Abstract
Tetraparesis is usually due to cerebral palsy (CP), inborn errors of metabolism, neurogenetic disorders and spinal cord lesions. However, literature data reported that about 10% of children with tetraparesis show a negative/non-specific neuroradiological findings without a specific etiological cause. Aicardi Goutières Syndrome (AGS) is a genetic encephalopathy that may cause tetraparesis. Interferon signature is a reliable biomarker for AGS and could be performed in sine-causa tetraparesis. The aim of the study was to examine the type I interferon signature and AGS related-genes in children with sine causa tetraparesis, to look for misdiagnosed AGS. A secondary aim was to determine which aspects of the patient history, clinical picture and brain imaging best characterize tetraparesis due to an interferonopathy.
Seven out of 78 patients affected by tetraparesis, characterized by unremarkable pre-peri-postnatal history and normal/non-specific brain magnetic resonance imaging (MRI) were selected and underwent anamnestic data collection, clinical examination, brain imaging review, peripheral blood interferon signature and AGS-related genes analysis.
At our evaluation time (mean age of 11.9 years), all the 7 patients showed spastic-dystonic tetraparesis. At clinical onset brain MRI was normal in 4 and with non-specific abnormalities in 3; at follow-up 3 patients presented with new white-matter lesions, associated with brain calcification in 1 case. Interferon signature was elevated in one subject who presented also a mutation of the IFIH1 gene.
AGS should be considered in sine-causa tetraparesis. Core features of interferonopathy-related tetraparesis are: onset during first year of life, psychomotor regression with tetraparesis evolution, brain white-matter lesions with late calcifications. A positive interferon signature may be a helpful marker to select patients with spastic tetraparesis who should undergo genetic analysis for AGS.
Keywords: aicardi–goutières syndrome, interferon signature, interferonopathy, tetraparesis
1. Introduction
Spastic tetraparesis is defined by spasticity of all four limbs. Spasticity was firstly defined by Lance[1] as "a velocity dependent increase of muscle tone with exaggerated tendon jerks resulting in hyperexcitability of the stretch reflex in association with other features of upper motor neuron syndrome”. Later, Young described positive and negative signs and symptoms of upper motor neuron damage. Positive ones consisted of increased muscle tone and tendon jerks, clonus, autonomic hyperreflexia, dystonia, and contractures. The negative ones were paresis, loss of dexterity and increased fatigability. Although Cerebral Palsy (CP) is considered a main cause of spastic tetraparesis in children,[2] recent studies suggested other possible etiologies, such as inborn errors of metabolism,[3] neurogenetic disorders,[4] and spinal cord lesions.[5] These disorders are usually characterized by pre-peri-postnatal history suggestive of CP and/or abnormal brain Magnetic Resonance Imaging (MRI) at clinical onset. However, literature data reported that about 10% of children with spastic tetraparesis show negative/non-specific neuroradiological findings without a specific etiological cause.[6,7]
In 2014 Crow and colleagues[8] reported 5 subjects with uneventful birth and perinatal period that developed spasticity signs and symptoms in lower limbs. Metabolic screening and nerve conduction study/electromyography were normal. Brain and spinal imaging were normal in 4 cases while a single patient presented diffuse nonspecific high signal on T2-weighted imaging associated with dilatation of lateral ventricles (patchy demyelination). Interferon signature analysis was performed in all patients, being elevated in 3 of them. Subsequent exome sequencing revealed mutations in Aicardi Goutières Syndrome (AGS) associated genes (Adenosine Deaminase RNA-Specific 1 -ADAR1-, Interferon Induced with Helicase C Domain 1 -IFIH1-, Ribonuclease H2 Subunit B -RNASEH2B-) in all cases. AGS is a genetic disorder that mainly strikes central nervous system and skin, caused by an upregulated type I interferon response.[9] To date 7 genes are known to be causative of this syndrome (AGS related genes): the DNA exonuclease (TREX1), the 3 nonallelic components of the RNaseH2 endonuclease complex RNASEH2A-2B-2C, SAM And HD Domain containing Deoxynucleoside triphosphate triphosphohydrolase (SAMHD1), the double stranded RNA editing enzyme (ADAR1) and the double-stranded cytosolic RNA sensor MDA5 (IFIH1).[10] All these proteins are involved in intracellular nucleic acid recognition and degradation and their mutations can led to an increased production of interferon alpha, which is thought to be the main cause of the clinical manifestations of the syndrome. Typical phenotype is characterized by secondary microcephaly, spasticity, dystonia, and developmental delay. Neuroradiological images usually show cerebral calcifications, cortical atrophy, and leukodystrophy.[11] The expression of specific type I interferon-stimulated genes in peripheral blood, called interferon signature, has been proved as a reliable biomarker for AGS and is elevated in 75% of patients with RNASEH2B mutations and in 100% of patients with mutations in any of the other AGS-related genes.[9]
The main aim of the study was to examine a sample of sine-causa spastic tetraparesis subjects through peripheral blood interferon signature and AGS-related genes analysis. A secondary aim was to determine which aspects of the patient history, clinical manifestations, and brain imaging best characterize tetraparesis due to an interferonopathy.
2. Methods
We retrospectively analyzed 78 clinical charts of tetraparesic patients seen at the Child Neurology and Psychiatry and Early Neurorehabilitation Center, ASST (Azienda socio-sanitaria territoriale) Spedali Civili of Brescia between 2011 and 2017. A group of patients was selected based on the following study inclusive criteria: clinical diagnosis of tetraparesis, uneventful pregnancy delivery and perinatal period, negative/non-specific brain MRI at signs/symptoms onset and normal metabolic exams.
All selected subjects underwent a detailed anamnestic data collection, an accurate clinical and neurological examination with verbal intelligence quotient evaluation (according to Leiter-R scores)[12] and brain imaging review. Interferon signature analysis in peripheral blood (according to Rice et al 2013 protocol)[9] was performed. Briefly, the expression of six interferon-stimulated genes was measured by quantitative polymerase chain reaction (PCR). The median fold change of the 6 interferon-stimulated genes, when compared with the median of healthy controls, was utilized to create an interferon score for each patient. Scores higher than the mean of controls plus 2 standard deviations (>3.5) were designated as positive. Patients were analyzed by Next Generation Sequencing technique using a gene panel containing the AGS related genes (TREX1, RNASEH2B, RNASEH2C, RNASEH2A, ADAR1, SAMHD1, and IFIH1); the identified gene mutations were confirmed by Sanger sequencing (according to Rice et al 2014 protocol).[13]
DNA extraction: Blood samples were collected in vacutainers containing EDTA. Genomic DNA extraction was performed using a semi-automated method. DNA was extracted from pellet using automatic standard procedures (Maxwell 16 System DNA Purification, Promega). DNA was quantified with NanoDrop ND1000 UV-Vis Spectrophotometer and Qubit fluorometer (Thermo Scientific).
Next generation sequencing and data analysis: Next Generation Sequencing analysis was performed using Nextera Rapid capture Custom enrichment kit (Illumina), according to the manufacturers’ instructions. The panel was composed of TREX1, RNASEH2B, RNASEH2C, RNASEH2A, ADAR1, SAMHD1, and IFIH1 genes. DNA processing and DNA-seq analysis have been carried out using Illumina MiSeq Sequencer. Samples were loaded on MiSeq instrument and the first steps of bioinformatic analysis (including base calling and demultiplexing) have been performed using MiSeq provided software (Real Time Analysis RTA v.1.18.54 and Casava v.1.8.2, Illumina, Inc., San Diego, CA). FastQ files provided for each sample, containing mate paired-end reads after demultiplexing and adapter removal, were used as input for an ad-hoc developed pipeline previously described.[14] Variant annotation was performed using Annovar software (table_annovar.pl). Mutations were considered pathogenic if they were not found in controls (ie, dbSNP, and 1000 Genomes databases), predicted to alter the sequence of the encoded protein (nonsynonymous, nonsense, splice-site, frameshift, and insertion/deletion mutations) and to adversely affect protein function, with the use of in silico prediction software (SIFT, PolyPhen,MutationTaster).
Written informed consent form was obtained from all the children's parents prior to beginning the data collection. The study was conducted in accordance with the ethical guidelines set forth by the Declaration of Helsinki, and was approved by the Ethics Committee of the ASST Spedali Civili of Brescia, Italy.
3. Results
Following the inclusive criteria, 7 (5 males, 2 females) out of 78 subjects affected by tetraparesis were selected for this study.
Mean age of our sample was 11.9 years (SD 7.7 years; range: 3 years and 4 months – 23 years and 2 months). Prenatal history (prenatal ultrasound and TORCH blood analysis) was normal for all of them. Birth was uneventful, with mean gestational age of 39+6 weeks (SD: 0.6; range: 39.2–41 weeks) and mean birth weight of 3381 grams (SD: 370.2; range: 2850–3700 grams). In all of the cases Apgar score was greater than 8.
Feeding problems were observed in 4 subjects (2 of them had frequent vomiting, associated with sucking and swallowing disorder in 1 case, 2 had coexisting sucking and swallowing disorders). Sleeping disorder characterized by several nocturnal awakenings was found in one subject and irritability in another one.
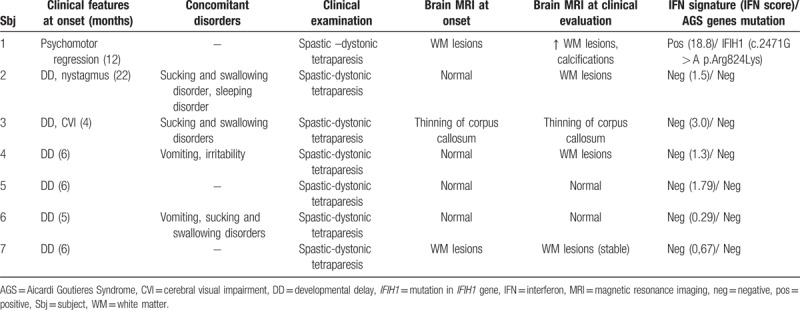
Accurate anamnestic history review showed that at a mean age of 6 months (SD: 1 month, range: 5–22 months) 6 subjects showed developmental delay (isolated in 4, associated with other neurological symptoms in other 2: nystagmus in one child, cerebral visual impairment in another one) and 1 subject had psychomotor regression. Metabolic exams (such as the evaluation of lactic acid, amino acid, long chain fatty acid, lysosomal enzymes, and oligosaccharides), muscle biopsy, electrophysiology analysis (nerve conduction and electromyography, auditory evoked potentials, visual evoked potentials, and electroretinogram) and genetic test (karyotype) were negative. Brain MRI was normal in 4 cases and altered in 3. One subject showed minimal thinning of corpus callosum while two had white matter damage (characterized by increased signal intensity on T2-weighted images in the periventricular areas in one case and patchy demyelination in the other one). At our evaluation (mean age: 11.9 years) all subjects showed spastic-distonic tetraparesis. Expressive language was absent in 4 subjects, dysarthric in 2 and normal in the last one. Intelligence quotient showed severe impairment in 3 cases, borderline level in 1 and normal scores in 3. Brain imaging follow-up was performed in all patients (mean time after first examination: 9 years and 10 months, SD: ± 6 years and 10 months, range: 2–16 years). From neuroimaging review 4 subjects showed stable findings while other 3 had an evolution of white matter injuries, with new subcortical white matter lesions in 2 cases and increased white matter lesions associated with brain calcifications (above all in lenticular nucleus, fronto-parietal and iuxta-cortical regions, cerebellar areas) in another one. Interferon signature was normal in 6 cases and elevated in the last 1 that presented an interferon score of 18.8 at the age of 14 years. AGS related genes analysis was performed in all patients, showing a de novo heterozygous IFIH1 mutation (c.2471G > A p.Arg824Lys) in the subject with increased interferon signature analysis. This mutation was subsequently confirmed by Sanger sequencing (Fig. 1). All above data have been summarized in Table 1.
Figure 1.
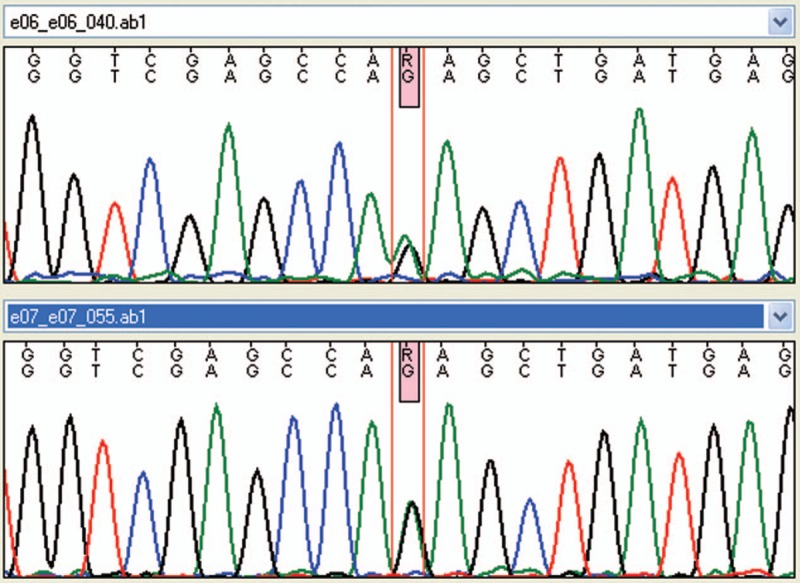
Forward and reverse sanger sequencing result for IFIH1 mutation (c.2471G > A p.Arg824Lys).
Table 1.
Clinical, neuroradiological, laboratoristic, and genetic data of the sample.
IFIH1 mutated patient is an Italian male born to non-consanguineous parents. He came to our attention at 13 years of age, with a diagnosis of tetraparesis of unknown origin. From the patient's history collection, it was found that pregnancy was uneventful and delivery was at full term, vaginal and with Apgar score 9/10. Birth weight was 2850 grams (10°p), length was 48 cm (10°p), and head circumference was 34.5 cm (10°p). Perinatal period was reported as normal. No feeding and sleeping disorders were described. Parents did not report irritability or recurrent fevers. Early developmental milestones were appropriately acquired (smile at age of 2 months, head control at age of 3 months; independent sitting at age of 6 months; grasping at age of 4 months and babbling at age of 8 months). At 12 months parents reported a psychomotor regression (documented with photographic material) with loss of acquired motor and expressive verbal milestones. Metabolic screening (lactic acid, amino acid, long chain fatty acid, lysosomal enzymes, oligosaccharides), neurotransmitter analysis in cerebrospinal fluid, skin and muscle biopsy, genetic analysis (karyotype, Methyl-CpG-binding protein 2 -MECP2- and SURF1 gene tests) and electrophysiology tests (nerve conduction and electromyography, auditory evoked potentials, visual evoked potentials, and electroretinogram) were performed, all with negative results. Brain MRI showed white matter lesions in periventricular areas only and enlarged ventricles as documented in Figure 2. At our evaluation he showed spastic-dystonic tetraparesis with normal head circumference, verbal expressive language was absent but he was able to express himself with informatics devices (personal computer and i-Pad). He was sociable and extrovert; non-verbal intelligence quotient was normal (97 standard scores at Leiter-R evaluation). Brain MRI and CT scans documented a mild progression of white matter lesions (above all in the frontal periventricular areas), associated with diffuse calcifications as documented in Figure 3.
Figure 2.
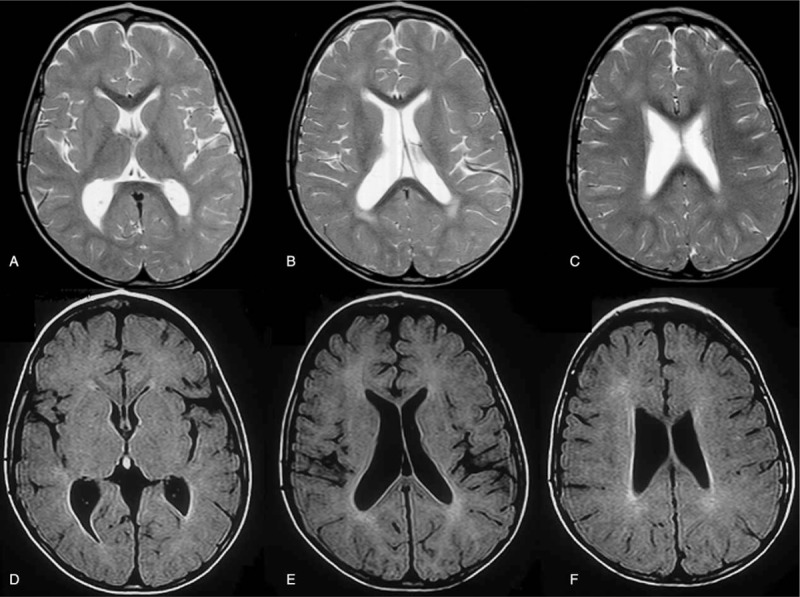
Brain MRI of patient No. 1 at the age of 25 months showing periventricular white matter lesions and enlarged ventricles. Axial T2 weighted TSE (A,B,C) and axial FLAIR T2-weighted (D,E, F). FLAIR = fluid-attenuated inversion recovery, MRI = magnetic resonance imaging, TSE = turbo spin Echo.
Figure 3.
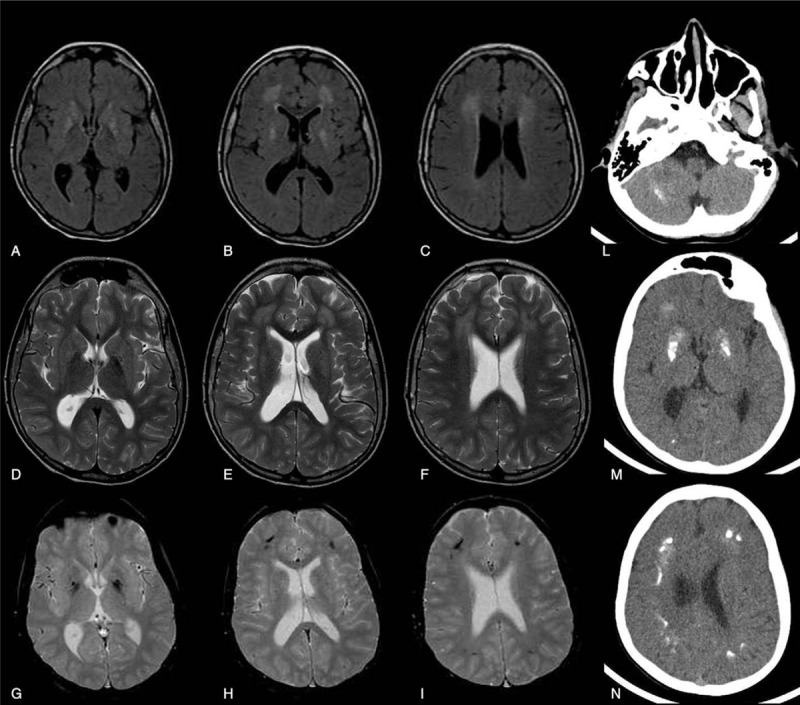
Neuroradiological findings (brain MRI and CT) of patient No. 1 at the age of thirteen years showing a mild progression of the periventricular white matter lesions (above all in the frontal area) and diffuse calcifications. Axial FLAIR T2-weighted (A,B,C), axial T2 weighted TSE (D,E,F), axial T2 weighted FFE (G,H,I) and CT images (L,M,N). CT = computed tomography, FFE = Fast Field Echo, FLAIR = Fluid-attenuated inversion recovery, MRI = magnetic resonance imaging, TSE = turbo spin Echo; FFE: Fast Field Echo.
4. Discussion
Our study aims to underline the importance of considering AGS as an etiology in sine-causa tetraparesis and type I interferon signature a useful marker to drive genetic analysis. This approach could avoid unnecessary and expensive examinations and allow to offer an early and specific genetic counseling to families. This study was oriented to tetraparesis population, in order to increase the knowledge on sine-causa patients and to determine which aspects of the patients’ history, clinical picture, and brain imaging might best characterize the tetraparesis due to an interferonopathy. Out of the 78 subjects with tetraparesis analyzed in our Center, 7 (9%) had uneventful pregnancy, delivery and perinatal period and normal or non-specific brain MRI. A diagnosis of AGS was made in one child (14%). In this patient typical early alarm signals of AGS as extreme irritability, disturbed sleep-wake patterns (including difficulty falling asleep and reversal of the sleep-wake cycle), feeding difficulties and recurrent sterile low-grade fevers[,15,16,17] were not observed. In children with AGS, these symptoms may indicate the onset of an initially sub-acute encephalopathy [16] and are followed by a psychomotor delay and/or regression, as well as signs of neurological impairment and a slowing head growth (secondary microcephaly). After this phase, which usually last a few months, the clinical picture typically stabilizes[10] as in our IFIH1 patient that showed AGS typical neurological symptoms characterized by psychomotor regression and evolution to spastic-dystonic tetraparesis but did not suffer from other symptoms usually associated with AGS (such as epilepsy, nystagmus, cerebral visual impairment, intelligence quotient deficit, acquired microcephaly, startle reactions). It is important to underline that other patients from our sample presented a delayed psychomotor skills acquirement associated with different neurologic problems.
In our study psychomotor regression has only been reported in the AGS patient. This symptom is atypical of CP (the main cause of tetraparesis in children) and might orient clinicians towards other diagnostic hypotheses, such as AGS.
Cardinal neuroradiological features of AGS (cerebral calcification, white matter abnormalities, and cerebral atrophy) were not present at clinical onset in our IFIH1 patient, who showed non-specific white matter damage. During brain imaging follow-up leukodystrophy and calcifications progressively appeared but no brain atrophy was documented. While leukodystrophy was found in 3 other subjects in our sample, no-one else showed brain calcification. Interferon signature is widely recognized as a biomarker for interferonopathies;[9] in our sample only 1 patient presented an elevated score. In consideration of literature data, reporting that not all the patients affected by AGS have an elevated interferon signature score, we performed genetic analysis of AGS related genes in all patients and revealed a de novo heterozygous IFIH1 mutation (c.2471G > A p.Arg824Lys) in the subject with increased interferon signature. IFIH1 mutations are often described as cause of a spectrum of severe neuroinflammatory phenotypes[13,18] with both motor and cognitive impairment.
Even though most patients affected by AGS present classic phenotypes, literature reports a heterogeneous spectrum of disease presentation, progression, and outcome.[10] As already discussed, some core features of AGS were missing in our IFIH1 patient, who presented strikingly with a relatively conserved cognitive functioning associated with normal head growth and absence of brain atrophy on MRI. Nonetheless, the mutation identified seems likely to be pathogenic and may orient towards the diagnosis of "tetraparesis due to an interferonopathy”.
A limitation of this study is the relatively small number of patients recruited. This was due to the difficulty to find subject affected by tetraparesis that has an uneventful pregnancy, delivery, and perinatal period associated with non-specific neuroimaging findings. The number of identified AGS patients is also relatively small (just one single case in our sample) given the extreme rarity of AGS (to date about 350 patients are described in literature).[16] To generalize the results further, future studies should be conducted in larger samples of participants.
In conclusion, our findings suggest considering AGS in any case of sine-causa tetraparesis. Core features of interferonopathy-related tetraparesis are: the onset during the first year of life, progressive psychomotor regression with upper motor neuron signs, normal non-verbal intelligence quotient, brain white-matter lesions with late calcifications. Interferon score analysis could be a useful tool in this context, to select those patients who should undergo AGS gene analysis.
Acknowledgments
We thank MD A. Filippini for the use of clinical information of patient No. 1. We thank also Golgi Foundation and Regione Lombardia.
Author contributions
J.G. and E.F. designed the study. J.G., F.G. and M.D. recruited and evaluated the patients and wrote the manuscript. J.G, M.V., and G.R. performed the genetic analysis. S.G. and D.V. performed the laboratory tests. M.C. and E.F. reviewed the manuscript. M.M. performed brain MRI and CT scan. The AGS study group members participated in different phases of the study and reviewed the manuscript before submission.
Conceptualization: Jessica Galli and Elisa Fazzi.
Data curation: Jessica Galli, Francesco Gavazzi, and Micaela De Simone.
Formal analysis: Silvia Giliani, Jessica Garau, Marialuisa Valente, Gillian Rice and Donatella Vairo.
Funding acquisition: Elisa Fazzi and Jessica Galli.
Investigation: Jessica Galli, Francesco Gavazzi, and Marzia Mortilla.
Methodology: Francesco Gavazzi and Jessica Galli.
Supervision: AGS Study group, Gillian Rice, and Elisa Fazzi.
Writing – original draft: Jessica Galli and Francesco Gavazzi.
Writing – review & editing: Micaela De Simone, Marco Cattalini, AGS Study group, Gillian Rice, and Elisa Fazzi.
Jessica Galli orcid: 0000-0002-3347-7548.
Footnotes
Abbreviations: ADAR1 = Adenosine Deaminase RNA-Specific, AGS = Aicardi Goutières Syndrome, ASST = azienda socio-sanitaria territoriale, CP = Cerebral Palsy, CT = computed tomography, CVI = cerebral visual impairment, DD = developmental delay, FFE = Fast Field Echo, FLAIR = fluid-attenuated inversion recovery, IFIH = Interferon Induced With Helicase C Domain 1, IFN = interferon, MRI = Magnetic Resonance Imaging, PCR = Polymerase Chain Reaction, RNASEH2A-2B-2C = Ribonuclease H2 Subunit A,B,C, SAMHD1 = deoxynucleoside triphosphate triphosphohydrolase, TREX1:three prime repair exonuclease 1, TSE = turbo spin Echo, WM = white matter.
The funding sources have not been involved in the planning of study design, the running of the study, the data analysis or the interpretation of the study findings.
Part of this work was supported by the Golgi Foundation (J.G.) and Regione Lombardia (E.F.).
All the authors declare absence of conflict of interest.
Contributor Information
Collaborators: AGS study group
References
- [1].Lance JW. The control of muscle tone, reflexes, and movement: Robert Wartenberg Lecture. Neurology 1980;30:1303–13. [DOI] [PubMed] [Google Scholar]
- [2].Awaad Y, Rizk T, Siddiqui I, et al. Complications of intrathecal baclofen pump: prevention and cure. ISRN Neurol 2012;2012:575168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Leach EL, Shevell M, Bowden K, et al. Treatable inborn errors of metabolism presenting as cerebral palsy mimics: systematic literature review. Orphanet J Rare Dis 2014;30:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lee RW, Poretti A, Cohen JS, et al. A diagnostic approach for cerebral palsy in the genomic era. Neuromolecular Med 2014;16:821–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rekand T. Clinical assessment and management of spasticity: a review. Acta Neurol Scand Suppl 2010;190:62–6. [DOI] [PubMed] [Google Scholar]
- [6].Bax M, Tydeman C, Flodmark O. Clinical and MRI correlates of cerebral palsy: the European Cerebral Palsy Study. JAMA 2006;296:1602–8. [DOI] [PubMed] [Google Scholar]
- [7].Krägeloh-Mann I, Horber V. The role of magnetic resonance imaging in furthering understanding of the pathogenesis of cerebral palsy. Dev Med Child Neurol 2007;49:948. [DOI] [PubMed] [Google Scholar]
- [8].Crow YJ, Zaki MS, Abdel-Hamid MS, et al. Mutations in ADAR1, IFIH1, and RNASEH2B presenting as spastic paraplegia. Neuropediatrics 2014;45:386–93. [DOI] [PubMed] [Google Scholar]
- [9].Rice GI, Forte GM, Szynkiewicz M, et al. Assessment of interferon-related biomarkers in Aicardi-Goutières syndrome associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, and ADAR: a case-control study. Lancet Neurol 2013;12:1159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Crow YJ, Chase DS, Lowenstein Schmidt J, et al. Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1. Am J Med Genet A 2015;167A:296–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Orcesi S, La Piana R, Fazzi E. Aicardi-Goutieres syndrome. Br Med Bull 2009;89:183–201. [DOI] [PubMed] [Google Scholar]
- [12].Roid G, Miller L, Pomplun M, et al. Leiter International Performance Scale. 3rd Edition; 2013. [Google Scholar]
- [13].Rice GI, Del Toro Duany Y, Jenkinson EM, et al. Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nat Genet 2014;46:503–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zucca S, Villaraggia M, Gagliardi S, et al. Analysis of amplicon-based NGS data from neurological disease gene panels: a new method for allele drop-out management. BMC Bioinformatics 2016;17Suppl 12:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pulliero A, Fazzi E, Cartiglia C, et al. The Aicardi-Goutières syndrome. Molecular and clinical features of RNAse deficiency and microRNA overload. Mutat Res 2011;717:99–108. [DOI] [PubMed] [Google Scholar]
- [16].Fazzi E, Cattalini M, Orcesi S, et al. Aicardi-Goutieres syndrome, a rare neurological disease in children: a new autoimmune disorder? Autoimmun Rev 2013;12:506–9. [DOI] [PubMed] [Google Scholar]
- [17].Cattalini M, Galli J, Andreoli L, et al. Exploring autoimmunity in a Cohort of children with genetically confirmed Aicardi-Goutières Syndrome. J Clin Immunol 2016;36:693–9. [DOI] [PubMed] [Google Scholar]
- [18].Della Mina E, Rodero MP, Crow YJ. Polymorphisms in IFIH1: the good and the bad. Nat Immunol 2017;18:708–9. [DOI] [PubMed] [Google Scholar]


