Abstract
The aim of this study was first to investigate associations between maternal dietary patterns and autism spectrum disorders (ASDs) and second to investigate association between maternal supplement intake and ASD.
We used a case-control study design to enroll typically developing (TD) children and children with ASD, and data were derived from the Autism Clinical and Environmental Database (ACED).
Three seventy four children with AUTISM and 354 age matched TD children were included. The multivariate logistic regression model revealed that maternal unbalanced dietary patterns before conception had a significant increased risk of ASD in offspring (mostly meat: adjusted OR, 4.010 [95% CI, 1.080, 14.887]; mostly vegetable: adjusted OR, 2.234 [95% CI, 1.009, 4.946]); maternal supplementation of calcium during pregnancy preparation was associated with decreased ASD risk (adjusted OR, 0.480 [95% CI, 0.276, 0.836]).
This study provided preliminary evidence that maternal unbalanced dietary patterns may be a risk factor for ASD and supplementation of calcium during pregnancy preparation may be inversely associated with ASD in offspring.
Keywords: autism spectrum disorder, diet, pregnancy, risk factor, supplement
1. Introduction
Autism spectrum disorders (ASDs) are a neurodevelopmental conditions characterized by social communication impairment, delayed development and social function deficit.[1] Approximately 1 in 59 children has ASD in the USA, and the incidence seems to be cumulatively increasing.[2] While the exact etiology of ASD remains unknown, both genetic and environmental factors are believed to be involved in its complex pathogenesis.[3] As for the environmental risk factors, the prenatal period is generally believed to be a suspected susceptibility period, and a growing number of studies have been focused on the nutritional status of mothers of children with ASD.[4–6]
Dietary patterns have been evidenced to be related to maternal nutritional status that might lead to different metabolic conditions,[7,8] and maternal metabolic dysfunction has been observed to be associated with ASD.[9] While extensive literature assessing associations between ASD and prenatal, perinatal and other maternal reproductive factors, have been published,[10] little is known regarding maternal dietary patterns before conception, during pregnancy or during lactation period. Also, previous studies reported supplementation of nutrients, such as folic acid,[4,11,12] around the time of conception may help to reduce the risk of ASD in children. However, those findings warrant replications. Moreover, calcium has been linked to ASD for its important role in neurodevelopment, for example, synaptic development,[13] but the association between maternal intake of calcium and ASD has not been reported.
Therefore, the aim of the present work based on a case-control study design was first to investigate association between maternal dietary patterns and ASD and second to investigate association between maternal supplement intake, folic acid and calcium, and ASD.
2. Methods
2.1. Study population
The data used in this study were derived from the Autism Clinical and Environmental Database (ACED) in China, which was approved by the ethics committee of the Second Xiangya Hospital. The database was building from January 2012 and had been described in our previous article.[14] Briefly, children with ASD were mainly recruited at special education schools in Changsha and Qingdao, China and from outpatient service of the Second Xiangya Hospital in Changsha, China. Typically developing (TD) children were recruited from ordinary kindergarten classes in Changsha, China. Informed consent was written by guardians of all participants prior to enrolment.
The inclusion criteria of children with ASD were as follows:
-
(1)
aged 3 to 6 years and diagnosed with autistic disorder, Asperger disorder, or PDD-NOS at a local hospital;
-
(2)
diagnosis was confirmed by a senior child psychiatrist according to the Diagnostic and Statistical Manual of Mental Disorders.
Fourth Edition, Text Revision (DSM-IV-TR) and an independent child psychiatrist confirmed the diagnosis with a semi-structured interview. DSM-IV-TR diagnosis of autistic disorder, Asperger disorder, or PDD-NOS have been acknowledged for a DSM-5 diagnosis of ASD.[2] The exclusion criteria were the following:
-
(1)
the child with severe physical diseases such as congenital heart disease, hematological system diseases, and others;
-
(2)
the child with any common chromosomal diseases, checked by chromosome karyotype analysis;
-
(3)
the child with total raw SRS scores lower than the suggested cut-off score of 56.5 in China child population.[15]
Age matched TD children were selected as controls and identified as following: the child performed well in classes with no report of serious learning, emotional, behavioral, or social problems from parents or teachers. A self-designed screening scale, filled out by parents or teachers, was used to check for these problems. The Social Responsiveness Scale (SRS) was also used to assess social problems in these children. Children with total raw SRS scores higher than 56.5 were excluded, as they may have experienced social problems.[15] Children with severe physical diseases such as congenital heart disease, hematological system diseases, and others were also excluded.
2.2. Measures
A structured investigative questionnaire was employed to collect demographic information about those included children and their family members, including child's age and gender, parental age and education levels, maternal body mass index (BMI) before conception and delivery, and delivery information. Besides, maternal information regarding dietary and nutritional supplement patterns according to prenatal period was collected by the questionnaire.
Maternal preference of food before conception, during pregnancy and during lactation were investigated by interviewing, and the answers included dietary patterns of mostly meat, mostly vegetables or both of them. Then, mothers were asked if they have taken nutrients, including folic acid, calcium and intestinal flora regulator, during pregnancy preparation, during pregnancy or during lactation. In addition, it should be noted that calcium supplements may also contain several other nutrients such as vitamin D3; and the most often used intestinal flora regulator is prebiotics.
SRS was used to measure social impairment in TD group and ASD group.[16] This tool surveys a child's current and past social behaviors correlating with the Autism Diagnostic Interview – Revised (ADI-R), it is also applicable to all children and is not affected by intelligence, age, race, or the education level of respondents.[16,17] The SRS has been translated to Chinese and have been utilized for examination in different local populations.[18] While the scale was original designed for 4- to 18-year-old children and adolescents, it also has been used as screening tools in children younger than 4 years old.[19] SRS includes total score and five specific symptom domains: social awareness, social cognition, social communication, social motivation, and autistic mannerisms. Higher SRS scores represent more ASD-related behaviors. Since norms is not available for China, raw scores were used in this study. The cut-off score 56.5, with high sensitivity and specificity in China child population, was used to discriminate ASD and TD control, children in TD group with SRS score higher than 56.5 and children in ASD group with SRS score lower than 56.5 were excluded.[15]
2.3. Statistical analyses
Statistical analyses were performed using SPSS 17.0 software. Descriptive statistics were used for demographics. Non-parametric median test, Chi-square test and Fisher exact tests were used for comparison of proportions as appropriate. Univariate logistic regression analyses were firstly used to test the significance between maternal dietary and nutrients variables and risk of ASD. Then, multivariable logistic regression analyses were performed with covariates including child's and parental age, child's gender, parental education, maternal BMI before conception and delivery, and premature delivery (yes or no) (Model 1). Also, multivariable logistic regression analysis additionally adjusted for other maternal dietary patterns or other supplements was used to decrease influence of other simultaneous factors (Model 2). Finally, to further explore the role of maternal intake of supplements, we performed multivariable logistic regression analysis according to different exposure period (during pregnancy preparation and pregnancy, during pregnancy preparation only, and during pregnancy only). All hypotheses tests were considered significant with a 2-sided P-value less than .05.
3. Results
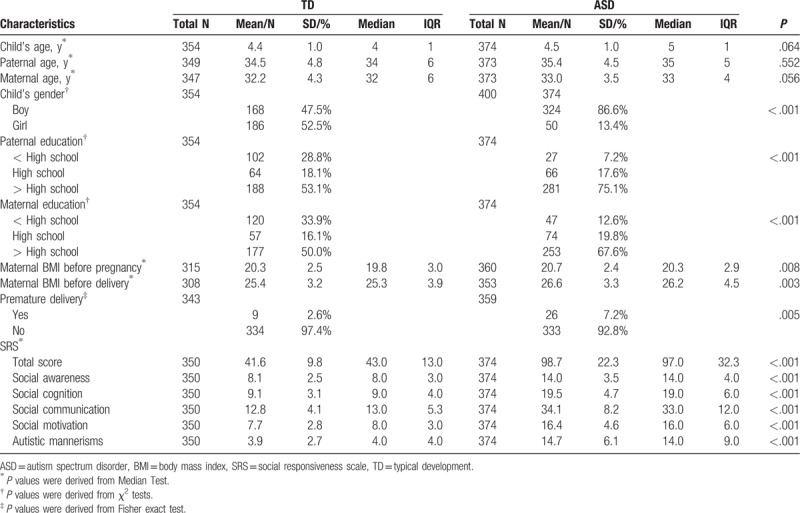
A total of 354 (168 boys) TD children and 374 (324 boys) children with ASD were recruited in this study. The mean age of TD children was 4.4 (SD: 1.0) years with no significant difference to that of ASD children [4.5 (SD: 1.00) years, P = .064]. Parental age between TD group and ASD group were not statistically different. However, parental education, maternal BMI and premature delivery (yes or no) between the 2 groups were significantly different. Therefore, child's age and gender, parental age, maternal BMI before conception and delivery, and premature delivery were adjusted in the multivariate logistic regression analysis as covariates. As for SRS measures, scores of ASD group were significantly higher than TD group in all aspects (Table 1).
Table 1.
Characteristics of children with autism spectrum disorder or typical development and their parents.
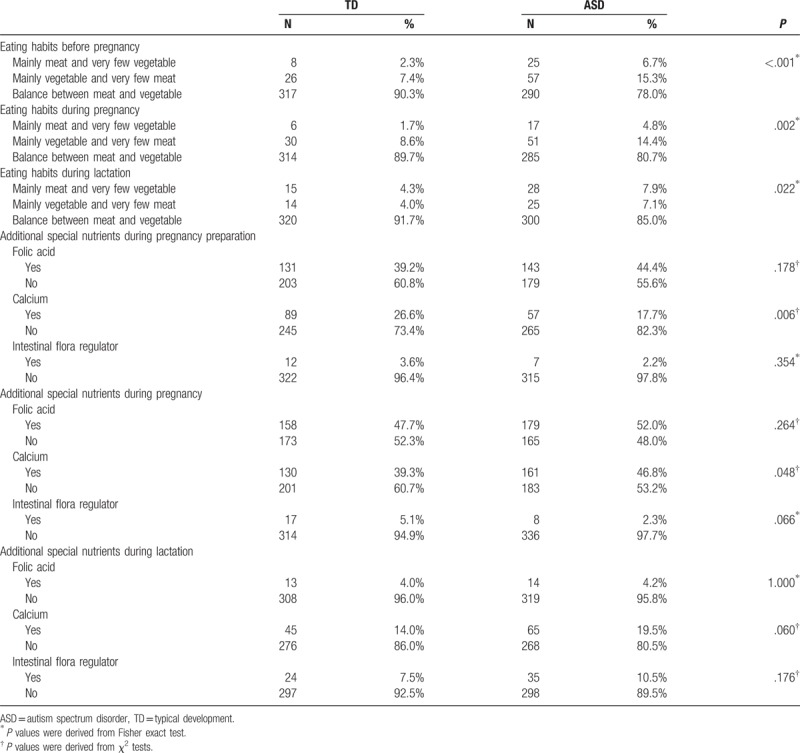
Results of comparisons of maternal dietary patterns and supplement intake between the 2 groups were summarized in Table 2. For maternal dietary patterns, ASD group had fewer rates of dietary of both meat and vegetables before conception (P < .001), during pregnancy (P = .002) or during lactation period (P = .028). For maternal supplement intake during pregnancy preparation, supplementation of calcium (P < .006) was significantly lower for ASD group than TD group. Also, for maternal supplement intake during pregnancy, intake of calcium was significantly lower for ASD group than TD group (P = .048). For maternal supplement intake during lactation period, no significant difference was observed.
Table 2.
Comparisons of maternal dietary patterns and supplement intake between TD and ASD groups.
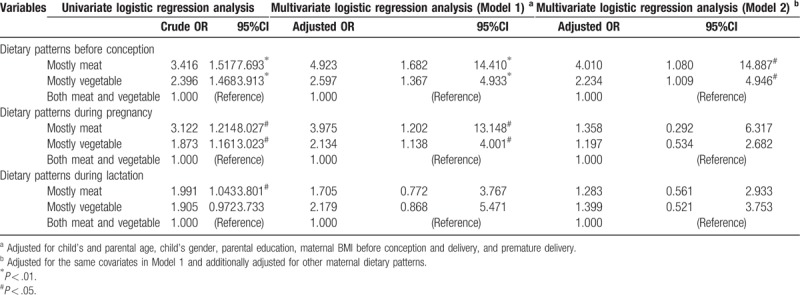
To further evaluate associations between maternal dietary patterns, supplement intake and risk of ASD in offspring, univariate and multivariate logistic regression analyses were performed (Table 3). Results from multivariable analyses (Model 1) revealed that maternal unbalanced dietary patterns before conception (mostly meat: adjusted OR, 4.923 [95% CI, 1.682, 14.410]; mostly vegetable: adjusted OR, 2.597 [95% CI, 1.367, 4.933]) and during pregnancy (mostly meat: adjusted OR, 3.975 [95% CI, 1.202, 13.148]; mostly vegetable: adjusted OR, 2.134 [95% CI, 1.138, 4.001]) were significantly associated with ASD. After additional adjustment for other dietary patterns and supplement intake (Model 2), maternal unbalanced dietary patterns before conception were also significantly associated with ASD (mostly meat: adjusted OR, 4.010 [95% CI, 1.080, 14.887]; mostly vegetable: adjusted OR, 2.234 [95% CI, 1.009, 4.946]); however, maternal unbalanced dietary patterns during pregnancy were not associated with ASD (mostly meat: adjusted OR, 1.358 [95% CI, 0.292, 6.317]; mostly vegetable: adjusted OR, 1.197 [95% CI, 0.534, 2.682]).
Table 3.
Univariate and multivariate logistic regression analysis on maternal dietary patterns and autism spectrum disorders.
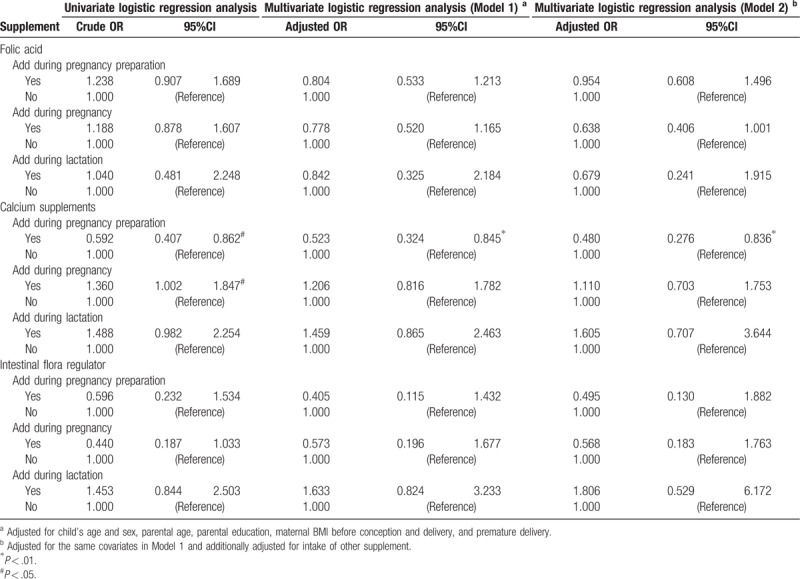
For supplements, we also applied 2 multivariate regression models (Table 4). As a result, in model 1, only calcium supplementation during pregnancy preparation was associated with significantly decreased risk of ASD (adjusted OR, 0.523 [95% CI, 0.324, 0.845. Similarly, in model 2, we only find that calcium supplementation during pregnancy preparation was associated with decreased ASD risk (fully adjusted OR, 0.480 [95% CI, 0.276, 0.836]). Associations of maternal intake of folic acid and intestinal flora regulator were not associated with ASD.
Table 4.
Multivariate logistic regression analysis on maternal supplement intake and autism spectrum disorders.
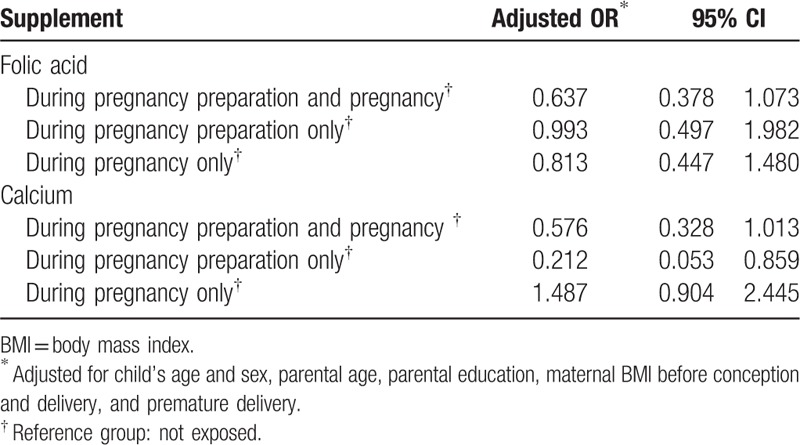
To further explore the role of maternal intake of folic acid and calcium in ASD, we performed analysis according to different exposure period (Table 5). Still, maternal intake of folic acid, during pregnancy preparation or pregnancy, was not associated with ASD as compared to non-exposed group. However, only calcium intake during pregnancy preparation showed lower risk of ASD (fully adjusted OR, 0.212 [95% CI, 0.053, 0.859]).
Table 5.
Multivariate logistic regression analysis on maternal folic acid and calcium intake and autism spectrum disorders according to different exposure period.
4. Discussion
In this study, we investigated the associations between maternal dietary patterns, supplements intake and ASD in offspring. Multivariate analysis showed that maternal unbalanced dietary patterns before conception were associated with increased risk of ASD in offspring, supplementation of calcium during pregnancy preparation was independently associated with decreased ASD risk.
Findings of this study revealed that maternal unbalanced dietary patterns might be a risk factor for ASD. Insufficient intake of vegetables may lead to deficiency in micronutrients such as vitamins and minerals, while less consumption of meat may result in dietary protein insufficiency, both would increase risk of metabolic conditions associated with nutrient deficiency. Unbalanced dietary patterns before conception may be related to long-term maternal metabolic conditions such as metabolic syndrome,[20] obesity[21] and diabetes.[22] Those conditions have also been identified as environmental risk factors for ASD as well as other neurodevelopmental disorders.[23–25]
The protective effect of calcium supplementation during pregnancy preparation was firstly reported. Previous studies have linked calcium to ASD. Children with ASD had significantly lower calcium levels in serum,[26] and children with ASD would consume less calcium than TD children,[27] suggesting calcium may be involved in the pathological process. Ca2+ could be transferred from the mother to the fetus through human placenta during pregnancy,[28] suggesting maternal calcium level may be associated with calcium level in fetus. Calcium is of great importance for neurodevelopment and may provide preventive and therapeutic strategies for ASD,[29] for example, calcium plays a vital role in regulating synapse development and function, therefore, increased calcium level may be beneficial for proper brain development as found abnormal synaptic development in ASD was observed.[30] Besides, results from previous randomized clinical trials[31,32] revealed that maternal calcium supplementation was associated with reductions in blood lead levels and may constitute an important secondary prevention effort to reduce circulating maternal lead through suppressing mobilization of lead and, consequently, decrease fetal lead exposure. The association between lead exposure and ASD have been identified.[33] Thus, we speculate calcium supplementation may prevent ASD through decreasing lead exposure. In addition, maternal supplementation of calcium may simultaneously take vitamin D in order to prevent calcium and vitamin D insufficiency during pregnancy. Although no study reported maternal calcium levels and ASD, maternal vitamin D deficiency was observed to be a risk factor for the development of ASD[34] and decreased vitamin D levels in children with ASD have been reported.[35] Hence, simultaneous supplementation of vitamin D may be involved in the association between maternal calcium supplementation and ASD.
Our findings did not replicate association of reduced risk of ASD with antenatal folic acid supplementation reported in existing literature.[4,11,12] This may be attributed to the high prevalence of folic acid supplementation (up to 40% during pregnancy preparation and up to 50% during pregnancy) in both ASD and TD group since mothers were well educated about the importance of nutrition and the health of the unborn baby during pregnancy. As a result, the effect of antenatal folic acid exposure was observed to be null for ASD. Another possibility of differences between studies, which have been discussed in a recently published study,[12] was the inconsistent amount of the nutrients resulted from background nutritional contexts of the countries.
The study has certain limitations. First, the children with ASD were enrolled using special school-based and hospital-based study design, not from an epidemiological sampling method, thus the sample may not adequately represent the whole population. Second, questions and items to collect information regarding maternal dietary patterns were ambiguous. It is uncertain whether mothers had taken adequate proteins (meat) or micronutrients (vegetables) or not because we focused on the dietary patterns instead of amount of nutrients. Third, the dose of supplements was unknown. A dose-response relationship is essential for determination of a causal association, but we failed to interpret the association based on current study results. Fourth, as maternal diets were reported retrospectively, accuracy of information may not be valid and the recall bias may have played a role in dietary patterns, categories of supplements and timing of exposure. Fifth, the dietary patterns in China may differ from other countries, which decreased the generalizability of our findings. Therefore, future prospective investigations with a more rigorous study design are warranted to further study underlying links and to improve our understanding of the mechanisms and replication studies in other countries are also encouraged.
5. Conclusions
In conclusion, our results provided preliminary evidence that maternal unbalanced dietary patterns before conception would increase risk of ASD, maternal supplementation of calcium during pregnancy preparation could reduce risk of ASD. However, those findings were preliminary and may not be conclusive. Future prospective studies with larger sample sizes and more detailed timing information should consider maternal nutritional status in association with ASD in offspring.
Acknowledgments
Many thanks to the Qingdao Elim and Changsha Star Garden autism training schools, and the Changsha Qihang Education Center for their assistance to this work.
Author contributions
Conceptualization: Jing-Ping Zhao, Jian-Jun Ou.
Data curation: Yi-Dong Shen.
Formal analysis: Jian-Jun Ou.
Funding acquisition: Jing-Ping Zhao, Jian-Jun Ou.
Investigation: Ya-Min Li, Yi-Dong Shen, Guang-Lei Xun, Huaqing Liu, Ren-Rong Wu, Kun Xia.
Methodology: Jian-Jun Ou.
Resources: Guang-Lei Xun, Huaqing Liu, Ren-Rong Wu, Kun Xia.
Software: Jian-Jun Ou.
Writing – original draft: Ya-Min Li, Yong-Jiang Li.
Writing – review & editing: Ya-Min Li, Yong-Jiang Li.
Footnotes
Abbreviations: ACED = autism clinical and environmental database, ADI-R = autism diagnostic interview – revised, ASDs = autism spectrum disorders, BMI = body mass index, DSM-IV-TR = diagnostic and statistical manual of mental disorders-fourth edition-text revision, SRS = social responsiveness scale, TD = typically developing.
This study was funded by the National Natural Science Foundation of China (No: 81601197) and the Major State Basic Research Development Program of China (973 Program, No. 2012CB517901).
The authors have no conflicts of interest to disclose.
References
- [1].American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub, Washington DC; 2013. [Google Scholar]
- [2].Baio J, Wiggins L, Christensen DL, et al. Prevalence of autism spectrum disorder among children aged 8 Years - autism and developmental disabilities monitoring network, 11 Sites, United States, 2014. MMWR Surveill Summ 2018;67:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lai M, Lombardo M, Baron-Cohen S. Autism. Lancet 2014;383:896–910. [DOI] [PubMed] [Google Scholar]
- [4].Surén P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA 2013;309:570–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schmidt RJ, Tancredi DJ, Krakowiak P, et al. Maternal intake of supplemental iron and risk of autism spectrum disorder. Am J Epidemiol 2014;180:890–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lyall K, Munger KL, O’reilly ÉJ, et al. Maternal dietary fat intake in association with autism spectrum disorders. Am J Epidemiol 2013;178:209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Martinez-Gonzalez MA, Bes-Rastrollo M. Dietary patterns, Mediterranean diet, and cardiovascular disease. Curr Opin Lipidol 2014;25:20–6. [DOI] [PubMed] [Google Scholar]
- [8].Ahluwalia N, Andreeva V, Kesse-Guyot E, et al. Dietary patterns, inflammation and the metabolic syndrome. Diabetes Metab 2013;39:99–110. [DOI] [PubMed] [Google Scholar]
- [9].Connolly N, Anixt J, Manning P, et al. Maternal metabolic risk factors for autism spectrum disorder–An analysis of electronic medical records and linked birth data. Autism Res 2016;9:829–37. [DOI] [PubMed] [Google Scholar]
- [10].Gardener H, Spiegelman D, Buka SL. Prenatal risk factors for autism: comprehensive meta-analysis. Br J Psychiatry 2009;195:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Levine SZ, Kodesh A, Viktorin A, et al. Association of maternal use of folic acid and multivitamin supplements in the periods before and during pregnancy with the risk of autism spectrum disorder in offspring. JAMA Psychiatry 2018;75:176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].DeVilbiss EA, Magnusson C, Gardner RM, et al. Antenatal nutritional supplementation and autism spectrum disorders in the Stockholm youth cohort: population based cohort study. Bmj-Brit Med J 2017;j4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron 2008;59:861–72. [DOI] [PubMed] [Google Scholar]
- [14].Chen C, Shen YD, Xun GL, et al. Aggressive behaviors and treatable risk factors of preschool children with autism spectrum disorder. Autism Res 2017;10:155–162. [DOI] [PubMed] [Google Scholar]
- [15].Cen CQ, Liang Y, Chen QR, et al. Investigating the validation of the Chinese Mandarin version of the social responsiveness scale in a Mainland China child population. BMC Psychiatry 2017;17:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Constantino JN, Davis SA, Todd RD, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord 2003;33:427–33. [DOI] [PubMed] [Google Scholar]
- [17].Reiersen AM, Constantino JN, Volk HE, et al. Autistic traits in a population-based ADHD twin sample. J Child Psychol Psychiatry 2007;48:464–72. [DOI] [PubMed] [Google Scholar]
- [18].Gau SS-F, Liu L-T, Wu Y-Y, et al. Psychometric properties of the Chinese version of the social responsiveness scale. Res Autism Spectr Disord 2013;7:349–60. [Google Scholar]
- [19].Moody EJ, Reyes N, Ledbetter C, et al. Screening for autism with the SRS and SCQ: variations across demographic, developmental and behavioral factors in preschool children. J Autism Dev Disord 2017;1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Esmaillzadeh A, Kimiagar M, Mehrabi Y, et al. Dietary patterns, insulin resistance, and prevalence of the metabolic syndrome in women. Am J Clin Nutr 2007;85:910–8. [DOI] [PubMed] [Google Scholar]
- [21].McNaughton SA, Ball K, Mishra GD, et al. Dietary patterns of adolescents and risk of obesity and hypertension. J Nutr 2008;138:364–70. [DOI] [PubMed] [Google Scholar]
- [22].Alhazmi A, Stojanovski E, McEvoy M, et al. The association between dietary patterns and type 2 diabetes: a systematic review and meta-analysis of cohort studies. J Hum Nutr Diet 2014;27:251–60. [DOI] [PubMed] [Google Scholar]
- [23].Li Y-M, Ou J-J, Liu L, et al. Association between maternal obesity and autism spectrum disorder in offspring: a meta-analysis. J Autism Dev Disord 2016;46:95–102. [DOI] [PubMed] [Google Scholar]
- [24].Xiang AH, Wang X, Martinez MP, et al. Association of maternal diabetes with autism in offspring. JAMA 2015;313:1425–34. [DOI] [PubMed] [Google Scholar]
- [25].Shen YD, Dong HX, Lu XZ, et al. Associations among maternal pre-pregnancy body mass index, gestational weight gain and risk of autism in the Han Chinese population. BMC Psychiatry 2018;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Meguid NA, Hashish AF, Anwar M, et al. Reduced serum levels of 25-Hydroxy and 1,25-Dihydroxy Vitamin D in egyptian children with autism. J Altern Complemt Med 2010;16:641–5. [DOI] [PubMed] [Google Scholar]
- [27].Herndon AC, DiGuiseppi C, Johnson SL, et al. Does nutritional intake differ between children with autism spectrum disorders and children with typical development? J Autism Dev Disord 2009;39:212–22. [DOI] [PubMed] [Google Scholar]
- [28].Belkacemi L, Bedard I, Simoneau L, et al. Calcium channels, transporters and exchangers in placenta: a review. Cell Calcium 2005;37:1–8. [DOI] [PubMed] [Google Scholar]
- [29].Palmieri L, Papaleo V, Porcelli V, et al. Altered calcium homeostasis in autism-spectrum disorders: evidence from biochemical and genetic studies of the mitochondrial aspartate/glutamate carrier AGC1. Mol Psychiatry 2010;15:38–52. [DOI] [PubMed] [Google Scholar]
- [30].Tang GM, Gudsnuk K, Kuo SH, et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron 2014;83:1131–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ettinger AS, Lamadrid-Figueroa H, Téllez-Rojo MM, et al. Effect of calcium supplementation on blood lead levels in pregnancy: a randomized placebo-controlled trial. Environ Health Perspect V 117 2009;26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hernandez-Avila M, Gonzalez-Cossio T, Hernandez-Avila JE, et al. Dietary calcium supplements to lower blood lead levels in lactating women: a randomized placebo-controlled trial. Epidemiology 2003;14:206–12. [DOI] [PubMed] [Google Scholar]
- [33].Wiesner L. Lead exposure and autism. in: encyclopedia of autism spectrum disorders. Child School Psychol 2013;705–1706. [Google Scholar]
- [34].Vinkhuyzen A, Eyles D, Burne T, et al. Gestational vitamin D deficiency and autism-related traits: the generation R study. Mol Psychiatry 2016;23:240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cannell JJ. Vitamin D and autism, what's new? Rev Endocr Metab Disord 2017;18:183–93. [DOI] [PubMed] [Google Scholar]


