Abstract
Rationale:
In the setting of metastatic or locally advanced adrenocortical carcinoma, a limited number of therapies are available and their efficacy is generally below modest. The backbone of treatment remains surgery, even for metastatic disease, whenever it is possible, and mitotane. Chemotherapy can be used with limited results. A small subset of patients with adrenocortical carcinoma may have high mutational burden and harbor mutations in mismatch-repair genes.
Patient concerns:
We report a 40-year old and a 28-year-old female patients with metastatic adrenocortical carcinoma refractory to multiple treatments.
Diagnosis:
Next-generation sequencing detected high mutational burden (>10 mutations/megabase) in both patients, one of them with MSH2 mutation.
Interventions:
They were treated with pembrolizumab (100 to 200 mg every 3 weeks).
Outcomes:
The patient harboring a MSH2 mutation experienced a long-term complete response after pembrolizumab, while the patient with high mutational burden and absence of mismatch repair deficiency did not have any response.
Lessons:
To the best of our knowledge, this is the first report in the literature of a durable complete response after pembrolizumab in a patient with metastatic adrenocortical carcinoma. Differences in therapy sequencing, possibly abscopal effect related to multiple previous radiotherapy exposition, predictive values of high mutational burden and mutations in mismatch-repair genes are discussed.
Keywords: adrenocortical carcinoma, high mutational burden, immunotherapy, metastatic, pembrolizumab
1. Introduction
Adrenocortical carcinoma is a rare disease affecting approximately 0.7 to 2 individuals per million.[1,2] Historically, localized disease is treated with surgical resection. Although controversies for adjuvant therapy still exists, surgery may be followed by adjuvant mitotane in those patients considered to be at high-risk (eg, tumor rupture, Ki67 immunoexpression in greater than 10% of tumor cells, positive lymph nodes or positive margins).[3–7]
Metastatic or inoperable disease is generally considered incurable, and treatment remains a challenge. The evidence to support everyday clinical decision-making process is still poor. Repeated resection of metastatic disease and/or other local treatments such as radiofrequency ablation are often attempted and might play a role in providing better clinical outcomes.[8–12] Patients with indolent inoperable disease are generally treated with high-dose mitotane, although toxicity may limit optimal dosing, and overall clinical responses are often poor.[13] Chemotherapy can be added to the adrenolytic agent, particularly in those patients considered to have more aggressive disease. In the FIRM-ACT trial, the first-line use of etoposide, doxorubicin and cisplatin (EDP) plus mitotane has shown increased rates of response and progression-free survival (PFS) when compared with streptozotocin plus mitotane.[14]
Molecularly, adrenocortical carcinomas are characterized by a high heterogeneity and low mutational burden, in which driver implicated mutations may include CTNNB1, TP53, ZNFR3, CCNE1, and PRKRAR1A.[15–17] Whole genome doubling is a typical event in a subset of adrenocortical carcinomas, and is associated with aggressiveness. At least 3 different prognostic groups can be identified using DNA methylation profiling.[16] Interestingly, a hypermutator phenotype is detected in a small subset of patients, which is associated with mutations in DNA mismatch repair genes. Associations with Lynch syndrome and mutations in MHS2, MSH6, MLH1, and POLE have been identified in approximately 3% of cases.[15,18,19]
Recently, anti-PD1/anti-PD-L1 agents have been shown effective for the treatment of many malignant neoplasms, such as melanoma, lung, kidney, and urothelial cancers. Strikingly, a fraction of these patients may experience solid long-term responses. Although the perfect predictive biomarker has not been identified, mutations in genes of DNA mismatch repair enzymes (or the lack of immunoexpression of these enzymes), high tumor mutational burden, increased ratios of tumor-infiltrating lymphocytes, or increased PD-L1 expression have been implicated in better outcomes after treatment with anti-PD-1/anti-PD-L1 agents in different clinical settings.[20,21]
In the present paper, we report the clinical cases of 2 patients with advanced adrenocortical carcinomas who received pembrolizumab. One of them, in which a splice mutation in MSH2 and high tumor mutational burden were detected, achieved a long-lasting complete response following pembrolizumab monotherapy, after the disease had progressed on multiple treatments, including radiotherapy and different chemotherapy regimens. The other patient had a progression after pembrolizumab treatment, although a high mutational burden was also detected in tumor samples. Insights on the predictive effect of mutational status of DNA repair-related genes, high tumor mutational burden, and possible abscopal effect after multiple radiotherapy treatments are discussed.
2. Methodology
2.1. Ethical statement
All procedures and protocols in this study were previously approved by the local Ethics Committee (protocol number: 2018-06) and were in accordance with the Declaration of Helsinki. Written informed consents were obtained from the patients for publication of the case reports and accompanying images.
2.2. Design and data acquisition
This is a retrospective series of 2 patients with metastatic adrenocortical carcinoma harboring high mutational burden treated with pembrolizumab. Clinical data was retrospectively reviewed using electronic charts. Next-generation sequencing (NGS) analysis (Foundation, Roche) was retrospectively assessed and high mutational burden was considered if tumor mutational burden was higher than 10 mutations per megabase. All responses to treatment were assessed by RECIST version 1.1 criteria.[22]
3. Case reports
3.1. Case #1
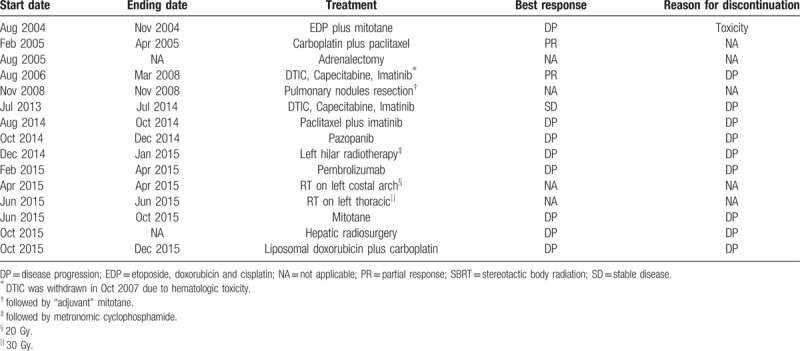
A 40-year old Latin female patient without any known comorbidities presented with a left adrenal mass on September 2008. 18F-FDG-PET/CT scans showed no metastatic disease, and she went through a left adrenalectomy with curative intent. Pathology analyses revealed a 9-cm adrenocortical carcinoma with vascular invasion. Table 1 summarizes the timeline of treatments for this patient.
Table 1.
Case #1: Timeline of administered treatment regimens.
Disease relapsed 3 months later as a 18F-FDG-PET/CT showed 2 hepatic hypermetabolic nodules. Systemic treatment with mitotane (maximum tolerated daily dose: 3 g) was started with disease progression after 3 months. Decision was made to start capecitabine, dacarbazine and mitotane. After 2 cycles, she had a new disease progression on the liver. On July 2009, a hepatic enucleation within the segments 2, 4, and 6 was performed and mitotane (maximum tolerated daily dose: 8 g) was started. A new hepatic lesion appeared on segment 4a, which was treated by radiofrequency ablation (RFA) on June 2010. Six months later, restaging 18F-FDG-PET/CT scans detected a 0.8 cm lung nodule and a 2 cm hepatic nodule (segment 8), which were treated by RFA. After 11 months (November 2011), another RFA procedure was performed due to a novel apical lung nodule.
On May 2013, a left lung metastasectomy was performed to treat a new lung nodule. Pathology analysis confirmed metastatic adrenocortical carcinoma. A Next-Generation Sequencing analysis (NGS, Foundation One, Roche) of this lesion identified mutations on the following genes: MSH2, ATM, APC, DAXX, KDM5LC, as shown in Table 2. Importantly, this patient had no familial history suggestive of Lynch disorder. HER-2 was not hyperexpressed, and PD-L1 expression was 10%. Tumor mutational burden was 32.65 mutations/Mb. On January 2014, she had a stereotatic body radiation therapy (SBRT, 45 Grays divided into 3 daily fractions) for a new lung nodule. On January 2015, 18F-FDG-PET/CT scans showed many hypermetabolic lung and pleural nodules, and a hepatic hypermetabolic lesion on segment 4. For the next 3 months, she was on curcumin with metronomic cyclophosphamide, which were discontinued due to disease progression. Pazopanib was started and maintained until a new disease progression on January 2016.
Table 2.
Next generation sequencing findings of Case #1 and Case #2.
On January 2016, decision was made to start pembrolizumab (100 mg every 3 weeks), and a complete radiologic and metabolic response was detected after 5 cycles, until April 2016. Figure 1 depicts the 18F-FDG-PET/CT scans before and after pembrolizumab treatment. After the fifth cycle, the patient developed progressive shortness of breath and dry cough, and chest imaging suggested a diffuse pulmonary inflammatory process. Bronchoscopy put away possible infectious complications and a transbronchial biopsy showed an organizing pneumonia. Grade III pneumonitis was diagnosed and pembrolizumab was no longer administered. Patient is currently on follow-up without any evidence of relapse, with her last 18F-FDG-PET/CT scans on March 2018, as depicted in Figure 2.
Figure 1.
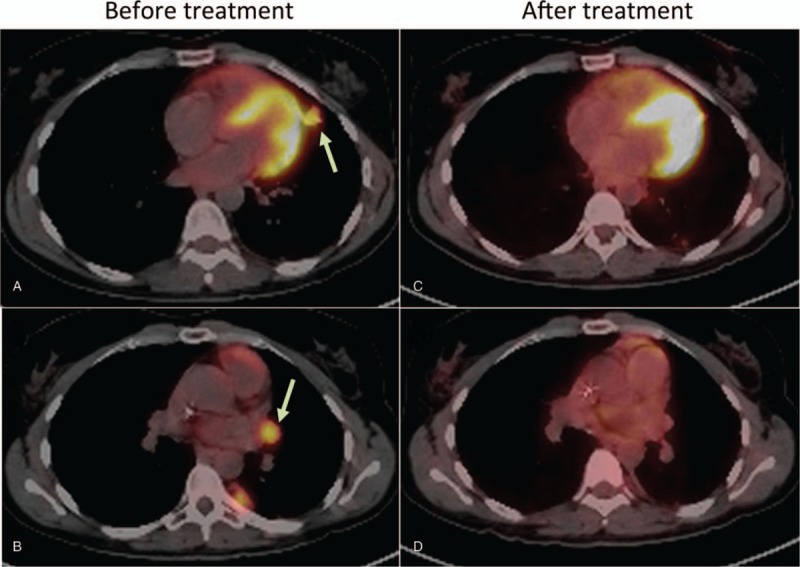
Representative cross-sectional fusion images of 18F-FDG PET scans before and after pembrolizumab treatment in Case #1. Cross-sectional images showing hypermetabolic pulmonary nodule and hilar lymph node before (A and B) and after (C and D) treatment with pembrolizumab. Arrows point towards hypermetabolic lesions.
Figure 2.
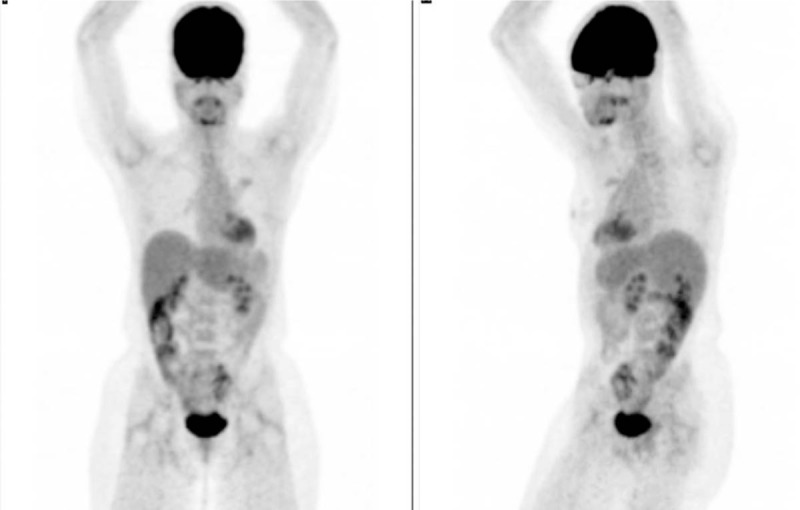
18F-FDG-PET scans of Case #1 on March/2018. As shown in the picture, 18F-FDG-PET-scans did not detect any hypermetabolic lesions 11 months after the last dose of pembrolizumab. This illustrates an unprecedented long-term response in adrenocortical carcinoma with an anti-PD1 agent.
3.2. Case #2
A 28-year-old Latin male patient without known comorbidities presented with a locally advanced adrenocortical carcinoma on August 2004. Combination of mitotane (8 mg daily) and chemotherapy (cisplatin, etoposide, and doxorubicin) was started. After 3 cycles, he developed limiting toxicity and treatment was changed to carboplatin plus paclitaxel, with partial response after 3 cycles. On August 2005, a successful right adrenalectomy was performed.
After 8 months, the patient had a pulmonary recurrence. On August 2006, a regimen containing dacarbazine, capecitabine and imatinib was started, with a partial response, followed by capecitabine plus imatinibe until March 2008, when 2 new pulmonary nodules were detected. A surgical resection of these nodules was performed followed by “adjuvant” mitotane from May to November 2008.
On July 2013, a new pulmonary and pleural recurrence was detected and the regimen with dacarbazine, capecitabine and imatinib was restarted, until disease progression on July 2014. He had paclitaxel plus imatinib for 2 months, until a new disease progression in mediastinal lymph nodes.
A NGS analysis (Foundation One, Roche) from the resected pulmonary nodule did not detect any predicted deleterious genomic alterations. Mutational tumor burden was 23 mutations/Mb. On October 2014, pazopanib 800 mg per day was started, without any detectable response. A left hilar radiotherapy was performed (30 Gy), followed by metronomic cyclophosphamide with disease progression after 3 months.
On February 2015, a decision was made to start pembrolizumab (200 mg every 3 weeks). After 5 cycles, there was hepatic, nodal, bone and pulmonary progression. On April 2015 he was treated with external beam radiotherapy (20 Gy) on the left costal arch, and on June 2015 he had radiotherapy on left thoracic wall (30 Gy). From June 2015 to October 2015, he had mitotane without clinical benefit. A hepatic radiosurgery was performed on October 2015, and patient had 2 cycles of liposomal doxorubicin plus carboplatin interrupted due to disease progression. On December 2015, the patient passed away due to progressive disease. Table 3 summarizes the treatments offered to this patient.
Table 3.
Case #2: Timeline of administered treatment regimens.
4. Discussion
In the present paper, we reported 2 patients with metastatic adrenocortical carcinomas who were treated with pembrolizumab. While the patient reported as Case #1 had a complete long-term metabolic and radiologic response, Case #2 progressed after pembrolizumab and passed away several months later. Both of them had high mutational burden (Case #1: 32 mutations/Mb; Case #2: 23 mutations/Mb), although only Case #1 had a known mutation in MSH2 gene.
Treatment of metastatic or locally advanced adrenocortical carcinoma usually relies on the use of the maximum tolerated dose of mitotane with or without chemotherapy. Whenever it is possible, local control of metastatic disease with surgery, radiofrequency ablation, or external beam radiotherapy is desired, and may be associated with better outcomes.[8–12] Patient described as Case #1 had hepatic enucleations, a lung metastasectomy, 3 procedures of radiofrequency ablation, and a SBRT of a lung lesion; while Case #2 had left hilar radiotherapy and hepatic radiosurgery to provide local control of metastatic disease, with long survivals.
Selection of patients for chemotherapy is usually made on the basis of tumor aggressiveness, performance status, and presence of comorbidities. The combination of etoposide, doxorubicin and cisplatin plus mitotane showed increased response rates and PFS when compared with mitotane plus streptozotocin in a phase 3 trial.[14] Case #2 had EDP plus mitotane in the neoadjuvant setting, which was discontinued due to intolerance and toxicity.
Different regimens have been tested as well, but solid evidence is still lacking, mainly due to disease rarity and high molecular complexity. In a phase I trial of the combination of imatinib, capecitabine, and dacarbazine in patients with advanced endocrine tumors, a response was seen in 1 of 6 patients with adrenocortical carcinomas.[23] The use of agents targeting the tyrosine kinase activity of the vascular endothelial growth factor receptor (VEGFR) tyrosine kinase has been tested in early clinical trials with only limited effectiveness.[24,25] Interestingly, Case #1 had a period of stable disease during treatment with pazopanib. Furthermore, treatment against insulin growth factor 1 receptor (IGF-1R) has also been attempted without success. A phase 3 trial comparing linsitinib, an IGF-1R inhibitor, versus placebo in patients with refractory metastatic or locally advanced adrenocortical carcinoma was early terminated due to lack of benefit.[26]
The recent introduction of anti-PD1/anti-PD-L1 agents changed the landscape of the treatment for many tumors, particularly those with known mutations in genes related to DNA repair. Tumors deficient of mismatch repair enzymes (ie, MLH1, MSH2, MSH6, PMS2) may derive the greatest benefit from these immune checkpoint inhibitors. In a seminal phase 2 trial, Le and colleagues evaluated the use of pembrolizumab, an anti-PD-1 antibody, in 41 patients with metastatic carcinomas. Immune-related response rate was 71% in the mismatch-repair-deficient-noncolorectal cancer cohort, 40% in the mismatch-repair-deficient colorectal cancer cohort, and no responses were observed in the cohort of mismatch-repair-proficient colorectal cancer. These compelling findings led to the approval of anti-PD1 agents by the Food and Drug Administration in the setting of tumors harboring mutations in genes related to mismatch-repair enzymes.[21] Other predictive findings, such as high mutational burden, increased PD-L1 expression, and high presence of tumor infiltrating lymphocytes may be directly linked to response and better outcomes, although the ideal predictive biomarker remains elusive.[27]
Case #1 had no familial history of Lynch, but NGS analysis showed a splice mutation in MSH2, along with a high mutational burden. This finding prompted us to treat the patient with pembrolizumab, and a complete long-term metabolic and radiologic response was achieved. To the best of our knowledge, this is the first report of a durable complete response after pembrolizumab in a patient with metastatic adrenocortical carcinoma. No mutations in mismatch repair genes were observed in Case #2. However, treatment with pembrolizumab was attempted supported by a high mutational burden detected by NGS analysis, without any success. This illustrates that tumor mutational burden might not be an impeccable biomarker for patient selection for anti-PD-1/PD-L1 agents, at least in the setting of advanced adrenocortical carcinomas.
Another difference between Case #1 and Case #2 was the number of radiotherapeutic treatments to which each case was submitted. Case #1 had 3 procedures of radiofrequency ablation, and a SBRT of a lung lesion before pembrolizumab; while Case #2 went through 1 procedure of left hilar radiotherapy before and 2 procedures after pembrolizumab. The abscopal effect is described as a T-cell-dependent response at tumor sites other than the site treated with radiotherapy. There is a growing body of evidence suggesting that the combination of radiotherapy and immune checkpoint inhibitors may boost the abscopal effect and promote greater antitumor responses.[28–30] Furthermore, Case #1 had received a low-dose cyclophosphamide for approximately 1 year before pembrolizumab treatment. Low-dose cyclophosphamide selective deplete T-regulatory lymphocytes, which might predispose one to an increased action of anti-PD1 antibodies in unleashing effector T cells, and possibly producing better outcomes.[31,32] Definitive conclusions cannot be drawn here, and we can only speculate if these differences could have affected the efficiency of pembrolizumab therapy in Case #1.
In summary, we described, for the first time in the literature, a clinical case of a patient with metastatic adrenocortical carcinoma who was treated with pembrolizumab and had a durable complete radiological, metabolic and clinical response. This patient had a splice mutation in MSH2 gene and a high mutational burden. The clinical case of another patient with high tumor mutational burden and no mutations in mismatch-repair genes had no response after pembrolizumab was here reported as well. These findings may help to support the role of immune checkpoint inhibitors in patients with adrenocortical carcinomas harboring mutations in mismatch-repair genes. Also, we speculate that the differences between the 2 cases described might help to improve the selection of patients with metastatic or locally advanced carcinoma for the treatment with current immunotherapeutic strategies.
Acknowledgments
The authors are grateful to Michele Artioli for her technical assistance.
Author contributions
Conceptualization: Jose Mauricio Mota, Luana Guimarães Sousa, Maria Ignez Braghiroli, João Evangelista Bezerra Neto, Ana A. de Oliveira Hoff, Paulo M. Hoff.
Data curation: Jose Mauricio Mota, Luana Guimarães Sousa, Paulo Chapchap.
Formal analysis: Jose Mauricio Mota, Luana Guimarães Sousa, Luiz Tenório Siqueira, Paulo Chapchap.
Investigation: Jose Mauricio Mota, Luana Guimarães Sousa, Luiz Tenório Siqueira, Paulo Chapchap.
Methodology: Jose Mauricio Mota, Luana Guimarães Sousa, Luiz Tenório Siqueira, Paulo Chapchap.
Project administration: Jose Mauricio Mota, Paulo M. Hoff.
Resources: Jose Mauricio Mota, Paulo Chapchap, Paulo M. Hoff.
Software: Jose Mauricio Mota.
Supervision: Jose Mauricio Mota, Maria Ignez Braghiroli, João Evangelista Bezerra Neto, Ana A. de Oliveira Hoff, Paulo M. Hoff.
Validation: Jose Mauricio Mota, Luiz Tenório Siqueira, Paulo M. Hoff.
Visualization: Jose Mauricio Mota, Luiz Tenório Siqueira.
Writing – original draft: Jose Mauricio Mota, Luana Guimarães Sousa, Luiz Tenório Siqueira.
Writing – review & editing: Jose Mauricio Mota, Maria Ignez Braghiroli, Luiz Tenório Siqueira, João Evangelista Bezerra Neto, Paulo Chapchap, Ana A. de Oliveira Hoff, Paulo M. Hoff.
Jose Mauricio Mota orcid: 0000-0001-9510-6956.
Footnotes
Abbreviations: EDP = Etoposide, doxorubicin, cisplatin, IGF-1R = Insulin growth factor 1 receptor, NGS = Next generation sequencing, PFS = Progression-free survival, RFA = Radiofrequency ablation, SBRT = Stereotatic body radiation therapy, VEGFR = Vascular endothelial growth factor receptor.
The authors declare no conflict of interest relevant to this manuscript.
References
- [1].Kerkhofs TM, Verhoeven RH, Van der Zwan JM, et al. Adrenocortical carcinoma: a population-based study on incidence and survival in the Netherlands since 1993. Eur J Cancer 2013;49:2579–86. doi:10.1016/j.ejca.2013.02.034. [DOI] [PubMed] [Google Scholar]
- [2].Golden SH, Robinson KA, Saldanha I, et al. Clinical review: Prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab 2009;94:1853–78. doi:10.1210/jc.2008-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Khorram-Manesh A, Ahlman H, Jansson S, et al. Adrenocortical carcinoma: surgery and mitotane for treatment and steroid profiles for follow-up. World J Surg 1998;22:605–11. discussion 611-2. [DOI] [PubMed] [Google Scholar]
- [4].Else T, Williams AR, Sabolch A, et al. Adjuvant therapies and patient and tumor characteristics associated with survival of adult patients with adrenocortical carcinoma. J Clin Endocrinol Metab 2014;99:455–61. doi:10.1210/jc.2013-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nowak KM, Samsel R, Cichocki A, et al. Prognostic factors in adrenocortical carcinoma: data from a large Polish series. Polish Arch Intern Med 2018;28:371–8. doi:10.20452/pamw.4260.1. [DOI] [PubMed] [Google Scholar]
- [6].Postlewait LM, Ethun CG, Tran TB, et al. Outcomes of adjuvant mitotane after resection of adrenocortical carcinoma: a 13-institution study by the US adrenocortical carcinoma group. J Am Coll Surg 2016;222:480–90. doi:10.1016/j.jamcollsurg.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Terzolo M, Angeli A, Fassnacht M, et al. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med 2007;356:2372–80. doi:10.1056/NEJMoa063360. [DOI] [PubMed] [Google Scholar]
- [8].Schulick RD, Brennan MF. Long-term survival after complete resection and repeat resection in patients with adrenocortical carcinoma. Ann Surg Oncol 1999;6:719–26. [DOI] [PubMed] [Google Scholar]
- [9].Datrice NM, Langan RC, Ripley RT, et al. Operative management for recurrent and metastatic adrenocortical carcinoma. J Surg Oncol 2012;105:709–13. doi:10.1002/jso.23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ripley RT, Kemp CD, Davis JL, et al. Liver resection and ablation for metastatic adrenocortical carcinoma. Ann Surg Oncol 2011;18:1972–9. doi:10.1245/s10434-011-1564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bauditz J, Quinkler M, Wermke W. Radiofrequency thermal ablation of hepatic metastases of adrenocortical cancer--a case report and review of the literature. Exp Clin Endocrinol Diabetes 2009;117:316–9. doi:10.1055/s-0028-1087178. [DOI] [PubMed] [Google Scholar]
- [12].Wood BJ, Abraham J, Hvizda JL, et al. Radiofrequency ablation of adrenal tumors and adrenocortical carcinoma metastases. Cancer 2003;97:554–60. doi:10.1002/cncr.11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Allolio B, Fassnacht M. Clinical review: Adrenocortical carcinoma: clinical update. J Clin Endocrinol Metab 2006;91:2027–37. doi:10.1210/jc.2005-2639. [DOI] [PubMed] [Google Scholar]
- [14].Fassnacht M, Terzolo M, Allolio B, et al. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med 2012;366:2189–97. doi:10.1056/NEJMoa1200966. [DOI] [PubMed] [Google Scholar]
- [15].Juhlin CC, Goh G, Healy JM, et al. Whole-exome sequencing characterizes the landscape of somatic mutations and copy number alterations in adrenocortical carcinoma. J Clin Endocrinol Metab 2015;100:E493–502. doi:10.1210/jc.2014-3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zheng S, Cherniack AD, Dewal N, et al. Comprehensive pan-genomic characterization of adrenocortical carcinoma. Cancer Cell 2016;29:723–36. doi:10.1016/j.ccell.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Vatrano S, Volante M, Duregon E, et al. Detailed genomic characterization identifies high heterogeneity and histotype-specific genomic profiles in adrenocortical carcinomas. Mod Pathol 2018;31:1257–69. [DOI] [PubMed] [Google Scholar]
- [18].Raymond VM, Everett JN, Furtado LV, et al. Adrenocortical carcinoma is a lynch syndrome-associated cancer. J Clin Oncol 2013;31:3012–8. doi:10.1200/JCO.2012.48.0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Medina-Arana V, Delgado L, González L, et al. Adrenocortical carcinoma, an unusual extracolonic tumor associated with Lynch II syndrome. Fam Cancer 2011;10:265–71. doi:10.1007/s10689-010-9416-8. [DOI] [PubMed] [Google Scholar]
- [20].Goodman AM, Kato S, Bazhenova L, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther 2017;16:2598–608. doi:10.1158/1535-7163.MCT-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–20. doi:10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. doi:10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- [23].Halperin DM, Phan AT, Hoff AO, et al. A phase I study of imatinib, dacarbazine, and capecitabine in advanced endocrine cancers. BMC Cancer 2014;14:561doi:10.1186/1471-2407-14-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].O'Sullivan C, Edgerly M, Velarde M, et al. The VEGF inhibitor axitinib has limited effectiveness as a therapy for adrenocortical cancer. J Clin Endocrinol Metab 2014;99:1291–7. doi:10.1210/jc.2013-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kroiss M, Quinkler M, Johanssen S, et al. Sunitinib in refractory adrenocortical carcinoma: a phase II, single-arm, open-label trial. J Clin Endocrinol Metab 2012;97:3495–503. doi:10.1210/jc.2012-1419. [DOI] [PubMed] [Google Scholar]
- [26].Fassnacht M, Berruti A, Baudin E, et al. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: a double-blind, randomised, phase 3 study. Lancet Oncol 2015;16:426–35. doi:10.1016/S1470-2045(15)70081-1. [DOI] [PubMed] [Google Scholar]
- [27].Topalian SL, Taube JM, Anders RA, et al. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 2016;16:275–87. doi:10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ngwa W, Irabor OC, Schoenfeld JD, et al. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer 2018;18:313–22. doi:10.1038/nrc.2018.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ko EC, Formenti SC. Radiotherapy and checkpoint inhibitors: a winning new combination? Ther Adv Med Oncol 2018;10:1758835918768240doi:10.1177/1758835918768240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Park SS, Dong H, Liu X, et al. PD-1 restrains radiotherapy-induced abscopal effect. Cancer Immunol Res 2015;3:610–9. doi:10.1158/2326-6066.CIR-14-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dimeloe S, Frick C, Fischer M, et al. Human regulatory T cells lack the cyclophosphamide-extruding transporter ABCB1 and are more susceptible to cyclophosphamide-induced apoptosis. Eur J Immunol 2014;44:3614–20. doi:10.1002/eji.201444879. [DOI] [PubMed] [Google Scholar]
- [32].Scurr M, Pembroke T, Bloom A, et al. Low-dose cyclophosphamide induces antitumor T-Cell responses, which associate with survival in metastatic colorectal cancer. Clin Cancer Res 2017;23:6771–80. doi:10.1158/1078-0432.CCR-17-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]


