Abstract
Nutritional deficiencies and malnutrition are considered to be related to ulcerative colitis (UC); however, the association between serum levels of micronutrients and UC is not well known. This study aimed to evaluate the serum levels of micronutrients in UC patients and investigate their association with disease activity.
This cross-sectional study was conducted on UC patients visiting the Department of Gastroenterology at 3 different teaching hospitals between January 2016 and January 2017. UC activity was measured based on Truelove and Witts’ severity index and guidelines for colonoscopy. A healthy gender- and age-matched group was also selected. Serum levels of zinc, copper, selenium, ceruloplasmin, albumin, and total protein were compared between the 2 groups of UC patients and healthy subjects using independent-samples t test. Also, the association between serum levels of micronutrients and UC activity was assessed by using Pearson and Spearman correlation coefficient tests. The data were analyzed by SPSS version 21, considering P ≤.05 as the statistical significance level.
Overall, 112 (54 male and 58 female) individuals with the mean age of 34.6 years were studied in the 2 groups of UC patients (n = 56) and healthy subjects (n = 56). The 2 groups were homogeneous in terms of age, gender, marital status, place of residence, and educational level (P >.05). The serum levels of total protein (6.41 ± 1.1 vs 7.41 ± 0.4 g/dL; P = .0001), albumin (4.72 ± 1.1 vs 5.19 ± 0.28 g/dL; P = .0001), zinc (679 ± 62 vs 1055 ± 156 μg/L; P = .0001), and selenium (81.85 ± 6.4 vs 108.4 ± 12.98 micg/L; P = .0001) were significantly lower in the UC patients. The serum level of copper did not differ significantly between the 2 groups (P = .1).
Considering the simultaneous reduction in nutritional criteria in the UC patient group, malnutrition appears to be a factor affecting micronutrient deficiency in patients with UC.
Keywords: colitis, inflammatory bowel disease, micronutrient, trace elements, ulcerative
1. Introduction
Ulcerative colitis (UC) is an idiopathic illness in immunocompromised patients that has a remarkable prevalence in the developed countries.[1] Recent studies have shown a significant upsurge in the incidence of this disease in the developing countries such as Iran.[2] The disease manifests itself with gastrointestinal disorders and chronic and debilitating symptoms including diarrhea, rectal bleeding, and abdominal cramps along with anorexia that may persist for weeks to months.[1]
Regarding the chronic nature of the disease, there is a likelihood of micronutrient deficiency due to poor nutritional support, reduced food intake (in the case of anorexia, abdominal pain, nausea, vomiting, and dietary restrictions), chronic diarrhea, increased intestinal excretion, bloody bowel movement, hypermetabolic state of the body (leading to an increase in the amount of energy needed in a resting state), and drug interactions.[1] Micronutrient deficiency may exacerbate the symptoms of UC.[1–3]
Previous studies reported discrepant results regarding the changes in the serum levels of micronutrients in UC patients. One of the most important reasons for this difference in results is the diversity in lifestyle and nutritional model in different populations. To the best of our knowledge, no studies have so far determined the prevalence of micronutrient deficiencies in UC patients and compared it with its prevalence in healthy individuals in northeastern Iran. Therefore, this study was conducted to investigate the levels of micronutrients in patients with UC and their relationship with the extent of disease activity.
2. Materials and methods
2.1. Study design and setting
This cross-sectional study was conducted on 2 groups of UC patients and healthy individuals between January 2016 and January 2017. Patients were selected from among those visiting the Department of Gastroenterology of 3 university-affiliated hospitals, namely Ghaem, Imam Reza, and Razavi Hospitals. UC patients referred to the Pazh Digestive Diseases Clinic were also considered eligible. Healthy individuals were selected from hospital personnel or patient caregivers who volunteered to participate in the study.
2.2. Inclusion and exclusion criteria
The inclusion criteria for the UC group were confirmed diagnosis of UC according to colonoscopic observations by a gastroenterologist and biopsy findings. Healthy individuals were included if they had no signs and symptoms of acute diseases. The exclusion criteria were use of nutritional supplements in the last 6 months, use of dietary treatments over the past 6 months, history of gastrointestinal surgery or malignancy, smoking, pregnancy, known diseases of the small intestine, such as celiac disease, and the presence of uncontrolled chronic diseases.
2.3. Sample size and sampling
The sample size was determined to be 112 in the 2 groups of healthy individuals (n = 56) and UC patients (n = 56), according to the results of Sturniolo et al[4] and based on the difference between 2 population means. The 2 groups were matched according to age and gender.
2.4. Data collection
Demographic characteristics of the participants including age, gender, marital status, place of residence, and level of education were recorded. All the UC patients underwent an accurate physical examination and history taking. The patients were classified into 3 groups of mild, moderate, and severe UC based on clinical evaluation by an expert gastroenterologist, as well as laboratory, colonoscopy, and histology findings using the Truelove and Witts’ severity index.
2.4.1. Blood samples
Blood samples were taken to measure serum zinc, copper, selenium, ceruloplasmin, total protein, and albumin in the patients and healthy subjects. Serum total protein content was obtained through the colorimetric Biuret method using the Human Assay Kit (Bionik Co., Iran). Serum albumin level was evaluated by photometric method using the Human Assay Kit (Pars Azmon Co., Iran). Ceruloplasmin level was measured using immunoturbidimetric assay with COBAS INTEGRA 400 plus systems and the Test CERU3 kit (Roche Co., Germany). The serum copper and selenium levels were assessed by Atomic Absorption Spectroscopy using Perkin Elmer Analyst 300. The serum level of zinc was measured with the Human Zinc Assay Kit (product code: CC02750, LTA Co., Italy). Moreover, erythrocyte sedimentation rate (ESR) and complete blood count test (CBC) were ordered for all the participants.
2.5. Ethical considerations
The study protocol was approved by the Ethics Committee of Mashhad University of Medical Sciences (IR.MUMS.fm.REC.1395.268). All the patients were explained about the study procedure before enrollment in the project, and if they signed the informed consent, their information was recorded. Confidentiality of the information and respect for the patients were also considered during the implementation of the project. The patients were free to withdraw from the study at any stage.
2.6. Statistical analysis
The collected data were analyzed by SPSS version 17. Continuous variables were expressed as mean ± SD and qualitative variables as frequencies and percentages. In data analysis, the normal distribution of the data was assessed by Kolmogorov-Smirnov test. Qualitative variables were compared between the 2 groups by Chi-square or Fisher exact test. Continuous variables including serum levels of micronutrients were compared between the 2 groups by independent-samples t test and Mann–Whitney U test. Moreover, analysis of variance (ANOVA) was used to compare the serum levels of micronutrients among the 3 subgroups of mild, moderate, and severe UC. Serum albumin and protein are markers of nutritional status and subsequently proofing the poor nutritional support, the association of serum levels of protein and albumin with zinc, ceruloplasmin, copper, and selenium levels was evaluated in the case and healthy individuals using Pearson correlation coefficient. Logistic regression analysis was performed for the study of the independent effect of micronutrients. P value less than .05 was considered statistically significant.
3. Results
3.1. Demographic characteristics
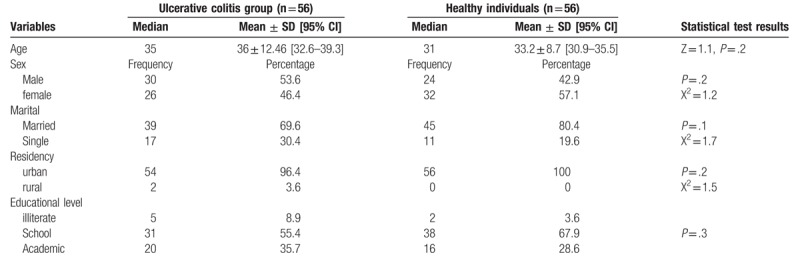
One hundred twelve (54 male, 58 female) individuals with the mean age of 34.6 years were studied in the 2 groups of UC (n = 56) and healthy (n = 56). The 2 groups were homogeneous in terms of age, gender, marital status, place of residence, and educational level (Table 1).
Table 1.
Comparison of demographic profiles between case and healthy individuals.
3.2. UC characteristics
The mean duration of disease was 5.8 ± 5.7 years. Of the 56 patients, disease activity was mild in 19 (33.92%), moderate in 18 (32.14%), and severe in 19 (33.92%) patients. The disease had progressed to proctitis (n = 3, 5.6%), proctosigmoiditis (n = 18, 33.3%), distal colitis (n = 11, 18.5%), extensive colitis (n = 11, 18.5%), and pancolitis (n = 13, 24.1%) based on the colonoscopic assessments. Most of the patients were under 5-aminosalicylic acid (5ASA) treatment (n = 31, in 63.3%), while immunosuppressive therapy coupled with 5ASA (n = 17, 34.7%), and anti-TNF (n = 1, 2.X) alone were other types of treatment. Seven patients were newly diagnosed and were not on any treatment yet.
3.3. Micronutrients in the 2 groups of UC patients and healthy individuals
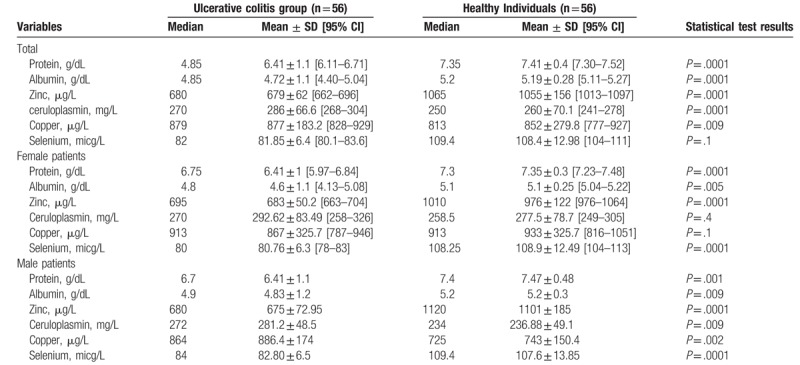
The serum levels of total protein, albumin, zinc, and selenium were significantly lower in the UC group; however, ceruloplasmin and copper levels were lower in the healthy individuals (Table 2), and the ceruloplasmin and copper levels were not significantly different between the 2 groups (Table 2).
Table 2.
Mean serum levels of the studied variables in case and healthy individuals.
3.4. Micronutrients in different groups of UC severity
The comparison of mean serum levels of zinc, ceruloplasmin, and copper in the 3 groups of mild, moderate, and severe UC showed no difference, while protein, albumin, and selenium levels of severe UC patients were significantly lower (Fig. 1). Post hoc test showed that the mean serum levels of protein, albumin, and selenium of the severe UC patients were significantly lower than those with mild activity (P = .01) and moderate activity (P = .0001). However, in terms of serum level of zinc, no difference was observed between patients with mild and moderate disease activity and the serum level of zinc did not correlate with disease severity (Fig. 1).
Figure 1.
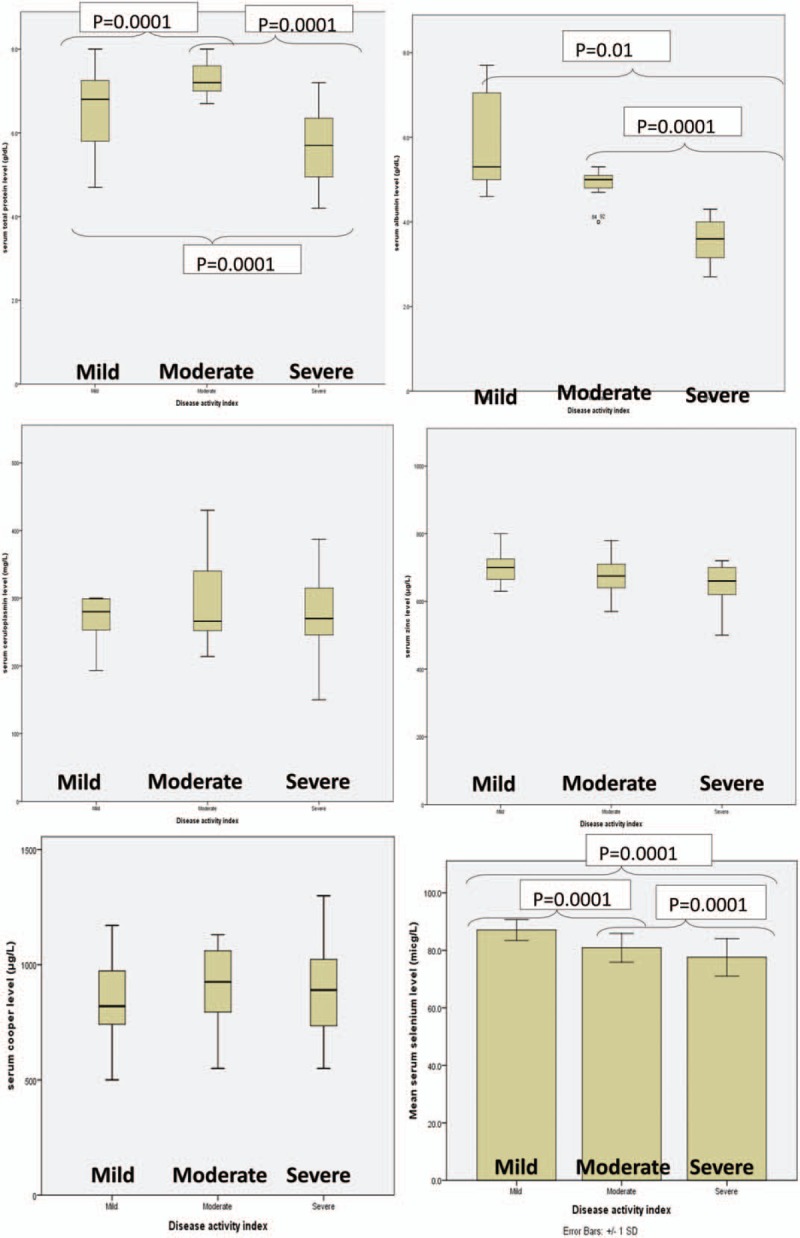
Micronutrients in different groups of UC severity. The comparison of mean serum levels of zinc, ceruloplasmin and copper in 3 groups of mild, moderate, and severe UC showed no difference; while protein, albumin, and selenium of severe UC patients were significantly lower. Post hoc test showed that the mean serum level of protein, albumin, and selenium of the severe UC patients were significantly lower than those with mild activity (P = .01) and moderate activity (P = .0001). However, there was no difference in mild and moderate activity when the serum protein was compared between the patients. The results were presented as following: the serum levels of zinc did not correlate with the disease severity. UC = ulcerative colitis.
3.5. Micronutrients based on gastrointestinal spread of UC
Disease spread (proctitis to pancolitis) was not significant according to Kruskal–Wallis test (Fig. 2). The post hoc test indicated that patients with proctosigmoiditis had lower selenium levels than those with extensive colitis (P = .03) and distal colitis (P = .02). The comparison of mean serum levels of protein, albumin, zinc, ceruloplasmin, and copper based on the disease spread (proctitis to pancolitis) was not significant based on Kruskal–Wallis test.
Figure 2.
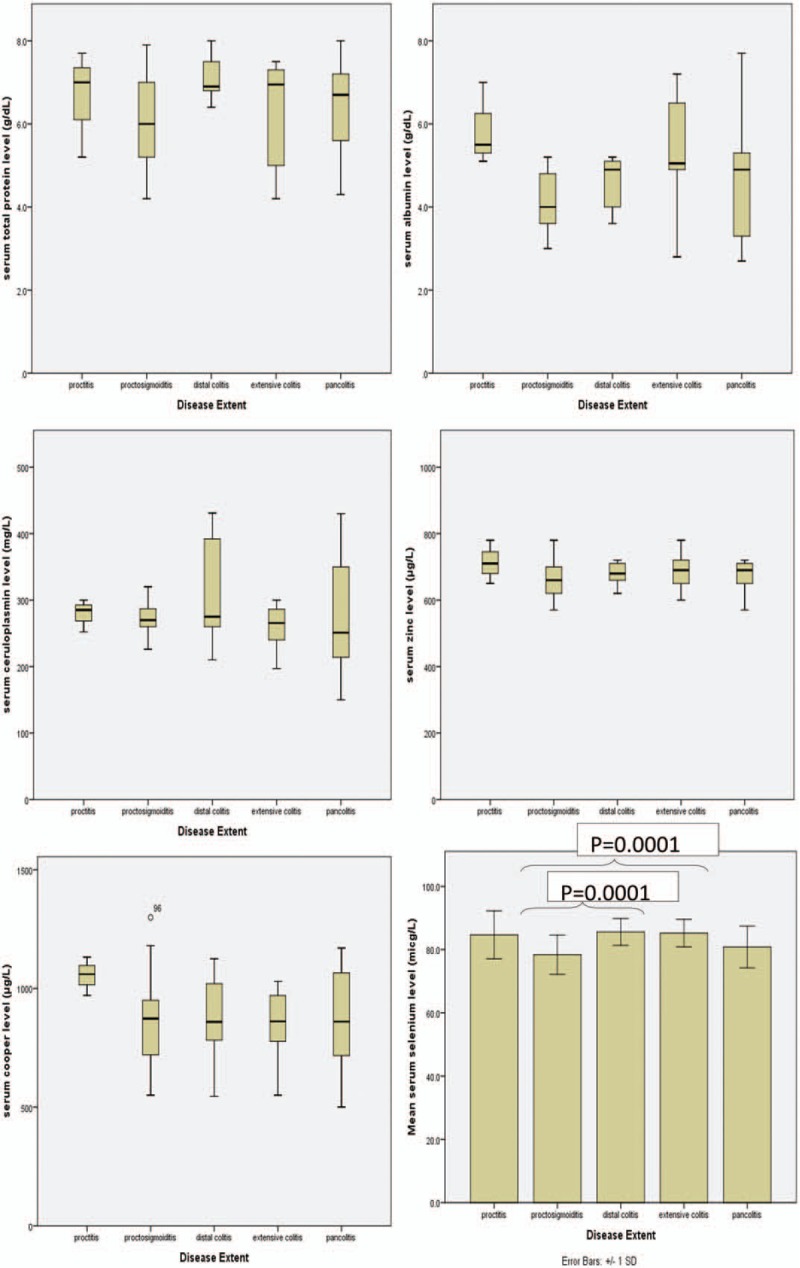
Micronutrients based on the UC gastrointestinal spread. Based on the disease spread (proctitis to pancolitis) was not significant in accordance with Kruskal–Wallis test. The Post Hoc test indicated that the patients with proctosigmoiditis had lower selenium level than in those with extensive colitis (P = .03) and distal colitis (P = .02). The comparison of mean serum levels of protein, albumin, zinc, ceruloplasmin, and copper based on the disease spread (proctitis to pancolitis) was not significant in accordance with Kruskal–Wallis test. UC = ulcerative colitis.
3.6. Micronutrients based on the UC treatment type
Comparison of the serum levels of protein, albumin, zinc, ceruloplasmin, copper, and selenium in patients treated with 5ASA alone and combination therapy of immunosuppressive and 5ASA showed no significant differences (Table 5).
3.7. Correlation of micronutrients
A positive correlation was found between the levels of albumin and protein and the levels of zinc (P = .0001, r = +0.495, and P = .001, r = +0.300, respectively) and selenium (P = .0001, r = +0.484 and P = .001, r = +0.332, respectively). Nonetheless, there was no correlation between the serum levels of albumin and protein and the serum level of copper (P = .5, r = −0.06, P = .7, r = +0.03, respectively).
3.8. Regression model
The patient and healthy groups were compared using logistic regression analysis and forward stepwise model. The results exhibited that only the serum level of zinc (95% CI = 0.962–0.984, OR = 0.98) was significant, which means that the odds ratio of allocation in the patient group was decreased by 2% per unit of zinc increment.
4. Discussion
In this study, we sought to investigate the levels of micronutrients in UC patients and determine their association with disease severity. Our results indicated significantly lower serum levels of protein and albumin along with serum levels of zinc and selenium in the patients with UC compared to healthy subjects. Despite the fact that ceruloplasmin level was significantly lower in the healthy subjects than in the UC patients, there was no significant difference in the serum level of copper between the 2 groups. Similar findings were noted in assessing these serum markers based on gender, though male patients had higher copper levels than their healthy counterparts.
The results showed significantly lower serum levels of protein, albumin, and selenium in the event of progression and increased severity of the disease, while the serum levels of zinc, ceruloplasmin, and copper were not associated with disease severity. Concerning the effect of colonoscopic development of the disease on changes in the serum levels of the studied elements, the results merely showed a significant decline in the serum level of selenium in patients with proctosigmoiditis. There was no link between the serum levels of the studied variables and treatment. According to the results, poor nutritional status was associated with a significant reduction in the serum levels of zinc and selenium, but not with the level of serum copper.
The frequency of reduced level of selenium in the study population was 80% and the serum level of this micronutrient in the UC patients was significantly lower than in healthy subjects, such that the reduction rate intensified with increased severity of the disease. Similar results were reported in previous studies. Han et al[5] reported that selenium level in UC patients was significantly lower than the respective values in patients with inflammatory bowel disease. Aguilar-Tablada et al[6] underscored that the mean level of serum selenium in patients with UC was significantly lower than that in healthy subjects. Conversely, Andoh et al[7] and El Muhtaseb et al,[8] based on 2 case–control studies in Japan and Norway,[7,9] reported equal serum levels of zinc and ceruloplasmin in healthy subjects and UC patients.
In general, the role of selenium in UC is more negligible than in Crohn's disease. Based on the findings of the present study, the level of this micronutrient was reduced in the UC patients, and this reduction rate deteriorated with increased severity of the disease.
Nutritional disorders seem to play a significant role in symptoms of UC. Studies have exhibited that UC patients had a poorer nutritional status due to symptoms such as pain and anorexia, which exacerbate with growing severity of the disease.[10] On the other hand, it has been shown that selenium level is directly related to nutritional status, not only in the patient population but also in healthy subjects.[11] It is worth mentioning that we found selenium deficiency associated with inadequate nutrition in 80% of our patients.
Moreover, in this study, significant zinc deficiency was observed in about 50% of the UC patients. Several epidemiological studies, clinical trials, and cellular studies have been conducted on the role of zinc in the development and activity of UC.
In line with our results, Siva et al,[12] Shokrzadeh et al,[13] and Mohammadi et al[14] emphasized that the deficiency of this micronutrient is frequently observed in patients with UC. Since zinc level was found to be associated with nutritional status in this study, nutritional disorder in UC patients seems to induce this micronutrient deficiency. There are other pathophysiological outcomes related to zinc deficiency, such as its absorption impairment or its role in inflammation associated with colitis, but there is no consensus among researcher in this regard.[15] At the molecular level, with the occurrence of neutrophil invasion, reactive oxygen species are produced, causing severe damage to the intestinal mucosa.
Zinc plays a role in reducing reactive oxygen species in its various ionic forms; for example, the copper/zinc isoform, as one of the components of superoxide dismutase (Cu/Zn SOD) scavengers or zinc-cytoprotective enzymes, including metallothioneins, removes reactive oxygen species from systematic inflammation in the body, thus, zinc in such processes may be consumed and its level may be reduced.[16–20]
Several studies have been conducted on the effects of zinc supplementation in UC patients. In a study by Di Leo et al,[18] zinc supplementation in rats with colitis improved weight gain and caused diarrhea, but this supplement did not affect neutrophil infiltration or tissue inflammation. Mulder et al[17] did not observe any changes in the disease activity index or inflammation in biopsy specimens of patients with CU after Zn supplementation. Tran et al[19] in a recent study concluded that zinc supplementation in UC patients suppressed colitis as shown by reduction in disease activity index, histological severity scores, and myeloperoxidase activity. Increased serum level of zinc at the microscopic level reduced inflammation in rat models.[21–24] Based on the available evidence, it can be concluded that zinc deficiency occurs in patients with UC, not only due to mucosal damage and malabsorption but also owing to poor nutritional support as a symptom of active disease.[25,26]
We found that the serum level of copper was non-significantly higher in the UC patients than in the healthy subjects. However, the serum level of copper was not correlated with disease activity or nutritional status of the patients. A few studies have been undertaken in this regard, whose results regarding increased serum level of copper in UC patients were consistent with the our findings. In a study by Shokrzadeh et al,[13] the mean serum copper concentration was 42.6 ± 138.3 μg/dL in UC patients and 38.5 ± 110 μg/dL in healthy subjects. Ringstad et al[9] reported that mean copper level was 18.47 ± 4.37 μmol/L in UC patients and 3.30 ± 15.74 μmol/L in healthy subjects, showing a significant difference between the 2 groups. Fernandez-Banares et al emphasized that the serum level of copper in 15 patients with active colitis was higher than in a healthy group.[27] The non-significant increase observed in the serum level of copper in the studied patients was probably due to an elevation in ceruloplasmin level in response to inflammation.[28]
The present study had some limitations, one of the most important of them being the lack of assessment of nutritional status of patients and healthy subjects by standard methods (and draw conclusions based solely on protein and albumin levels) along with the study of other nutritional indexes such as iron, beta-globulins, and cholesterol. Another limitation of our study was the inability to precisely examine the spread of the disease in severe phases due to the impossibility of complete colonoscopy. Additionally, the patients were not evaluated for socioeconomic status. Given the cross-sectional nature of this study, we could not establish causal relationships between the data collected during a certain period.
Apart from these limitations, this study had some strength as it is one of the first studies on the population of UC patients in northeastern Iran. Due to the lifestyle and cultural diversities in various regions of the country, the implementation of this study was of significance as it was conducted with an acceptable sample size and a matched healthy group.
5. Conclusion
The present study aimed to investigate the levels of micronutrients in UC patients and their association with the extent of disease activity. Our results indicated significant zinc and selenium deficiency in the patients with UC in comparison with the healthy subjects, which could be attributed to the malnutrition associated with this disease. We recommend physicians to closely monitor the nutritional status of patients with UC and perform the necessary serum tests to detect possible deficiencies. In addition, future studies are suggested to take into account the nutritional and socioeconomic status of individuals. Assessment of patients at the cellular level (and their healthy counterparts who have experienced biopsy for reasons other than inflammatory bowel disease) may also be beneficial. Considering the high prevalence of selenium and zinc deficiencies in UC patients, which is sometimes associated with high disease severity, performing clinical trials in this regard is strongly recommended.
Author contributions
Conceptualization: Mitra Ahadi, Ali Beheshti Namdar, Reza Ziaolhagh.
Data curation: Hassan Vosoughinia, Mohammad R Farzanehfar.
Formal analysis: Maryam Salehi.
Investigation: Hassan Vosoughinia, Mohammad R Farzanehfar.
Methodology: Maryam Salehi, Ali Beheshti Namdar, Reza Ziaolhagh, Bahram Memar.
Project administration: Mitra Ahadi.
Resources: Farid Poursadegh, Reza Ziaolhagh.
Software: Farid Poursadegh, Maryam Salehi.
Supervision: Mitra Ahadi.
Validation: Farid Poursadegh, Bahram Memar.
Visualization: Farid Poursadegh, Bahram Memar.
Writing – original draft: Farid Poursadegh.
Writing – review & editing: Farid Poursadegh, Mitra Ahadi, Hassan Vosoughinia, Maryam Salehi, Ali Beheshti Namdar.
Footnotes
Abbreviations: 5ASA = 5-aminosalicylic acid, UC = ulcerative colitis.
The study was funded by Mashhad University of Medical Sciences (grant number: 941810). The authors declare that there were no conflicts of interest
The authors declare that there were no conflicts of interest.
References
- [1].Rocha R, Santana GO, Almeida N, et al. Analysis of fat and muscle mass in patients with inflammatory bowel disease during remission and active phase. Br J Nutr 2009;101:676–9. [DOI] [PubMed] [Google Scholar]
- [2].Van Patter WN, Bargen JA, Dockerty MB, et al. Regional enteritis. Gastroenterology 1954;26:347–450. [PubMed] [Google Scholar]
- [3].Lanfranchi GA, Brignola C, Campieri M, et al. Assessment of nutritional status in Crohn's disease in remission or low activity. Hepatogastroenterology 1984;31:129–32. [PubMed] [Google Scholar]
- [4].Sturniolo GC, Mestriner C, Lecis PE, et al. Altered plasma and mucosal concentrations of trace elements and antioxidants in active ulcerative colitis. Scand J Gastroenterol 1998;33:644–9. [DOI] [PubMed] [Google Scholar]
- [5].Han YM, Yoon H, Lim S, et al. Risk factors for vitamin D, Zinc, and selenium deficiencies in korean patients with inflammatory bowel disease. Gut Liver 2017;11:363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Castro Aguilar-Tablada T, Navarro-Alarcon M, Quesada Granados J, et al. Ulcerative colitis and Crohn's disease are associated with decreased serum selenium concentrations and increased cardiovascular risk. Nutrients 2016;8:780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Andoh A, Hirashima M, Maeda H, et al. Serum selenoprotein-P levels in patients with inflammatory bowel disease. Nutrition (Burbank, Los Angeles County, Calif) 2005;21:574–9. [DOI] [PubMed] [Google Scholar]
- [8].El Muhtaseb MS, Duncan A, Talwar DK, et al. Assessment of dietary intake and trace element status in patients with ileal pouch-anal anastomosis. Dis Colon Rectum 2007;50:1553–7. [DOI] [PubMed] [Google Scholar]
- [9].Ringstad J, Kildebo S, Thomassen Y. Serum selenium, copper, and zinc concentrations in Crohn's disease and ulcerative colitis. Scand J Gastroenterol 1993;28:605–8. [DOI] [PubMed] [Google Scholar]
- [10].Takaoka A, Sasaki M, Kurihara M, et al. Comparison of energy metabolism and nutritional status of hospitalized patients with Crohn's disease and those with ulcerative colitis. J Clin Biochem Nutr 2015;56:208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Alfthan G, Eurola M, Ekholm P, et al. Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: from deficiency to optimal selenium status of the population. J Trace Elem Med Biol 2015;31:142–7. [DOI] [PubMed] [Google Scholar]
- [12].Siva S, Rubin DT, Gulotta G, et al. Zinc deficiency is associated with poor clinical outcomes in patients with inflammatory bowel disease. Inflamm Bowel Dis 2017;23:152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shokrzadeh M, Taghvaei T, Haghighi-hasanalideh P, et al. The relationship between copper and zinc levels in the serum and urine and the risk of ulcerative colitis. J Mazandaran Univ Med Sci 2013;23:78–84. [Google Scholar]
- [14].Mohammadi E, Qujeq D, Taheri H, et al. Evaluation of serum trace element levels and superoxide dismutase activity in patients with inflammatory bowel disease: translating basic research into clinical application. Biol Trace Elem Res 2017;177:235–40. [DOI] [PubMed] [Google Scholar]
- [15].Skrovanek S, DiGuilio K, Bailey R, et al. Zinc and gastrointestinal disease. World J Gastrointest Pathophysiol 2014;5:496–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mulder TP, van der Sluys Veer A, Verspaget HW, et al. Effect of oral zinc supplementation on metallothionein and superoxide dismutase concentrations in patients with inflammatory bowel disease. J Gastroenterol Hepatol 1994;9:472–7. [DOI] [PubMed] [Google Scholar]
- [17].Tran CD, Ball JM, Sundar S, et al. The role of zinc and metallothionein in the dextran sulfate sodium-induced colitis mouse model. Dig Dis Sci 2007;52:2113–21. [DOI] [PubMed] [Google Scholar]
- [18].Tran CD, Butler RN, Philcox JC, et al. Regional distribution of metallothionein and zinc in the mouse gut: comparison with metallothionien-null mice. Biol Trace Elem Res 1998;63:239–51. [DOI] [PubMed] [Google Scholar]
- [19].Xia B, Deng CS, Chen DJ, et al. Role of copper zinc superoxide dismutase in the short-term treatment of acetic acid-induced colitis in rats. Acta Gastroenterol Latinoam 1996;26:227–30. [PubMed] [Google Scholar]
- [20].Di Leo V, D’Inca R, Barollo M, et al. Effect of zinc supplementation on trace elements and intestinal metallothionein concentrations in experimental colitis in the rat. Dig Liver Dis 2001;33:135–9. [DOI] [PubMed] [Google Scholar]
- [21].Chen BW, Wang HH, Liu JX, et al. Zinc sulphate solution enema decreases inflammation in experimental colitis in rats. J Gastroenterol Hepatol 1999;14:1088–92. [DOI] [PubMed] [Google Scholar]
- [22].Luk HH, Ko JK, Fung HS, et al. Delineation of the protective action of zinc sulfate on ulcerative colitis in rats. Eur J Pharmacol 2002;443:197–204. [DOI] [PubMed] [Google Scholar]
- [23].Ohkawara T, Takeda H, Kato K, et al. Polaprezinc (N-(3-aminopropionyl)-L-histidinato zinc) ameliorates dextran sulfate sodium-induced colitis in mice. Scand J Gastroenterol 2005;40:1321–7. [DOI] [PubMed] [Google Scholar]
- [24].Yoshikawa T, Yamaguchi T, Yoshida N, et al. Effect of Z-103 on TNB-induced colitis in rats. Digestion 1997;58:464–8. [DOI] [PubMed] [Google Scholar]
- [25].Ainley CC, Cason J, Carlsson LK, et al. Zinc status in inflammatory bowel disease. Clin Sci (Lond, Engl 1979) 1988;75:277–83. [DOI] [PubMed] [Google Scholar]
- [26].Geerling BJ, Badart-Smook A, Stockbrugger RW, et al. Comprehensive nutritional status in recently diagnosed patients with inflammatory bowel disease compared with population controls. Eur J Clin Nutr 2000;54:514–21. [DOI] [PubMed] [Google Scholar]
- [27].Fernandez-Banares F, Mingorance MD, Esteve M, et al. Serum zinc, copper, and selenium levels in inflammatory bowel disease: effect of total enteral nutrition on trace element status. Am J Gastroenterol 1990;85:1584–9. [PubMed] [Google Scholar]
- [28].Chapman AL, Mocatta TJ, Shiva S, et al. Ceruloplasmin is an endogenous inhibitor of myeloperoxidase. J Biol Chem 2013;288:6465–77. [DOI] [PMC free article] [PubMed] [Google Scholar]


