Abstract
Carotid plaque is an aggregate marker of exposure to vascular risk factors, which are linked to structural brain changes. We investigated prestroke global and regional changes in brain volume in a carotid plaque population of cognitively healthy individuals and the association between carotid plaque characteristics and these changes.
A total of 76 participants were divided into healthy control (HC, n = 28), vulnerable plaque (n = 27) and stable plaque groups (n = 21). All subjects underwent carotid ultrasound and brain magnetic resonance imaging (MRI). Voxel-based morphometry (VBM) was used to examine differences in regional gray matter volumes (rGMVs) among the different groups.
The plaque group had a significantly lower mean total cerebral brain volume (TCBV) than the HC group (P = .03). Carotid intima-media thickness (CIMT) was negatively correlated with TCBV (r = -0.311, P = .006) and rGMV in the right thalamus (r = -0.589, P = .001). The rGMVs of the right middle occipital gyrus and bilateral lingual gyrus were significantly different between the unstable and stable groups. The gray-scale median (GSM) of the plaque and the total plaque risk score (TPRS) were correlated with the volume of the right middle occipital gyrus (r=-0.478, P = .001; r = 0.541, P = .001) and bilateral lingual gyrus (r = -0.419, P = .003; r = 0.288, P = .04).
Carotid plaque is related to the volume of the brain parenchyma and right thalamus. The rGMVs of the right middle occipital gyrus and bilateral lingual gyrus differed between the vulnerable plaque and stable plaque groups, and the characteristics of carotid plaques may serve as indexes that reflect these changes
Keywords: brain volume, carotid plaques, magnetic resonance imaging, stroke, voxel-based morphometry
1. Introduction
Stroke is a major cause of death and disability worldwide,[1] with ischemic stroke representing approximately 70% to 80% of cases.[2,3] One of the main mechanisms of ischemic cerebrovascular disease is atherosclerotic plaque rupture, particularly of high-risk carotid atherosclerotic plaques (vulnerable or unstable plaque).[4] The severity of carotid luminal stenosis has been used in clinical settings as an imaging marker for the risk level of atherosclerotic plaques and stroke,[5] and some studies have shown that subjects with carotid stenosis had significant structural brain changes[6] and poor cognitive performance.[7,8] Whether brain structural changes are present in populations with carotid atherosclerotic plaques without carotid stenosis is unknown.
Vascular dementia occurs in approximately 30% of stroke survivors, and the incidence of vascular cognitive impairment is high after stroke.[9–11] Many studies have shown that poor cardiovascular health is associated with brain atrophy and an increased risk of developing Alzheimer dementia. Freedman et al[12] found that calcified atherosclerotic plaques might cause smaller gray matter volumes and poor cognitive performance in African Americans with diabetes. One imaging study suggested that blood homocysteine (Hcy) levels were related to cortical gray matter thickness, cortical gray matter volume and cortical gray matter surface area.[13] Moreover, chronic inflammation plays an important role in the development of atherosclerosis, and high-sensitivity C-reactive protein (hsCRP) is one of the inflammatory markers. Research has shown that there is a correlation between regional gray matter volume (rGMV) and hsCRP.[14] Therefore, the brain structures of patients with a high risk of ischemic stroke have most likely changed before the occurrence of ischemic stroke. Previous studies have shown that carotid atherosclerosis may be associated with cognitive impairment in stroke-free individuals,[15,16] but the mechanism has not yet been fully elucidated. Therefore, we speculated that carotid plaque, as a cardiovascular risk factor, may induce changes in brain structure, which may be a potential mechanism for cognitive decline.
The term plaque stability has been used to describe the propensity of a plaque to induce local thrombosis and/or embolism distally and cause stroke. Carotid plaques are divided into vulnerable plaques and stable plaques. The gray-scale median (GSM) is a statistical analysis of the gray values of all pixels in a region of interest selected on ultrasound gray-scale image by using a computer-aided program. Previous studies have demonstrated that the GSM may be a good indicator of carotid plaque behavior, and carotid plaques with low and medium GSM values have been linked to an increased risk of cerebrovascular events.[17,18] In this study, plaques were classified according to the GSM value of plaque. The components of vulnerable plaques and those of stable plaques are different, and their effects on brain structure may also be different. In fact, few studies have investigated the different effects of various types of plaques on brain changes.
The purpose of this study was to evaluate the changes in brain volume in people of normal intelligence with carotid plaques who are within a high-risk population for stroke and to explore whether there are different changes in brain structure between vulnerable plaque groups and stable plaque groups.
2. Materials and methods
2.1. Study population
The subjects were selected from among the participants of the Stroke Screening and Prevention Project, a comprehensive project assessing stroke risk in a population aged over 40 years old. This assessment project was conducted by the Xiangya Hospital base, and all subjects were from the Wujialing community. Subjects were interviewed by researchers according to specific questionnaires to gather information regarding the age, sex, race, education level, alcohol consumption, smoking, past medical history and medications of the participants. The project enrolled 2015 subjects, all from the Wujialing community, 1154 of whom underwent carotid ultrasound. After inclusion and exclusion criteria screening, a total of 107 subjects (66 women and 41 men; age 60.55 ± 6.62 years old, mean ± SD) willing to undergo brain magnetic resonance imaging (MRI) were ultimately recruited for our study between December 2015 and June 2017. The inclusion criteria were as follows:
-
(1)
subjects aged from 40 to 75 years old;
-
(2)
Mini-Mental State Examination (MMSE) scores ≥ 27;
-
(3)
a single type of bilateral carotid atherosclerotic plaques, hypoechoic, isoechoic or hyperechoic;
-
(4)
carotid stenosis <50%;
-
(5)
right-handedness;
-
(6)
no previous diagnosis of transient ischemic attack or stroke;
-
(7)
no organic or mental illness and
-
(8)
no drug dependence.
Persons with MRI contraindications, plaque in the subclavian artery, a history of alcohol or drug abuse, previous carotid endarterectomy, intracranial artery stenosis >50% (magnetic resonance angiography), history of or current atrial fibrillation (AF), severe liver and kidney disease, inflammatory diseases and pregnancy were excluded. All procedures were approved by the Ethics committee of Xiangya School of Medicine, Central South University.
2.2. Blood analysis
Venous blood was collected from the antecubital vein in the morning before the participants ate breakfast, following an overnight fast of 12 to 14 h. All subjects underwent routine laboratory tests, including assays for blood sugar, serum total cholesterol, serum triglycerides, serum high-density lipoprotein cholesterol, serum low-density lipoprotein cholesterol and Hcy.
2.3. Carotid ultrasound acquisition
Examinations of the carotid plaques were carried out with an ultrasound scanner (5-MHz Sector Array transducer; iU22, Philips Ultrasound, Bothell, WA) in both the right and left common carotid arteries (CCAs) and bifurcations by the same experienced radiologist. The distance from the media-adventitia interface to the intima-lumen interface was defined as the intima-media thickness (IMT).[19] A plaque was defined as a focal structure with an IMT >1.5 mm. The carotid intima-media thickness (CIMT) was defined as the average IMT and was calculated from the bilateral IMTs of the CCAs, carotid bulb and bifurcations in a region free of plaque.
2.4. GSM analysis
The digital images of the patients from the ultrasound device were exported by using a mobile hard disk. GSM analysis was performed by using the method previously described by El-Atrozy et al[20]. The images were assessed as demonstrating a 3-fold increase in the initial size. First, the entire image was normalized by a process involving linear scaling based on blood regions and the adventitia region with computer software (Adobe Photoshop CS6). The GSM of the blood was set to 0, the GSM of the adventitia was set to 195, and the gray-scale range of the image was 0 to 255 (black = 0, white = 255). After normalization, each whole plaque was completely outlined within its longitudinal section close to the edge, and the local area of the sternocleidomastoid muscle was selected from the same image to obtain the GSM value. The GSM of the sternocleidomastoid was the reference point, and the plaque was classified as one of 3 types, namely, hypoechoic, isoechoic or hyperechoic. The GSM values of the hyperechoic and hypoechoic plaques were lower and higher than that of the reference point, respectively, and the GSM values of isoechoic plaques were close to the GSM of the sternocleidomastoid. The GSM measurements of each plaque were performed by 3 independent researchers, and the reproducibility of these measurements was also assessed. The GSM values of the plaque was the mean.
2.5. Total plaque risk score
The plaque score criteria were defined in 4 parts. The first part was the severity of stenosis, graded 0 if lumen stenosis was <40% cross-sectionally and 1 if >40%. The second part was the type of echogenicity and was graded from 0 to 2: 0 for no plaque, 1 for hyperechoic plaque, and 2 for hypoechoic and isoechoic plaques. The third part was the texture, graded 0 if the echo pattern was uniform and 1 if the echo pattern was complex. The fourth part consisted of the characteristics of the surface according to the plaque contour, graded 0 if the surface was smooth or 1 if the surface was irregular.[21,22] All plaques were evaluated by 3 independent researchers and were defined according to strict criteria. The Total Plaque Risk Score (TPRS) served as a summary score for these 4 parameters and is considered to be a feasible way to reflect the characteristics of a single plaque. The TPRS ranges from 1 to 5, and subjects with no plaques had a TPRS equal to 0. According to Prati research,[23] the highest risk of plaque represents the plaque risk of a subject. We computed the TPRS of all plaques for each subject and identified the plaque with the highest score in each subject.
2.6. Image acquisition and processing
All MRI data were acquired on a 3T General Electric MRI scanner with a 12-channel head coil. The MRI scanning included T1-weighted imaging (T1WI), T2-weighted imaging (T2WI) and fluid attenuated inversion recovery (FLAIR) sequences. Each scan involved a three-dimensional sagittal brain volume imaging (BRAVO) sequence with the following parameters: repetition time (TR) = 7.792ms, echo time (TE) = 2.984ms, flip angle = 7°, 188 slices, slice thickness = 1 mm, slice spacing = 1 mm, acquisition matrix = 256 × 256, voxel size = 1 × 1 × 1 mm3. Data preprocessing was performed using the VBM8 toolbox of SPM8 (Statistical Parametric Mapping, Wellcome Trust Center for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) in MATLAB (Mathworks, Natick, MA). First, the T1 images were normalized to the Montreal Neurological Institute (MNI) space, and the reorientation of the T1 images was manually set for better registration. Second, the T1 images were segmented into white matter, gray matter (GM) and cerebrospinal fluid through the VBM8 toolbox. Then, the GM maps were inspected to ensure the success of the segmentation algorithm. Finally, the GM and white matter images were smoothed with an 8-mm full-width at half-maximum Gaussian kernel to increase the signal-to-noise ratio. To correct the differences in head size, the total cerebral brain volume (TCBV) value was used as an indicator to explore whether atherosclerosis has an effect on brain structure. TCBV is the ratio of the total brain parenchymal volume to the total cranial volume. Without cerebrospinal fluid, the total brain parenchymal volume is the sum of global GM and global white matter obtained through the VBM8 toolbox.
2.7. Statistical analysis
Continuous variables with normal distributions are expressed as the mean (SD), and categorical variables are expressed as percentages. A one-way analysis of variance (ANOVA) was performed to investigate the differences in the demographic characteristics, plaque characteristics and TCBV values among the three groups, and separate 2-tailed, 2-sample t tests were used to assess the differences between each pair of groups. Chi-square tests were used to analyze categorical variables. Pearson correlation analysis and Spearman correlation analysis were used to explore the relationships between the characteristics of the carotid plaques and the TCBV values. These analyses were performed using the SPSS 22.0 software. P values <.05 were considered statistically significant. The correlation between the characteristics of the carotid plaques and rGMV was investigated with multiple linear regression analysis after adjusting for age, gender, body mass index (BMI), and intracranial volume by using SPM8. The primary significance threshold was set at a P value of .001, and the FDR-corrected and FWE-corrected thresholds were set at a P value <.05.
3. Results
3.1. Characteristics of the study population
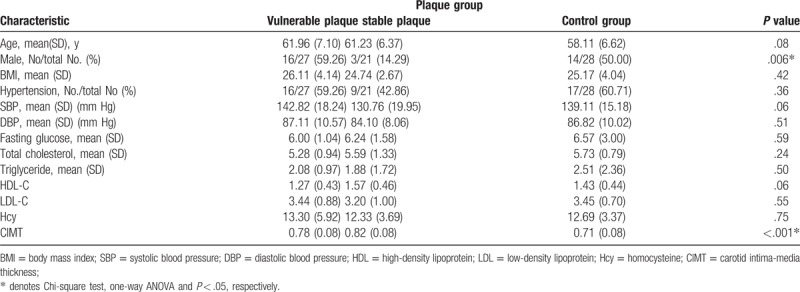
One hundred seven subjects were enrolled in the study. Sixteen people were excluded because of plaques in the subclavian artery, and 15 people were excluded because of the presence of both stable and unstable plaques. Thus, 76 subjects were included in the analysis. Figure 1 describes criteria for selection of subjects. The demographic and clinical characteristics of the study population are summarized in Table 1. There were 21 subjects in the stable (hyperechoic) plaque subgroup, 27 subjects in the vulnerable (hypoechoic and isoechoic) plaque subgroup and 28 subjects in the healthy control (HC) subgroup. A one-way ANOVA showed that there were significant differences among the three groups in terms of the CIMT (P<.001, F = 11.35, df = 2). The CIMT values of the vulnerable plaque (P = .002) subgroup and the stable plaque subgroup (P<.001) were significantly lower than that of the HC subgroup, but the difference in the CIMT values between the 2 plaque subgroups was not significant (P = .11). No significant differences were found in age, BMI or traditional risk factors of atherosclerosis, such as systolic blood pressure, LDL-C, HDL-C, triglycerides, fasting glucose and Hcy, among the 3 groups. The proportion of females in the stable plaque subgroup was significantly higher than that in the other 2 subgroups (P = .006).
Figure 1.
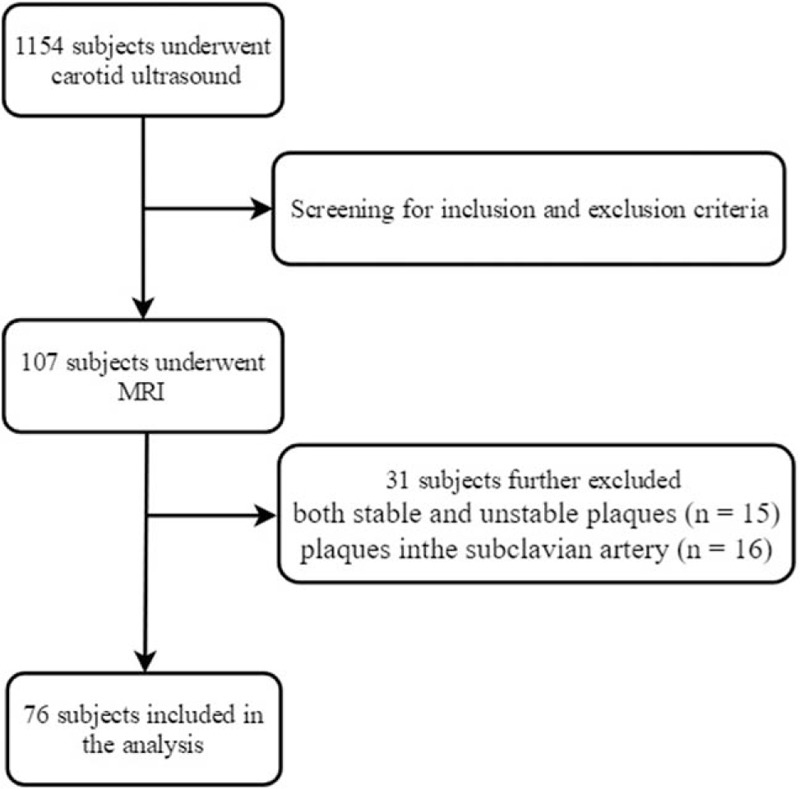
The flow diagram illustrating the selection of patients for the study.
Table 1.
Demographic and clinical characteristics of the participants.
3.2. Characteristics of carotid plaques
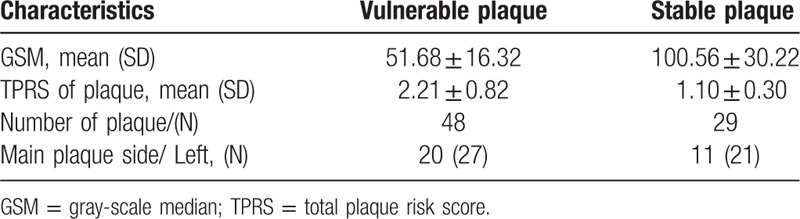
Some of the subjects in the plaque group had more than 1 plaque. Specifically, 48 patients with 77 plaques were included in the study, but only the main plaque was included in the analyses. Table 2 summarizes the characteristics of the plaques in the 2 plaque subgroups. The difference between the mean whole GSM value of the unstable group (51.68 ± 16.32) and that of the stable subgroup (100.56 ± 30.22) was significant (P<.001). Similarly, the TPRS of the unstable subgroup (2.21 ± 0.82) presented significantly higher mean values than the stable subgroup (1.10 ± 0.3) (P<.001).
Table 2.
Differences in the carotid ultrasound characteristics between the vulnerable plaque patients and the stable plaque patients.
3.3. Correlations between the characteristics of carotid plaques and brain volume
It was found that persons with plaque (0.816 ± 0.19), including those in the unstable subgroup and the stable subgroup, had significantly lower mean TCBV values (P = .03) than the HCs (0.826 ± 0.21) after controlling for age, sex, BMI and vascular risk factors, such as blood pressure, diabetes, total cholesterol, HDL, triglycerides, LDL and Hcy, and there was no significant difference in the mean TCBV values between the unstable subgroup and the stable subgroup (0.816 ± 0.13 versus 0.818 ± 0.18, respectively; P = .81). The associations between the characteristics of the carotid plaques and TCBV are presented in Figure 1. There were significant negative correlations between the CIMT and TCBV values (r = -0.311, P = .006, Pearson's correlation). Furthermore, multiple linear regression analysis showed that CIMT was significantly negatively correlated with the rGMV of the right thalamus (primary threshold = 0.000; P < 0.05, FWE-corrected) (Figs. 2 and 3). However, no correlations were observed among the GSM of each plaque, TPRS, number of plaques or TCBV.
Figure 2.
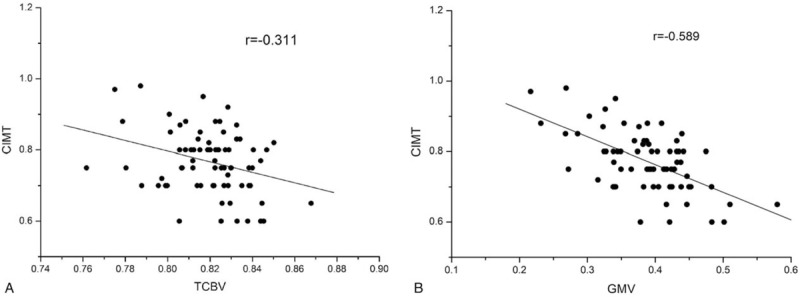
Scatter plots of the relationship between the characteristics of carotid plaques and the total cerebral brain volume (TCBV). (a) Scatter plot showing the negative relationship between carotid intima-media thickness (CIMT) and the TCBV. (b) Scatter plot showing the negative relationship between CIMT and GMV in the right thalamus.
Figure 3.
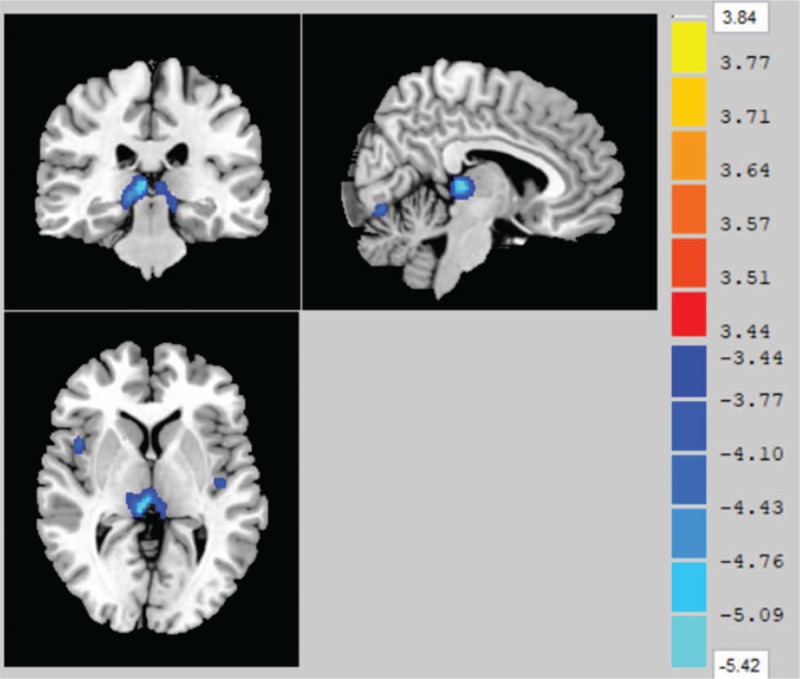
Association between carotid intima-media thickness (CIMT) and the gray matter volume (GMV). The figure shows that there was a negative correlation between the CIMT and the GMV of the right thalamus after controlling for age, sex and intracranial volume. (P < .05, FWE-corrected, with a 30-voxel clustering criterion).
3.4. Differences in rGMV among three groups
After entering age, sex, BMI and intracranial volume as covariates, the differences in rGMV and regional whiter matter volume (rWMV) were not statistically significant between the plaque group and the HC group. However, there were significant differences in the GMV of the right middle occipital gyrus (primary threshold = 0.000; P < .05, FWE-corrected) and the bilateral lingual gyrus (primary threshold = 0.000; P < .05, FDR-corrected) between the unstable subgroup and the stable subgroup. There was a significant positive correlation between the GSM of the plaque and the GMV in a cluster that included the right middle occipital gyrus (r = -0.478, P = .001) and the bilateral lingual gyrus (r = -0.419, P = .003). The TPRS of the plaque was negatively correlated with the GMV in the same areas (r = 0.541, P<.001; r = 0.288, P = .04, respectively; Spearman's correlation), and this finding was statistically significant (Figs. 4and 5, and Table 3). The difference in the rWMV values between the 2 plaque subgroups was also not significant.
Figure 4.
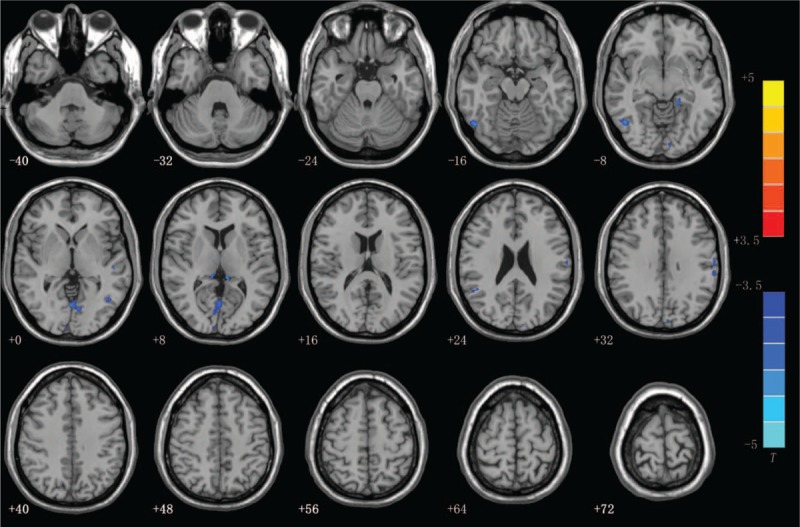
Axial slices overlaid with regions showing significant differences in gray matter volume (GMV) between the vulnerable plaque patients and the stable plaque patients after controlling for age, sex and intracranial volume. Blue areas represent that the GMVs of stable plaque patients in the right middle occipital gyrus and the bilateral lingual gyrus were less than those of the vulnerable plaque patients. (P < .05, FWE-corrected and FDR-corrected, with a 30-voxel clustering criterion).
Table 3.
VBM results of the differences in gray matter between the vulnerable plaque group and the stable plaque group.

Figure 5.
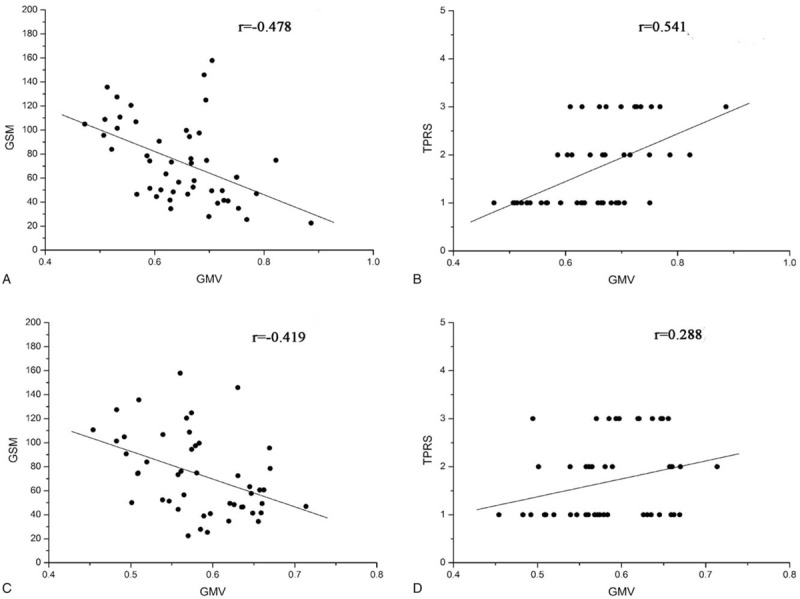
Scatter plots of the relationship and between the characteristics of the carotid plaques and regional gray matter volume (rGMV). (a) shows a scatter plot of the negative relationship between the gray-scale median (GSM) and the GMV of the right middle occipital gyrus. (b) shows a scatter plot of the positive relationship between the total plaque risk score (TPRS) and the GMV of the right middle occipital gyrus. (c) shows a scatter plot of the negative relationship between the GSM and the GMV of the bilateral lingual gyrus. (d) shows a scatter plot of the positive relationship between the TPRS and the GMV of the bilateral lingual gyrus.
4. Discussion
Our study demonstrated that the plaque group (including the stable plaque and vulnerable plaque subgroups) had significantly lower TCBV values than the HC group. The CIMT was negatively correlated with the TCBV. The results were consistent with those of previous studies, specifically those that showed that carotid atherosclerosis is associated with structural brain changes.[24,25] Our data support the findings of the Framingham Study, which observed associations among carotid atherosclerosis and decreases in global brain volumes and increases in WMHV; however, we also explored the relationship between the volume of all parts of the whole brain and the CIMT, not just the volume of the hippocampus.[26] The result showed a correlation between the CIMT and GMV of the right thalamus. As we know, the thalamus is a nerve center that regulates body temperature, blood sugar, fat metabolism,[27] feeding habits, sleep and the autonomic nervous system. Evidence suggested that the hypothalamus is also involved in the regulation of atherosclerosis. Damage to the hypothalamus in rats led to the appearance of early AS, including endothelial cell degeneration, the formation of foam cells, etc.[28] Another study showed that the inhibitory central neurotransmitter 5- serotonin (5-HT), which is secreted by the hypothalamus, is negatively correlated with carotid IMT and that 5-HT dysfunction increase the risk of stroke.[29] The thalamus may play a role in regulating the formation of carotid atherosclerotic plaques. Nevertheless, our study cannot determine the causal relationship between carotid atherosclerosis and structural changes in the thalamus.
The degree of carotid stenosis has previously been linked to cerebral damage.[25,30,31] In addition, studies have suggested that the CIMT may be a marker of intracranial atherosclerosis[32] and overall atherosclerotic plaque burden.[33] Given that no participants in the plaque group had hemodynamically significant stenosis ≥ 50%, the hemodynamic reduction in cerebral flow may not be the mechanism of brain injury. The mechanism may instead involve microcirculation, inflammation,[34,35] systemic atherosclerosis, etc. Our results suggested that the CIMT may be a preliminary indicator of brain damage in atherosclerotic patients. Our results suggested that the CIMT may be a preliminary indicator of brain damage in atherosclerotic patients.
Interestingly, we further applied VBM to analyze the difference in rGMV between the plaque group and the HC group, and there were no significant differences. In other words, the difference in the global changes in brain volume could not be demonstrated for the regional volumes. Similarly, the results showed that the difference in the rGMV between the 2 plaque subgroups was significant, but there was no significant difference in the TCBV values between the vulnerable plaque and stable plaque subgroups. There was an inconsistency between the global changes in brain volume and the regional changes in brain volume. From a statistical point of view, the combination of each slight change without statistical significance may become a meaningful difference, and a small local structural change may not be enough to demonstrate changes in the TCBV. However, we can only speculate. The reason for this phenomenon has not yet been fully elucidated. We cannot focus on only one aspect while observing the changes in brain structure. Rather, we need to pay attention to both global and regional changes. Although the CIMT was significantly different between the plaque and HC groups, the volume of the thalamus did not differ in the 2 groups, which may implicate other factors in the structural changes of the thalamus in addition to the CIMT.
Vascular dementia is defined as a kind of cognitive impairment with notable vascular events or diseases and/or risk factors. Sang Won Seo et al found that the cortexes of patients with subcortical vascular dementia became thinner in frontal, temporal, and occipital regions.[36] Our results showed that the stable plaque subgroup had smaller GMVs in the right middle occipital gyrus than the vulnerable plaque subgroup after entering age, sex, BMI and intracranial volume as covariates. Although there were significant differences in the sex ratios between the 2 groups, the possible effects of gender on the results were corrected for. The GSM and TPRS values of the plaques were associated with the rGMV in the right occipital middle gyrus. Previous experimental and clinical studies have suggested the axonal damage in the brain would be harmful to both proximal and distal neurons.[37,38] For these reasons, ischemic injury to fibers that begins and terminates in the occipital middle gyrus may cause cortical atrophy in the middle occipital gyrus and the lingual gyrus. Future studies may use diffusion tensor imaging (DTI) to prove this hypothesis. As the GSM and TPRS values of the plaques were associated with the rGMV in the right occipital middle gyrus, these 2 indexes may reflect the degree of structural brain changes in occipital regions. In addition, we also found that the GMV decreased in the bilateral lingual gyrus and the visual cortexes. One visuoperceptual task study suggested that visuospatial and perceptual skills were impaired in patients with vascular dementia.[39] Although the risk of stroke was lower in the stable plaque subgroup than in the vulnerable plaque subgroup, the visual damage may have been more severe, and the risk for vascular dementia is likely to be higher in the stable plaque group than in the vulnerable plaque subgroup. Longitudinal evaluations of these individuals with plaques will be necessary to assess whether brain GM atrophy in the middle occipital gyrus and the lingual gyrus predicts a higher risk for future dementia. On the other hand, the potential pathophysiologic mechanism underlying the regulation of plaque vulnerability remains speculative, and regional GM atrophy may be a new research direction.
There were several potential limitations to our study. First, the study was a cross-sectional observational study with a small sample size. Cross-sectional, observational studies are prone to intrinsic bias and will limit the possibilities of identifying causal relationships between structural brain changes and plaque. The sample was undersized, and some effects may not have been demonstrated because of insufficient power in the study, but this was an exploratory study for further research. In addition, this study focused on the structural changes in the brain, and there was no analysis conducted of the changes in brain function or in specific white matter fibers. Lastly, we cannot exclude the possibility that an unmeasured factor, such as genes, behavior or environment, may have had an effect on brain structure. A longitudinal cohort study with a larger sample size is necessary to confirm our findings.
5. Conclusions
The CIMT is associated with whole-brain structural changes and GMV in the right thalamus. Subjects in the stable plaque subgroup had low GMVs in occipital regions and may have had a high risk for visuospatial skill impairment and/or even vascular dementia. Plaque characteristics may be used as indexes to reflect the extent of brain damage. The causal and temporal relationships between atherosclerosis and brain changes is still unknown, and these relationships are a topic of continued research in our laboratory. In addition, our exploratory study provides a possible new research direction for exploring the mechanism that affects plaque vulnerability, namely, structural brain changes. We hope to determine whether targeted intervention for high-risk individuals can modulate cognitive decline in the future.
Author contributions
Investigation: Jia Tuo, Shuai Yang, Jie Feng, Yanbin Wen, Wei He.
Methodology: Jia Tuo, Wenping Gu, Xinglin Tan, Tao Tang, Jie Feng, Yanbin Wen, Wei He.
Software: Jia Tuo, Weihua Liao, Shuai Yang, Jie Feng, Yanbin Wen, Wei He.
Validation: Jia Tuo.
Writing – original draft: Jia Tuo.
Writing – review & editing: Jia Tuo, Yunhai Liu, Wenping Gu, Qing Huang.
Data curation: Yunhai Liu, Weihua Liao.
Formal analysis: Yunhai Liu, Xinglin Tan, Tao Tang, Qing Huang.
Project administration: Yunhai Liu, Weihua Liao, Tao Tang.
Supervision: Yunhai Liu, Weihua Liao, Wenping Gu, Xinglin Tan, Tao Tang, Hua Chen, Qing Huang.
Resources: Weihua Liao, Shuai Yang, Hua Chen.
Visualization: Shuai Yang, Jie Feng, Yanbin Wen, Wei He.
Conceptualization: Qing Huang.
Funding acquisition: Qing Huang.
Footnotes
Abbreviations: AF = atrial fibrillation, ANOVA = analysis of variance, BMI = body mass index, BRAVO = three- dimensional sagittal brain volume imaging, CIMT = carotid intima-media thickness, CCAs = common carotid arteries, DTI= diffusion tensor imaging, FLAIR = fluid attenuated inversion recovery, GM = gray matter, GSM = the gray-scale median, HC = healthy control, Hcy = homocysteine, hsCRP = high-sensitivity C-reactive protein, 5-HT = 5-serotonin, IMT = intima-media thickness, MRI = Magnetic Resonance Imaging, MMSE = Mini-Mental State Examination, MNI = Montreal Neurological Institute, rGMVs = regional gray matter volumes, rWMV = regional whiter matter volume, TCBV = total cerebral brain volume, TE = echo time, TPRS = Total Plaque Risk Score, TR = repetition time, T1WI = T1-weighted imaging, T2WI = T2-weighted imaging, VBM = Voxel-based morphometry, WMHV = white matter hyperintensity volume.
Funding: This study was funded by the National Natural Science Foundation of China grant funded by the China Government (grant number: 8140051197) and the Natural Science Foundation of Hunan Province, China (grant number: 2018JJ2643).
The authors have no conflicts of interest to disclose.
References
- [1].Johnston SC, Mendis S, Mathers CD. Global variation in stroke burden and mortality: estimates from monitoring, surveillance, and modelling. Lancet Neurol 2009;8:345–54. [DOI] [PubMed] [Google Scholar]
- [2].Tsai CF, Thomas B, Sudlow CL. Epidemiology of stroke and its subtypes in Chinese vs white populations: a systematic review. Neurology 2013;81:264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sun Xingang, Wang Yanli, Zhang Ning, et al. Incidence and trends of stroke and its subtypes in Changsha, China from 2005 to 2011. J Clin Neurosci 2014;21:436–40. [DOI] [PubMed] [Google Scholar]
- [4].Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685–95. [DOI] [PubMed] [Google Scholar]
- [5].Halliday A, Harrison M, Hayter E, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet (London, England) 2010;376:1074–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Avelar WM, D’Abreu A, Lima FO, et al. Brain structures anormalites detected by voxel based morphometry in individuals with asymptomatic carotid stenosis. Stroke 2013;44:1197–203. [Google Scholar]
- [7].Lal BK, Dux MC, Sikdar S, et al. Asymptomatic carotid stenosis is associated with cognitive impairment. J Vasc Surg 2017;66:1083In press. [DOI] [PubMed] [Google Scholar]
- [8].Sztriha LK, Nemeth D, Sefcsik T, et al. Carotid stenosis and the cognitive function. J Neurol Sci 2009;283:36–40. [DOI] [PubMed] [Google Scholar]
- [9].Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol 2009;8:1006–18. [DOI] [PubMed] [Google Scholar]
- [10].Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011;42:2672–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tu Qiuyun, Ding Binrong, Yang Xia, et al. The current situation on vascular cognitive impairment after ischemic stroke in Changsha. Arch Gerontol Geriatr 2014;58:236–47. [DOI] [PubMed] [Google Scholar]
- [12].Freedman BI, Divers J, Whitlow CT, et al. Subclinical atherosclerosis is inversely associated with gray matter volume in african americans with type 2 diabetes. Diabetes Care 2015;38:2158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Madsen SK, Rajagopalan P, Joshi SH, et al. Higher homocysteine associated with thinner cortical gray matter in 803 participants from the Alzheimer's Disease Neuroimaging Initiative. Neurobiol Aging 2015;36:S203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Taki Y, Thyreau B, Kinomura S, et al. Correlation between high-sensitivity C-reactive protein and brain gray matter volume in healthy elderly subjects. Hum Brain Mapp 2013;34:2418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhong W, Cruickshanks KJ, Schubert CR, et al. Carotid atherosclerosis and 10-year changes in cognitive function. Curr Med 2012;224:506–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Suemoto CK, Santos IS, Bittencourt MS, et al. Subclinical carotid artery atherosclerosis and performance on cognitive tests in middle-aged adults: Baseline results from the ELSA-Brasil. Atherosclerosis 2015;243:510–5. [DOI] [PubMed] [Google Scholar]
- [17].Nakamura T, Tsutsumi Y, Shimizu Y, et al. Ulcerated carotid plaques with ultrasonic echolucency are causatively associated with thromboembolic cerebrovascular events. J Stroke Cerebrovasc Dis 2013;22:93–9. [DOI] [PubMed] [Google Scholar]
- [18].Sztajzel R, Momjian S, Momjian-Mayor I, et al. Stratified gray-scale median analysis and color mapping of the carotid plaque: correlation with endarterectomy specimen histology of 28 patients. Stroke 2005;36:741–5. [DOI] [PubMed] [Google Scholar]
- [19].Pignoli P, Tremoli E, Poli A, et al. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation 1986;74:1399–406. [DOI] [PubMed] [Google Scholar]
- [20].Elatrozy T, Nicolaides A, Tegos T, et al. The effect of B-mode ultrasonic image standardisation on the echodensity of symptomatic and asymptomatic carotid bifurcation plaques. Int Angiol 1998;17:179–86. [PubMed] [Google Scholar]
- [21].Gray-Weale AC, Graham JC, Burnett JR, et al. Carotid artery atheroma: comparison of preoperative B-mode ultrasound appearance with carotid endarterectomy specimen pathology. J Cardiovasc Surg 1988;29:676–81. [PubMed] [Google Scholar]
- [22].Nicolaides AN. Asymptomatic carotid stenosis and risk of stroke. Identification of a high risk group (ACSRS). A natural history study. Int Angiol 1995;14:21–3. [PubMed] [Google Scholar]
- [23].Prati P, Tosetto A, Casaroli M, et al. Carotid plaque morphology improves stroke risk prediction: usefulness of a new ultrasonographic score. Cerebrovasc Dis (Basel, Switzerland) 2011;31:300–4. [DOI] [PubMed] [Google Scholar]
- [24].Aparicio HJ, Petrea RE, Massaro JM, et al. Association of descending thoracic aortic plaque with brain atrophy and white matter hyperintensities: the Framingham heart study. Atherosclerosis 2017;265:305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Muller M, Van dGY, Algra A, et al. Carotid atherosclerosis and progression of brain atrophy: the SMART-MR study. Ann Neurol 2011;70:237–44. [DOI] [PubMed] [Google Scholar]
- [26].Romero JR, Beiser A, Seshadri S, et al. Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment: the framingham study. Stroke 2009;40:1590–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Su Tao, Wu Jing, Liu Weifang, et al. Expression change of SH2B1, SOCS3, PTP1B and NPY in mice hypothalamus and its relation with obesity. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2014;39:43–8. [DOI] [PubMed] [Google Scholar]
- [28].Kai Yun WU, Gao SY, Xiong JP, et al. Influence of transplanted embryonic arcuate nucleus in destroyed nucleus on atherosclerosis. Chin J Arteriosc Lerosis 2000. [Google Scholar]
- [29].Muldoon MF, Mackey RH, Sutton-Tyrrell K, et al. Lower central serotonergic responsivity is associated with preclinical carotid artery atherosclerosis. Stroke 2007;38:2228–33. [DOI] [PubMed] [Google Scholar]
- [30].Baradaran H, Gialdini G, Mtui E, et al. Silent brain infarction in patients with asymptomatic carotid artery atherosclerotic disease. J Vasc Surg 2016;64:534–5. [DOI] [PubMed] [Google Scholar]
- [31].Ammirati E, Moroni F, Magnoni M, et al. Relation between characteristics of carotid atherosclerotic plaques and brain white matter hyperintensities in asymptomatic patients. Sci Rep 2017;7:10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cohen-Manheim I, Pinchas-Mizrachi R, Doniger GM, et al. Measures of carotid atherosclerosis and cognitive function in midlife: The Jerusalem LRC longitudinal study. Intelligence 2016;57:73–80. [Google Scholar]
- [33].Sibal L, Agarwal SC, Home PD. Carotid intima-media thickness as a surrogate marker of cardiovascular disease in diabetes. Diabetes Metab Syndr Obes 2011;4:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liu Tiantian, Liu Hengfang, Feng Li, et al. Kinin B1 receptor as a novel, prognostic progression biomarker for carotid atherosclerotic plaques. Mol Med Rep 2017;16:8930–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liu Huamin, Yao Yan, Wang Youxin, et al. Association between high-sensitivity C-reactive protein, lipoprotein-associated phospholipase A2 and carotid atherosclerosis: A cross-sectional study. J Cell Mol Med 2018;22:5145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sang WS, Yoon AU. Cortical thinning in vascular mild cognitive impairment and vascular dementia of subcortical type. J Neuroimag 2010;20:37–45. [DOI] [PubMed] [Google Scholar]
- [37].Ginsberg SD, Martin LJ. Axonal transection in adult rat brain induces transsynaptic apoptosis and persistent atrophy of target neurons. J Neurotrauma 2002;19:99–109. [DOI] [PubMed] [Google Scholar]
- [38].Johnson H, Cowey A. Transneuronal retrograde degeneration of retinal ganglion cells following restricted lesions of striate cortex in the monkey. Exp Brain Res 2000;132:269–75. [DOI] [PubMed] [Google Scholar]
- [39].Graham NL, Emery T, Hodges JR. Distinctive cognitive profiles in Alzheimer's disease and subcortical vascular dementia. J Neurol Neurosurg Psychiatry 2004;75:61–71. [PMC free article] [PubMed] [Google Scholar]


