Abstract
Background:
This study aimed to evaluate the effectiveness of neuromuscular electrical stimulation (NMES) therapy in patients with urinary incontinence after stroke (UIAS).
Methods:
A total of 82 patients with UIAS were randomly assigned to 2 groups that received NMES therapy (NMES group) or sham NMES (sham group) for 10 weeks. The primary efficacy endpoints were measured by urodynamic values, and Overactive Bladder Symptom Score (OABSS). The secondary efficacy endpoints were assessed by International Consultation on Incontinence Questionnaire-Short Form (ICIQ-SF) score, Barthel Index (BI) scale, and adverse events. All outcomes were evaluated at baseline and at the end of 10 weeks treatment.
Results:
After 10-week treatment, the patients received NMES therapy showed better efficacy in primary endpoints of urodynamic values (P <.01) and OABSS (P <.01), and secondary endpoints of ICIQ-SF (P <.01) and BI (P <.01), compared with patients who underwent sham NMES. No adverse events were recorded in both groups.
Conclusions:
In summary, we demonstrated that 10 weeks of NMES therapy was efficacious in patients with UIAS.
Keywords: neuromuscular electrical stimulation, randomized controlled trial, stroke, urinary incontinence
1. Introduction
Urinary incontinence after stroke (UIAS) is a common condition for patients with stroke.[1–3] It has been estimated that this condition affects more than 50% of stroke survivors,[1,4–5] with prevalence ranges from 32% to 79%.[5–8] Of these patients, 25% to 28% of them experience UIAS upon discharge from the hospital, and about 15% of them experience this condition 1 year later after the discharge.[6–9] Although sometimes this condition can recover very well, it is still a persistent tricky problem in many stroke survivors. Additionally, it is also associated with some psychological problems, such as anxiety, depression.
Management for UIAS mainly include behavioral techniques (bladder training, double voiding, scheduled toilet trips, and fluid and diet management),[10] pelvic floor muscle exercises,[11] electrical stimulation, such neuromuscular electrical stimulation (NMES),[12,13] medications (anticholinergics, mirabegron, alpha blockers, and topical estrogen),[14,15] medical devices (urethral insert, and pessary),[16] interventional therapies (bulking material injections, botulinum toxin type A, and nerve stimulators),[17] surgery (sling procedures, bladder neck suspension, prolapse surgery, and artificial urinary sphincter),[18] and absorbent pads and catheters (pads and protective garments, and catheter).[16] However, most interventions have their own limitations and insufficient efficacy.
Although a previously published study investigated the effects of NMES for treating patients with post-stroke urinary incontinence,[3] limited data are available to support the evidence that NMES can treat UIAS. Therefore, in the present randomized sham-controlled study, we hypothesized that the effectiveness of NEMS would be superior to the sham NMES for patients with UIAS.
2. Methods
2.1. Design
This randomized 2-arm sham-controlled trial was approved by the Medical Ethical Committee of Yanan University Affiliated Hospital, and The First People's Hospital of Xianyang City. All the included patients were recruited at these 2 hospitals. It was performed between November 2016 and April 2018. A total of 82 patients with UIAS were randomly allocated to the NMES group (received NMES therapy) or Sham group (received sham NMES) for 10 weeks, with 41 subjects each group. All outcomes were measured at baseline and at the end of 10 weeks treatment.
2.2. Inclusion and exclusion criteria
Both men and women with UIAS were diagnosed according to the Diagnosis Criteria of the American Stroke Association and International Continence Society.[19,20] All patients aged 40 to 75 years were included in this study. In addition, all patients had more than 6 months duration of stroke; and urinary incontinence after the stroke; normal consciousness, effectively communication; and written informed consent.
The exclusion criteria included urinary retention; UIAS caused by other diseases (such as spinal injury, multiple sclerosis); acute or chronic urinary incontinence before the stroke; severe diseases of important organs, such as heart, liver, kidney; psychological disorders; taken other medications that affected the urinary incontinence; pregnancy or breastfeeding; received electrical stimulation, such as NMES, or electroacupuncture 2 months before the study; or patients who did not agree to continue the study.
2.3. Randomization and blinding
To minimize the selection bias, the patients were allocated randomly to a NMES group or a sham group by a statistician using the SAS software (version 9.1; SAS Institute, Inc., Cary, NC). All randomization and allocation information were concealed in opaque, sealed envelopes. All investigators were masked to the randomization assignment and allocation. The outcome assessors and data analysts were also blinded in this study.
2.4. Intervention
Patients in the NMES group received NMES therapy. It was performed by a portable NMES stimulator (Globus ACTIVA 600 Pro, Globus, Italy) with 2 sets of electrode pads. The positive pad was placed at region of the second sacral level on opposite sides of the vertebral column. On the other hand, the negative pad was placed at the inside of the middle and lower third of the junction between the posterior superior iliac spine and the ischial node according to the published study.[13]Each individual was treated with 50 Hz frequency, 250μs pulse duration, and 10 seconds on and 30 seconds off for 30 minutes each session, once daily, 5 sessions weekly for a total of 10 weeks. The current intensity was gradually increased to each patient's maximum tolerance. The participants in the Sham group were administered sham NEMS at the same location, treatment protocol, using same NMES device, but without an active probe.
2.5. Efficacy endpoints assessment
The primary efficacy endpoints were measured by the urodynamic outcome, and Overactive Bladder Symptom Score (OABSS).[21,22] The total score varies from 0 to 15, with higher score indicating more severe symptom. The secondary efficacy endpoints were assessed by the International Consultation on Incontinence Questionnaire-Short Form (ICIQ-SF) score,[23] and Barthel Index (BI) scale.[24–26] The ICIQ-SF score ranges from 0 to 21, with a higher score indicating more severity urinary leakage.[23] BI scale ranges from 0 to 20, with higher scores indicating lower disability.[24,25] In addition, adverse events related to the NMES were also recorded in this study. All primary and secondary efficacy endpoints were measured at baseline and at the end of 10 weeks treatment.
2.6. Statistical analysis
All outcome data were analyzed by a statistician using the SAS software. The intention-to-treat (ITT) approach was applied. Chi-square test was utilized to analyze the categorical data; while the t test or Mann–Whitney U test was performed to analyze the continuous data. The statistical significance level was defined with P <.05.
3. Results
A total of 116 patients with UIAS were initially entered for eligibility (Fig. 1). Of these subjects, 34 were excluded because they did not meet the study criteria. Thus, 82 patients were equally allocated into the NMES and sham groups. The outcome data of all 82 included patients were analyzed by using the ITT approach, although 4 patients lost to follow-up, and 4 participants withdrew.
Figure 1.
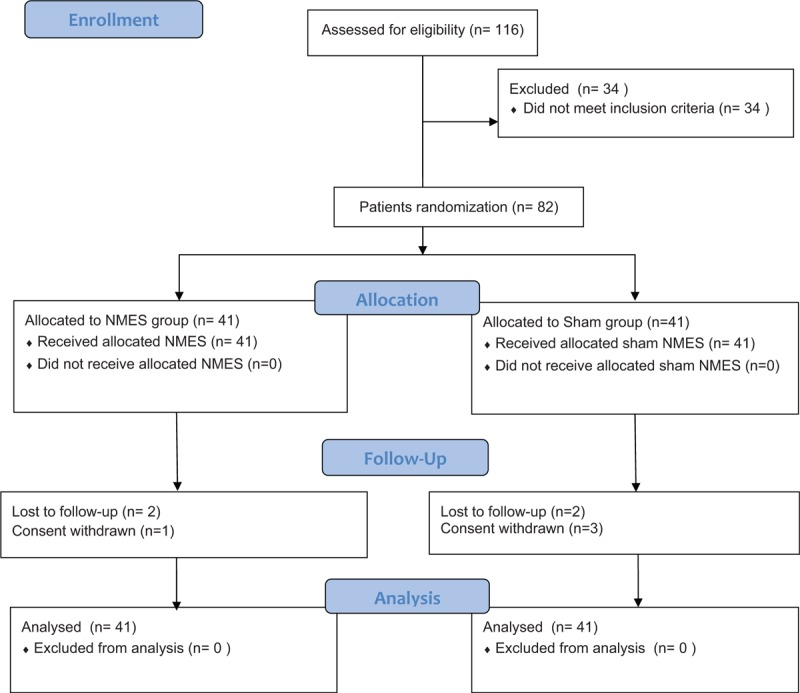
Flowchart of participant selection.
The demographics and characteristics of all included patients with UIAS at baseline are listed in Table 1. There were no significant differences regarding all baseline values between 2 groups. These values included age, sex, body mass index, duration of stroke, duration of urinary incontinence, disease types, region, co-morbidities, and outcome measurements at baseline.
Table 1.
Patients demographics and characteristics at baseline.
Results of this study showed that NMES had more promising efficacy for the treatment of patients with the UIAS when compared with sham NMES. After 10-week treatment, patients in the NMES group showed better outcomes both in the primary efficacy endpoint of urodynamic values (P <.01, Table 2) and OABSS (P <.01, Table 3), and also the secondary endpoints of ICIQ-SF (P <.01, Table 4) and BI (P <.01, Table 5), compared with patients in the sham group.
Table 2.
Comparison of urodynamic values after 10-week treatment (change from baseline).
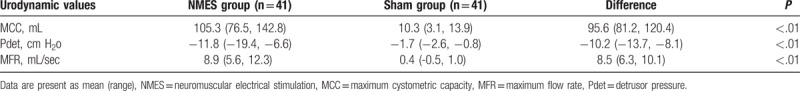
Table 3.
Comparison of OABSS after 10-week treatment (change from baseline).

Table 4.
Comparison of ICIQ-SF after 10-week treatment (change from baseline).

Table 5.
Comparison of BI after 10-week treatment (change from baseline).

During the period of 10-week treatment, no adverse effects, such as discomfort related to the NMES or sham NMES occurred in either group.
4. Discussion
To the best of our knowledge, this study is the first 10-week, randomized sham-controlled trial that has been conducted with NMES therapy in Chinese patients with UIAS. The results of this study demonstrated that NMES can not only enhance the symptoms of patients with UIAS but also can improve their quality of life after 10-week treatment.
Previous study has also investigated the efficacy of NMES for treating patients with post-stroke urinary incontinence.[3] However, that study specifically focused on the female patients, which is different from the present study, including both males and females. In addition, that study is a retrospective study without applying the randomization and blinding procedure, which may have higher risk of patient selection. On the other hand, the present study was designed as the randomized sham-controlled trial, which can provide much higher level of evidence than the previous study.[3]
The results of the present study confirmed our hypothesis that NMES therapy resulted in better treatment efficacy in all endpoints of urodynamic values, OABSS, ICIQ-SF, and BI, compared to sham NEMS in the treatment of UIAS. These results indicate the positive efficacy of NMES on the symptoms of patients with UIAS. Furthermore, NMES treatment also appears to be promising for the improvement of quality of life in patients with UIAS.
This study had several limitations. First, all included patients are Chinese Han, thus, it may be influenced its finding generalized to the other ethnicities in China. Second, this study included 10-week treatment duration and no further follow-up when the treatment ceased. Thus, longer term of follow-up after 10 weeks are still needed to be explored in the future studies. Third, patients were failed to blinded, which may increase the selection risk in this study. Overall, further studies should avoid the above limitations.
5. Conclusion
The results of this study revealed that NMES can benefit patients with UIAS after 10-week treatment. However, longer-term clinical trials with follow-up assessment are still needed to warrant these findings.
Author contributions
Conceptualization: Yong-gang Kang, Gai-yan Guo.
Data curation: Yong-gang Kang, Gai-yan Guo.
Formal analysis: Gai-yan Guo.
Investigation: Gai-yan Guo.
Methodology: Gai-yan Guo.
Project administration: Yong-gang Kang.
Resources: Yong-gang Kang, Gai-yan Guo.
Software: Gai-yan Guo.
Supervision: Yong-gang Kang.
Validation: Yong-gang Kang.
Visualization: Yong-gang Kang.
Writing – original draft: Yong-gang Kang, Gai-yan Guo.
Writing – review & editing: Yong-gang Kang, Gai-yan Guo.
Footnotes
Abbreviations: BI = Barthel Index, ICIQ-SF = International Consultation on Incontinence Questionnaire-Short Form, NMES = neuromuscular electrical stimulation, OABSS = Overactive Bladder Symptom Score, UIAS = urinary incontinence after stroke.
The authors have no conflicts of interest to disclose.
References
- [1].Tuong NE, Klausner AP, Hampton LJ. A review of post-stroke urinary incontinence. Can J Urol 2016;23:8265–70. [PubMed] [Google Scholar]
- [2].Gibson JM, Thomas LH, Harrison JJ, et al. Stroke survivors’ and carers’ experiences of a systematic voiding programme to treat urinary incontinence after stroke. J Clin Nurs 2018;27:2041–51. [DOI] [PubMed] [Google Scholar]
- [3].Shen SX, Liu Y. A retrospective study of neuromuscular electrical stimulation for treating women with post-stroke incontinence. Medicine (Baltimore) 2018;97:e11264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nakayama H, Jørgensen HS, Pedersen PM, et al. Prevalence and risk factors of incontinence after stroke. the Copenhagen stroke study. Stroke 1997;28:58–62. [DOI] [PubMed] [Google Scholar]
- [5].Williams MP, Srikanth V, Bird M, et al. Urinary symptoms and natural history of urinary continence after first-ever stroke-a longitudinal population-based study. Age Ageing 2012;41:371–6. [DOI] [PubMed] [Google Scholar]
- [6].Cai W, Wang J, Wang L, et al. Prevalence and risk factors of urinary incontinence for post-stroke inpatients in southern china. Neurourol Urodyn 2015;34:231–5. [DOI] [PubMed] [Google Scholar]
- [7].Pilcher M, MacArthur J. Patient experiences of bladder problems following stroke. Nurs Stand 2012;26:39–46. [DOI] [PubMed] [Google Scholar]
- [8].Brittain KR, Perry SI, Peet SM, et al. Prevalence and impact of urinary symptoms among community-dwelling stroke survivors. Stroke 2000;31:886–91. [DOI] [PubMed] [Google Scholar]
- [9].Hankey GJ. Potential new risk factors for ischemic stroke what is their potential. Stroke 2006;37:2181–8. [DOI] [PubMed] [Google Scholar]
- [10].Schreiber Pedersen L, Lose G, Høybye MT, et al. Predictors and reasons for help-seeking behavior among women with urinary incontinence. Int Urogynecol J 2018;29:521–30. [DOI] [PubMed] [Google Scholar]
- [11].Nilsen I, Rebolledo G, Acharya G, et al. Mechanical oscillations superimposed on the pelvic floor muscles during Kegel exercises reduce urine leakage in women suffering from stress urinary incontinence: A prospective cohort study with a 2-year follow up. Acta Obstet Gynecol Scand 2018;97:1185–91. [DOI] [PubMed] [Google Scholar]
- [12].Guo ZF, Liu Y, Hu GH, et al. Transcutaneous electrical nerve stimulation in the treatment of patients with poststroke urinary incontinence. Clin Interv Aging 2014;9:851–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liu Y, Xu G, Luo M, et al. Effects of transcutaneous electrical nerve stimulation at two frequencies on urinary incontinence in poststroke patients: a randomized controlled trial. Am J Phys Med Rehabil 2016;95:183–93. [DOI] [PubMed] [Google Scholar]
- [14].Thomas LH, Cross S, Barrett J, et al. Treatment of urinary incontinence after stroke in adults. Cochrane Database Syst Rev 2008;CD004462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mehdi Z, Birns J, Bhalla A. Post-stroke urinary incontinence. Int J Clin Pract 2013;67:1128–37. [DOI] [PubMed] [Google Scholar]
- [16].John G, Primmaz S, Crichton S, et al. Urinary incontinence and indwelling urinary catheters as predictors of death after new-onset stroke: a report of the South London stroke register. J Stroke Cerebrovasc Dis 2018;27:118–24. [DOI] [PubMed] [Google Scholar]
- [17].Zeuner KE, Knutzen A, Kühl C, et al. Functional impact of different muscle localization techniques for Botulinum neurotoxin A injections in clinical routine management of post-stroke spasticity. Brain Inj 2017;31:75–82. [DOI] [PubMed] [Google Scholar]
- [18].Baztán JJ, Arias E, González N, et al. New-onset urinary incontinence and rehabilitation outcomes in frail older patients. Age Ageing 2005;34:172–5. [DOI] [PubMed] [Google Scholar]
- [19].Saver JL, Wasiak H. Stroke council and American stroke association update. Stroke 2011;42:830–1. [DOI] [PubMed] [Google Scholar]
- [20].Warren JW, Meyer WA, Greenberg P, et al. Using the international continence society's definition of painful bladder syndrome. Urology 2006;67:1138–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Homma Y, Yoshida M, Seki N, et al. Symptom assessment tool for overactive bladder syndrome—overactive bladder symptom score. Urology 2006;68:318–23. [DOI] [PubMed] [Google Scholar]
- [22].Blaivas JG, Panagopoulos G, Weiss JP, et al. Validation of the overactive bladder symptom score. J Urol 2007;178:543–7. [DOI] [PubMed] [Google Scholar]
- [23].Twiss CO, Fischer MC. Nitti VW. Comparison between reduction in 24-hour pad weight, International Consultation on Incontinence-Short Form (ICIQ-SF) score, International Prostate Symptom Score (IPSS), and Post-Operative Patient Global Impression of Improvement (PGI-I) score in patient evaluation after male perineal sling. Neurourol Urodyn 2007;26:8–13. [DOI] [PubMed] [Google Scholar]
- [24].Quinn TJ, Langhorne P, Stott DJ. Barthel index for stroke trials: development, properties, and application. Stroke 2011;42:1146–51. [DOI] [PubMed] [Google Scholar]
- [25].Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol 1989;42:703–9. [DOI] [PubMed] [Google Scholar]
- [26].Sainsbury A, Seebass G, Bansal A, et al. Reliability of the Barthel Index when used with older people. Age Ageing 2005;34:228–32. [DOI] [PubMed] [Google Scholar]


