Supplemental Digital Content is available in the text
Keywords: aquatic exercise, knee osteoarthritis, land-based exercise, meta-analysis
Abstract
Background:
This study aimed to systemically review the effectiveness of aquatic exercise (AQE) compared to land-based exercise (LBE) in treating knee osteoarthritis (OA).
Methods:
The Medline, Embase, Web of Science, Cochrane Central Register of Controlled Clinical Trials, CINAHL, and psyclNFO databases were comprehensively searched for randomized controlled trials (RCTs) evaluating the effectiveness of AQE and LBE for knee OA from their inception date to September 24, 2018. The risk of bias was examined using the Cochrane Collaboration Tool, and Review Manager 5.3 was used for data collation and analysis.
Results:
Eight RCTs were included, involving a total of 579 patients. The meta-analysis showed that there was no significant difference between AQE and LBE for pain relief, physical function, and improvement in the quality of life, for both short- and long-term interventions, in patients with knee OA. However, the adherence and satisfaction level for AQE was higher than for LBE. Compared to no intervention, AQE showed a mild effect for elevating activities of daily living (standardized mean difference [SMD]: −0.55, 95% confidence interval [CI] [−0.94, −0.16], P = .005) and a high effect for improving sports and recreational activities (SMD: −1.03, 95% CI [−1.82, −0.25], P = .01).
Conclusion:
AQE is comparable to LBE for treating knee OA.
1. Introduction
Osteoarthritis (OA) is a common joint disorder which frequently affects the knee joint, especially in middle aged and elderly people. Recently, a Chinese population-based observation study estimated that the prevalence of symptomatic knee OA was nearly 8.1%.[1] With an increasing aged population in China, the prevalence of knee OA is still rising rapidly. Clinically, patients with knee OA usually present with radiographic narrowing of the joint space accompanied by articular degradation, subchondral bone sclerosis, and osteophyte formation. These result in chronic knee joint pain, stiffness, and physical disability.[2] This chronic and disabling condition not only diminishes an individual's quality of life (QOL), but it also enhances anxiety, fear, and even depression.[3] To date, the signs and symptoms of knee OA can only be alleviated with a joint replacement.[4]
Land-based exercise (LBE), as a nonpharmacologic intervention, is highly recommended for the treatment of knee OA, since it can improve muscle strength, relieve pain, reduce stiffness, and ameliorate physical function.[5–7] Exercise is a broad concept that encompasses many forms including resistance, isokinetic, and aerobic exercise. All types of exercise could significantly relieve knee OA joint pain and improve physical function.[8–11] A Cochrane systematic review further testified that LBE provides short-term pain control and improves physical function which is sustained for at least 2 to 6 months after the exercise intervention in patients with knee OA.[12] Despite the importance of LBE, excessive exercise dosage may worsen arthritis symptoms by increasing weight-bearing or load.[4] Statistical analysis indicated that arthritic patients present a lower level of physical activity compared to the general population,[13] and nearly 50% of OA individuals were reluctant to do extra exercise due to pain.[4,14] Even if they participated in a physical exercise program, long-term adherence is problematic. Therefore, it is important to explore other treatment options for patients with knee OA.
Aquatic exercise (AQE) or hydrotherapy refers to exercise performed in the water, and it has been used in the treatment of diseases for more than 18 years.[15] It has many advantages compared to LBE. Firstly, the relatively constant water temperature and hydrostatic pressure may facilitate blood circulation, ease soft-tissue contracture, and relieve muscle spasms and fatigue. Secondly, since water resistance acts in the opposite direction to body motion, greater muscle activity is required which may enhance muscular strengthening. Thirdly, water buoyancy can reduce the likelihood of injury, and protect against joint degradation by reducing weight bearing.[16–18] In addition, AQE provides a more comfortable and suitable environment for patients with knee OA who are reluctant to exercise.[19] Therefore, AQE may be a beneficial treatment for knee OA. Studies that have examined the effectiveness of AQE for treating OA have suggested that AQE can relieve joint pain, and improve physical function and QOL.[14,19]
Although there are many advantages of AQE compared to LBE, it is still unclear which is more effective in treating knee OA. Many studies have compared the effectiveness of AQE and LBE; however, consistent conclusions have not been drawn. It is therefore necessary to determine which type of exercise is more efficient. In this study, a systematic review and meta-analysis of randomized controlled trials (RCTs) was conducted to compare AQE and LBE nonpharmacologic treatments for knee OA. Pain relief, symptoms, physical function, and improvement in the QOL were assessed, with the aim of determining the most effective type of exercise for knee OA management.
2. Materials and methods
This research is reported according to the PRISMA statement guidelines.[20] No ethical approval is required as the research is based on previously published articles.
2.1. Search strategy
Six databases, including Medline (via PubMed), Embase, Web of Science, Cochrane Central Register of Controlled Clinical Trials, CINAHL, and psyclNFO, were searched from their inception date to September 24, 2018. The search strategy was based on combinations of medical subject headings and keywords. The Medline search strategy is described in the supplementary material (Table S1). Strategies for the other databases were adjusted to meet the requirements of each database. In addition, to achieve a full-scale search, the references of relevant articles were searched. The systematic review details were registered in PROSPERO: the International prospective register of systematic reviews (no: CRD42018095026).
2.2. Study selection
All included studies met the following inclusion criteria: the study design was a RCT; patients were diagnosed with knee OA according to symptoms and radiologic findings without any invasive intervention; The RCT compared AQE to LBE. All types of exercise developed in a therapeutic/heated indoor/outdoor pool were eligible; and the experimental group which received AQE combined with the certain therapy (e.g., nonsteroidal anti-inflammatory drugs) and the control group with the same certain therapy were also included.
Studies were excluded if they met the following criteria: the study included animal experiments, and it was a review, cross-over study, cohort study, PICO protocol, or conference abstract; the study included an AQE experimental group, but the control group did not perform any exercise (e.g., no intervention or just education) and there was no LBE group; the study focused not only on knee OA, but other joint diseases as well (e.g., hip OA), precluding the ability to separate the outcomes from the patients with knee OA; the study had been published previously; and the study was not published in the English language.
2.3. Data extraction
Two authors (RD and YW) independently extracted data from each study using a predefined data extraction form which included: the first author's name, the year of publication, the patient characteristics, methodologic features of the studies, research country, intervention and duration, follow-up period, main outcome measurements, withdraw, and quality of trial design. Email was used to contact the original authors when the above information could not be obtained from the full-text of the included studies. Uncertainty or disagreement was resolved by discussion or consensus with a 3rd author (PT).
2.4. Quality assessment and bias analysis
The quality of all of the studies which met the inclusion criteria was assessed using the Modified Jadad score.[21] It contains four main sections examining almost all of the important elements of RCTs. It is a 7-point system with a score ≥4 considered as high quality, and a score <3 deemed as low quality. The bias of each study was evaluated using the Cochrane Handbook for Systematic Reviews of Interventions.[22] It mainly assesses selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases. The bias of each domain was defined as low risk, unclear risk, or high risk. Two authors (RD and YW), independently assessed the quality and the bias of the included research studies, and disagreement was resolved by discussion or consensus with a 3rd author (PT).
2.5. Outcome measurement
The main outcomes that were examined included: pain relief, physical function, and symptom and QOL improvement. Across the studies, pain relief was measured using the visual analog scale [23](VAS), the Western Ontario and McMaster Universities Osteoarthritis Index[24] (WOMAC) pain score, and the knee injury and OA outcome score[24] (KOOS). Symptoms were measured using the KOOS for symptoms, physical function was measured using the medical outcomes study 36-item short form health survey (SF-36),[25] the KOOS for activities of daily living (ADL), and sports and recreational activities (sport&rec). QOL was measured using the KOOS for QOL.
2.6. Statistical analysis
Review Manager (RevMan) software (Computer program, version 5.3, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) was used for data analysis. All continuous outcomes were presented as the standardized mean difference (SMD) with 95% confidence intervals (CIs). Heterogeneity was defined using the P-value and I2 from the standard Chi-squared test. A fixed-effect model was performed when the relevant data showed low heterogeneity (P > .1, I2 < 50%); otherwise, a random-effect model was conducted.[26] The differences between groups were considered as significant when the 95% CI did not include zero. Effect size was defined as small (SMD > 0.2), moderate (SMD > 0.5), or large (SMD > 0.8).
3. Results
3.1. Study selection and study characteristics
A total of 1264 studies were identified from the 6 databases. After removing duplicates and reviewing the titles and abstracts of 755 records, the full text of 45 records was reviewed. Finally, 8 RCTs with 579 participants were included in the meta-analysis (Fig. 1).
Figure 1.
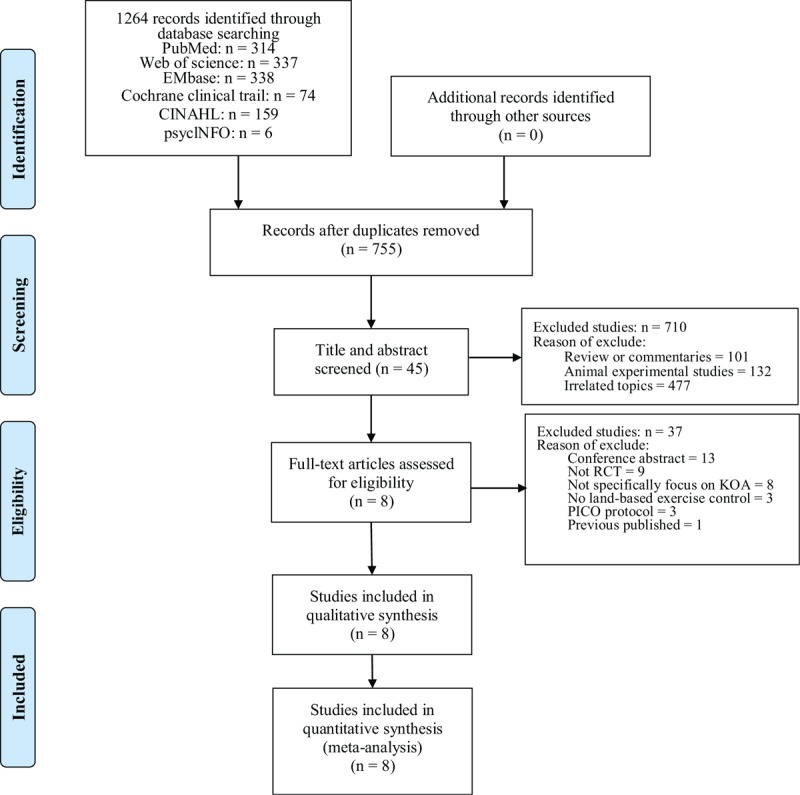
The flowchart of literature selection procedure.
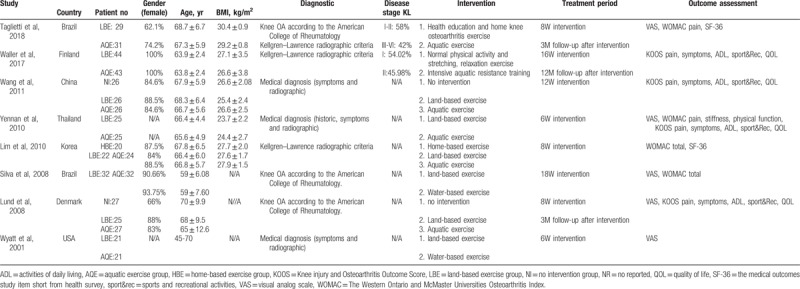
The characteristics of each study are summarized and presented in Table 1. All of the studies were published in English between 2001 and 2018, and they were conducted in Brazil,[16,27] China,[28] Finland,[29] Denmark,[30] Korea,[31] Thailand,[32] and the United States.[33] Patients who participated in these studies were diagnosed with knee OA according to the American College of Rheumatology criteria,[16,27,30] or the Kellgren–Lawrence radiographic criteria for defining the disease stage.[27,29,31] Other studies which diagnosed knee OA according to symptoms and radiographic findings did not specify the exact diagnostic criteria.[28,32,33] There were no significant differences between the AQE and LBE groups at baseline for all of the included studies. Gender distribution, which is essential for knee OA research, was described in most of the included records.[16,27–31] Each research design included a LBE group as a positive control; moreover, 3 of them involved a negative control group with no intervention[28,30] or home-based exercise.[31]
Table 1.
Characteristic of the included studies.
The exercise program for the AQE group in each study is summarized in Table 2. AQE was performed for 40 to 65 minutes, 2 to 3 times a week, for 6,[32,33] 8,[27,30,31] 12,[28] 16,[29] or 18[16] weeks. Water depth was 1.15 to 1.5 m and water temperature was 30°C to 34°C. The baseline outcome measurements for all of the included studies are presented in the supplementary material (Table S2) (Table 3).
Table 2.
The prescription of AQE in each study.
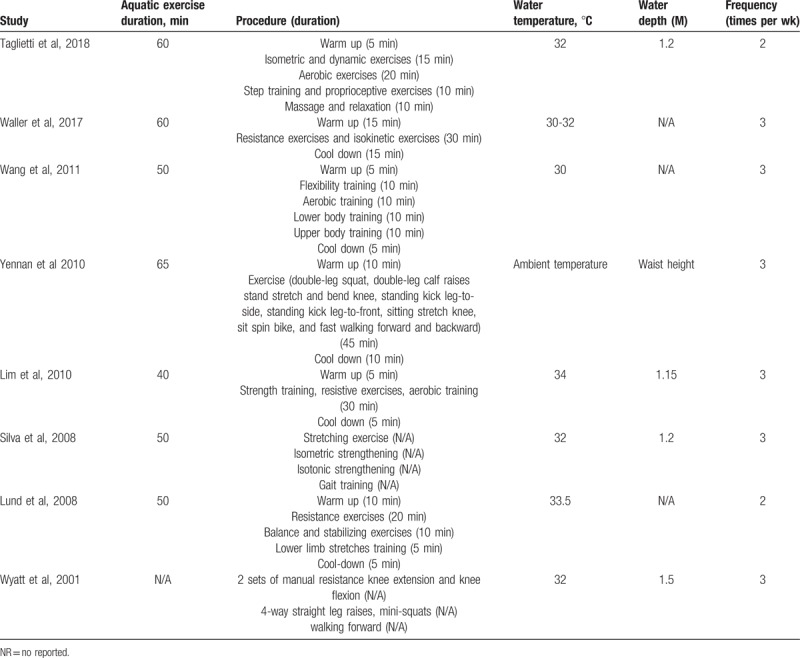
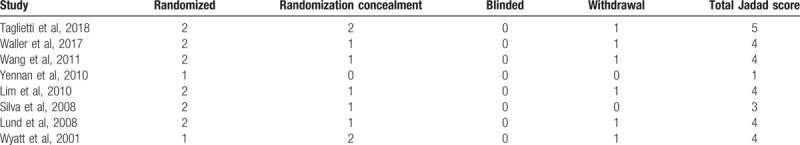
Table 3.
Modified Jadad score for each literature.
3.2. Risk of bias assessment
The risk of bias assessment is presented in Figure 2. Selection bias existed in most trials, and although these trials reported randomization, only 6 of the included records described the randomization method. These studies used random sequence generation,[27,28] block randomization,[29–31] and drawing lots[16]; the other trials did not specify the exact randomization method.[32,33] Two records[27,30] reported using opaque envelopes to conceal group allocation; this was not detailed in any of the other studies. All studies had a high risk of performance bias due to the nature of the interventions. Most trials reported that a blinded investigator performed the outcome measurements, and only 1 study[32] had detection bias. There were no other biases across the included trials.
Figure 2.
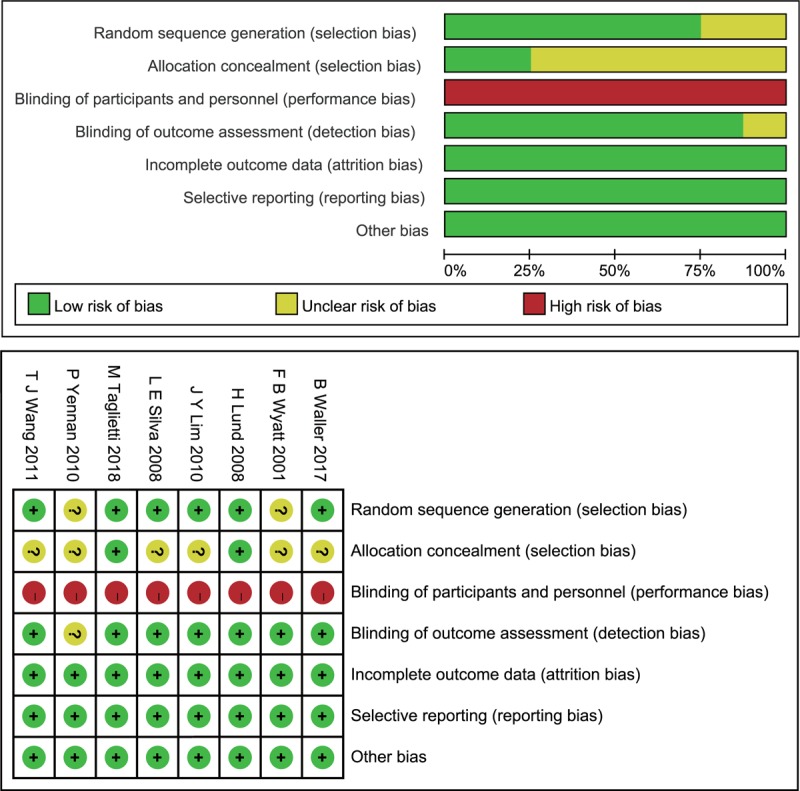
(A) Risk of bias graph. (B) Risk of bias summary.
3.3. Effect of intervention
3.3.1. Pain control
Since joint pain is the primary symptom described by patients with knee OA, all of the included studies assessed pain as the primary outcome. VAS score,[16,27,30,32,33] WOMAC pain,[27,32] and KOOS pain[28,29,32,33] were used to measure pain. Studies which used VAS and WOMAC pain showed high heterogeneity (VAS: P < .001, I2 = 85%, WOMAC pain: P < .001, I2 = 98%), whereas KOOS pain showed moderate heterogeneity (P = .20, I2 = 36%). There were no significant differences in VAS (SMD: −0.62, 95% CI [−1.27, 0.03], P = .06), WOMAC pain (SMD: −1.66, 95% CI [−4.90, 1.58], P = .31), and KOOS pain (SMD: 0.19, 95% CI [−0.07, 0.45], P = .15) in the AQE group compared to the LBE group (Fig. 3).
Figure 3.
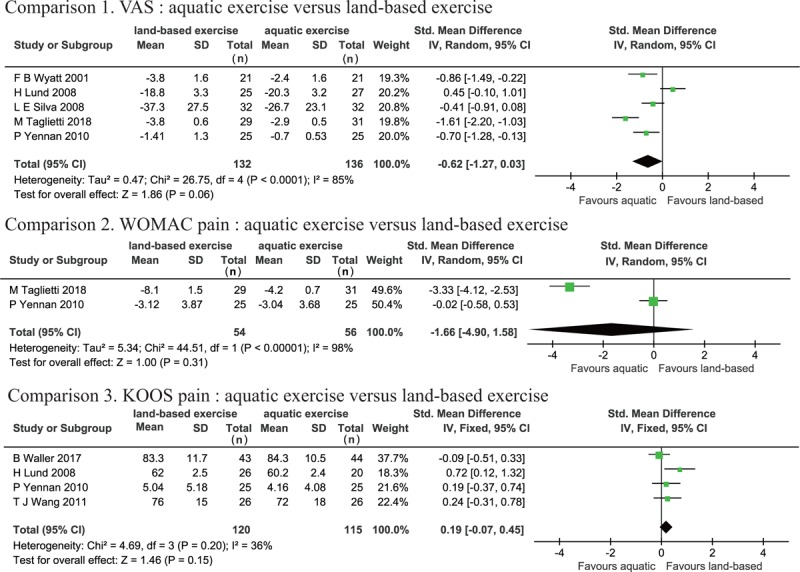
Forest plot of aquatic exercise (AQE) vs land-based exercise (LBE) interventions in pain.
3.3.2. Symptoms
Three records assessed symptoms using KOOS symptom.[28,29,33] A random effects model was used for data analysis due to the high degree of heterogeneity (P = .009, I2 = 74%). The meta-analysis (SMD: 0.19, 95% CI [−0.32, 0.71], P = .46) showed that there was no significant difference between the AQE and LBE groups for symptom improvement (Fig. 4).
Figure 4.
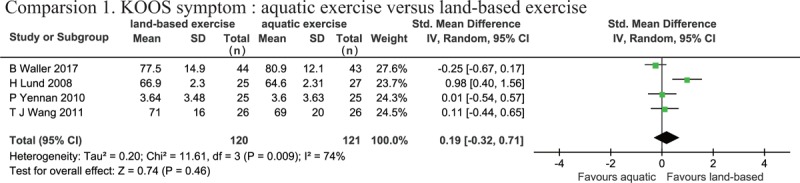
Forest plot of aquatic exercise (AQE) vs land-based exercise (LBE) interventions in symptom.
3.3.3. Physical function improvement
Physical function was measured using KOOS ADL,[28–30,32] KOOS sport&rec,[28–30,32] and SF-36 physical function.[27,31] Heterogeneity was not apparent for KOOS ADL (P = .16, I2 = 41%); however, KOOS sport&rec and SF-36 physical function demonstrated high heterogeneity (KOOS sport&rec: P = .04, I2 = 64%, SF-36 physical function: P < .001, I2 = 98%). There were no significant differences in KOOS ADL (SMD: 0.17, 95% CI [−0.08, 0.43], P = .19), KOOS sport&rec (SMD: 0.24, 95% CI [−0.19, 0.67], P = .27), or SF-36 physical function (SMD: −1.68, 95% CI [−5.38, 2.03], P = .38) between the AQE and LBE groups. Therefore, the meta-analysis revealed that there was no difference in the improvement of physical function between the 2 interventions (Fig. 5).
Figure 5.
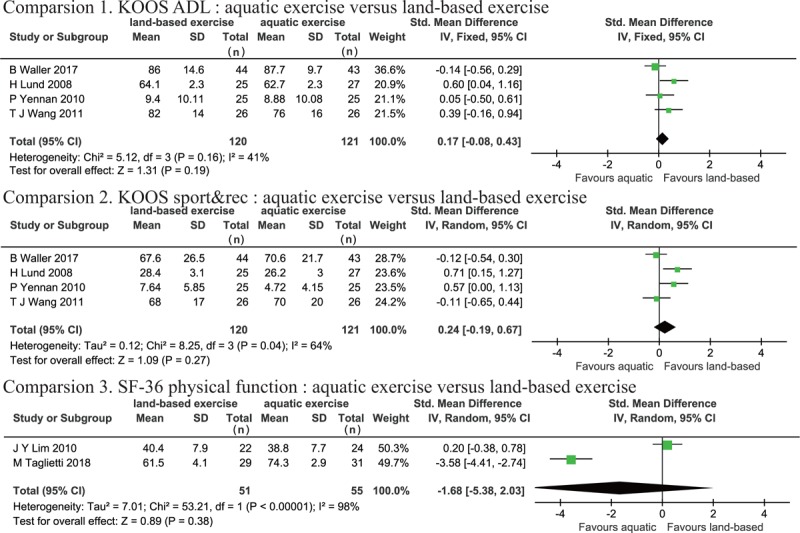
Forest plot of aquatic exercise (AQE) vs land-based exercise (LBE) interventions in physical function.
3.3.4. Quality of life
Four studies assessed QOL using KOOS QOL.[28–30,32] Heterogeneity was not observed in the analyses for QOL (P = .80, I2 = 0%), and the meta-analysis (SMD: 0.19, 95% CI [−0.07, 0.44], P = .15) demonstrated that there was no significant difference in the improvement of QOL between the 2 groups (Fig. 6).
Figure 6.
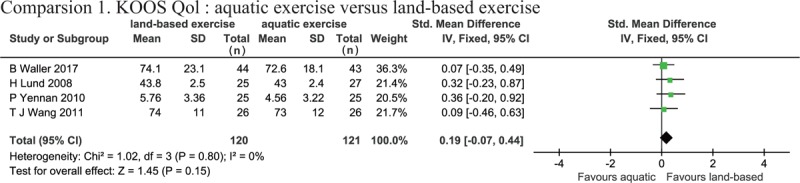
Forest plot of aquatic exercise (AQE) vs land-based exercise (LBE) interventions in quality of life.
3.3.5. AQE compared to no intervention
Two studies[28,30] included 3 groups (AQE, LBE, and no intervention), so a meta-analysis was conducted to compare the AQE group to the no intervention group. KOOS pain (P = .50, I2 = 0%), KOOS ADL (P = .46, I2 = 0%), and KOOS QOL (P = .19, I2 = 42%) presented negligible heterogeneity. KOOS symptom (P = .02, I2 = 81%) and KOOS sport&rec (P = .006, I2 = 72%) demonstrated high heterogeneity. The results showed a significant improvement in KOOS ADL (SMD: −0.55 95% CI [−0.94, −0.16], P = .005) and sport&rec (SMD: −1.03, 95% CI [−1.82, −0.25], P = .01) in the AQE group compared to the no intervention group, but no significant differences were observed for KOOS pain (SMD: −0.09, 95% CI [−0.47, 0.30], P = .66), symptom (SMD: −0.89, 95% CI [−1.81, 0.04], P = .06), or QOL (SMD: −0.21, 95% CI [−0.59, 0.18], P = .29) (Fig. 7).
Figure 7.
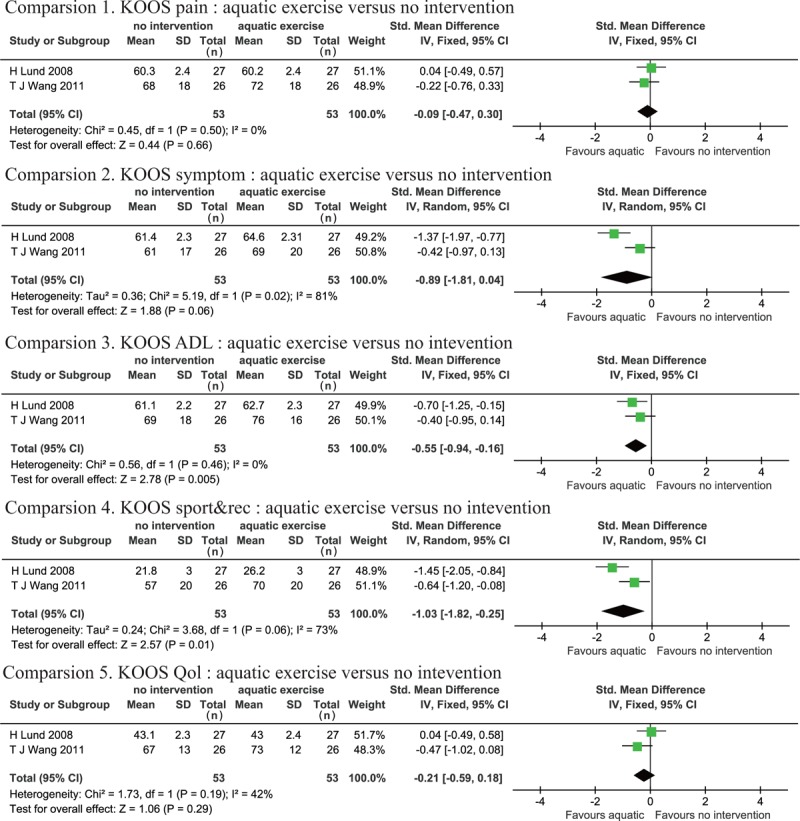
Forest plot of AQE versus CON interventions in all outcomes.
3.3.6. Outcome follow-up
Three trials reported follow-up durations of 3 months[27,30] and 12 months[29] after the intervention, which can be considered to be relatively long-term follow-up periods for the study outcomes. Pain, physical function, and QOL improvement was measured using VAS[16,27,30] and KOOS.[29,30] We observed high heterogeneity in VAS (P < .001, I2 = 97%) and KOOS sport&rec (P < .001, I2 = 94%). Heterogeneity was not observed for KOOS pain (P = 1.0, I2 = 0%), symptom (P = .78, I2 = 0%), ADL (P = .50, I2 = 0%) or QOL (P = .29, I2 = 11%). No statistically significant effects were evident for VAS (SMD: −0.25, 95% CI [−2.57, 2.06], P = .83), KOOS pain (SMD: −0.15, 95% CI [−0.49, 0.20], P = .40), KOOS symptom (SMD: −0.23, 95% CI [−0.57, 0.11], P = .19), KOOS ADL (SMD: −0.16, 95%CI [−0.51, 0.18], P = .35), KOOS sport&rec (SMD: 0.74, 95% CI [−0.95, 2.44], P = .39), or KOOS QOL (SMD: 0.20, 95% CI [−0.15, 0.54], P = .26). The pooled results revealed that there was no significant difference between AQE and LBE groups in knee OA long-term outcomes (Fig. 8).
Figure 8.
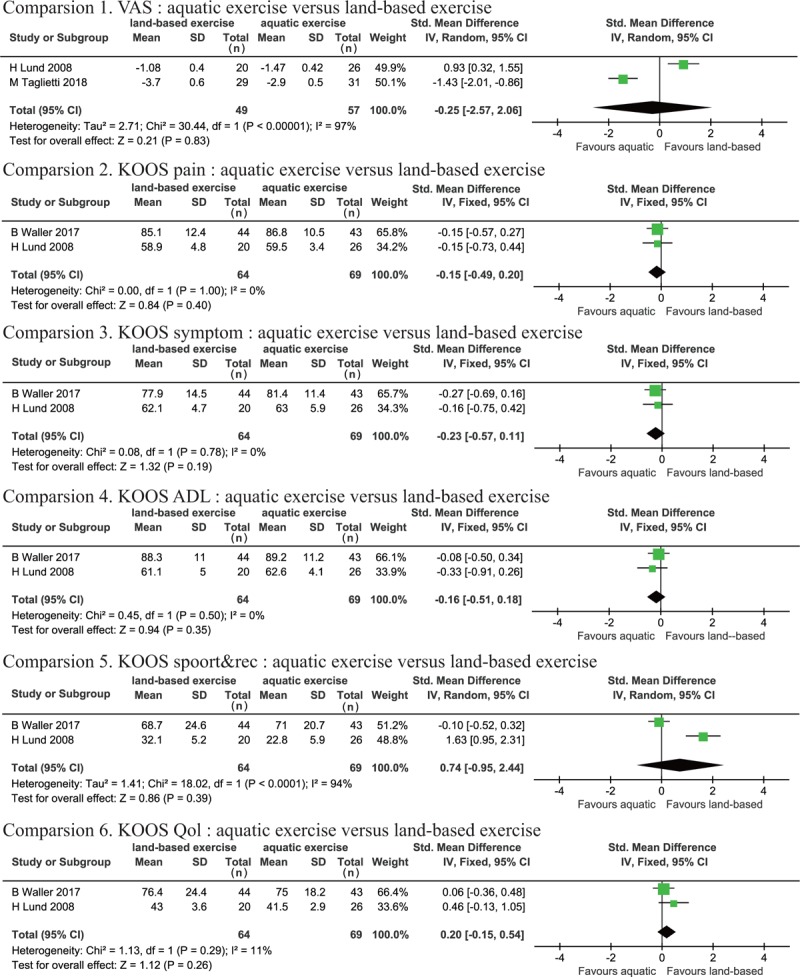
Forest plot of aquatic exercise (AQE) vs land-based exercise (LBE) interventions in long-term outcome.
3.4. Adverse events
Three of the 8 studies reported mild adverse effects in the AQE group, including pain,[29,30] dyspnea,[29] and dizziness.[28] However, the adverse effects were more frequent and severe for the LBE group. One mentioned a 44% incidence of adverse effects in the LBE group, including pain and joint swelling; 3 participants even dropped out,[30] another record reported 2 patients increased pain after exercise.[28]
3.5. Publication bias analysis
The sample size of this meta-analysis was too small to detect publication bias via a funnel plot.
3.6. Level of evidence
The quality of the RCTs was assessed using the modified Jadad scoring system. A score of ≥4 was obtained for 6 studies[27–31,33] and 2 studies scored <3.[16,32] According to these results, the majority of the studies included can be considered to be high quality.
4. Discussion
Increasing evidence suggests that knee OA is not only a type of joint disorder, but it is also a risk factor for other diseases.[34–36] Patients with painful knee OA have a higher risk of cardiovascular disease-specific and all-cause mortality compared to non-OA individuals.[37] Furthermore, substantial medical resources and costs are involved in the treatment of knee OA, so there is an urgent need to explore methods of slowing down or even attenuating the progression of this disease. LBE is a highly cost-effective method for treating knee OA, as patients are often obese and they have poor muscle strength. Regular exercise can efficiently decrease fat mass[29] while enhancing muscular strength. AQE describes an environment for structured physical activity rather than a type of exercise.[38] It has many advantages compared to LBE, and it is also recommended for post-total knee arthroplasty patient rehabilitation.[39,40] Accordingly, we hypothesized that AQE would be more effective than LBE in improving pain and physical function associated with knee OA.
This review synthesized data from 8 trials and summarized the effectiveness of AQE compared to LBE in the treatment of knee OA. For all of the assessment outcomes, no statistically significant differences were found between the 2 interventions over a short period of time, which is consistent with previous studies.[41,42] Furthermore, assessments conducted over a long period of time showed that AQE was comparable to LBE in the treatment of knee OA. The effectiveness of AQE compared to no intervention was also analyzed, although there were limited data. The results indicated that AQE had little effect on pain management and improvement of QOL, and only a small effect on the improvement of physical function.
Although blinding of the participants was impossible due to the nature of the interventions, 3 quarters of the included trials were deemed to be of high quality according to the modified Jadad score. The results indicated that there were no significant differences in pain relief between the 2 interventions. However, a single study involving an 18 week intervention, showed that AQE significantly decreased pain (measured using a VAS) before and after a 50-foot Walk Test compared to LBE.[16] Two studies also reported that AQE significantly improved walking speed compared to LBE.[28,29] This review showed only mild adverse effects in the AQE group after the intervention and no participants dropped out of this group. Thus, a high level of adherence and satisfaction for this type of intervention is indicated.
The lack of effectiveness may be ascribed to the heterogeneity of the included studies. Following a review of the characteristics of each study, various factors may have affected data collation and analysis, including: the age and body mass index of the patients, the diagnostic criteria and disease stage, the prescription and duration of exercise, and the use of different outcome assessments. The exercise prescription appeared to be the greatest source of heterogeneity. All of the 8 records included in this study reported totally different exercise prescriptions. Although the majority of the prescribed exercises consisted of strength and aerobic training, different exercise programs and durations may exert different effects. In addition, the thermal energy, resistance, and buoyancy provided by the water at different temperatures and depths may also directly impact the exercise outcomes.
The AQE interventions consist of many factors. Since the 1930s, it has been suggested that regulation of the dosage, character, frequency, and duration of AQE may be beneficial for restoring muscle function; however, relevant guidelines are still unavailable. For example, the optimal exercise mode, intensity, duration and frequency, and the optimal water characteristics (i.e., temperature and depth) are still unclear. Various modes of AQE have shown positive effects on knee OA symptoms and function[43–45]; however, the most efficient type or combination of AQE is still unknown. The intensity of an exercise program is typically described as high, moderate, or low. The effect of exercise intensity on the efficacy of interventions cannot be ignored, even though a recent study reported no significant difference between high-intensity and low-intensity exercise programs on improving pain and physical function in the short term.[46] The assessment of AQE intensity is quite different to LBE due to water resistance and buoyancy. Compared to LBE, the same effect on aerobic capacity may therefore be achieved with a lower intensity of AQE.[15] The precise assessment and management of AQE intensity remains to be elucidated.
Water properties, such as temperature and depth, are important in AQE. A temperature range of 33.5°C to 35.5°C is suitable because it allows lengthy immersion and thus enables sufficient exercise to be performed to achieve therapeutic effects without participants becoming cold or over-heating.[15] Different depths of water provide different buoyancy effects. A greater water depth may significantly reduce joint load-bearing by improving buoyancy.[47] Three of the included trials described a water depth of 1.15 to 1.2 m. When immersed to the xiphoid, approximately 50% of body weight is offloaded. One trial reported a water depth of 1.5 m, with immersion almost to the cervical region. In this study, the buoyancy would counteract approximately 60% of body weight. There is no doubt that all of the above-mentioned factors will increase the heterogeneity of clinical outcomes. Therefore, to facilitate the development of AQE guidelines, we recommend that future studies include detailed descriptions of the exercise program used, including the exercise mode, intensity and duration, and the water temperature and depth. High-quality, multi-center, large-sample RCTs are also required to evaluate the efficiency and safety of AQE for knee OA.
4.1. Limitation
There are several limitations of this review. Firstly, the inclusion of only a small number of studies with limited sample size, precluded the ability to draw definitive conclusions. In addition, all of the records failed to include an estimation of sample size. Secondly, all of the included studies used different modes and durations of AQE. Variations in the exercise program may potentially affect its efficiency and outcomes. Thirdly, due to the nature of the intervention, sufficient blinding was impossible to achieve.
5. Conclusion
Overall, AQE and LBE show comparable effects for treating knee OA. The results of this meta-analysis favored neither AQE nor LBE interventions for improving pain relief, physical function, and QOL both in the short and long term. Compared to no exercise, AQE can effectively improve physical function. We failed to provide a convincing conclusion due to the small sample of patients and the limited number of appropriate research studies. Further high quality RCTs with long follow-up periods are required.
Acknowledgment
The authors thank the National Natural Science Foundation of China and Zhejiang Chinese Medical University for their funds and support.
Author contributions
Conceptualization: Peijian Tong.
Data curation: Shibing Xu, Lei Zhang.
Formal analysis: Yunyao Wu.
Funding acquisition: Hongting Jin.
Investigation: Rui Dong, Peijian Tong.
Methodology: Rui Dong, Hongting Jin.
Resources: Peijian Tong.
Software: Yunyao Wu, Jun Ying.
Validation: Rui Dong, Peijian Tong.
Writing – original draft: Rui Dong, Yunyao Wu.
Writing – review & editing: Pinger Wang, Luwei Xiao, Peijian Tong.
Supplementary Material
Footnotes
Abbreviations: ADL = activities of daily living, AQE = aquatic exercise, CI = confidence interval, KOOS = knee injury and osteoarthritis outcome score, LBE = land-based exercise, OA = osteoarthritis, QOL = quality of life, RCT = randomized controlled trials, SF-36 = the medical outcomes study item short from health survey, SMD = standardized mean difference, sport&rec = sports and recreational activities, VAS = visual analog scale, WOMAC = the Western Ontario and McMaster Universities Osteoarthritis Index.
RD and YW contributed equally to this work.
This study was supported by the National Natural Science Foundation of China (no: 81774332, 81774346, 81873324, and 81873325).
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Tang X, Wang S, Zhan S, et al. The prevalence of symptomatic knee osteoarthritis in china: results from the china health and retirement longitudinal study. Arthritis Rheumatol 2016;68:648–53. [DOI] [PubMed] [Google Scholar]
- [2].Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II Arthritis Rheum 2008;58:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Scopaz KA, Piva SR, Wisniewski S, et al. Relationships of fear, anxiety, and depression with physical function in patients with knee osteoarthritis. Arch Phys Med Rehabil 2009;90:1866–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gay C, Chabaud A, Guilley E, et al. Educating patients about the benefits of physical activity and exercise for their hip and knee osteoarthritis. Systematic literature review. Ann Phys Rehabil Med 2016;59:174–83. [DOI] [PubMed] [Google Scholar]
- [5].Hochberg MC, Altman RD, Toupin AK, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res 2012;64:465–74. [DOI] [PubMed] [Google Scholar]
- [6].Fernandes L, Hagen KB, Bijlsma JWJ, et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis 2013;72:1125–35. [DOI] [PubMed] [Google Scholar]
- [7].McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 2014;22:363–88. [DOI] [PubMed] [Google Scholar]
- [8].Coudeyre E, Jegu AG, Giustanini M, et al. Isokinetic muscle strengthening for knee osteoarthritis: a systematic review of randomized controlled trials with meta-analysis. Ann Phys Rehabil Med 2016;59:207–15. [DOI] [PubMed] [Google Scholar]
- [9].Li Y, Su Y, Chen S, et al. The effects of resistance exercise in patients with knee osteoarthritis: a systematic review and meta-analysis. Clin Rehabil 2016;30:947–59. [DOI] [PubMed] [Google Scholar]
- [10].Brosseau L, Taki J, Desjardins B, et al. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part three: aerobic exercise programs. Clin Rehabil 2017;31:612–24. [DOI] [PubMed] [Google Scholar]
- [11].Brosseau L, Taki J, Desjardins B, et al. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part two: strengthening exercise programs. Clin Rehabil 2017;31:596–611. [DOI] [PubMed] [Google Scholar]
- [12].Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br J Sports Med 2015;49:1554–1554. [DOI] [PubMed] [Google Scholar]
- [13].Rosemann T, Kuehlein T, Laux G, et al. Factors associated with physical activity of patients with osteoarthritis of the lower limb. J Eval Clin Pract 2008;14:288–93. [DOI] [PubMed] [Google Scholar]
- [14].Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev 2016;3:CD005523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Becker BE. Aquatic therapy: scientific foundations and clinical rehabilitation applications. PM R 2009;1:859–72. [DOI] [PubMed] [Google Scholar]
- [16].Silva LE, Valim V, Pessanha AP, et al. Hydrotherapy versus conventional land-based exercise for the management of patients with osteoarthritis of the knee: a randomized clinical trial. Phys Ther 2008;88:12–21. [DOI] [PubMed] [Google Scholar]
- [17].Foley A, Halbert J, Hewitt T, et al. Does hydrotherapy improve strength and physical function in patients with osteoarthritis–a randomised controlled trial comparing a gym based and a hydrotherapy based strengthening programme. Ann Rheum Dis 2003;62:1162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Suomi R, Collier D. Effects of arthritis exercise programs on functional fitness and perceived activities of daily living measures in older adults with arthritis. Arch Phys Med Rehabil 2003;84:1589–94. [DOI] [PubMed] [Google Scholar]
- [19].Kim IS, Chung SH, Park YJ, et al. The effectiveness of an aquarobic exercise program for patients with osteoarthritis. Appl Nurs Res 2012;25:181–9. [DOI] [PubMed] [Google Scholar]
- [20].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary. Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [22].Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Langley GB, Sheppeard H. The visual analogue scale: its use in pain measurement. Rheumatol Int 1985;5:145–8. [DOI] [PubMed] [Google Scholar]
- [24].Collins NJ, Misra D, Felson DT, et al. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS). Arthritis Care Res (Hoboken) 2011;63Suppl 11:S208–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Laucis NC, Hays RD, Bhattacharyya T. Scoring the SF-36 in orthopaedics: a brief guide. J Bone Joint Surg Am 2015;97:1628–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Khoshdel A, Attia J, Carney SL. Basic concepts in meta-analysis: a primer for clinicians. Int J Clin Pract 2006;60:1287–94. [DOI] [PubMed] [Google Scholar]
- [27].Taglietti M, Facci LM, Trelha CS, et al. Effectiveness of aquatic exercises compared to patient-education on health status in individuals with knee osteoarthritis: a randomized controlled trial. Clin Rehabil 2018;32:766–76. [DOI] [PubMed] [Google Scholar]
- [28].Wang TJ, Lee SC, Liang SY, et al. Comparing the efficacy of aquatic exercises and land-based exercises for patients with knee osteoarthritis. J Clin Nurs 2011;20:2609–22. [DOI] [PubMed] [Google Scholar]
- [29].Waller B, Munukka M, Rantalainen T, et al. Effects of high intensity resistance aquatic training on body composition and walking speed in women with mild knee osteoarthritis: a 4-month RCT with 12-month follow-up. Osteoarthritis Cartilage 2017;25:1238–46. [DOI] [PubMed] [Google Scholar]
- [30].Lund H, Weile U, Christensen R, et al. A randomized controlled trial of aquatic and land-based exercise in patients with knee osteoarthritis. J Rehabil Med 2008;40:137–44. [DOI] [PubMed] [Google Scholar]
- [31].Lim JY, Tchai E, Jang SN. Effectiveness of aquatic exercise for obese patients with knee osteoarthritis: a randomized controlled trial. PM R 2010;2: 723-731. [DOI] [PubMed] [Google Scholar]
- [32].Yennan P, Suputtitada A, Yuktanandana P. Effects of aquatic exercise and land-based exercise on postural sway in elderly with knee osteoarthritis. Asian Biomed (Res Rev News) 2010;4:739–45. [Google Scholar]
- [33].Wyatt FB, Milam S, Manske RC, et al. The effects of aquatic and traditional exercise programs on persons with knee osteoarthritis. J Strength Cond Res 2001;15:337–40. [PubMed] [Google Scholar]
- [34].Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol 2018;30:160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Schieir O, Tosevski C, Glazier RH, et al. Incident myocardial infarction associated with major types of arthritis in the general population: a systematic review and meta-analysis. Ann Rheum Dis 2017;76:1396–404. [DOI] [PubMed] [Google Scholar]
- [36].Courties A, Sellam J, Maheu E, et al. Coronary heart disease is associated with a worse clinical outcome of hand osteoarthritis: a cross-sectional and longitudinal study. RMD Open 2017;3:e000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kluzek S, Sanchez-Santos MT, Leyland KM, et al. Painful knee but not hand osteoarthritis is an independent predictor of mortality over 23 years follow-up of a population-based cohort of middle-aged women. Ann Rheum Dis 2016;75:1749–56. [DOI] [PubMed] [Google Scholar]
- [38].Heywood S, McClelland J, Mentiplay B, et al. Effectiveness of aquatic exercise in improving lower limb strength in musculoskeletal conditions: a systematic review and meta-analysis. Arch Phys Med Rehabil 2017;98:173–86. [DOI] [PubMed] [Google Scholar]
- [39].Kuster MS. Exercise recommendations after total joint replacement: a review of the current literature and proposal of scientifically based guidelines. Sports Med 2002;32:433–45. [DOI] [PubMed] [Google Scholar]
- [40].Harmer AR, Naylor JM, Crosbie J, et al. Land-based versus water-based rehabilitation following total knee replacement: a randomized, single-blind trial. Arthritis Rheum 2009;61:184–91. [DOI] [PubMed] [Google Scholar]
- [41].Lu M, Su Y, Zhang Y, et al. Effectiveness of aquatic exercise for treatment of knee osteoarthritis: systematic review and meta-analysis. Z Rheumatol 2015;74:543–52. [DOI] [PubMed] [Google Scholar]
- [42].Batterham SI, Heywood S, Keating JL. Systematic review and meta-analysis comparing land and aquatic exercise for people with hip or knee arthritis on function, mobility and other health outcomes. BMC Musculoskelet Disord 2011;12:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wang TJ, Belza B, Elaine TF, et al. Effects of aquatic exercise on flexibility, strength and aerobic fitness in adults with osteoarthritis of the hip or knee. J Adv Nurs 2007;57:141–52. [DOI] [PubMed] [Google Scholar]
- [44].Alkatan M, Baker JR, Machin DR, et al. Improved function and reduced pain after swimming and cycling training in patients with osteoarthritis. J Rheumatol 2016;43:666–72. [DOI] [PubMed] [Google Scholar]
- [45].So BCL, Kong ISY, Lee RKL, et al. The effect of Ai Chi aquatic therapy on individuals with knee osteoarthritis: a pilot study. J Phys Ther Sci 2017;29:884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Regnaux JP, Lefevre-Colau MM, Trinquart L, et al. High-intensity versus low-intensity physical activity or exercise in people with hip or knee osteoarthritis. Cochrane Database Syst Rev 2015;CD010203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kutzner I, Richter A, Gordt K, et al. Does aquatic exercise reduce hip and knee joint loading? In vivo load measurements with instrumented implants. PLoS One 2017;12:e0171972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


