (4-Amino-N-(pyridin-2-yl)benzenesulfonamide), (4-amino-N-(phenanthridin-9-yl)benzenesulfonamide), (4-amino-N-(6-hydroxypyridin-2-yl)benzenesulfonamide), and (4-amino-N-(pyrimidin-4-yl)benzenesulfonamide). Sulfonamide derivatives with good docking score such as, carbazole, isoindole, indoxyl, isobenzofuran, pyrrole and indole are the derivatives, those passed the Lipinski’s and ADMET filters. Literature search of structure similarity has been done by simplified molecular-input line-entry system (SMILES) in Pubchem and ZINC database, and no exact similar structures are found in the databases. Most of the sulfonamide derivatives have passed the Lipinski’s Filter but failed the ADMET filter, because they are Caco2 negative.
Conclusion
Sulfonamides show potent anti-microbial activity and also active against MTBs in vivo. Molecular docking interaction of 102 sulfonamides with DHPS and DHFR proteins of MTBs show that two derivatives 4-amino-N-(6-hydroxypyridin-2-yl)benzenesulfonamide (M1) and 4-amino-N-(9H-carbazol-2-yl)benzenesulfonamide (M2) are potentially active and of low toxicity as per ADMET data. This results will encourage chemists to synthesize substituted sulfonamides for future drug demonstration.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We sincerely thank the Council of Scientific and Industrial Research, New Delhi, India [Grant number 01(2894)/17/EMR-II] for funding.
Compliance with ethical standards
Conflict of interest
The authors confirm that this article content has no conflicts of interest.
References
- Ajay, Bemis GW, Murcko MA. Designing libraries with CNS activity. J Med Chem. 1999;42(24):4942–4951. doi: 10.1021/jm990017w. [DOI] [PubMed] [Google Scholar]
- Ames BN, Gurney EG, Miller JA, Bartsch H. Carcinogens as frameshift mutagens: metabolites and derivatives of 2-acetylaminofluorene and other aromatic amine carcinogens. Proc Natl Acad Sci USA. 1972;69(11):3128–3132. doi: 10.1073/pnas.69.11.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becke AD. Density-functional thermochemistry. I. The role of exact exchange. J Chem Phys. 1993;98(7):5648–5652. doi: 10.1063/1.464913. [DOI] [Google Scholar]
- Cheah H-L, Lim V, Sandai D. Inhibitors of the glyoxylate cycle enzyme ICL1 in Candida albicans for potential use as antifungal agents. PLoS One. 2014;9(4):e95951. doi: 10.1371/journal.pone.0095951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F, Li W, Zhou Y, Shen J, Wu Z, Liu G, Lee PW, Tang Y. admetSAR: a comprehensive source and free tool for assessment of chemical ADMET properties. J Chem Inf Model. 2012;52(11):3099–3105. doi: 10.1021/ci300367a. [DOI] [PubMed] [Google Scholar]
- Chung BK, Dick T, Lee DY. In silico analyses for the discovery of tuberculosis drug targets. J Antimicrob Chemother. 2013;68(12):2701–2709. doi: 10.1093/jac/dkt273. [DOI] [PubMed] [Google Scholar]
- Finch A, Pillans P. P-glycoprotein and its role in drug–drug interactions. Aust Prescr. 2014;37(4):137–139. doi: 10.18773/austprescr.2014.050. [DOI] [Google Scholar]
- Freilich EB, Coe GC, Wien NA. The use of sulfanilamide in pulmonary tuberculosis; preliminary report. Ann Intern Med. 1939;13(6):1042–1045. doi: 10.7326/0003-4819-13-6-1042. [DOI] [Google Scholar]
- Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Jr, Peralta JE, Ogliaro F, Bearpark MJ, Heyd J, Brothers EN, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09. Wallingford: Gaussian Inc.; 2009. [Google Scholar]
- Gill PM, Johnson BG, Pople JA, Frisch MJ. The performance of the Becke–Lee–Yang–Parr (B–LYP) density functional theory with various basis sets. Chem Phys Lett. 1992;197(4–5):499–505. doi: 10.1016/0009-2614(92)85807-M. [DOI] [Google Scholar]
- Hou T, Wang J. Structure—ADME relationship: still a long way to go? Expert Opin drug Metab Toxicol. 2008;4(6):759–770. doi: 10.1517/17425255.4.6.759. [DOI] [PubMed] [Google Scholar]
- Lionta E, Spyrou G, Vassilatis DK, Cournia Z. Structure-based virtual screening for drug discovery: principles, applications and recent advances. Curr Top Med Chem. 2014;14(16):1923–1938. doi: 10.2174/1568026614666140929124445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1–3):3–26. doi: 10.1016/S0169-409X(00)00129-0. [DOI] [PubMed] [Google Scholar]
- Masters PA, O’Bryan TA, Zurlo J, Miller DQ, Joshi N. Trimethoprim–sulfamethoxazole revisited. Arch Intern Med. 2003;163(4):402–410. doi: 10.1001/archinte.163.4.402. [DOI] [PubMed] [Google Scholar]
- Minato Y, Thiede JM, Kordus SL, McKlveen EJ, Turman BJ, Baughn AD. Mycobacterium tuberculosis folate metabolism and the mechanistic basis for para-aminosalicylic acid susceptibility and resistance. Antimicrob Agents Chemother. 2015;59(9):5097–5106. doi: 10.1128/AAC.00647-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitcheson JS. hERG potassium channels and the structural basis of drug-induced arrhythmias. Chem Res Toxicol. 2008;21(5):1005–1010. doi: 10.1021/tx800035b. [DOI] [PubMed] [Google Scholar]
- Mortelmans K, Zeiger E. The Ames Salmonella/microsome mutagenicity assay. Mutat Res. 2000;455(1–2):29–60. doi: 10.1016/S0027-5107(00)00064-6. [DOI] [PubMed] [Google Scholar]
- Myers S, Baker A. Drug discovery—an operating model for a new era. Nat Biotechnol. 2001;19(8):727–730. doi: 10.1038/90765. [DOI] [PubMed] [Google Scholar]
- O’Hagan S, Kell DB. The apparent permeabilities of Caco-2 cells to marketed drugs: magnitude, and independence from both biophysical properties and endogenite similarities. PeerJ. 2015;3:e1405. doi: 10.7717/peerj.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomino JC, Martin A. The potential role of trimethoprim–sulfamethoxazole in the treatment of drug-resistant tuberculosis. Future Microbiol. 2016;11(4):539–547. doi: 10.2217/fmb.16.2. [DOI] [PubMed] [Google Scholar]
- Pradhan S, Mondal S, Sinha C. In search of Tuberculosis drug design: an in silico approach to azoimidazolyl derivatives as antagonist for cytochrome P450. J Indian Chem Soc. 2016;93(9):1067–1084. [Google Scholar]
- Rengarajan J, Sassetti CM, Naroditskaya V, Sloutsky A, Bloom BR, Rubin EJ. The folate pathway is a target for resistance to the drug para-aminosalicylic acid (PAS) in mycobacteria. Mol Microbiol. 2004;53(1):275–282. doi: 10.1111/j.1365-2958.2004.04120.x. [DOI] [PubMed] [Google Scholar]
- Rozhenko AB. Density functional theory calculations of enzyme-inhibitor interactions in medicinal chemistry and drug design. In: Gorb L, Kuz’min V, Muratov E, editors. Application of computational techniques in pharmacy and medicine. Dordrecht: Springer; 2014. pp. 207–240. [Google Scholar]
- Sacchettini JC, Rubin EJ, Freundlich JS. Drugs versus bugs: in pursuit of the persistent predator Mycobacterium tuberculosis. Nat Rev Microbiol. 2008;6(1):41–52. doi: 10.1038/nrmicro1816. [DOI] [PubMed] [Google Scholar]
- Sandhu GK. Tuberculosis: current situation, challenges and overview of its control programs in India. J Glob Infect Diseases. 2011;3(2):143–150. doi: 10.4103/0974-777X.81691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Cowan JA. DNA cleavage by copper-ATCUN complexes. Factors influencing cleavage mechanism and linearization of dsDNA. J Am Chem Soc. 2005;127(23):8408–8415. doi: 10.1021/ja0503985. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440(7083):463–469. doi: 10.1038/nature04710. [DOI] [PubMed] [Google Scholar]
- Sauvant N, Pepin D, Piccinni E. Tetrahymena pyriformis: a tool for toxicological studies. A review. Chemosphere. 1999;38(7):1631–1669. doi: 10.1016/S0045-6535(98)00381-6. [DOI] [PubMed] [Google Scholar]
- Shen J, Cheng F, Xu Y, Li W, Tang Y. Estimation of ADME properties with substructure pattern recognition. J Chem Inf Model. 2010;50(6):1034–1041. doi: 10.1021/ci100104j. [DOI] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7(1):539–545. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirajuddin M, Ali S, Badshah A. Drug–DNA interactions and their study by UV–visible, fluorescence spectroscopies and cyclic voltametry. J Photochem Photobiol B. 2013;124:1–19. doi: 10.1016/j.jphotobiol.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Soga S, Shirai H, Kobori M, Hirayama N. Use of amino acid composition to predict ligand-binding sites. J Chem Inf Model. 2007;47(2):400–406. doi: 10.1021/ci6002202. [DOI] [PubMed] [Google Scholar]
- Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem. 1994;98(45):11623–11627. doi: 10.1021/j100096a001. [DOI] [Google Scholar]
- Summan M, Cribb AE. Novel non-labile covalent binding of sulfamethoxazole reactive metabolites to cultured human lymphoid cells. Chem Biol Interact. 2002;142(1–2):155–173. doi: 10.1016/S0009-2797(02)00060-1. [DOI] [PubMed] [Google Scholar]
- Szymański P, Markowicz M, Mikiciuk-Olasik E. Adaptation of high-throughput screening in drug discovery—toxicological screening tests. Int J Mol Sci. 2012;13(1):427–452. doi: 10.3390/ijms13010427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem. 2010;31(2):455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breemen RB, Li Y. Caco-2 cell permeability assays to measure drug absorption. Expert opinion on drug metabolism & toxicology. 2005;1(2):175–185. doi: 10.1517/17425255.1.2.175. [DOI] [PubMed] [Google Scholar]
- Vasanthanathan P, Taboureau O, Oostenbrink C, Vermeulen NP, Olsen L, Jorgensen FS. Classification of cytochrome P450 1A2 inhibitors and noninhibitors by machine learning techniques. Drug Metab Dispos Biol Fate Chem. 2009;37(3):658–664. doi: 10.1124/dmd.108.023507. [DOI] [PubMed] [Google Scholar]
- Walker PL, McKinstry HA, Wright CC. X-ray diffraction studies of a graphitized carbon—changes in interlayer spacing and binding energy with temperature. Ind Eng Chem. 1953;45(8):1711–1715. doi: 10.1021/ie50524a033. [DOI] [Google Scholar]
- Winum J-Y, Dogné J-M, Casini A, de Leval X, Montero J-L, Scozzafava A, Vullo D, Innocenti A, Supuran CT. Carbonic anhydrase inhibitors: synthesis and inhibition of cytosolic/membrane-associated carbonic anhydrase isozymes I, II, and IX with sulfonamides incorporating hydrazino moieties. J Med Chem. 2005;48(6):2121–2125. doi: 10.1021/jm0494826. [DOI] [PubMed] [Google Scholar]
- Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008;36(Database issue):D901–D906. doi: 10.1093/nar/gkm958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Robertson DH, Brooks CL, 3rd, Vieth M. Detailed analysis of grid-based molecular docking: a case study of CDOCKER-A CHARMm-based MD docking algorithm. J Comput Chem. 2003;24(13):1549–1562. doi: 10.1002/jcc.10306. [DOI] [PubMed] [Google Scholar]
- Wyss PC, Gerber P, Hartman PG, Hubschwerlen C, Locher H, Marty H-P, Stahl M. Novel dihydrofolate reductase inhibitors. Structure-based versus diversity-based library design and high-throughput synthesis and screening. J Med Chem. 2003;46(12):2304–2312. doi: 10.1021/jm020495y. [DOI] [PubMed] [Google Scholar]
- Zhan C-G, Nichols JA, Dixon DA. ionization potential, electron affinity, electronegativity, hardness, and electron excitation energy: molecular properties from density functional theory orbital energies. J Phys Chem A. 2003;107(20):4184–4195. doi: 10.1021/jp0225774. [DOI] [Google Scholar]
- Zhang L, Brett CM, Giacomini KM. Role of organic cation transporters in drug absorption and elimination. Annu Rev Pharmacol Toxicol. 1998;38:431–460. doi: 10.1146/annurev.pharmtox.38.1.431. [DOI] [PubMed] [Google Scholar]
- Zhao YH, Le J, Abraham MH, Hersey A, Eddershaw PJ, Luscombe CN, Boutina D, Beck G, Sherborne B, Cooper I. Evaluation of human intestinal absorption data and subsequent derivation of a quantitative structure–activity relationship (QSAR) with the Abraham descriptors. J Pharm Sci. 2001;90(6):749–784. doi: 10.1002/jps.1031. [DOI] [PubMed] [Google Scholar]
- Zheng J, Rubin EJ, Bifani P, Mathys V, Lim V, Au M, Jang J, Nam J, Dick T, Walker JR, Pethe K, Camacho LR. para-Aminosalicylic acid is a prodrug targeting dihydrofolate reductase in Mycobacterium tuberculosis. J Biol Chem. 2013;288(32):23447–23456. doi: 10.1074/jbc.M113.475798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.