Supplemental Digital Content is available in the text
Keywords: major depressive disorder, meta-analysis, systematic review, transcutaneous auricular vagus nerve stimulation
Abstract
Background:
Transcutaneous auricular vagus nerve stimulation (taVNS), as a noninvasive intervention, has beneficial effects on major depressive disorder based on clinical observations. However, the potential benefits and clinical role of taVNS in the treatment of major depressive disorder are still uncertain and have not been systematically evaluated. Therefore, we performed a systematic review and meta-analysis to evaluate the effectiveness and safety of taVNS in treating major depressive disorder.
Methods:
Four electronic databases, namely, Embase, MEDLINE, the Cochrane Library and PsycINFO, were searched for all related trials published through May 1, 2018. We extracted the basic information and data of the included studies and evaluated the methodological quality with the Cochrane risk of bias tool and the nonrandomized studies-of interventions (ROBINS-I) tool. A meta-analysis of the comparative effects was conducted using the Review Manager 5.3 software.
Results:
A total of 423 citations from the databases were searched, and 4 studies with 222 individuals were included in the meta-analysis. The taVNS technique could decrease 24-item HAMD scores more than the sham intervention (MD: −4.23, 95% CI: −7.15, −1.31; P = .005) and was also more effective in decreasing Self-Rating Depression Scale scores ((MD: −10.34, 95% CI: −13.48, −7.20; P < .00001), Beck Depression Inventory scores (MD: −10.3, 95% CI: −18.1, −2.5; P = .01) and Self-Rating Anxiety Scale scores (MD: −6.57, 95% CI: −9.30, −3.84; P < .00001). However, there was no significant difference in the Hamilton Anxiety Rating Scale scores between the taVNS and sham taVNS groups (MD: −1.12, 95% CI: −2.56, 0.32; P = .13). No obvious adverse effects of taVNS treatment were reported in the included studies.
Conclusion:
The results of the analysis preliminarily demonstrated that taVNS therapy can effectively ameliorate the symptoms of major depressive disorder, providing an alternative technique for addressing depression. However, more well-designed RCTs with larger sample sizes and follow-ups are needed in future studies to confirm our findings.
1. Introduction
Major depressive disorder (MDD) is a mental disorder that does harm to the physical and psychological health of an individual and, even worse, may lead to suicide. The global prevalence of major depressive disorder was estimated to be approximately 216,047,000 people in 2015 according to the Global Burden of Disease (GBD) study, representing an increase of 17.8% from the measurement in 2005.[1] MDD are characterized by the symptoms of low mood, sadness, isolation and accompanied by several psychophysiological changes that last at least 2 weeks. Although both bipolar depression and unipolar depression are associated with depressive symptoms and functional impairment, bipolar depression accompanies with the feature of mania or hypomania and is observed with more white matter abnormalities in the brain,[2] which needs to be differentiated in order to treat properly. According to the Canadian network for mood and anxiety treatments (CANMAT) clinical guidelines for the management of major depressive disorder, there are many interventions for treating major depressive disorder, including pharmacotherapy, psychotherapy, neurostimulation, and complementary and alternative interventions.[3] However, previous studies found that approximately 30% of patients would resist antidepressants, even though antidepressant medicines are widely used in clinical practice.[4,5] Neurostimulation, such as vagus nerve stimulation (VNS), deep brain stimulation (DBS), or electroconvulsive therapy (ECT), has been recommended and is effective for treatment-resistant depression; however, these interventions also possess a certain risk of developing infections and other potential side effects due to surgical implantation.[6–8] Therefore, it is necessary to find a safe and effective method to address major depressive disorder.
Transcutaneous auricular vagus nerve stimulation (taVNS), as a noninvasive method, has a good efficacy in treating neuropsychiatric disorders.[9] Transcutaneous electrical stimulation of the concha or the lower half of the back ear (afferent vagus nerve distribution), can produce a similar modulatory effect to that of invasive nerve stimulation (iVNS).[10] In recent years, several clinical trials were involved in exploring the therapeutic effects of taVNS for managing major depressive disorder; however, the potential benefits and clinical role of taVNS in the treatment of major depressive disorder are still uncertain and have not been systematically evaluated. Therefore, we performed a systematic review and meta-analysis to assess the efficiency and advantages of taVNS in the treatment of major depressive disorder.
2. Methods
2.1. Search strategy
Four electronic databases, namely, Embase (via OVID), MEDLINE (via OVID), the Cochrane Library/Central Register of Controlled Trials and PsycINFO (via OVID), were searched for all citations published through May 1, 2018. The combinations of medical subject heading terms (MeSH) and free text terms related to major depressive disorder, transcutaneous auricular vagus nerve stimulation and clinical trials were searched for potentially eligible citations. The specific search strategies of each database are listed in appendix 1.
2.2. Selection and exclusion criteria
All clinical trials that met the following criteria were included in the meta-analysis: patients were diagnosed with major depressive disorder; transcutaneous auricular vagus nerve stimulation was used as an intervention; placebo or other non-taVNS were used as a comparison; and randomized controlled trials or nonrandomized controlled trials were used as the study design.
Studies that reported insufficient data or nontarget outcomes were excluded. Conference abstracts, editorials, case reports, and letters were also excluded.
2.3. Data extraction and quality assessment
Data and relevant information were extracted by 2 reviewers (PHL and HLF) independently. Detailed information of the basic characteristics of each study's population, intervention, comparisons and outcomes was extracted. Another 2 reviewers (WTC and CXW) checked for the accuracy of the data and related information and then evaluated the methodological quality of the included studies according to the Cochrane risk of bias tool and the ROBINS-I bias tool.[11,12] Any disagreement was resolved via discussion or was adjudicated by a third reviewer (LML) if necessary.
2.4. Outcomes
The primary outcomes of our study were depression scales, including the 24-item Hamilton Depression Rating Scale (HAMD), the Self-Rating Depression Scale (SDS), and the Beck Depression Inventory (BDI). The secondary outcomes were major depressive disorder-related scales, including the Hamilton Anxiety Rating Scale (HAMA) and the Self-Rating Anxiety Scale (SAS).
2.5. Statistical analysis
The meta-analysis was conducted with Review Manager 5.3 (Cochrane Collaboration, Oxford, UK)). The continuous outcomes were reported as the mean value and standard deviation and were analysed by using the mean difference (MD) with 95% CIs. I-square (I2), as an index, was used to assess heterogeneity and to determine which statistical model to use to analyse the results. If I2 exceeded 50% and the P-value was <.1, a random-effects model was selected; otherwise, a fixed-effects model was used to analyse the results. Moreover, if the pooled results showed clinical heterogeneity, a subgroup analysis or sensitivity analysis was conducted to solve this issue. Publication bias was estimated by funnel plots or Egger's test. If the number of included studies was <10 or if it was difficult assess publication bias in a study, then Egger's test was performed. Conversely, funnel plots were used to evaluate publication bias.
3. Results
3.1. Study Identification and Selection
The titles and abstracts of a total of 423 citations from 4 databases were screened for initial review. After removing 61 duplicates and 327 studies with unrelated target topics, 35 articles remained for full-text reviews. Three studies (n = 222) met the inclusion criteria and were eligible for further quantitative analyses. Figure 1 shows the specific screening procedure of the PRISMA flowchart.
Figure 1.
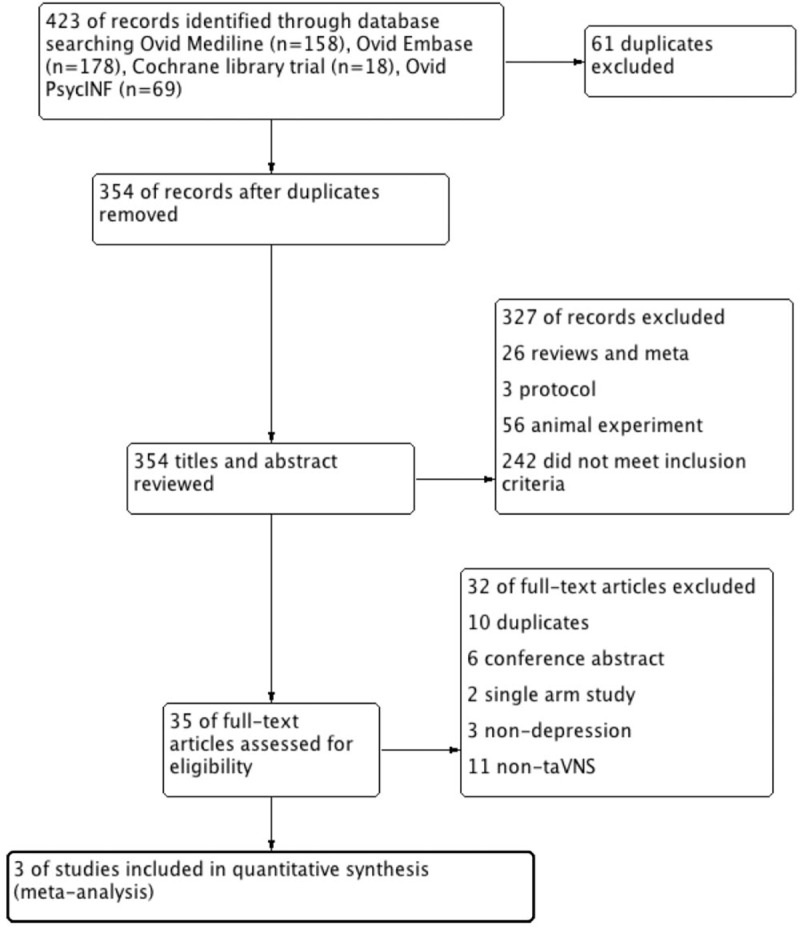
Screening procedure of the PRISMA flowchart.
3.2. Characteristics of the included studies
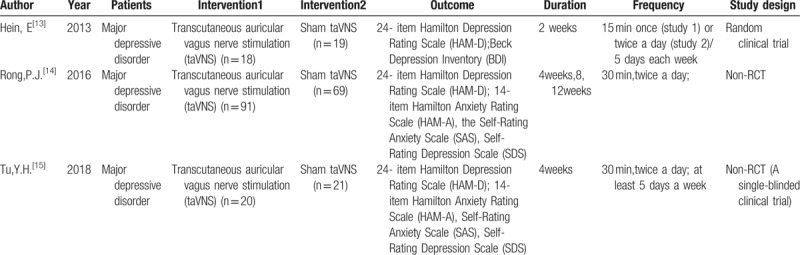
Among the 3 included studies, there was one randomized controlled trial and 2 nonrandomized controlled trials.[13–15] The 4 included studies were published between 2013 and 2018. The clinical trials of the included studies were conducted in Germany and China. The sample sizes of the included studies ranged from 37 to 160 patients.
The population of the included studies were all major depressive disorder patients according to the ICD-10 (World Health Organization 1992), and the patients were all in a stable stage. The interventions used in the control groups were all sham taVNS.[13–15] The therapy duration ranged from 2 weeks to 4 weeks. In addition, the frequency of treatment was mostly twice a day or at least 5 days a week. The specific characteristics of the included studies are presented in Table 1.
Table 1.
The characteristics of included studies.
3.3. Quality assessment of the included studies
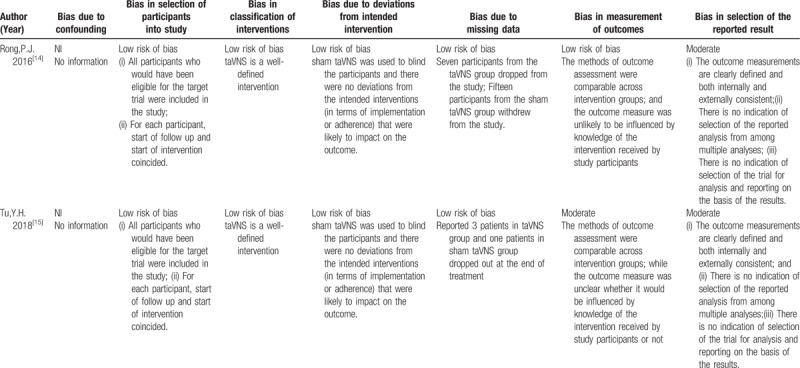
We assessed the quality of the included studies according to the Cochrane risk of bias tool and the risk of bias of nonrandomized studies-of interventions (ROBINS-I) tool. One randomized controlled trial[13] reported adequate random sequence generation (selection bias), while concealment of allocation was unclear in this RCT study. In addition, this RCT did not use blinding of either the participants or personnel. The attrition bias and reporting bias of this RCT were low risk. The other 2 clinical trials[14,15] were evaluated with the ROBINS-I tool. All the non-RCT studies did not report confounding biases since the studies were not cohort studies. Two studies[14,15] had a low risk of bias in the selection of participants for the study due to all the eligible subjects for the target trials being included in the study and the interventions being consistent from the start to the end of treatment. Since taVNS was a well-defined intervention in these 2 trials, the bias in classification of the intervention was low. The deviation bias from the intended intervention was low in all 2 studies, as all the studies used a blinding method to mask participants and to reduce the chance of an impact on the outcome. Two trials[14,15] reported drop-out rates that had a low-risk bias of missing data. One study[14] reported that the outcome assessments might not have been influenced by the knowledge of the participants, while the other one trials[15] was unclear as to whether the outcome measures could have been influenced by knowledge of the intervention received by the participants, resulting in a moderate risk of bias. All the non-RCT studies[14,15] had a moderate risk of selection report bias. The detailed quality assessments of the RCT study and the non-RCT studies are presented in Tables 2 and 3.
Table 2.
Risk of bias summary for RCT study: review authors’ judgements about each risk of bias item for each included study.
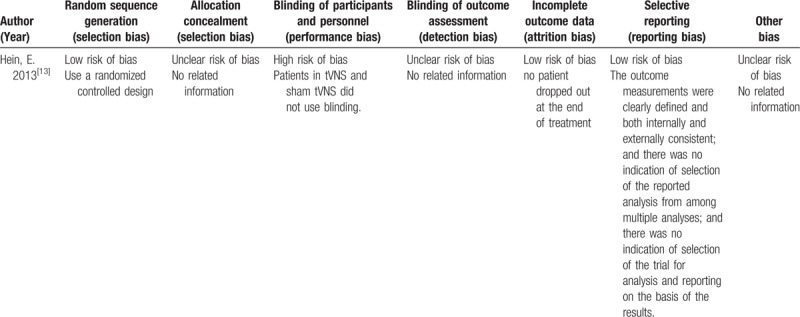
Table 3.
Risk of bias summary for non-RCTs studies: review authors’ judgements about each risk of bias item for each included study.
3.4. Analysis of outcomes
3.4.1. Primary outcomes
3.4.1.1. 24-item Hamilton Depression Rating Scale (HAMD)
Four studies used the 24-item HAMD as their primary outcome. Since the heterogeneity index, namely, I2, of the pooled results of the 3 studies was 64%, and P-value equalled .06, we selected a random-effects model to analyse the pooled results. The 24-item HAMD score decreased more in the taVNS group at the end of treatment than in the sham group (MD: −4.23, 95% CI: −7.15, −1.31; P = .005) (Fig. 2).
Figure 2.
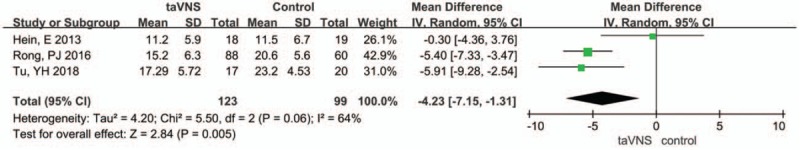
Forest plot of comparison for transcutaneous vagus auricular nerve stimulation (taVNS) versus sham treatment (HAMD outcome). CI = confidence interval, IV = inverse variance, SD = standard deviation.
3.4.1.2. Self-Rating Depression Scale (SDS)
Two studies used the Self-Rating Depression Scale (SDS) as a measured outcome. We selected a fixed-effects model since the heterogeneity index I2 was 0%, and P = .5. The pooled results of the SDS score differed between the taVNS group and the sham group at the end of treatment (MD: −10.34, 95% CI: −13.48, −7.20; P < .00001) (Fig. 3).
Figure 3.
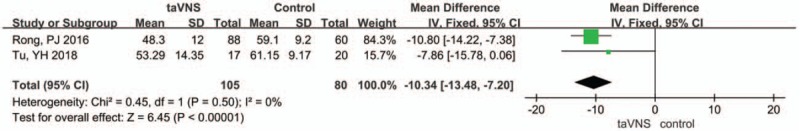
Forest plot of comparison for transcutaneous vagus auricular nerve stimulation (taVNS) versus sham treatment (SDS outcome). CI = confidence interval, IV = inverse variance, SD = standard deviation.
3.4.1.3. Beck Depression Inventory (BDI)
One study used the Beck Depression Inventory (BDI) as a measured outcome. The BDI score was significantly decreased in the taVNS group compared to that in the sham group (MD: −10.3, 95% CI: −18.1, −2.5; P = .01) (Fig. 4).
Figure 4.
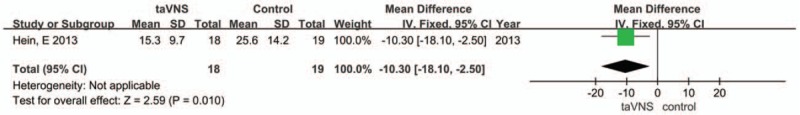
Forest plot of comparison for transcutaneous vagus auricular nerve stimulation (taVNS) versus sham treatment (BDI outcome). CI = confidence interval, IV = inverse variance, SD = standard deviation.
3.4.2. Secondary outcomes
3.4.2.1. Hamilton Anxiety Rating Scale (HAMA)
Two studies reported the Hamilton Anxiety Rating Scale (HAMA) as a measured outcome. A fixed-effects model was chosen to analyse the pooled results due to the heterogeneity index I2 being 0%, and P = .47. The pooled results showed that the HAMA score was lower (MD: −1.12, 95% CI: −2.56, 0.32; P = .13) in the taVNS group post-intervention than in the sham group. While there were no significant differences between the taVNS and sham taVNS (Fig. 5)
Figure 5.
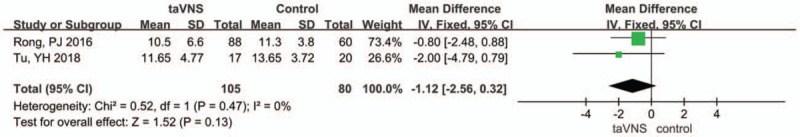
Forest plot of comparison for transcutaneous vagus auricular nerve stimulation (taVNS) versus sham treatment (HAMA outcome). CI = confidence interval, IV = inverse variance, SD = standard deviation.
3.4.2.2. Self-Rating Anxiety Scale (SAS)
The Self-Rating Anxiety Scale (SAS) was used as an assessment outcome in 2 studies. The heterogeneity index I2 was 0%; thus, we selected a fixed-effects model. After combining the results, the pooled results showed that there was a significant difference between the taVNS group and the sham group at the end of treatment (MD: −6.57, 95% CI: −9.30, −3.84; P < .00001) (Fig. 6).
Figure 6.
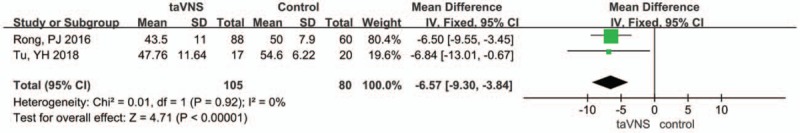
Forest plot of comparison for transcutaneous vagus auricular nerve stimulation (taVNS) versus sham treatment (SAS outcome). CI = confidence interval, IV = inverse variance, SD = standard deviation.
3.5. Publication bias
Due to the small number of included studies, a funnel plot did not allow assessment of the publication bias. Therefore, we used Egger's test to evaluate the publication bias. There was no obvious publication bias in included studies when performing Egger's test (P = .773). The specific Egger's tests are shown in Table 4.
Table 4.
Egger's test of publication bias of all included trials comparing taVNS with control interventions Egger's test.
![]()
3.6. Adverse outcomes
One studies recorded the side effects of transcutaneous auricular vagus nerve stimulation in treating major depressive disorder but did not report the adverse outcomes.[15] One study reported that 2 patients who underwent taVNS and 3 patients who underwent sham taVNS had mild tinnitus side effects but recovered quickly after cessation of the taVNS intervention.[14] Another study reported that there were no adverse side effects after the taVNS intervention.[13]
4. Discussion
4.1. Summary of findings
We conducted this meta-analysis by mainly comparing transcutaneous auricular vagus nerve stimulation with sham taVNS. The analysis consisted of 2 study designs, namely, RCTs, and non-RCTs. Normally, RCTs are difficult to combine with other study designs in analysing the results. However, since taVNS is a new and non-invasive intervention for major depressive disorder and considering the ethical and safety concerns, there were few RCT studies involved in studying taVNS. Therefore, it seemed reasonable to combine the results of RCTs and non-RCTs together to explore the potential effects of taVNS on major depressive disorder. After performing a systematic review and meta-analysis, there were several findings as follows.
First, the pooled results of our meta-analysis demonstrated that taVNS could significantly reduce HAMD, SDS, SAS, and BDI scores. The HAMD is the most frequently used and is considered the gold standard for assessing depressive symptoms that includes evaluating the mood, suicide ideation, feelings of guilt, insomnia and other somatic symptoms of depression patients.[16,17] The BDI scale mainly assesses depression patients from a psychodynamic perspective.[18,19] These measured scales can comprehensively and typically evaluate the symptoms of depression. Therefore, the pooled results suggested that taVNS, as a noninvasive therapy, could alleviate the symptoms of major depressive disorder effectively. As the Hamilton Anxiety Rating Scale (HAMA) scores between the taVNS and sham taVNS groups were not significantly different, transcutaneous auricular vagus nerve stimulation might be less effective for ameliorating anxiety symptoms. Previous researchers demonstrated that vagus nerve stimulation was effective for refractory or medication-resistant depression.[20,21] Transcutaneous auricular vagus nerve stimulation intervention also stimulates the auricular vagus nerve (afferent vagus nerve distribution) via transcutaneous auricular electric stimulation without surgical implantation. This intervention is safe and has few side effects compared to vagus nerve stimulation with surgical implantation. One researcher also analysed and summarized the treatment effects and potential mechanism of taVNS on major depressive disorder, indicating that taVNS had beneficial effects of reducing multiple symptoms of depression patients according to the changes of subscores of the 24-item HAMD scale.[22] A portion of major depressive disorder patients may be resistant to antidepressants and may need a variety of therapies to address major depressive disorder.[23,24] Therefore, based on our analysis results, healthcare professionals could recommend that depressive patients select taVNS as an alternative intervention when confronted with resistant or refractory depression.
Second, the adverse events of taVNS intervention were mostly reported to be safe for individuals with major depressive disorder. Only one study[14] reported that 2 patients in the taVNS group and 3 patients in the sham taVNS had tinnitus side effects, which fully recovered after self-adjustment. The side-effect reports of these studies demonstrated that taVNS was a safe therapy for major depressive disorder.
Third, the quality of the included studies showed that only one study used the random clinical trial design,[13] while the other 2 trials used non-RCT designs, and we evaluated the quality using the ROBINS-I tool.[14,15] The 2 non-RCT studies had a low risk of bias in the selection of the participants, classification of interventions, deviations, outcome assessments, and attrition and a moderate risk of reporting selection bias. In contrast, the confounding bias was not reported in any of these 2 trials.[14,15]
4.2. Findings in relation to previous studies and reviews
To our knowledge, our current study was a first systematic review and meta-analysis that evaluated the effectiveness of transcutaneous auricular vagus nerve stimulation in the treatment of major depressive disorder. The previous meta-analyses mainly focused on assessing the effectiveness of vagus nerve stimulation via surgical implantation for managing major depressive disorder.[25–27] Another review conducted a systematic review to assess auricular therapy, including ear buried seeds and transcutaneous vagus nerve stimulation, for treating major depressive disorder.[28] Although this previous systematic review involved taVNS therapy, the review was not comprehensive and included few taVNS studies in the analysis. The above systematic reviews mainly focus on evaluating the effectiveness of vagus nerve stimulation (surgical implantation) or auricular therapy in treating depression, while studies in systematically estimating effectiveness and safety of transcutaneous auricular vagus nerve stimulation in addressing major depressive disorder were still lacking in the current. We only analysed one type of vagus nerve stimulation (transcutaneous auricular vagus nerve stimulation) for treating depression and did not combine with other therapies, thus allowing us to evaluate the clinical effects of taVNS accurately without other confounding factors.
4.3. Limitations
Several limitations in this meta-analysis need to be taken into consideration. First, most of the included studies were non-RCTs, and only one RCT with a small sample size was included in the analysis, which may have weakened the strength of the evidence. Second, all the included studies only blinded patients and did not blind the therapists or the outcome assessors. Although it is difficult to blind the therapists, the outcome assessors could have been blinded to reduce detection bias. Third, only one study reported follow-up surveys, which may influence the evaluation of the long-term effectiveness of transcutaneous auricular vagus nerve stimulation in treating major depressive disorder.
4.4. Implications for clinical practice
We summarized the effectiveness of transcutaneous auricular vagus nerve stimulation for major depressive disorder and determined that taVNS could alleviate the symptom of depression, specifically reducing 24-item Hamilton Depression Rating Scale, Self-Rating Depression Scale, Self-Rating Anxiety Scale and Beck Depression Inventory scores, which may provide clinicians and patients with an alternative intervention for major depressive disorder. However, the evidence was not strong enough since the inclusion of only 3 studies into quantitative synthesis, which encouraged researchers to do more clinical research about taVNS in order to provide robust evidence. In addition, the current conditions and characteristics of taVNS in the treatment of major depressive disorder that we systematically reviewed are convenient for researchers to do in the future clinical research.
5. Conclusion
In conclusion, our systematic review and meta-analysis preliminarily demonstrated that transcutaneous auricular vagus nerve stimulation is an effective and safe method for treating major depressive disorder. The taVNS technique could alleviate the symptoms of depression, providing an alternative technique for patients who suffer a stable depressive disorder and are unwilling to select other invasive therapies. However, more well-designed RCTs with larger sample sizes and follow-ups are needed in future studies to confirm our findings.
Author contributions
TCZ, LLM participated in the study concept and design and manuscript authorization. WCX, LPH, FHL and CWT extracted the data and assessed studies. CSY and LLM analyzed the data. WCX wrote the manuscript and ensured the integrity of the data.
All authors read and approved the final manuscript.
Conceptualization: Chunzhi Tang.
Data curation: Peihui Liu, Huaili Fu, Wentao Chen.
Formal analysis: Shaoyang Cui, Liming Lu.
Writing – original draft: Chunxiao Wu.
Writing – review & editing: Liming Lu, Chunzhi Tang.
Supplementary Material
Footnotes
Abbreviations: BDI = Beck Depression Inventory, CANMAT = Canadian network for mood and anxiety treatments, DBS = deep brain stimulation, ECT = electroconvulsive therapy, GBD = Global Burden of Disease, HAMA = Hamilton Anxiety Rating Scale, HAMD = Hamilton Depression Rating Scale, iVNS = invasive nerve stimulation, MD = mean difference, MDD = major depressive disorder, ROBINS-I = risk of bias of nonrandomized studies-of interventions, SAS = Self-Rating Anxiety Scale, SDS = Self-Rating Depression Scale, taVNS = transcutaneous auricular vagus nerve stimulation (taVNS), VNS = vagus nerve stimulation.
CW is the first author. CT is the correspondence and LL is the co-correspondence. No funding was received for this meta-analysis.
Competing interests: The authors declare no conflict of interests regarding the publication of this paper.
Supplemental Digital Content is available for this article.
References
- [1].GBD, 2015 Disease, Injury Incidence, Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1545–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Serafini G, Pompili M, Borgwardt S, et al. Brain changes in early-onset bipolar and unipolar depressive disorders: a systematic review in children and adolescents. Eur Child Adolesc Psychiatry 2014;23:1023–41. [DOI] [PubMed] [Google Scholar]
- [3].Milev RV, Giacobbe P, Kennedy SH, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 4. Neurostimulation Treatments. Can J Psychiatry 2016;61:561–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Conway CR, George MS, Sackeim HA. Toward an evidence-based, operational definition of treatment-resistant depression: when enough is enough. JAMA Psychiatry 2017;74:9–10. [DOI] [PubMed] [Google Scholar]
- [5].Peterson K, Dieperink E, Anderson J, et al. Rapid evidence review of the comparative effectiveness, harms, and cost-effectiveness of pharmacogenomics-guided antidepressant treatment versus usual care for major depressive disorder. Psychopharmacology 2017;234:1649–61. [DOI] [PubMed] [Google Scholar]
- [6].Sackeim HA, Rush Aj Fau-George MS, George Ms Fau-Marangell LB, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology 2001;25:713–28. [DOI] [PubMed] [Google Scholar]
- [7].Moksnes KM, Ilner SO. Electroconvulsive therapy—efficacy and side-effects. Tidsskrift den Norske Lægeforening Tidsskrift Praktisk Medicin Række 2010;130:2460–4. [DOI] [PubMed] [Google Scholar]
- [8].Saleh C, Fontaine D. Deep brain stimulation for psychiatric diseases: what are the risks? Curr Psychiatry Rep 2015;17:33. [DOI] [PubMed] [Google Scholar]
- [9].Shiozawa P, da Silva ME, de Carvalho TC, et al. Transcutaneous vagus and trigeminal nerve stimulation for neuropsychiatric disorders: A systematic review. Arq Neuropsiquiatr 2014;72:542–7. [DOI] [PubMed] [Google Scholar]
- [10].Carreno FR, Frazer A. The allure of transcutaneous vagus nerve stimulation as a novel therapeutic modality. Biol Psychiatry 2016;79:260–1. [DOI] [PubMed] [Google Scholar]
- [11].Savovic J, Weeks L, Fau - Sterne JAC, et al. Evaluation of the Cochrane Collaboration's tool for assessing the risk of bias in randomized trials: focus groups, online survey, proposed recommendations and their implementation. Syst Rev 2014;3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hein E, Nowak M, Kiess O, et al. Auricular transcutaneous electrical nerve stimulation in depressed patients: A randomized controlled pilot study. J Neural Transm (Vienna) 2013;120:821–7. [DOI] [PubMed] [Google Scholar]
- [14].Rong P, Liu J, Wang L, et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: A nonrandomized controlled pilot study. J Affect Disord 2016;195:172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tu Y, Fang J, Cao J, et al. A distinct biomarker of continuous transcutaneous vagus nerve stimulation treatment in major depressive disorder. Brain Stimul 2018;11:501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Trajkovic G, Starcevic V, Fau-Latas M, et al. Reliability of the Hamilton Rating Scale for depression: a meta-analysis over a period of 49 years. Psychiatry Res 2011;189:1–9. [DOI] [PubMed] [Google Scholar]
- [17].Hedlund JL, Viewig BW. The Hamilton rating scale for depression: a comprehensive review. J Operational Psychiatry 1979;10:149–65. [Google Scholar]
- [18].Wang YP, Gorenstein C. Psychometric properties of the Beck Depression Inventory—II: a comprehensive review. Rev Bras Psiquiatr 2013;35:416–31. [DOI] [PubMed] [Google Scholar]
- [19].Jackson-Koku G. Beck Depression Inventory. Occup Med (Lond) 2016;66:174–5. [DOI] [PubMed] [Google Scholar]
- [20].Berry SM, Broglio K, Bunker M, et al. A patient-level meta-analysis of studies evaluating vagus nerve stimulation therapy for treatment-resistant depression. Med Devices (Auckl) 2013;6:17–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Carreno FR, Frazer A. Vagal nerve stimulation for treatment-resistant depression. Neurotherapeutics 2017;14:716–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kong J, Fang J, Park J, et al. Treating depression with transcutaneous auricular vagus nerve stimulation: state of the art and future perspectives. Front Psychiatry 2018;9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].McIntyre RS, Filteau MJ, Martin L, et al. Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J Affect Disord 2014;156:1–7. [DOI] [PubMed] [Google Scholar]
- [24].Ionescu DF, Rosenbaum JF, Alpert JE. Pharmacological approaches to the challenge of treatment-resistant depression. Dialogues Clin Neurosci 2015;17:111–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Martin JLR, Martín-Sánchez E. Systematic review and meta-analysis of vagus nerve stimulation in the treatment of depression: variable results based on study designs. Eur Psychiatry 2012;27:147–55. [DOI] [PubMed] [Google Scholar]
- [26].Daban C, Martinez-Aran A, Cruz N, et al. Safety and efficacy of Vagus Nerve Stimulation in treatment-resistant depression. A systematic review. J Affect Disord 2008;110:1–5. [DOI] [PubMed] [Google Scholar]
- [27].Cimpianu CL, Strube W, Falkai P, et al. Vagus nerve stimulation in psychiatry: a systematic review of the available evidence. J Neural Transm (Vienna) 2017;124:145–58. [DOI] [PubMed] [Google Scholar]
- [28].Zhang ZX, Li CR, Rong PJ, et al. Efficacy and safety of auricular therapy for depression. Med Acupunct 2016;28:256–67. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


