Abstract
Rationale:
Cisplatin monotherapy-induced cardiotoxicity is rare, and the prevalence remains unknown. It's extremely important to stop cisplatin when cardiotoxicity is considered.
Patient concerns:
A 53-year-old woman developed cervical cancer. She was administered cisplatin (37 mg/m2/wk) for 3 weeks, but the left ventricular ejection fraction (LVEF) declined from 70% to 48%.
Diagnosis:
Electrocardiogram showed first-degree atrioventricular block and ST-segment depression by 0.05 mv on leads II, III, and V3–5. Neither cardiac markers nor N-terminal pro-B-type natriuretic peptide (NT-pro BNP) was elevated. After a careful physical examination and laboratory investigation, we confirmed that cervical cancer did not progress and no other cause was evident. So we figured cardiotoxicity might be induced by cisplatin.
Interventions:
Cisplatin was stopped and cardioprotective therapies were given to the patient.
Outcomes:
After discontinuing cisplatin and adding cardioprotective therapies, the LVEF increased to 50% and 53%, respectively (M-mode echocardiography) after 17 and 90 days, which further confirmed our diagnosis.
Lessons:
According to this case and literature review, cisplatin-induced cardiotoxicity should be considered for the patient. When necessary, we should discontinue the suspected drug to confirm diagnosis. Cardioprotective therapies would minimize the drug-induced cardiovascular adverse events and improve patients’ outcome.
Keywords: cardiotoxicity, cisplatin, ejection fraction
1. Introduction
Cisplatin is one of the first-generation platinum-based cell-cycle nonspecific antineoplastic agents with a wide range of anticancer activities. The major adverse events of cisplatin include nephrotoxicity, neurotoxicity, ototoxicity, and gastrointestinal toxicities (>50 mg/m2). Cisplatin-induced cardiotoxicity is rare, and the prevalence remains unknown. Jakubowski and Kemeny[1] showed that cardiotoxicity occurs in 6% of the patients receiving cisplatin and 5-fluorouracil (5-FU) and in 1.6% of the patients receiving 5-FU alone. In conventional multidrug regimens, investigating the effects of cisplatin on cardiotoxicity is rather challenging.[2] Here, we report a case that developed cardiotoxicity during the use of cisplatin alone. We also reviewed the literature with respect to clinical characteristics, pathophysiological mechanisms, and treatments of cisplatin-induced cardiotoxicity.
2. Case report
A 53-year-old woman was diagnosed with cervical squamous cell carcinoma stage IIB 1 month ago. After radiotherapy, the patient received cisplatin 37 mg/m2 per week. Five days after the third course of treatment, the patient complained of retrosternal burning. Hypotension and abnormal electrocardiogram (ECG) were also observed. Left ventricular ejection fraction (LVEF) declined from 70% to 48% (see Figs. 1 and 2). Electrocardiogram showed first-degree atrioventricular block and ST-segment depression by 0.05 mv on leads II, III, and V3–5. Neither cardiac markers nor N-terminal pro-B-type natriuretic peptide (NT-proBNP) was elevated. We inferred a causative relationship between cisplatin and cardiotoxicity. The clinical characteristics were complied with the diagnostic criteria of cardiotoxicity with midrange ejection fraction listed in the 2016 European Society of Cardiology (ESC) guidelines for the diagnosis and treatment of acute and chronic cardiotoxicity.[3] The comparisons of echocardiographic findings before and after cisplatin were listed in Fig. 1.
Figure 1.
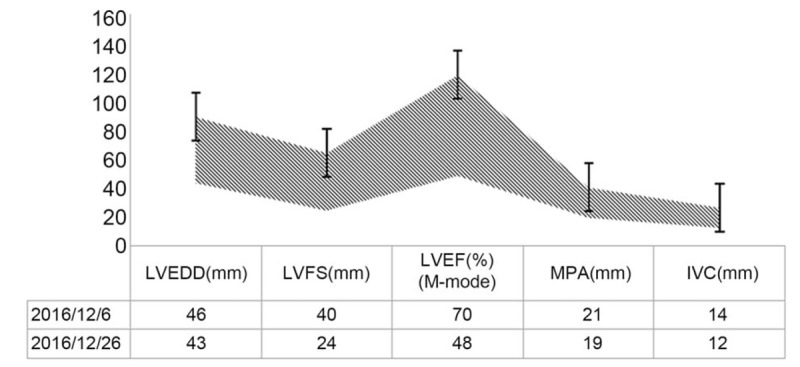
Comparisons of echocardiographic findings before and after DDP use. LVEDD stands for left ventricular end-diastolic ejection fraction; LVFS stands for left ventricular fractional shortening; LVEF stands for left ventricular ejection fraction (M-mode); MPA stands for main pulmonary artery; IVC stands for inferior vena cava.
Figure 2.
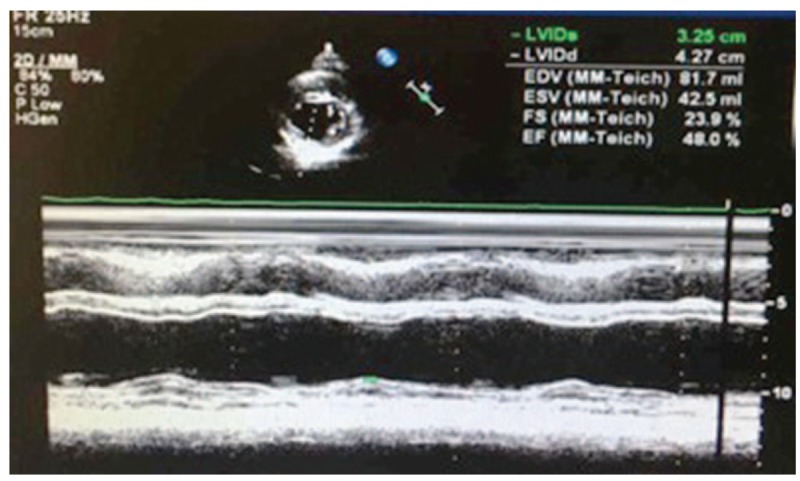
Parasternal short axis view at the mid-left ventricle (papillary muscle) of echocardiogram.
Therefore, the cisplatin was stopped and LVEF reduced by 22%. Coenzyme Q10 (Ubidecarenone, 10 mg tid) and trimetazidine (Vasorel, 20 mg tid) were administered to protect cardiomyocytes. After 3 weeks, M-mode echocardiography showed that the left ventricular end-diastolic ejection fraction (LVEDD) was 46 mm, LVEF 50%, and E/A [eathy atrial (mitral diastolic filling)] ratio 1.0. Due to cisplatin-induced cardiotoxicity, the fourth course of cisplatin was not started. And the gynecologist suggested continued radiotherapy without cisplatin. The antigen level of squamous cell carcinoma was 1.0 ng/mL. She was in stable condition and was advised a repeat check by echocardiography after 3 months; the LVEF was found to be increased to 53% (M-mode echocardiography).
3. Discussion
The patient in this study did not have a history of hypertension, coronary heart diseases, chest distress, and chest pain. Other risk factors such as smoking or drinking were also absent. In addition, we found no direct relationships between cardiotoxicity and comorbidities and the use of other drugs. We confirmed that the patient's cardiotoxicity was caused by cisplatin.
According to the criteria of adverse drug reactions recommended by the World Health Organization (WHO),[4] we identified the “likely/probable” relationship between cisplatin and cardiotoxicity with HF with midrange HF (HFmrEF). The occurrence of HFmrEF is attributable to the accumulation of cisplatin. Based on the criteria of cardiac adverse events (NCI CTC4.0), the patient was graded 2 due to the left ventricular systolic dysfunction with 40% to 50% LVEF.
Although current guidelines of European Society of Cardiology appropriate diagnostic tool to diagnose cardiotoxicity is calculation of ejection fraction using 2D Simpson method, 3-D based or Global longitudinal strain (GLS). In our department of cardiology, physicians always calculate ejection fraction using M-mode echocardiography in our hospital depend on local conditions. Based on M-mode echocardiography before and after the use of cisplatin, we can identify the “likely/probable” relationship.
Elevated NT-proBNP was not been found in this case, because sometimes it doesn’t occur in early stage of cardiotoxicity.[3]
Due to the less number of the prevalent studies, the mechanisms of cisplatin-induced cardiotoxicity remain unclear. Jakubowski and Kemeny[1] reported that cardiotoxicity occurs in 6% of the patients receiving cisplatin and 5-FU. In this case, the following manifestations might contribute to the occurrence of cardiotoxicity after the use of cisplatin. Firstly, the patient with malignant tumor (cervical cancer stage IIB) receiving multiple courses of chemotherapy putatively developed emboli and thrombi and exhibited an imbalanced stress response. Fukuhara et al[5] demonstrated dose-dependent risks of adverse events with respect to the tumor stage; however, they did not indicate the specific risks for different stages. Secondly, the patient displayed abnormal ECG findings with ST-T segment depression and a PR interval of 0.231 seconds on multiple leads. Subsequently, we observed the newly occurred first-degree atrioventricular block, ST-segment depression by 0.05 mv on leads II, III, and V3–5, and a PR interval of 0.21 seconds. Although ECG abnormalities were not related to cisplatin, the usage might impair the proximal tubules, reduce the reabsorptions of potassium and magnesium, shorten the duration of the action potential, decrease calcium inflow, and finally result in early systolic dysfunction.[5] Thirdly, the patient received 3 courses cisplatin. Cisplatin activates the transmembrane protein kinase RNA (PKR)-like ER kinase (PERK) signaling pathway, phosphorylates IF2a, activates caspase 3, promotes the formation of apoptosome, and induces apoptosis of the cardiomyocytes.[6] Furthermore, cisplatin combines with histone-lacking mitochondrial DNA (mtDNA) to form various complexes,[7,8] disabling the nucleotide excision repair pathways that can remove such complexes. As a result, myocardial mtDNA is impaired, and mitochondria are unable to produce adenosine triphosphate. Cardiomyocytes suffer from dysfunction and subsequently hypoxic necrosis,[6] resulting in cardiotoxicity.
Few strategies have been proposed to prevent and treat the cisplatin-induced cardiotoxicity effectively. Firstly, we dynamically monitored the echocardiography and ECG of the patient before and during the therapy. Secondly, we stopped the usage of cisplatin at a relatively early stage. Thirdly, we used coenzyme Q10 and trimetazidine to prevent the cardiac function from deterioration, based on potential pathophysiological mechanisms of cisplatin-induced cardiotoxicity.[9,10] Our experience might provide an example for time management for the use of medications for cardioprotection following cisplatin-induced cardiotoxicity.
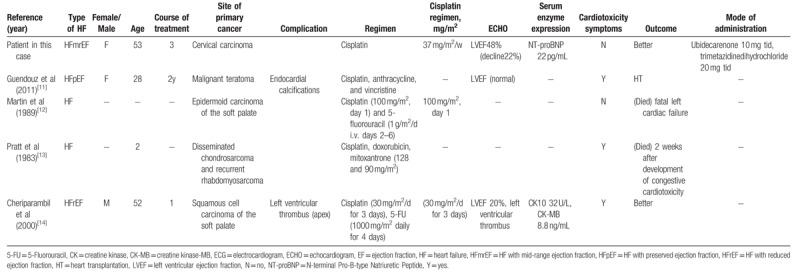
Cardiotoxicity has rarely been reported in patients receiving cisplatin. Only 4 cases reported (from January 1, 1980, through April 1, 2017) cisplatin-induced cardiotoxicity or reduced ejection fraction. Table 1 summarizes the clinical characteristics of 5 cases. We reported the first case of cisplatin-induced cardiotoxicity exhibiting a distinct causative relationship between cisplatin use and cardiotoxicity. All the 4 previous cases received a combination therapy containing cisplatin. Two cases finally succumbed to mortality: 1 case was autopsied, and 1 case underwent heart transplantation. The lethal consequences of cisplatin should not be neglected in clinical practice. Cardiotoxicity with midrange ejection fraction and arrhythmias (first-degree atrioventricular block) might be induced by cisplatin in patients without any risk factors for coronary heart disease. The patients receiving cisplatin should be monitored closely for LVEF, cardiac markers, and ECG to identify cardiac dysfunction at an early stage. Once cardiotoxicity occurs, cisplatin should be reduced or discontinued. Consecutively, cardioprotective therapies should be administered to the patient, in particular for those with risk factors for cardiovascular diseases. Currently, neither a dose-dependent nor a temporal relationship between cisplatin and cardiotoxicity is observed. In addition, the 2016 ESC position study on cancer treatments and cardiovascular toxicity does not discuss the cisplatin-induced cardiovascular diseases.[3] The clinical staff is encouraged to accumulate and exchange relevant experience in order to minimize the drug-induced impairments, prevent disease progression, reduce drug-associated cardiovascular complications, and improve the patient's outcomes.
Table 1.
Summary of case reports of cisplatin-induced cardiotoxicity.
Acknowledgments
This work was supported by Peking Union Medical College Hospital.
Author contributions
Conceptualization: Yang Hu, Bin Sun, Bin Zhao.
Formal analysis: Yang Hu.
Investigation: Bin Zhao, Dan Mei.
Methodology: Yang Hu, Bin Sun, Qing Gu.
Supervision: Bin Sun, Dan Mei, Zhuang Tian.
Visualization: Bin Zhao.
Writing – original draft: Yang Hu.
Writing – review & editing: Yang Hu, Bin Sun, Bin Zhao, Dan Mei, Qing Gu, Zhuang Tian.
Footnotes
Abbreviations: ECG = electrocardiogram/electrocardiographic, ESC = European Society of Cardiology, GLS = global longitudinal strain, HF = heart failure, HFmrEF = HF with midrange HF, LVEF = left ventricular ejection fraction, MtDNA = mitochondrial DNA, NT-proBNP = N-terminal Pro-B-type natriuretic peptide, PERK = Protein Kinase RNA (PKR)-like ER Kinase, WHO = World Health Organization.
Informed consent: The informed consent has been obtained from the patient.
The authors have no conflicts of interest to declare.
References
- [1].Jakubowski AA, Kemeny N. Hypotension as a manifestation of cardiotoxicity in three patients receiving cisplatin and 5-fluorouracil. Cancer 1988;62:266–9. [DOI] [PubMed] [Google Scholar]
- [2].Labianca R, Beretta G, Clerici M, et al. Cardiac toxicity of 5-fluorouracil: a study on 1083 patients. Tumori 1982;68:505–10. [DOI] [PubMed] [Google Scholar]
- [3].Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Rev Esp Cardiol (Engl Ed) 2016;69:1167. [DOI] [PubMed] [Google Scholar]
- [4].Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet 2000;356:1255–9. [DOI] [PubMed] [Google Scholar]
- [5].Fukuhara H, Yagi M, Ando K, et al. Long-term administration of single-agent carboplatin (AUC 4) for advanced testicular seminoma safely achieved complete response in an 80-year-old man with chronic heart failure: a case report. Can Urol Assoc J 2014;8:E931–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ma H, Jones KR, Guo R, et al. Cisplatin compromises myocardial contractile function and mitochondrial ultrastructure: role of endoplasmic reticulum stress. Clin Exp Pharmacol Physiol 2010;37:460–5. [DOI] [PubMed] [Google Scholar]
- [7].Jamieson ER, Lippard SJ. Structure, recognition, and processing of cisplatin-DNA adducts. Chem Rev 1999;99:2467–98. [DOI] [PubMed] [Google Scholar]
- [8].Perez RP. Cellular and molecular determinants of cisplatin resistance. Eur J Cancer 1998;34:1535–42. [DOI] [PubMed] [Google Scholar]
- [9].Fotino AD, Thompson-Paul AM, Bazzano LA. Effect of coenzyme Q(1)(0) supplementation on heart failure: a meta-analysis. Am J Clin Nutr 2013;97:268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang L, Lu Y, Jiang H, et al. Additional use of trimetazidine in patients with chronic heart failure: a meta-analysis. J Am Coll Cardiol 2012;59:913–22. [DOI] [PubMed] [Google Scholar]
- [11].Guendouz S, Buicuic O, Kirsch M, et al. Restrictive cardiomyopathy associated with left ventricle and left atria endocardial calcifications following chemotherapy. J Am Coll Cardiol 2011;57:1633. [DOI] [PubMed] [Google Scholar]
- [12].Martin M, Diaz-Rubio E, Furio V, et al. Lethal cardiac toxicity after cisplatin and 5-fluorouracil chemotherapy. Report of a case with necropsy study. Am J Clin Oncol 1989;12:229–34. [DOI] [PubMed] [Google Scholar]
- [13].Pratt CB, Crom DB, Wallenberg J, et al. Fatal congestive heart failure following mitoxantrone treatment in two children previously treated with doxorubicin and cisplatin. Cancer Treat Rep 1983;67:85–8. [PubMed] [Google Scholar]
- [14].Cheriparambil KM, Vasireddy H, Kuruvilla A, et al. Acute reversible cardiomyopathy and thromboembolism after cisplatin and 5-fluorouracil chemotherapy–a case report. Angiology 2000;51:873–8. [DOI] [PubMed] [Google Scholar]


