Abstract
Although stroke is one of the most common causes of dysphagia, no studies have investigated the radionuclide salivagram as a predictor of aspiration pneumonia in patients with stroke. In addition, few researches on the risk factors of aspiration pneumonia in patients with subacute and chronic stroke undergoing rehabilitation in the rehabilitation unit have been rarely conducted. In this study, therefore, we investigated whether a radionuclide salivagram could predict aspiration pneumonia, and tried to find other clinical factors that may be helpful in predicting aspiration pneumonia in stroke patients undergoing rehabilitation in the rehabilitation department.
From March 2013 and January 2018, a retrospective review of the medical records of 1182 subacute and chronic stroke patients who were admitted to rehabilitation department (South Korea) was carried out. We included 117 stroke patients with swallowing difficulties who were admitted to our rehabilitation department and satisfied our criteria retrospectively. Stroke lesion, the degree of paralysis, sex, age, onset duration, feeding methods, the Mini-Mental State Examination (MMSE), the Global Deterioration Scale (GDS), the presence of aspiration in VFSS or salivagram, the penetration-aspiration scale (PAS), and the total score of the Modified Barthel Index (MBI) were investigated by reviewing medical records.
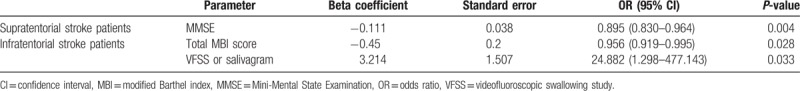
To evaluate the predictor of aspiration pneumonia for patients with stroke, multivariate logistic regression analysis with forward stepwise was performed. In the results of this study, only MMSE was significant as a clinical predictor, but not aspiration in VFSS or salivagram in multivariate analysis of supratentorial stroke patients (OR, 0.895) (95% CI, 0.830–964). In multivariate analysis of infratentorial stroke patients, combined results of salivagram and VFSS (aspiration in a salivagram or VFSS) (OR, 0.956) (95% CI, 0.919–995), and total MBI scores were significant as clinical predictors (OR, 24.882) (95% CI, 1.298–477.143).
In conclusion, MMSE can be a clinical predictor of the occurrence of aspiration pneumonia in patients with supratentorial stroke. In contrast, total MBI score and combined results of a salivagram and VFSS can be clinical predictors of the occurrence of aspiration pneumonia in patients with infratentorial stroke.
Keywords: deglutition, deglutition disorders, MMSE, modified Barthel index, salivagram, VFSS
1. Introduction
Dysphagia, which is one of the common complications affecting patients with stroke, is considered to be a major risk factor for post-stroke pneumonia.[1] In diagnosis of dysphagia in stroke patients, videofluoroscopic swallowing study (VFSS) is considered as the golden standard and has become the most common method to assess swallowing ability and aspiration in clinical practice.[2,3] However, despite this usefulness of VFSS in evaluating dysphagia, previous studies using VFSS have reported significant false-negative results in predicting aspiration pneumonia.[4] In those studies, the authors estimated that the significant false-negative results of VFSS in predicting aspiration pneumonia may be due to episodic aspiration that may not be accurately reflected during examination, or salivary aspiration, which cannot be detected in VFSS.[5,6]
In order to overcome these limitations of VFSS in predicting aspiration pneumonia, the radionuclide salivagram has been investigated as a tool to complement VFSS in previous studies.[3,5–7] In addition to aspiration in VFSS, multiple previous cerebral infarctions, brainstem stroke, oral hygiene, previous cardiopulmonary disease (e.g., chronic obstructive pulmonary disease and congestive heart failure), salivary aspiration, masticatory muscle paralysis, abolition of gag reflex, and impaired cough reflex have been reported as another risk factor of the aspiration pneumonia in the previous studies.[8–11] However, although stroke is one of the most common causes of dysphagia, no studies have investigated the radionuclide salivagram as a predictor of aspiration pneumonia in patients with stroke. Moreover, considering that pneumonia is the most common respiratory complication, accounting for approximately one-third of all deaths after stroke,[12,13] investigating the clinical predictor of aspiration pneumonia and its use in prevention and early treatment may help to reduce the mortality rate of stroke patients. In addition, few researches on the risk factors of aspiration pneumonia in patients with subacute and chronic stroke undergoing rehabilitation in the rehabilitation unit have been rarely conducted. In this study, therefore, we investigated whether a radionuclide salivagram could predict aspiration pneumonia, and tried to find other clinical factors that may be helpful in predicting aspiration pneumonia in stroke patients undergoing rehabilitation in the rehabilitation department.
2. Method
2.1. Patients
This study received Institutional Review Board approval of Daegu Fatima Hospital. From March 2013 and January 2018, a retrospective review of the medical records of 1182 subacute and chronic stroke patients, who were admitted to rehabilitation department of our hospital, was carried out. Subacute stroke in this study was defined from 1 week to 3 months after stroke onset.[14] And chronic stroke was defined greater than 3 months after stroke onset.[14] The inclusion criteria for the study were adult patients (≥20 years old) diagnosed with stroke and referred for VFSS and radionuclide salivagram who had swallowing difficulty. We excluded patients who had a more than seven-day interval between the radionuclide salivagram and VFSS, and those with idiopathic Parkinson's disease, motor neuron disease, high cervical spinal cord injury, or brain tumors (Fig. 1).
Figure 1.
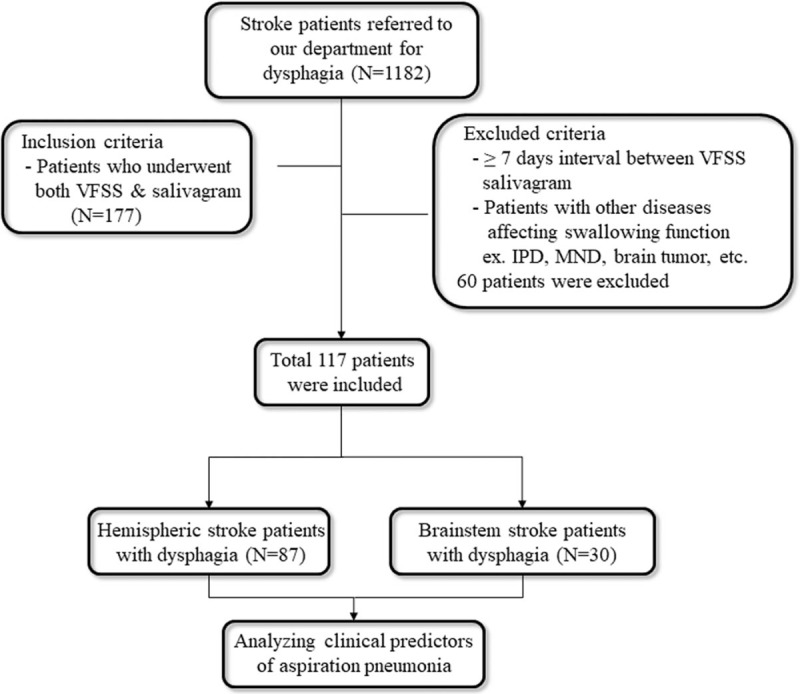
Flow chart of inclusion and exclusion criteria of the study sample. VFSS = videofluoroscopic swallowing study. IPD = idiopathic parkinson's disease, MND = motor neuron disease.
2.2. Sample size calculation
The sample size calculation was based on the author's preliminary data of 35 patients by using the value and those standard deviation of parameters correlated with aspiration pneumonia.[3] The largest SD was 2.0. With α of less than 0.05 in two-tailed tests and a power of 80%, the authors calculated that their target sample size was 84 stroke patients with dysphagia.
2.3. Clinical parameters
To investigate the correlation between the development of aspiration pneumonia and clinical parameters, we reviewed stroke lesion (supratentorial stroke vs. infratentorial stroke), the degree of paralysis (hemi- or quadriplegia), sex, age, onset duration of stroke, feeding methods (oral feeding, Levin tube feeding, percutaneous endoscopic gastrostomy feeding), Mini-Mental State Examination (MMSE), Global Deterioration Scale (GDS),[15] and the total score of the Modified Barthel Index (MBI).[16] Based on the previous study that has shown that salivary aspiration was associated with MMSE, GDS, and total score of the MBI, these clinical parameters were investigated in this study.[3] We excluded patients who had a more than 7-day interval between the VFSS and the measurement of clinical parameters. Therefore, in all patients included in this study, the radionuclide salivagram, VFSS, and the measurement of clinical parameters were performed within a week.
2.4. Aspiration pneumonia
A retrospective review was performed to confirm the development of aspiration pneumonia in the patients with stroke. We investigated the occurrence of aspiration pneumonia within 1 month before and after VFSS. The following data were collected: symptoms such as coughing during feeding; presence of sputum, dyspnea, or fever; findings of chest X-ray; blood laboratory findings (white blood cell (WBC) counts, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels); and use of antibiotics.[5]
Although a definitive diagnosis of aspiration is difficult and the diagnostic criteria for aspiration pneumonia differ slightly across studies,[5,17–19] patients who met all the following criteria were considered to have aspiration pneumonia in this study: (1) the presence of both objective signs suggesting pneumonia (presence of lung infiltration on chest X-ray, coarse lung sounds, and systemic inflammation based on blood laboratory findings such as increased WBC counts and CRP levels) and subjective symptoms (cough, fever, and increased purulent sputum), (2) clinical suspicion of aspiration (coughing during swallowing or delayed swallowing), and (3) no evidence of microorganisms, such as legionella or mycoplasma, which are consistent with atypical pneumonia.[19,20] In addition, the clinical reports of infection and/or respiratory physicians were used for diagnosis of aspiration pneumonia.
2.5. Videofluoroscopic swallowing study
The VFSS exams were performed with a fluoroscopy unit and recorded with computerized recording systems. The dynamic fluoroscopic images were obtained with anterior, posterior, and lateral views and stored at 30 frames per second. All examinations were analyzed by two physicians at the department of rehabilitation medicine of our hospital. The VFSS was performed using sequential swallowing of the following materials mixed with barium: thin fluids, thick fluids (51–350 cP), pureed rice (351–1750 cP), and solids (greater than 1750 cP).[21–23] Patients were evaluated in an upright seated position and were not instructed about any compensation techniques. The saved images were analyzed by one of the authors. VFSS findings were described according to the Penetration-Aspiration scale (PAS, Appendix), and considered positive for aspiration if the PAS score was greater than 5.[24]
2.6. Radionuclide salivagram
Radionuclide salivagrams were performed simultaneously with the VFSS tests within seven days. All salivagrams were performed at the Department of Nuclear medicine in our hospital. Technetium 99m sulfur colloid solution (0.5 mL of 0.3 mCi) was administered into the mouth, and sequential supine posterior images were obtained for one hour routinely, at 1, 5, 10, 20, 30, and 60 min after the instillation. Images were taken with a gamma camera (GE Healthcare, 102 Discovery NM630, Buckinghamshire, England).[3,25,26] The radionuclide salivagram images were analyzed by an experienced nuclear medicine doctor. Aspiration was reported to be present when radiopharmaceutical activity was detected in tracheobronchial fields.
2.7. Statistical analysis
Descriptive and frequency analyses of the data were presented as mean with standard deviation. Group comparisons according to the presence of aspiration pneumonia were performed using the Wilcoxon rank sum test or the Mann–Whitney and the Pearson's chi-squared test where applicable. Multivariate logistic regression analysis with forward stepwise was performed to evaluate the predictor of aspiration pneumonia for patients with stroke. The area under the curve (AUC) was calculated from the receiver operating characteristic (ROC) curve to assess the accuracy of the predictive factor for aspiration pneumonia. P-values <.05 were considered to denote statistical significance. Statistical analyses were performed using SPSS software, version 22.0 (SPSS, Chicago, IL), and MedCalc program for Windows.
3. Results
3.1. Patient characteristics
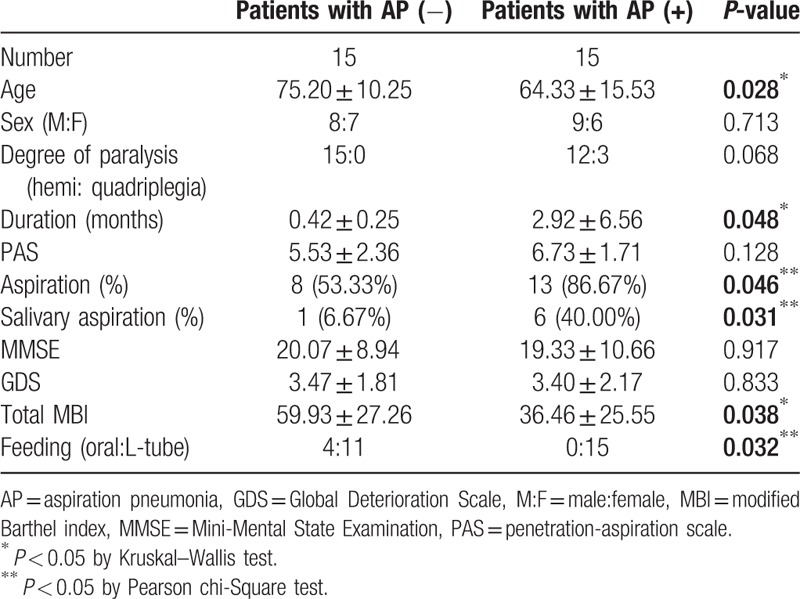
A total of 177 stroke patients with swallowing difficulties were referred for both VFSS and radionuclide salivagram. This study included 117 patients who satisfied our criteria (The mean time of the intervals between the radionuclide salivagram and VFSS was 3.61 ± 1.11 days. The mean time of the intervals between the measurement of clinical parameters and VFSS was 4.84 ± 1.42 days). The patients’ demographic data are presented in Table 1. We divided the subjects into two groups according to the occurrence of aspiration pneumonia. Thirty-one out of 117 patients showed signs of aspiration pneumonia in a retrospective review of their medical records. To investigate the correlations between clinical parameters and the development of aspiration pneumonia, the patients were also analyzed as two groups according to stroke lesions (supratentorial stroke vs. infratentorial stroke group). Their demographic data are presented in Tables 2 and 3.
Table 1.
Characteristics of the patients in this study.
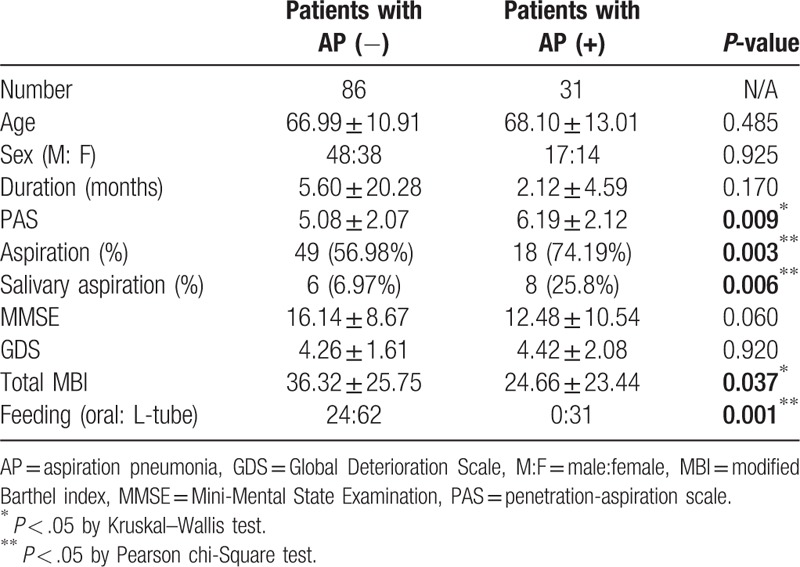
Table 2.
Characteristics of the supratentorial stroke patients in this study.
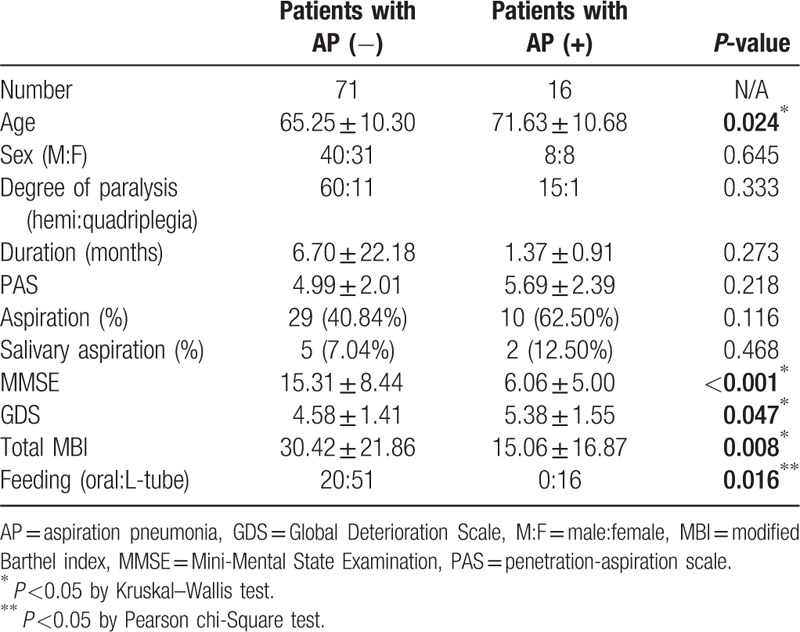
Table 3.
Characteristics of the infratentorial stroke patients in this study.
3.2. Comparison between stroke (both supratentorial and brain stem stroke) patients with and without aspiration pneumonia
Age, duration of disease, PAS, MMSE, GDS, and total MBI were compared between the two groups, using the Wilcoxon rank sum test or the Mann–Whitney test. Statistically significant differences were observed in PAS and total MBI score (Table 2, P-value <.05). Differences in the sex ratio, feeding method and aspiration in VFSS, and salivary aspiration in the radionuclide salivagram between the two groups were analyzed by Pearson's chi-squared test. There were no significant differences between the sex ratios (P-value = .925) of the two groups. However, aspiration in VFSS and salivary aspiration in the radionuclide salivagram were significantly high in group of stroke patients with aspiration pneumonia (Table 2) (P < .05). There were more stroke patients with L-tube feeding in the aspiration pneumonia group (Table 2) (P < .05).
3.3. Comparison between supratentorial stroke patients with and without aspiration pneumonia
Age, duration of disease, PAS, MMSE, GDS, and the total MBI were compared between the two groups, using the Wilcoxon rank sum test or the Mann–Whitney test. Statistically significant differences were observed in Age, MMSE, GDS, and total MBI score (Table 1, P-value <0.05). Differences of sex ratio, feeding method and aspiration in VFSS, and salivary aspiration in the radionuclide salivagram between the two groups were analyzed by Pearson's chi-squared test. There were no significant differences between sex ratio (P-value = .925), degree of paralysis (P-value = .333), aspiration in VFSS (P-value = .116), salivary aspiration in the radionuclide salivagram (P-value = .468) in the two groups. However, use of the L-tube feeding method was significantly high in supratentorial patients with aspiration pneumonia (Table 1) (P < .05). There were more supratentorial stroke patients with L-tube feeding in the aspiration pneumonia group.
3.4. Comparison between infratentorial stroke patients with and without aspiration pneumonia
Age, duration of disease, PAS, MMSE, GDS, and total MBI were compared between the two groups, using the Wilcoxon rank sum test or the Mann–Whitney test. Statistically significant differences were observed for age, duration of stroke onset, and total MBI score (Table 3, P-value <.05). Differences of the sex ratio, feeding method and aspiration in VFSS, and salivary aspiration in the radionuclide salivagram between the two groups were analyzed by Pearson's chi-squared test. There were no significant differences between the sex ratio (P-value = .925) and degree of paralysis (P-value = .068) of the two groups. However, aspiration in VFSS and salivary aspiration in the radionuclide salivagram were significantly high in the group of infratentorial stroke patients with aspiration pneumonia (Table 3) (P < .05). There were more stroke patients with L-tube feeding in the aspiration pneumonia group (Table 3) (P < .05).
3.5. Relationship between aspiration pneumonia and findings in VFSS and/or the radionuclide salivagram
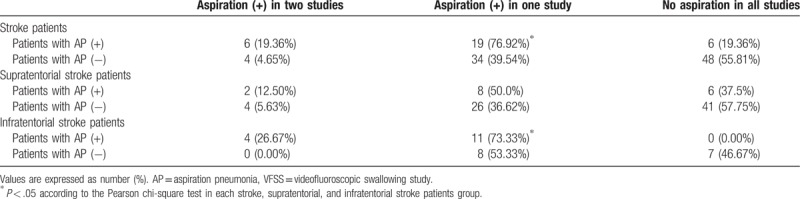
When the aspiration findings were positive in both of the two tests (VFSS and radionuclide salivagram) in stroke patients, the probability of aspiration pneumonia was 19.36%. But if the aspiration findings were positive in one or more of the two tests, the probability increased to 76.92% with statistical significance. If the aspiration findings were negative in both tests, aspiration pneumonia did not occur with a probability of 19.36% (Table 4). When the aspiration findings were positive in both of the tests in supratentorial stroke patients, the probability of aspiration pneumonia was 12.50%. But if the aspiration findings were positive in one or more of the two tests, the probability increased to 50.00% with statistical significance. If the aspiration findings were negative in both tests, aspiration pneumonia did not occur with a probability of 37.75% (Table 4). When the aspiration findings were positive in both tests in infratentorial stroke patients, the probability of aspiration pneumonia was 26.67%. But if the aspiration findings were positive in one or more of the two tests, the probability increased to 73.33% with statistical significance. If the aspiration findings were negative in both tests, aspiration pneumonia did not occur with a probability of 0.00% (Table 4). In a Pearson chi-square test with stroke patients, supratentorial stroke patients, and infratentorial stroke patients separately, only positive findings of aspiration in one or more of the two tests were significant between patients with and without aspiration pneumonia (Table 4). So, combined results of VFSS and a salivagram (positive findings of aspiration in one or more of the two tests) were included in the multivariate analysis of each stroke, supratentorial, and infratentorial stroke patients.
Table 4.
Correlation of radionuclide salivagram and/or VFSS with AP in each stroke, supratentorial, and infratentorial stroke patients group.
3.6. Clinical predictor of aspiration pneumonia for patients with stroke
In multivariate analysis to evaluate clinical predictors of aspiration pneumonia in stroke patients, the total MBI score and combined results of VFSS and salivagram (positive findings of aspiration in one or more of two tests) were significantly correlated with the occurrence of aspiration pneumonia in stroke and infratentorial stroke patients group (Table 5). However, in multivariate analysis of supratentorial stroke patients, only MMSE was significantly correlated with the occurrence of aspiration pneumonia (Table 5).
Table 5.
Multivariate logistic regression analysis with forward stepwise method of clinical characteristics associated with aspiration pneumonia.
3.7. MMSE score for developing aspiration pneumonia in supratentorial stroke patients
In the ROC curve analysis, the areas under the ROC curves (AUCs) for developing aspiration pneumonia were 0.809 (95% CI, 0.710–0.885; P < .0001). The optimal cut-off values obtained from the maximal Youden's index were a score of 8 or less on MMSE (sensitivity 75.00%, specificity 80.28%) for developing aspiration pneumonia (Fig. 2-A).
Figure 2.
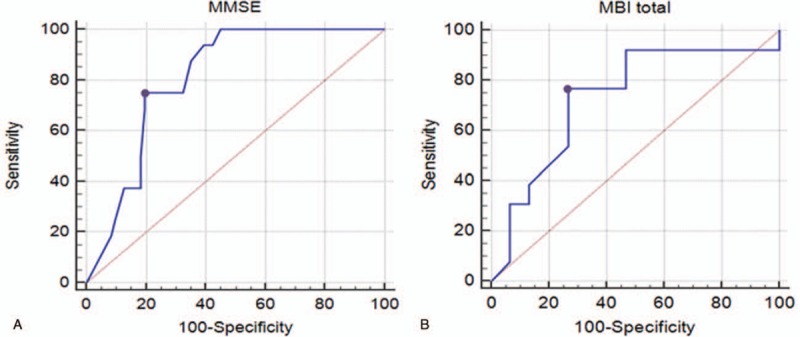
(A) ROC curve of MMSE score for developing aspiration pneumonia in supratentorial stroke patients with dysphagia. The optimal cut-off value (dots on the curves) for MMSE score, which was obtained from the maximal Youden's index, was 18 or less for development of aspiration pneumonia (AUC, 0.707; 95% confidential interval, 0.600–0.800; P = .0023; sensitivity 93.75%, specificity 47.89%). (B) ROC curve of total modified Bathel index (MBI) score for developing aspiration pneumonia in infratentorial stroke patients with dysphagia. The optimal cut-off value (dots on the curves) for total MBI score, which was obtained from the maximal Youden's index, was 52 or less for development of aspiration pneumonia (AUC, 0.731; 95% confidential interval, 0.531–0.880; P = .0243; sensitivity 76.92%, specificity 73.33%). AUC = area under the ROC curve, MBI = modified Barthel index, MMSE = Mini-Mental State Examination, ROC = receiver operating characteristic.
3.8. Total MBI score for developing aspiration pneumonia in infratentorial stroke patients
In the ROC curve analysis, the areas under the ROC curves (AUCs) for developing aspiration pneumonia were 0.731 (95% CI, 0.531–0.880; P = .0243). The optimal cut-off values obtained from the maximal Youden's index were a score of 52 or less on the total MBI score (sensitivity 76.92%, specificity 73.33%) for developing aspiration pneumonia (Fig. 2-B).
4. Discussion
The combination of salivagram and VFSS has shown good results in predicting aspiration pneumonia in previous studies, for example on children and adults with a brain lesion.[5,6,27] However, there have been no studies about the usefulness of salivagram and VFSS in the prediction of aspiration pneumonia in stroke patients. In addition, the results of this study showed that the aspiration pneumonia predictability of salivagram and VFSS in stroke patients differed according to stroke lesion. In the results of our study, total MBI score, MMSE, and feeding method (L-tube feeding) showed significant differences between supratentorial stroke patients with aspiration pneumonia and without aspiration pneumonia. In multivariate analysis of supratentorial stroke patients, only MMSE was significant as a clinical predictor, but not aspiration in VFSS or salivary aspiration in a salivagram. In contrast, infratentorial stroke patients showed significant differences in aspiration in VFSS, salivary aspiration in a salivagram, total MBI, and feeding method (L-tube feeding) showed significant differences between infratentorial stroke patients with and without aspiration pneumonia. In multivariate analysis of infratentorial stroke patients, the combined results of the salivagram and VFSS, and total MBI scores were significant as a clinical predictor, but not MMSE.
These totally different clinical predictors of aspiration pneumonia according to stroke lesion may be related to the direct involvement of the cough reflex pathway. The cough reflex has both sensory (afferent) components (mainly through the vagus nerve) and motor (efferent) components.[28,29] The lung irritant receptors (cough receptors) in the epithelium of the respiratory tract are sensitive to both mechanical and chemical stimuli.[28,30] The cough receptors, or rapidly adapting irritant receptors, are located mainly on the posterior wall of the trachea, pharynx, and at the carina of the trachea.[28,30] When an irritant receptor is triggered, impulses travel through the internal laryngeal nerve, a branch of the superior laryngeal nerve which stems from the vagus nerve (CN X) to the medullar of the brain.[31] The efferent neural pathway then follows, with relevant signals transmitted back from the cerebral cortex and medulla through the vagus and superior laryngeal nerves to the glottis, external intercostals, diaphragm, and other inspiratory and expiratory muscles.[29] Therefore, in supratentorial stroke patients, the aspiration findings of VFSS and the salivagram were not significantly correlated with aspiration pneumonia because there was no direct involvement of the cough reflex pathway in patients with supratentorial stroke. In other words, a conserved cough reflex in supratentorial stroke patients can send aspirated material out of the lungs.
On the other hand, unlike supratentorial stroke patients, combined results of the salivagram and VFSS may predict the aspiration pneumonia due to direct involvement of the cough reflex pathway in brain stem stroke patients. However, the down-regulation of the cough reflex in patients with aspiration pneumonia could be mediated by both cortical facilitatory pathways for cough and medullary reflex pathways.[32,33] In supratentorial stroke patients, the decrease of MMSE was significantly correlated with aspiration pneumonia instead of aspiration in VFSS and the salivagram. This may be because the degree of MMSE reduction reflects a decrease in the supra-medullary cough reflex (cortical facilitatory pathway). These results can be explained by the suppression of the supra-medullary cough reflex, which may lead to a reduction in the cough reflex, even if there is no involvement of the cough reflex pathway at the medullary level.
A cough is usually referred to as a reflex defense mechanism mediated at the infratentorial level and is processed by the medullary respiratory network to produce the motor pattern of a cough.[34] However, there is an accumulating evidence indicating that the human cough is under voluntary control and that higher centers such as the cerebral cortex or subcortical regions have an important role in both initiating and inhibiting a reflexive cough.[33,35] A cough is typically preceded by an awareness of an irritating stimulus and recognized as a need to cough, termed the urge-to-cough.[34] A recent functional magnetic resonance imaging (fMRI) study revealed that the urge-to-cough was associated with activations in a variety of brain regions, including the insula cortex, anterior mid-cingulate cortex, primary sensory cortex, orbitofrontal cortex, supplementary motor area, and cerebellum.[36] Moreover, previous studies have shown that urge-to-cough is proportional to cognition.[37] Therefore, in our study, the correlation between MMSE and aspiration pneumonia in supratentorial stroke patients can be explained by the proportional relationship between urge-to-cough and cognition.
There are several limitations to our study. First, the number of patients with aspiration pneumonia was not large in our study. For this reason, it seems that the results of this study are not enough to make a general conclusion. This is probably because we investigated only aspiration pneumonia within a month before and after VFSS to reducing bias due to change in the clinical condition over time. In addition, we tried to investigate the occurrence of aspiration pneumonia through the diagnostic criteria, but false-positive or negative aspiration pneumonia may not be completely excluded. However, the correlations between aspiration pneumonia and various clinical features of stroke patients of this study are considered to be sufficient for a preliminary study, although further cohort studies with larger patients group are warranted to confirm these correlations. Second, this was a retrospective study, so it was not possible to investigate the factors considered as important clinical predictors of aspiration pneumonia, such as cough reflex and urge-to-cough. In addition, we exclude patients with insufficient medical records, which caused bias in the analysis. Therefore, further prospective studies involving various clinical parameters such as cough reflex and urge-to-cough should be performed to identify the important clinical predictors of aspiration pneumonia in stroke patients.
5. Conclusion
MMSE can be a clinical predictor of the occurrence of aspiration pneumonia in patients with supratentorial stroke, and total MBI score and combined results of VFSS and salivagram (positive findings of aspiration in one or more of the two tests) can be a clinical predictor of the occurrence of aspiration pneumonia in patients with infratentorial stroke. Therefore, care should be taken to prevent aspiration pneumonia when MMSE is less than 8 in patients with supratentorial stroke, and total MBI score is less than 52 or there are positive findings of aspiration in one or more of the VFSS and salivagram in patients with infratentorial stroke.
Author contributions
Data curation: Kwang Jae Yu, Hyunseok Moon.
Formal analysis: Kwang Jae Yu.
Writing – original draft: Donghwi Park.
Writing – review & editing: Donghwi Park.
Footnotes
Abbreviations: AUC = area under the curve, GDS = Global Deterioration Scale, MBI = Modified Barthel Index, MMSE = Mini-Mental State Examination, ROC = receiver operating characteristic, VFSS = videofluoroscopic swallowing study.
Disclosure: This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (NRF-2017R1D1A1B03033127).
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the authors or upon any organization with which the authors are associated.
The authors have no conflicts of interest to disclose.
References
- [1].Katzan IL, Cebul RD, Husak SH, et al. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology 2003;60:620–5. [DOI] [PubMed] [Google Scholar]
- [2].Kim SB, Lee SJ, Lee KW, et al. Usefulness of early videofluoroscopic swallowing study in acute stroke patients with dysphagia. Ann Rehabil Med 2018;42:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Park D, Woo SB, Lee DH, et al. The correlation between clinical characteristics and radionuclide salivagram findings in patients with brain lesions: a preliminary study. Ann Rehabil Med 2017;41:915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Smith Hammond CA, Goldstein LB, Horner RD, et al. Predicting aspiration in patients with ischemic stroke: comparison of clinical signs and aerodynamic measures of voluntary cough. Chest 2009;135:769–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kim GE, Sung IY, Ko EJ, et al. Comparison of videofluoroscopic swallowing study and radionuclide salivagram for aspiration pneumonia in children with swallowing difficulty. Ann Rehabil Med 2018;42:52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jang DH, Choi KH, Kim DH, et al. Comparison between the radionuclide salivagram and videofluoroscopic swallowing study methods for evaluating patients with aspiration pneumonia. Ann Nucl Med 2013;27:247–52. [DOI] [PubMed] [Google Scholar]
- [7].Bar-Sever Z, Connolly LP, Treves ST. The radionuclide salivagram in children with pulmonary disease and a high risk of aspiration. Pediatr Radiol 1995;25Suppl 1:S180–3. [PubMed] [Google Scholar]
- [8].Marik PE, Kaplan D. Aspiration pneumonia and dysphagia in the elderly. Chest 2003;124:328–36. [DOI] [PubMed] [Google Scholar]
- [9].Ebihara S, Sekiya H, Miyagi M, et al. Dysphagia, dystussia, and aspiration pneumonia in elderly people. J Thorac Dis 2016;8:632–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xu Z, Gu Y, Li J, et al. Dysphagia and aspiration pneumonia in elderly hospitalization stroke patients: risk factors, cerebral infarction area comparison. J Back Musculoskelet Rehabil 2018;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [11].Ding R, Logemann JA. Pneumonia in stroke patients: a retrospective study. Dysphagia 2000;15:51–7. [DOI] [PubMed] [Google Scholar]
- [12].Yu YJ, Weng WC, Su FC, et al. Association between pneumonia in acute stroke stage and 3-year mortality in patients with acute first-ever ischemic stroke. J Clin Neurosci 2016;33:124–8. [DOI] [PubMed] [Google Scholar]
- [13].Koton S, Tanne D, Green MS, et al. Mortality and predictors of death 1 month and 3 years after first-ever ischemic stroke: data from the first national acute stroke Israeli survey (NASIS 2004). Neuroepidemiology 2010;34:90–6. [DOI] [PubMed] [Google Scholar]
- [14].Gittler M, Davis AM. Guidelines for adult stroke rehabilitation and recovery. JAMA 2018;319:820–1. [DOI] [PubMed] [Google Scholar]
- [15].Reisberg B, Ferris SH, de Leon MJ, et al. Global Deterioration Scale (GDS). Psychopharmacol Bull 1988;24:661–3. [PubMed] [Google Scholar]
- [16].Shah S, Muncer S. Sensitivity of Shah, Vanclay and Cooper's modified Barthel Index. Clin Rehabil 2000;14:551–2. [DOI] [PubMed] [Google Scholar]
- [17].Lagos-Guimaraes HN, Teive HA, Celli A, et al. Aspiration pneumonia in children with cerebral palsy after videofluoroscopic swallowing study. Int Arch Otorhinolaryngol 2016;20:132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Guidelines for prevention of nosocomial pneumonia. Centers for Disease Control and Prevention. MMWR Recommendations and Reports 1997;46:1–79. [PubMed] [Google Scholar]
- [19].Kitamura T, Nakase H, Iizuka H. Risk factors for aspiration pneumonia after percutaneous endoscopic gastrostomy. Gerontology 2007;53:224–7. [DOI] [PubMed] [Google Scholar]
- [20].Jo H, Park JG, Min D, et al. Incidence of pneumonia after videofluoroscopic swallowing study and associated factors. Dysphagia 2016;31:41–8. [DOI] [PubMed] [Google Scholar]
- [21].Strowd L, Kyzima J, Pillsbury D, et al. Dysphagia dietary guidelines and the rheology of nutritional feeds and barium test feeds. Chest 2008;133:1397–401. [DOI] [PubMed] [Google Scholar]
- [22].Park D, Lee HH, Lee ST, et al. Normal contractile algorithm of swallowing related muscles revealed by needle EMG and its comparison to videofluoroscopic swallowing study and high resolution manometry studies: a preliminary study. J Electromyogr Kinesiol 2017;36:81–9. [DOI] [PubMed] [Google Scholar]
- [23].Park D, Oh Y, Ryu JS. Findings of abnormal videofluoroscopic swallowing study identified by high-resolution manometry parameters. Arch Phys Med Rehabil 2016;97:421–8. [DOI] [PubMed] [Google Scholar]
- [24].Rosenbek JC, Robbins JA, Roecker EB, et al. A penetration-aspiration scale. Dysphagia 1996;11:93–8. [DOI] [PubMed] [Google Scholar]
- [25].Lee DH, Kim JM, Lee Z, et al. The effect of radionuclide solution volume on the detection rate of salivary aspiration in the radionuclide salivagram: a STROBE-compliant retrospective study. Medicine 2018;97:e11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lee ZI, Yu KJ, Lee DH, et al. The effect of nebulized glycopyrrolate on posterior drooling in patients with brain injury: two cases of different brain lesions. Am J Phys Med Rehabil 2017;96:e155–8. [DOI] [PubMed] [Google Scholar]
- [27].Kang Y, Chun MH, Lee SJ. Evaluation of salivary aspiration in brain-injured patients with tracheostomy. Ann Rehabil Med 2013;37:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nasra J, Belvisi MG. Modulation of sensory nerve function and the cough reflex: understanding disease pathogenesis. Pharmacol Ther 2009;124:354–75. [DOI] [PubMed] [Google Scholar]
- [29].Widdicombe JG. Neurophysiology of the cough reflex. Eur Respir J 1995;8:1193–202. [DOI] [PubMed] [Google Scholar]
- [30].Hegland KW, Bolser DC, Davenport PW. Volitional control of reflex cough. J Appl Physiol 2012;113:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Reflex cough in bronchoscopic work. Can Med Assoc J 1922;12:904–5. [PMC free article] [PubMed] [Google Scholar]
- [32].Ebihara S, Ebihara T. Cough in the elderly: a novel strategy for preventing aspiration pneumonia. Pulm Pharmacol Ther 2011;24:318–23. [DOI] [PubMed] [Google Scholar]
- [33].Widdicombe J, Eccles R, Fontana G. Supramedullary influences on cough. Respir Physiol Neurobiol 2006;152:320–8. [DOI] [PubMed] [Google Scholar]
- [34].Yamanda S, Ebihara S, Ebihara T, et al. Impaired urge-to-cough in elderly patients with aspiration pneumonia. Cough 2008;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ebihara S, Saito H, Kanda A, et al. Impaired efficacy of cough in patients with Parkinson disease. Chest 2003;124:1009–15. [DOI] [PubMed] [Google Scholar]
- [36].Mazzone SB, McLennan L, McGovern AE, et al. Representation of capsaicin-evoked urge-to-cough in the human brain using functional magnetic resonance imaging. Am J Respir Crit Care Med 2007;176:327–32. [DOI] [PubMed] [Google Scholar]
- [37].Ebihara S, Ebihara T, Kanezaki M, et al. Aging deteriorated perception of urge-to-cough without changing cough reflex threshold to citric acid in female never-smokers. Cough 2011;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]


