Abstract
Background
Abnormal neutrophils are involved in many chronic endocrine diseases, including type 2 diabetes mellitus (T2DM), and in periodontitis (PD), which is a chronic inflammatory disease in which neutrophils play a vital role. The p38 mitogen-activated protein kinase (MAPK) signaling pathway participates in the apoptosis of many inflammatory cells. Additionally, 1,25-dihydroxyvitamin-D3 (1,25VitD3) as a regulator can induce responses to infection and tumor cell apoptosis. However, the effect of 1,25VitD3 in the pathogenic relationship between T2DM and PD remains unclear. The aim of this study was to assess the effect of 1,25VitD3 on neutrophil apoptosis in patients with T2DM and PD and the p38-MAPK-relevant signaling pathway mechanism in this process in vitro.
Methods
Neutrophils were stained with Wright's stain, and apoptosis was detected by flow cytometry and Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) staining. Apoptosis- and p38-related mRNAs and proteins were examined by real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR), Western blotting and ELISA. The internal relationships were analyzed using a linear regression equation and Pearson's correlation coefficient.
Results
The highest rate of neutrophil apoptosis occurred in cultures treated with 10–8 mol/L 1,25VitD3 in the T2DM-PD group. The apoptosis rate in the T2DM-PD-p38 inhibitor group was higher than that in the healthy control group. Western blot, ELISA and qRT-PCR results showed that the mRNA and protein expression profiles of Caspase-3 and Bax were highly up-regulated and that Bcl-2 was down-regulated in the T2DM-PD-p38 inhibitor group. The expression levels of apoptotic mRNAs and proteins in the T2DM and T2DM-PD groups were significantly higher than those in the T2DM-p38 and T2DM-PD-p38 inhibitor groups. 1,25VitD3-induced neutrophil apoptosis and phosphorylated p38 (p-p38) expression were partially inhibited by the p38 inhibitor. Expression levels of apoptosis-related genes and p-p38 in neutrophils were positively associated with increasing concentrations of 1,25VitD3. p-p38 protein expression was positively associated with the level of serum 1,25VitD3.
Conclusion
1,25VitD3 could promote peripheral blood neutrophil apoptosis in patients with T2DM and PD through activation of the p38-MAPK signaling pathway in vitro.
Keywords: 1,25-dihydroxyvitamin-D3; neutrophil apoptosis; p38 MAPK signaling pathway; periodontitis; type 2 diabetes mellitus
1. Introduction
Periodontitis (PD), an omnipresent disease and the sixth most widespread oral disease, has affected from 45% to 60% of the adult population in today's world, primarily due to tooth loss. [1–3] PD is an inflammatory disease mainly marked by attachment loss, periodontal pus pocket formation and gingival bleeding, even involving tooth loosening or displacement. Bacterial plaques proliferating on the teeth are the primary risk factor for PD, but the mechanism of PD in soft or solid tissues around the teeth mainly relies on inflammation induced by the bacteria and their metabolites.[4–6] Present research from the National Health and Nutrition Examination Surveys (NHANES) has reported the wide range of PD prevalence in male adults aged 65 years and older, which was greater than that of male adults 30 to 44 years of age; the prevalence was even greater in women.[7] According to recent research, PD is more likely to occur in adults with uncontrolled type 2 diabetes mellitus (T2DM).[8]
A recent survey reported that approximately 400 million individuals have been diagnosed with diabetes worldwide.[9] T2DM is a chronic and metabolic disease that affects the majority of diabetes patients and is characterized by hyperglycemia attributed to defective secretion of insulin or low insulin sensitivity and insulin resistance.[10–12] Diabetes has become a worldwide health problem due to its high morbidity and death rate combined with its multiple complications, including cardiovascular and cerebrovascular diseases, diabetic nephropathy and oral diseases.[13,14] Moreover, prolonged exposure to hyperglycemia is considered as the main diabetic complication.[15] Hence, the severity and clinical manifestations of PD in diabetes patients rely on the affected individual's level of blood glucose control. PD is the sixth major complication of T2DM. The pathogenesis of PD with T2DM may contribute to leukocyte chemotaxis and phagocytosis defects, lesions in the vascular basement membranes in tissues, reductions in collagen synthesis and bone matrix formation and hindrance of wound healing.[16,17] It is gradually becoming evident that T2DM and PD are intimately associated and closely linked by underlying biologic mechanisms within each individual as a function of the interplay between innate and acquired immune responses, genetic and epigenetic factors and external environmental factors. In particular, the inflammatory reaction plays a vital role in the progress of T2DM with PD.[18] Peripheral blood neutrophils (PMNs) or neutrophils as significant inflammatory cells are the front line of host defense in the innate immune system and have core positions in inflammatory reactions. Neutrophils are activated to identify invasive microorganisms and to clean dead host cells and their fragments. Once adhered, the chemotaxis and phagocytosis capacities of neutrophils are impaired and the release of inflammatory cytokines is increased, hindering the removal of pathologic bacterial microorganisms in periodontal tissues and aggravating tissue destruction, which may further lead to amplification of the systemic inflammatory impacts related to the course of PD with T2DM.[19,20]
Neutrophils are removed from inflammatory lesions via the initiation and activation of constitutive apoptosis (i.e., a procedure involving spontaneous apoptosis), which mediates the clearance of the apoptotic neutrophils through macrophages. Hence, at the last stage, efficient clearance of the neutrophils can alleviate the inflammatory reaction and restore the internal homeostasis.[21]
The main active form of vitamin D is 1,25-dihydroxyvitamin-D3 (1,25VitD3). In an early study of 1,25VitD3 treatment, this form of vitamin D was determined to be primarily involved in calcium phosphate metabolism. However, current evidence suggests that the immune-regulation effects of 1,25VitD3 can be widely applied as anti-inflammatory and immune-regulatory treatments, particularly in innate immunity, including the up-regulation of the expression of antimicrobial peptides, the promotion of phagocytic killing of pathogenic microorganisms, the down-regulation of inflammatory factor release and the reduction of inflammation.[22] Vitamin D deficiency is common in many parts of the world and is frequently combined with chronic disease. Recent studies have shown that vitamin D deficiency results in increased bacterial burden and neutrophil infiltration.[23–24] The major biological functions of 1,25VitD3 are primarily mediated via nuclear vitamin D receptor (nVDR) with high specificity and compatibility through the regulation of gene expression. Previous studies have demonstrated that nVDR is found in multiple immune cells, including neutrophils, macrophages, dendritic cells, and lymphoid B cells.[25] Recent research has also highlighted the significance of 1,25VitD3 as a locally active immune modulator for antigen-activated inflammatory cells.[26] However, the connection between 1,25VitD3 and the apoptosis of neutrophils in PD patients with T2DM remains to be determined.
In this study, we investigated the effect of 1,25VitD3 on the apoptosis of neutrophils, and we hypothesized that 1,25VitD3-induced neutrophil apoptosis is associated with the p38 mitogen-activated protein kinase (MAPK) pathway in PD patients with T2DM.
2. Materials and methods
2.1. Subjects and groups
This study sample consisted of a total of 107 participants (68 males, 39 females): 67 patients with PD and T2DM, 20 patients with simple T2DM and 20 healthy volunteers. This study was approved by the Research Ethics Committee of Zunyi Medical University Affiliated Hospital. All patients and volunteers were recruited from the Periodontology Department of Zunyi Medical University, and individuals’ informed consent was provided before drawing blood. All experiments were repeated 3 times.
All of the subjects were grouped as follows: a healthy control group (neutrophils of healthy individuals), a T2DM group (neutrophils of patients with T2DM), a T2DM-SB203580 group (neutrophils of patients with T2DM cultured with the p38 inhibitor SB203580), a T2DM-PD group (neutrophils of patients with T2DM, and PD), a T2DM-PD-SB203580 group (neutrophils of patients with T2DM and PD cultured with SB203580), a T2DM-PD-VitD3 group (neutrophils of patients with T2DM and PD cultured with 1,25VitD3), and a T2DM-PD-VitD3-SB203580 group (neutrophils of patients with T2DM and PD cultured with 1,25VitD3 and SB203580).
2.2. Inclusion criteria for patients with T2DM and PD
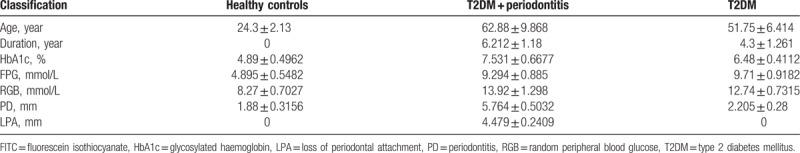
The subjects qualifying as healthy individuals were enrolled with normoglycemic status and had no systemic and partial infectious diseases (e.g., PD, furuncles, and other symptoms). The patients were diagnosed with simple T2DM based on the criteria of the WHO consultation with no other diabetic complications (Table 1),[27] and T2DM subjects with PD were selected according to the criteria of the American Academy of Periodontology (Table 2).[28] Clinical history and demographic data for all patients, including age, durations of T2DM, and PD, glycosylated hemoglobin (HbA1c), fasting plasma glucose (FPG), random peripheral blood glucose (RGB), PD, and loss of periodontal attachment (LPA) were recorded (Table 3).
Table 1.
Diagnostic criteria for T2DM.
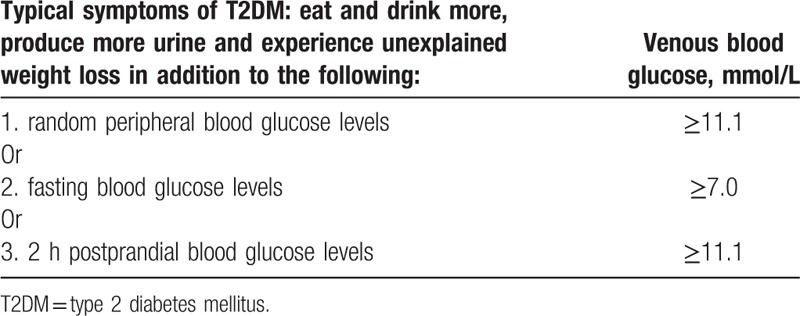
Table 2.
Diagnostic criteria for PD.

Table 3.
Clinical history and demographic properties of the study individuals (Mean ± Std).
2.2.1. Criteria of patients with T2DM
-
I.
The patients had been diagnosed with T2DM for at least 1 year and did not have inflammatory infections in their respiratory, digestive or urinary systems or in other organs;
-
II.
The recent status of diabetes mellitus and treatment dosage had not changed significantly;
-
III.
The patients did not have kidney, eye, or peripheral neuropathy complications;
-
IV.
The patients did not take vitamin D;
-
V.
The patients met the diagnostic criteria shown in Table 1 for T2DM.
2.2.2. Criteria of patients with PD (Table 2)
The peripheral blood samples were strictly collected from patients who met the inclusion and exclusion criteria. The peripheral blood neutrophils of these participants were collected and separated by density gradient centrifugation. The 10 mL samples of fasting venous blood were stored in ethylenediaminetetraacetic acid (EDTA) anticoagulant tubes, and the tubes were immediately transferred to the laboratory at 4°C.
2.3. Isolation, culture, and staining of neutrophils
2.3.1. Isolation and culture of neutrophils
After peripheral venous blood was collected into vacutainer tubes containing 10 U/mL heparin, the blood samples were delivered to the laboratory; blood was transferred into a centrifuge tube, and 10 mL of PolymorphprepTM separation medium was slowly added to the tube, after which the medium was centrifuged at a speed of 500 × g for 30 minutes at 20°C. The neutrophil layer was extracted with a sterile plastic straw and transferred to another centrifuge tube. Cells were centrifuged at 400 × g for 10 minutes at 20°C before the liquid was washed with 10 mL of phosphate buffer saline (PBS). After discarding the PBS, the cells were gently mixed with red blood cell lysis buffer and then washed with PBS. Then, RPMI 1640 culture medium (HyClone Company) supplemented with 10% fetal bovine serum (FBS) (Gibco Company) was added to suspend the cells, which were counted using a counting plate. The cells in each group were transferred into wells 15.6 mm in diameter. Every well in each group was examined 4 times: for flow cytometry, Western blot analysis, real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) and ELISA. Neutrophils were collected for subsequent experiments after being treated for 24 hours.
2.3.2. Wright's and trypan blue staining of neutrophils
Peripheral blood neutrophils were fixed with 4% paraformaldehyde for 15 minutes at 37°C. After the cells were washed with PBS 3 times and centrifuged, the neutrophils were resuspended in Wright's staining solution, and cover slips were slowly mounted onto the slides with 90% glycerol. The neutrophils were observed under an inverted phase contrast microscope. Trypan Blue staining was performed according to the manufacturer's instructions to observe the survival rate of neutrophils.
2.4. Treatment with 1,25VitD3 and the p38-specific inhibitor of 1,25VitD3 (SB203580)
Manipulations were performed at a sterile laboratory bench. Sixty microliters of sterile deionized water were added after 10 μg of 1,25VitD3 (Sigma Company) was completely solubilized in 250 μL of absolute ethyl alcohol. The 1,25VitD3 was then solubilized for 24 hours at 4°C; the total solution volume was brought to 25 mL, and the final concentration of 1,25VitD3 was adjusted to 1 × 10–4 mol/L. The 1,25VitD3 was stored in the dark at 4°C. When other concentrations were needed, the stock solution was diluted as described. dimethyl sulfoxide (DMSO) was used to dissolve the inhibitor, which was then stored at 4°C according to the manufacturer's instructions. Neutrophils of patients with T2DM and PD were pretreated with 1 × 10–6, 1 × 10–8 and 1 × 10–10 mol/L 1,25VitD3 in the preliminary experiments, and a dose of 1 × 10–8 mol/L 1,25VitD3 was used for additional experiments. The abovementioned groups of neutrophils were cultured for 24 hours at 37°C. The doses of SB203580 were chosen based on previous studies and preliminary experiments.
2.5. Neutrophil intervention assay using different concentrations of 1,25VitD3
Preliminary experiments were performed to identify the effects of different concentrations of 1,25VitD3 on neutrophils from patients with T2DM and PD, and very small differences between 2 adjacent concentrations were observed. Different concentrations of 1,25VitD3 (10–6, 10−8, and 10−10 mol/L) were chosen, and 10−8 mol/L 1,25VitD3 had a better effect on neutrophils than the other concentrations. Hence, the neutrophils in all of the groups were treated with the concentration of 10−8 mol/L 1,25VitD3 for 24 hours before experimentation.
2.6. Neutrophil apoptotic rate assay
The percentages of apoptotic neutrophils were determined using the Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) kit (Beckman Company) and flow cytometry. The data were analyzed using the Modfit and Cell Quest software programs (Becton Company). The apoptosis rate represents the proportion of apoptotic neutrophils measured minus the baseline value and was utilized for further analysis. Briefly, neutrophils in all the groups were collected and resuspended in binding buffer ([pH 7.5] 10 mM HEPES, 2.5 mM CaCl2 and 140 mM NaCl) after isolation and incubation with cell-culture media for 24 hours. The cellular density was adjusted to 106 cells/mL with the binding buffer, and 100 μL of cells was transferred to labeled flow cytometry tubes. Each group consisted of the following 4 parallel tubes: unlabeled, 5 μL of Annexin V-FITC only, 5 μL of PI only and 5 μL each of Annexin V-FITC and PI. After the samples were incubated with the reagents for 15 minutes at 25°C in the dark, 200 μL of binding buffer was added to every tube, and samples were analyzed using flow cytometry. In the SB203580 intervention groups, the neutrophils were pretreated with 10 μmol/L SB203580 for 0.5 hours followed by 24 hours incubation; the neutrophils in the T2DM-PD-1,25VitD3 group were incubated with 10−8 mol/L 1,25VitD3. Approximately 104 cells from every sample were counted. Neutrophils in the early stage of apoptosis stained positively for Annexin V-FITC; cells in the late stage stained positively for both Annexin V-FITC and PI.
2.7. Serum 1,25VitD3 analysis
The sera of patients with T2DM and PD were collected and extracted by density gradient centrifugation and adjusted to 20 μL of isotope-labeled internal standard to 200 μL of serum. After the samples were shaken, centrifuged and dried, the mixture was resuspended in 100 μL of 75% methanol solution. The 1,25VitD3 in serum was detected using the high-performance liquid chromatography-mass spectrometry (HPLC-MS) method (Shimadzu Company). Methanol with 0.2% formic acid and double distilled water was used as the flow medium for gradient eluting, and the flow rate was set at 1.0 mL/min. A mass spectrometer was used for quantitative analysis.
2.8. Western blot, ELISA, and qRT-PCR analyses of relative protein and gene expression levels
2.8.1. Western blot assay
2.8.1.1. Whole protein extraction from PMNs
The collected neutrophils were centrifuged at 500 × g for 5 minutes and washed with precooled PBS. An appropriate amount of radioimmunoprecipitation assay (RIPA) buffer containing phenylmethylsulfonyl fluoride (PMSF) was added to the cells, and the suspension was shaken on an oscillator at the highest speed for 15 seconds. The mixture was incubated on ice for 30 minutes; the cells were centrifuged at 16,000 × g for 5 minutes, and the supernatant containing whole proteins from neutrophils was retained. The protein concentration was detected using the bicinchoninic acid (BCA) protein assay kit (Thermo Company). SDS-PAGE loading buffer was added to the proteins and boiled for 5 minutes, and aliquots were stored at -80°C until use in Western blot analysis.
2.9. Nucleoprotein extraction from PMN
CER-I buffer and PMSF were added to the extracted neutrophils in 1.5 mL eppendorf tube (EP) tubes and shaken at the highest speed for 15 seconds. After 10 minutes of incubation on ice, the appropriate quantity of CER-II buffer was added; then, the cells were shaken, incubated for 1 minute, and centrifuged at 16,000 × g for 5 minutes. Most of the supernatant containing plasmocin was removed. Then, NER buffer and PMSF were added to mix the sediments, and the mixture was shaken and incubated on ice for 10 minutes 3 times. The admixture was centrifuged at 16,000 × g for 5 minutes. The nucleoprotein-containing supernatant was collected, and the nucleoprotein concentrations were detected using a BCA protein assay kit as described above.
The levels of p38 and phosphorylated-p38 in the protein samples from each group were measured using Western blot analysis. The monoclonal mouse anti-human GAPDH (ab8245; Abcam) served as a control for equal protein loading. Protein samples were transferred to precast SDS-PAGE gels using Tris-glycine buffer. Subsequently, the proteins in the gels were carefully transferred onto polyvinylidene difluoride (PVDF) membranes using the semidry method. The membranes were blocked with 5% evaporated milk for 2 hours at room temperature. PVDF membranes were incubated with mouse monoclonal antihuman phosphorylated-p38 (ab45381; Abcam) and p38 (ab31828; Abcam) antibodies overnight at room temperature. After the PVDF membranes were washed 3 times with TBST for 10 minutes, they were probed with fluorescently labeled mouse polyclonal secondary antibodies (LI-COR, Odyssey Company). The fluorescent signal was captured by the LI-COR Odyssey Infrared Imaging System. The relative signal intensities of the bands were measured using ImageJ analysis software.
2.9.1. Expression of apoptosis-related protein by enzyme-linked immunosorbent assay (ELISA)
The peripheral blood neutrophils in each group were isolated and collected by density gradient centrifugation at a speed of 500 × g for 30 minutes at 20°C. The cells were washed with PBS 2 times for 5 minutes each at room temperature. Then, the whole proteins in each group of neutrophils were repeatedly frozen and thawed using liquid nitrogen at -196°C and water bath at 37°C 2 times. Human Bax (E09344 h, Cusabio), Caspase-3 (E08856 h, Cusabio), Bcl-2 (EL002613HU, Cusabio), and 1,25VitD3 (E08097 h, Cusabio) protein levels were detected by double antibody sandwich ELISA in each group. Standard and sample were added per well and covered with the adhesive strip. The plate was then incubated for 30 minutes at 37°C. Standard concentration curves were generated as follows: Bax: 0, 1.25, 2.5, 5.0, 10.0, 20.0, 40.0, 80.0 ng/mL; Caspase-3: 0, 0.312, 0.625, 1.25, 2.5, 5.0, 10.0, 20.0 ng/mL; Bcl-2: 0, 0.625, 1.25, 2.5, 5.0, 10.0, 20.0, 40.0 ng/mL; and 1,25VitD3: 0, 20, 40, 80, 100 ng/mL in strict accordance with the kit instructions. All wells in the plate were aspirated and washed, repeating the process 2 times for a total of 3 washes. The wells were washed by filling with 200 μL of washing buffer and left to stand for 2 minutes. Any remaining washing buffer was removed by aspirating after the last wash. The plate was inverted and blotted against clean paper towel. To each well was added 100 μL of HRP-conjugate; the plate was then covered with a new adhesive strip, and after being left to incubate at 37°C for 30 minutes, the wells were aspirated and washed 5 times. To the wells was added 90 μL of TMB, and the substrate was then incubated at 37°C for 20 minutes in the dark. Then, 50 μL of stop solution was added to each well, and the plate was then tapped to ensure thorough mixing for 5 minutes. An enzyme mark instrument measured the ceiling light value at 450 nm and used the standard logarithmic concentrations as the abscissae and the measured OD values as the ordinates to draw the standard curves according to the OD values to calculate the concentration of each index.
2.9.2. qRT-PCR test
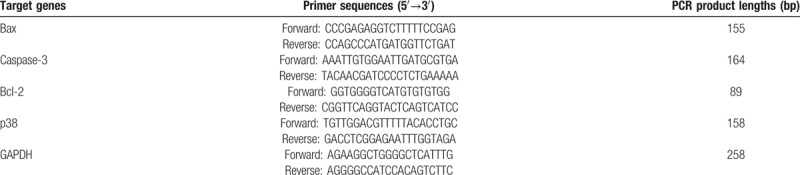
The peripheral blood neutrophils in all of the groups were isolated by density gradient centrifugation at a speed of 500 × g for 30 minutes at 20°C. Cells were washed 3 times with precooled PBS, and then the cells were collected. Total RNA was extracted from the 107 cells using 1 mL of RNAiso plus (TAKARA Company) and shaken vigorously for 15 seconds. Then, 200 μL of chloroform was added to the tube, inverted, shaken for 15 seconds and incubated for 5 minutes at room temperature. The tube was then centrifuged at 10000 g for 10 minutes at 4°C without vortexing. The supernatant liquid was extracted, and the same volume of isopropanol was added. The mixture was then vortexed violently for 15 seconds, then stand for 15 minutes at room temperature. The cells were centrifuged at 10000 g for 10 minutes at 4°C. The sediment was washed with 1 mL of 75% ethanol 2 times. The supernatant was removed, and the tube was inverted on a clean Kimwipe for drying. The total RNA collected was dissolved in DEPC water, and the concentration was normalized to 1000 ng/mL. The cDNAs were generated by reverse transcription from total RNA using the “PrimeScript RT reagent Kit” (RR037A, TAKARA Company). The following reaction mixture was placed on ice. Gene expression levels of Bax, Caspase-3, Bcl-2, and p38 were detected by RT-PCR. The amplification conditions were as follows: predenaturation at 95°C for 30 seconds, denaturation at 95°C for 5 seconds and annealing at 60°C for 30 seconds; the human GAPDH gene was used as the internal reference. The sequences and product lengths for the PCR primers are displayed in Table 4. The relative mRNA expression levels between groups were analyzed using the 2ΔΔCT method.
Table 4.
Primer sequences and product lengths of apoptosis-related genes.
2.10. Statistical analysis
A 3-way analysis of variance was performed to examine the differences. All experiments were conducted in triplicate, and the data are expressed as mean ± standard deviations (std). Statistical analyses were conducted using the SPSS 14.0 software package, and Fisher and Student-Newman-Keuls q tests were conducted for comparisons between groups. Pearson equation and coefficient were used to estimate the fitting level. The threshold for statistical significance was set at P <.05.
3. Results
3.1. Culture and morphology of peripheral blood neutrophils
Isolated neutrophils cultured for 24 hours were stained with Wright's stain and observed under an inverted phase contrast microscope; the purity was greater than 95%. Intracellular granulocyte granules were observed by the Wright's staining. The cytoplasm of neutrophils exhibited neutral and slightly red color in which many particles presenting neutral pink and purple colors after staining were distributed. Large amounts of neutral nonbasophilic and noneosinophilic granules were observed in the cytoplasm of neutrophils. The nuclei of neutrophils exhibited a multilobulated shape, and the nuclei had a characteristic lobed appearance, mostly consisting of nuclei divided into 2 to 5 lobes; multiple nuclei fusions were commonly observed. Trypan Blue exclusion analysis revealed that 95% to 98% of the cells were alive (Fig. 1A).
Figure 1.
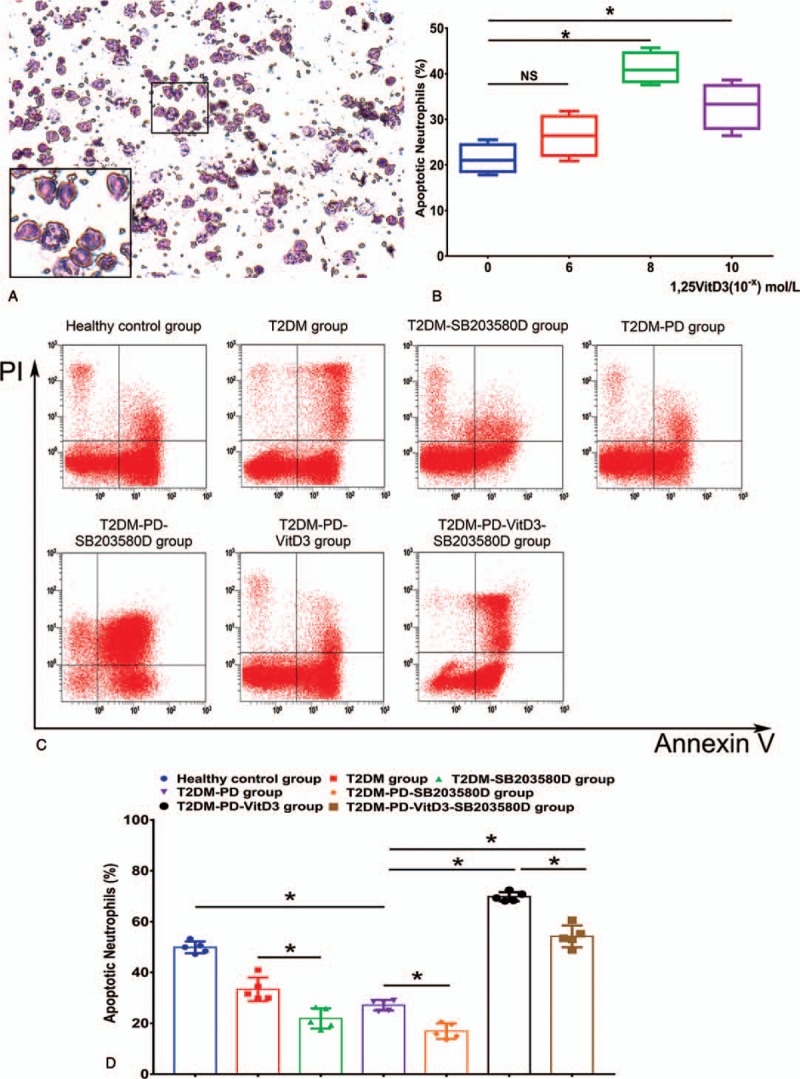
Morphology of isolated peripheral blood neutrophils; 1,25VitD3-induced apoptosis of peripheral blood neutrophils from patients with T2DM and PD in a dose-dependent manner, and flow cytometry of 1,25VitD3-induced apoptosis of neutrophils in each group. Peripheral blood neutrophils were stained with Wright's stain and presented a slightly red color in which many particles distributed in the cells presented a neutral pink and purple color. The nuclei of neutrophils exhibited a multilobulated shape. Large amounts of neutral nonbasophilic and noneosinophilic granules were observed in the cytoplasm of neutrophils. The nuclei had a characteristic lobed appearance, mostly containing a nucleus divided into 2 to 5 lobes; multiple nuclei fusions were commonly observed (Fig. 1A). (Original magnification 200 × , scale bar in Fig. 1A: 200 μm). Neutrophils were cultured for 24 hours in the presence of 0, 1 × 10–6, 1 × 10–8, or 1 × 10–10 mol/L 1,25VitD3 as indicated, and neutrophil apoptosis was assessed by flow cytometry. Treatment with 1,25VitD3 at the dose 1 × 10–8 mol/L induced the highest rate of neutrophil apoptosis compared with the other groups (Fig. 1B) (∗P <.05, NS: P>.05). A total of 1 × 106 of peripheral blood neutrophils were pretreated with 10 μmol/L SB203580 in the T2DM-PD, T2DM-PD-SB203580, and T2DM-PD-VitD3-SB203580 groups for 0.5 hours, and neutrophils in the T2DM-PD-VitD3-SB203580 group were then incubated with 1 × 10–8 mol/L 1,25VitD3 for 24 hours. According to the analysis data, the RUQ represents the terminal apoptotic and necrotic neutrophils (FITC [+], PI [+]). The RLQ represents the early apoptotic neutrophils with positive Annexin V and negative PI (FITC [+], PI [-]). The LLQ represents the vital and living neutrophils with negative Annexin V and PI expression (FITC [+], PI [-]). The counted numbers of apoptotic neutrophils in the right lower quadrant are displayed (Fig. 1C). The total cell counts are shown as the % of 106 cells. Neutrophils in the T2DM-PD-VitD3 group expressed the highest apoptotic level among all of the groups, whereas neutrophils in the T2DM-PD-SB203580 group expressed the lowest apoptotic level (Fig. 1D). The data are presented as the mean ± std (∗P <.05, NS: P >.05). 1,25VitD3 = 1,25-Dihydroxyvitamin-D3, FITC = fluorescein isothiocyanate, LLQ = lower left quadrant, PD = periodontitis, PI = propidium iodide, RLQ = right lower quadrant, RUQ = right upper quadrant, T2DM = type 2 diabetes mellitus.
3.2. 1,25VitD3 induces neutrophil apoptosis in T2DM and PD patients
The rate of neutrophil apoptosis exhibited a dose-dependent relationship with 1,25VitD3, and the apoptotic rate was upregulated with the increasing 1,25VitD3 level in the range of 1 × 10–0 to 1 × 10–8 mol/L. The higher or lower (i.e., 1 × 10–0 and 1 × 10–10mol/L) dose of 1,25VitD3 did not significantly affect apoptosis compared with that of other groups (P >.05). However, median doses (i.e., 1 × 10–8 mol/L) of 1,25VitD3 significantly increased the neutrophil apoptosis rate in patients with T2DM and PD (P <.05) (Fig. 1B). Thus, we used a 1 × 10−8 mol/L dose of 1,25VitD3 in the subsequent experiments.
3.3. Analysis of neutrophil apoptosis in groups by flow cytometry
The Annexin V data illustrate the differences in the apoptotic rates of neutrophils in each group. First, the neutrophil apoptosis rate was apparently lower in the T2DM-PD group than that in the healthy control group (Fig. 1C) (P <.05). When using SB203580, the neutrophil apoptosis rate in the T2DM group was significantly higher than that in the T2DM-SB203580 group (P <.05). This difference was also similar in other groups; the neutrophil apoptosis rate in the T2DM-PD group was markedly higher than that in the T2DM-PD-SB203580 group, and the apoptotic rate in the T2DM-PD-1,25VitD3 group was higher than that in the T2DM-PD-1,25VitD3-SB203580 group with significant differences (P <.05). The neutrophil apoptosis rate in the T2DM-PD-1,25VitD3 group was significantly increased compared with that in the T2DM-PD group, and the T2DM-PD-1,25VitD3 group showed the highest apoptotic rate after 24 hours of treatment using 1,25VitD3 compared with the other groups (P <.05). With the combination of 1,25VitD3 and SB203580, the neutrophil apoptosis rate in the T2DM-PD-1,25VitD3-SB203580 group was significantly higher than that in the T2DM-PD group (P <.05) (Fig. 1D).
3.4. Western blot analysis
The Western blot data show variations in phosphorylated p38 (p-p38) and p38 expression levels in neutrophils in each group and show the effect of a p38 MAPK inhibitor on 1,25VitD3-induced neutrophil apoptosis. We also observed the effects of the p38-specific inhibitor SB203580 cultured for 24 hours on 1,25VitD3-induced neutrophil apoptosis. The p38 expression levels were essentially the same and were not significantly different among all of the groups (P >.05) (Fig. 2A). p-p38 expression was lower in the T2DM group than in the healthy control group, with a more apparent, significantly different reduction in the T2DM-PD group. The p-p38 expression level in peripheral blood neutrophils was markedly increased upon treatment with 1,25VitD3 compared to that in the nontreated groups (P <.05). When using SB203580, the p-p38 expression level in neutrophils was notably reduced in the T2DM-SB203580 group compared with that in the T2DM group; a similar pattern of neutrophil apoptosis reduction was observed for the T2DM-PD-SB203580 group compared with that of the T2DM-PD group (P <.05). Moreover, the p-p38 protein level in the T2DM-PD-1,25VitD3-SB203580 group was markedly lower than that in the T2DM-PD-1,25VitD3 group with p-p38 expression being the highest in the T2DM-PD-1,25VitD3 group (P <.05) (Fig. 2B).
Figure 2.
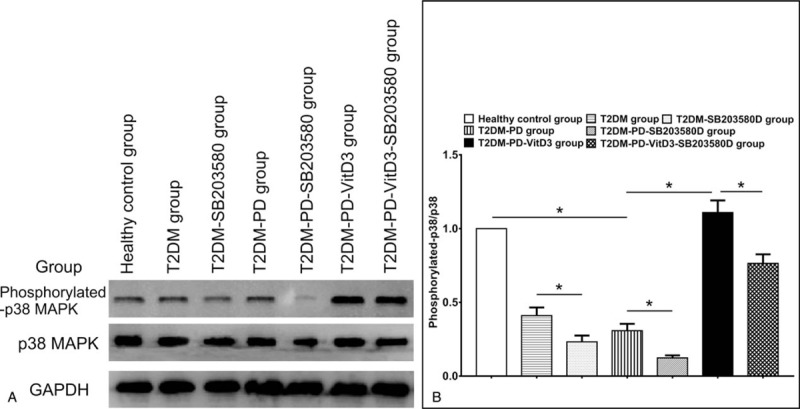
Effects of 1,25VitD3 on p38 and phosphorylated-p38 MAPK protein expression levels in peripheral blood neutrophils in each group. Expression levels of p38 and p-p38 MAPK in all of the groups as determined by Western blot (Fig. 2A). The p38 protein expression did not show significant differences in neutrophils among any of the groups. The p-p38 MAPK protein expression was higher in the healthy control group than in the T2DM and T2DM-PD groups, and the T2DM-PD-VitD3 group expressed the highest level among the groups. The p38 and GAPDH served as a control for equal protein loading (Fig. 2B). Representative results of at least 3 experiments are shown (∗P <.05, NS: P >.05). 1,25VitD3 = 1,25-Dihydroxyvitamin-D3, MAPK = mitogen-activated protein kinase, p-p38 = phosphorylated p38, T2DM = type 2 diabetes mellitus.
3.5. Gene expression levels of Bax, Caspase-3, Bcl-2, and p38 by qRT-PCR
The qRT-PCR results show that the mRNA expression levels of Bax and Caspase-3 had increased the most in the T2DM-PD-1,25VitD3 group, which expressed the highest levels among the other groups; upon treatment with 1,25VitD3 and SB203580, Bax and Caspase-3 gene expression levels were greatly reduced in the T2DM-PD-1,25VitD3-SB203580 group and were expressed the lowest in the T2DM-PD-SB203580 group (P <.05) (Fig. 3A, B). Upon treatment with SB203580, Bax and Caspase-3 gene expression levels in the T2DM-SB203580 and T2DM-PD-SB203580 groups were reduced significantly compared with those in the T2DM and T2DM-PD groups, respectively (P <.05). The mRNA expression level of the apoptosis inhibitory factor Bcl-2 was contrary to those of Bax and Caspase-3; upon treatment with 1,25VitD3, the Bcl-2 expression level in the T2DM-PD-1,25VitD3 group was downregulated greatly and was the lowest compared with that in the other groups (P <.05) (Fig. 3C). The T2DM-PD-SB203580 group had the highest Bcl-2 mRNA expression level of all of the groups following treatment with SB203580 (P <.05). Compared with healthy individuals, that is, the control group, after a 24 hours treatment with 1,25VitD3, the p38 mRNA expression levels in each group were not significantly different (P >.05) (Fig. 3 D).
Figure 3.
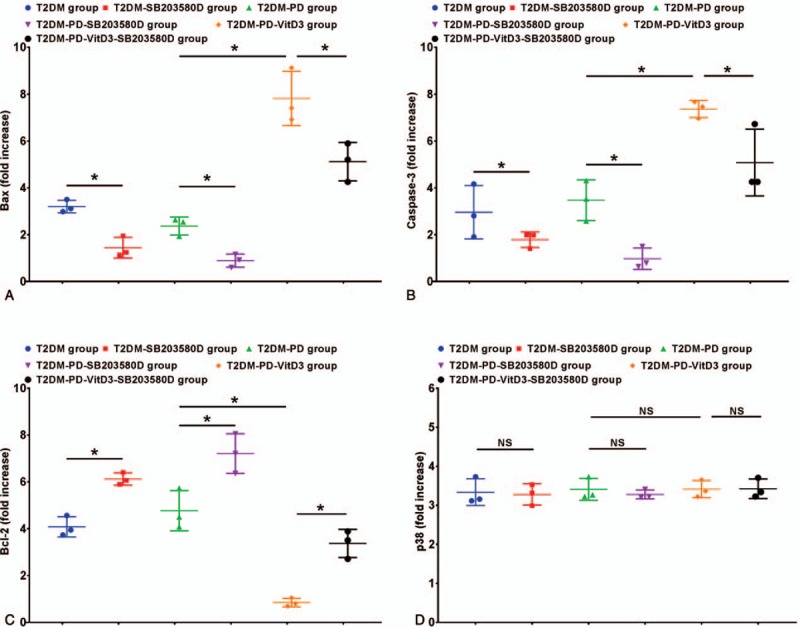
Expression of apoptosis-related mRNAs of peripheral blood neutrophils in each group. The expression levels of the apoptosis-related mRNAs Bax (Fig. 3A) and Caspase-3 (Fig. 3B) were significantly increased upon treatment with 1,25VitD3 compared with the other groups. Among all of the groups, the T2DM-PD-VitD3 group showed the greatest increase in Bax mRNA expression. Caspase-3 expression in the T2DM-PD-VitD3 group differed significantly from that in the other groups; the T2DM-PD-VitD3 group also showed higher expression of Caspase-3 than that in the other groups. The mRNA expression levels of the antiapoptotic protein Bcl-2 (Fig. 3 C) were different in all of the groups; the Bcl-2 expression level in the T2DM-PD-SB203580 group was higher than that in all the other groups. Bcl-2 expression was decreased upon treatment with 1,25VitD3; the T2DM-PD-VitD3 group expressed the lowest Bcl-2 expression level among all of the groups. The expression of p38 mRNA did not show significant differences among the groups (Fig. 3D). The data are presented as the mean ± std (∗P <.05, NS: P >.05). 1,25VitD3 = 1,25-Dihydroxyvitamin-D3, T2DM = type 2 diabetes mellitus.
3.6. Detection of Bax, Caspase-3, Bcl-2 and 1,25VitD3 proteins in neutrophils by ELISA
The Bax, Caspase-3, Bcl-2, and 1,25VitD3 protein expression levels in peripheral blood neutrophils in each group were detected by ELISA. Similar patterns were observed for Bax and Caspase-3 protein expression in neutrophils in the T2DM-PD-1,25VitD3 group, with higher expression than in the other groups (P <.05). Compared with the Bax and Caspase-3 protein expression levels in the healthy control, the levels of these proteins were significantly reduced in the T2DM and T2DM-PD groups with the reduction tendency being more apparent in the T2DM-PD group (P <.05). Following treatment with SB203580, Bax and Caspase-3 protein levels in the T2DM-SB203580 and T2DM-PD-SB203580 groups were reduced significantly compared with those in the T2DM and T2DM-PD groups, respectively; the T2DM-PD-SB203580 group expressed the lowest protein levels of Bax and Caspase-3 among the other groups (P <.05). The Bax and Caspase-3 expression levels in T2DM-PD-1,25VitD3-SB203580 group were subsequently reduced following treatment with 1,25VitD3 and SB203580, but the expression levels of the 2 proteins in the T2DM-PD-1,25VitD3-SB203580 group were still higher than those in the groups without 1,25VitD3 treatment (P <.05) (Fig. 4A, B). In contrast, the expression levels of the antiapoptotic protein Bcl-2 increased markedly in the T2DM-PD-SB203580 group, which showed the highest expression (P <.05) (Fig. 4C). Treatment with 1,25VitD3 decreased the Bcl-2 protein expression level, which was the lowest and significantly different compared with the level in the other groups (P <.05). The 1,25VitD3 levels in 3 types of subjects were measured. Healthy individuals had the highest 1,25VitD3 levels, and the 1,25VitD3 levels in the T2DM group were significantly higher than those in the T2DM-PD group (P <.05) (Fig. 4D).
Figure 4.
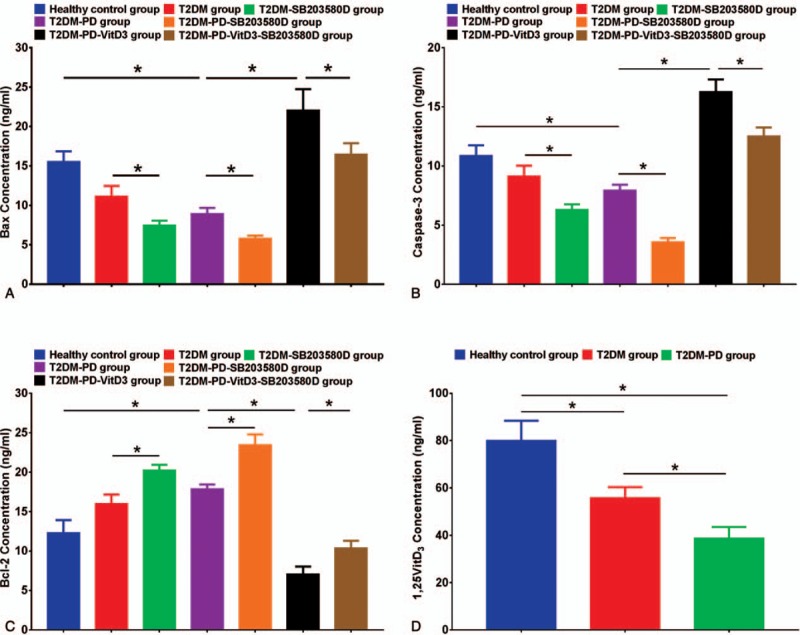
Apoptosis-related protein expression in peripheral blood neutrophils and 1,25VitD3 levels in each group based on ELISA. The expression level of the apoptosis-related protein Bax was significantly increased after treatment with 1,25VitD3, and Bax expression was highest in the T2DM-PD-VitD3 group compared with that in the other groups. The T2DM-PD-VitD3-SB203580 group showed limited relative Bax expression. Bax protein levels were significantly greater in the T2DM and T2DM-PD groups than those in the T2DM-SB203580 and T2DM-PD-SB203580 groups, respectively. Compared with the T2DM, T2DM-SB203580 and T2DM-PD groups, the healthy control group showed strong expression of Bax (Fig. 4A). A similar pattern was observed for Caspase-3 protein expression with expression being the highest in the T2DM-PD-VitD3 group (Fig. 4B). Bcl-2 expression was significantly increased following treatment with the p38 inhibitor with expression being the highest in the T2DM-PD-SB203580 group. However, Bcl-2 protein expression was decreased after application of 1,25VitD3 with the T2DM-PD-VitD3 group exhibiting lower Bcl-2 expression than the other groups (Fig. 4C). The 1,25VitD3 levels in neutrophils were highest in healthy individuals among the groups, and the 1,25VitD3 levels in simple T2DM patients were higher than those in T2DM with periodontitis patients (Fig. 4D). The data are presented as the mean ± std deviations (P <.05, NS: P >.05). 1,25VitD3 = 1,25-Dihydroxyvitamin-D3, ELISA = enzyme-linked immunosorbent assay, T2DM = type 2 diabetes mellitus.
3.7. Relevance analysis of 1,25VitD3 and the p38 pathway
The linear regression analysis showed that the rate of neutrophil apoptosis was positively correlated with serum 1,25VitD3 (R2 = 0.65) (Fig. 5A-a) and p-p38 protein (R2 = 0.71) levels in PD patients with T2DM (Fig. 5A-b) (P <.05) in which the connection was closer to a dose-dependent relationship. Moreover, p-p38 protein was positively correlated with the serum 1,25VitD3 level in PD patients with T2DM (Fig. 5A-c) (R2 = 0.77, P <.05).
Figure 5.
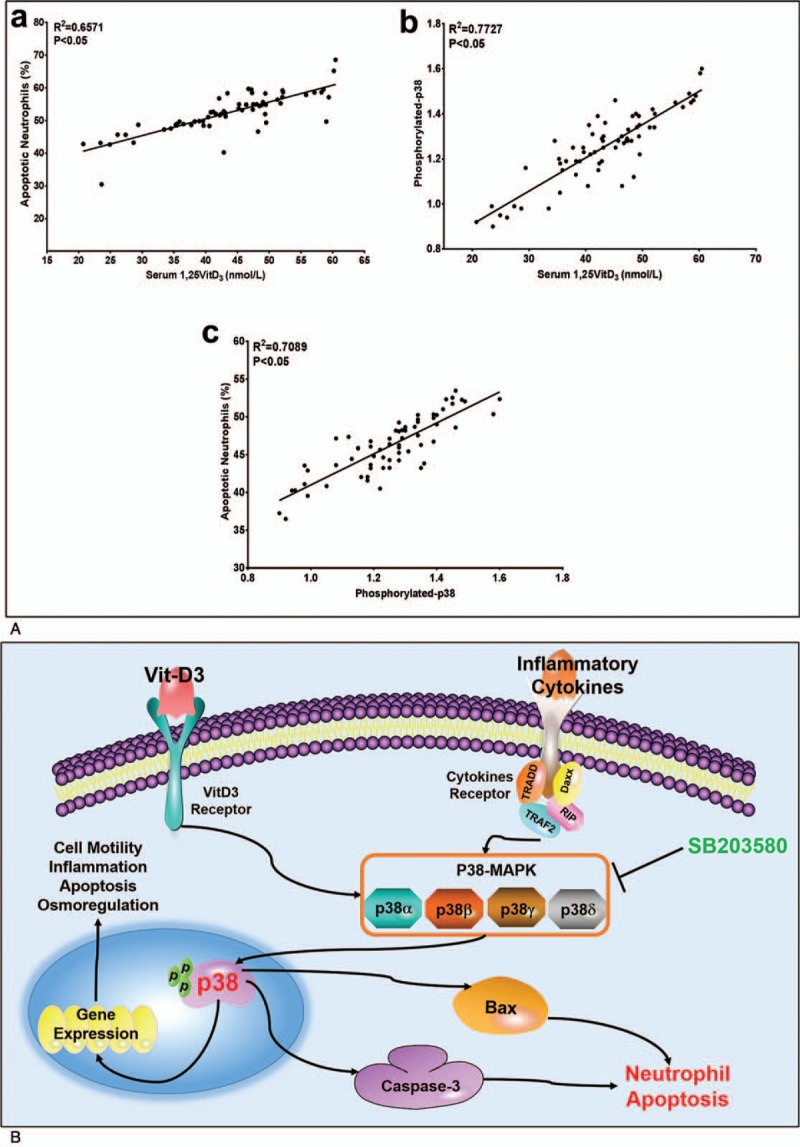
Correlation and regression analysis among 1,25VitD3 level, neutrophil apoptotic rate and p-p38 expression in T2DM and periodontitis patients. Serum 1,25VitD3 levels were associated with apoptotic neutrophils (Fig. 5A-a) and phosphorylated-p38 expression in T2DM and periodontitis patients (Fig. 5A-b). The relationship between phosphorylated-p38 and apoptotic neutrophils presents a linear correlation in T2DM and periodontitis patients (Fig. 5A-c). The R-square and P values were assessed using Pearson's correlation analysis. A proposed model of action is that p38 signaling upregulates 1,25VitD3-induced apoptosis in neutrophils, which in turn induces multiple apoptotic factors. A convergence of 1,25VitD3 and p38 signaling leads to efficient neutrophil reduction (Fig. 5B). 1,25VitD3 = 1,25-Dihydroxyvitamin-D3, T2DM = type 2 diabetes mellitus.
4. Discussion
T2DM is a disease characterized by chronic high blood glucose and other metabolic abnormalities. The major complications of T2DM include peripheral vascular disease, coronary artery and cerebrovascular diseases, delayed wound healing and PD, which are primarily recognized as due to prolonged exposure to high blood glucose levels.[29] PD as an inflammatory complication plays an essential role in the pathological process of T2DM. However, neutrophils in a state of malfunction, with abnormal adherence, accumulation, chemotaxis, and phagocytosis as potential mechanisms, are linked to PD due to impaired host immune resistance to inflammation.[30,31] Our demographic data support the viewpoint of the literature: the rates of spontaneous apoptosis of neutrophils in healthy individuals are higher than those in the T2DM-PD group. Due to the increasing recruitment of neutrophils, the rates of neutrophil apoptosis in the T2DM-PD group are lower than those in the T2DM group. Hence, treatments for T2DM can have beneficial effects on oral diseases: maintaining normal blood sugar levels could slow the speed of attachment loss, thus reducing the symptoms of PD. Based on previous studies, systematic treatment of PD can effectively decrease HbA1c levels by 10.8% in patients with T2DM.[32–34] The sterilizing functions of neutrophils are important factors that contribute to the high incidence of morbidity and death of patients with T2DM.[35]
Some functions of neutrophils are preserved in patients with T2DM, including phagocytosis and bactericidal functions.[36] In addition, neutrophils contribute to inflammation due to the release and synthesis of inflammatory cell factors, which may cause the degradation and bone resorption of paradental soft tissue.[37] Neutrophils remove invading pathogenic microorganisms but may induce immunological injury to periodontal tissues due to overreaction.[38] Based on this evidence, neutrophils could protect the periodontia;[39,40] thus, our data showed that the neutrophil apoptosis rates and the expression levels of apoptotic proteins and genes were higher in the healthy control group than in the T2DM and T2DM-PD groups and that the expression of Bcl-2, an early state antiapoptotic protein, was higher in the T2DM-PD-SB203580 group than in other groups. Recent work illustrated that 1,25VitD3 deficiency correlates with severity in many types of inflammatory and infectious diseases, including joint infection, chronic obstructive pulmonary disease, and PD.[41–43] A recent study showed that serum vitamin-25D deficiency has the potential to be an easily modifiable risk factor in the prevention of PD.[44–46] Taking this information into account, we performed in vitro treatment with 1,25VitD3 on neutrophils from T2DM-PD patients to
-
1)
evaluate the expression levels of apoptotic mRNAs and proteins in neutrophils and
-
2)
explore p38 MAPK as a potential signaling pathway participating in 1,25VitD3-induced neutrophil apoptosis.
In our study, the apoptosis rate of neutrophils in the T2DM-PD-1,25VitD3 group was apparently higher than that in the T2DM-PD group, indicating that the 1,25VitD3 levels may play a significant role in modulating apoptosis in patients with T2DM and PD. However, the mechanisms underlying the effects of 1,25VitD3 on T2DM and the pathogenesis of PD are poorly understood. MAPK is a classical signal transduction pathway regulating multiple cytokines and has been a hot spot in a number of research fields, and p38 is a type of MAPK pathway protein that is mainly located in the cytoplasm in the resting state. After activation, p38 is transferred into the nucleus, where it regulates the expression levels of various mRNAs and proteins via phosphorylated transcription factors.[47] Based on previous studies, the p38 MAPK pathway is a vital signaling pathway involved in regulating inflammatory responses.[48] As shown in a study by Frasch et al,[49] the p38 MAPK signaling pathway plays an important role in human neutrophil apoptosis. The most attractive characteristic of the p38 MAPK signal transduction pathway is the inflammatory cytokines, which are upstream of the p38 MAPK target signal. These cytokines include interleukin-1β (IL-1β), TNF-α, IL-6, and prostaglandin E2, which are generated by the periodontal tissue. Moreover, cells that synthesize prostaglandin, including activated monocytes and macrophages, are regulated by p38 MAPK.[50–53] These cytokines induce the secretion of other inflammatory mediators, such as matrix metalloproteinase, prostaglandin, and receptor activator of nuclear factor kappa-B ligand (RANKL), which ultimately lead to osteoclast proliferation and tissue destruction.[54] Caspase-3, Bax, and Bcl-2 are the significant transcription markers of p38 MAPK that regulate the expression of inflammation-related cytokines, proteases, adhesion molecules and receptors. Activated p38 MAPK can inhibit Caspase-3 and Bax mRNA transcription functions via a protein-to-protein model that modulates IL-1, IL-6, IL-2, IL-8, and TNF-α and other inflammatory factors’ expression levels.[55,56] On the basis of the data in our study, p-p38 MAPK protein and apoptotic mRNAs, including Caspase-3 and Bax, were significantly increased in neutrophils following treatment with 1,25VitD3; in contrast, expression of the antiapoptosis protein Bcl-2 was decreased in the T2DM-PD-1,25VitD3 group, illustrating that the p38 MAPK signaling pathway participates in the induction of neutrophil apoptosis with 1,25VitD3. However, upon using a p38 MAPK inhibitor, neutrophil apoptosis decreased in the T2DM-SB203580 and T2DM-PD-SB203580 groups compared with that in the T2DM and T2DM-PD groups regardless of the mRNA or protein expression levels, demonstrating that the p38 MAPK pathway plays a key role in the induction of neutrophil apoptosis via 1,25VitD3. Remarkably, after treatment with SB203580, the apoptotic index in the T2DM-PD-1,25VitD3-SB203580 group was higher than that in the T2DM-PD group, indicating that either the p38 MAPK signaling pathway did not completely regulate the pathological mechanism or a unique molecular mechanism exists in patients with T2DM and PD. Thus, further studies are required to determine whether other apoptotic signaling pathways affect neutrophil apoptosis in patients with T2DM and PD.
1,25VitD3 insufficiency and deficiency may affect the phosphorylation of p38 MAPK and subsequently decrease neutrophil apoptosis, which may be one possible important pathogenic mechanism of the combination of T2DM and PD. Wang et al used vitamin D3 to treat rats with T2DM and PD and found that vitamin D3 controlled the expression of Toll-like receptor 4 (TLR4) in gingival inflammatory cells and reduced the expression of tumor necrosis factor-α (TNF-α).[57,58] In another study, vitamin D3 intervention increased the proliferation of bone marrow-derived stem cells and enhanced bone formation in rats with T2DM and PD.[59] Poon et al studied the combination of vitamin D3 and vitamin K2 to determine whether it increased the quantity and migration of osteoblasts in rats with T2DM; the expression levels of osteogenic transcription factors and related metabolic markers were upregulated, potentially reducing the absorption of alveolar bone.[60–62] 1,25VitD3 restrains the hyperactive immunological response, upregulates anti-inflammatory cytokines, and downregulates inflammatory factors through multiple signaling pathways that exert anti-inflammatory and protective effects on islet cells. As a result, 1,25VitD3 and its derivatives might be used to treat elderly patients with T2DM and PD.[63] As shown in our study, upon treatment with 1,25VitD3, the apoptotic indexes in the T2DM-PD-1,25VitD3 and T2DM-PD-1,25VitD3-SB203580 groups increased significantly compared with those of the other groups. Therefore, 1,25VitD3 and its analogs might have a supplementary role in the treatment of both T2DM and PD (Fig. 5B).
Our experiments have some limitations. First, we determined the apoptosis rate of neutrophils treated with 1,25VitD3 in vitro but did not evaluate the efficiency of apoptosis in neutrophils in which p38 MAPK was activated by 1,25VitD3. Therefore, we did not determine the number of apoptotic cells or their rate of apoptosis. Efficient apoptosis is considered one of the most important effects of 1,25VitD3 and the main reason for the enhanced curative outcomes associated with treatment. Nonetheless, neutrophils underwent apoptosis after a 24 hours treatment with 1,25VitD3. A longer study should be conducted to determine whether the p38 MAPK signaling pathway is the major mechanism by which 1,25VitD3 mediates apoptosis of neutrophils from patients with T2DM and PD over time.
In summary, 1,25VitD3 induced the apoptosis of peripheral blood neutrophils from patients with T2DM and PD, and the apoptosis mechanism was related to the p38 MAPK signaling pathway.
Acknowledgments
The authors thank Pro. Luchuan Liu from Daping Hospital, the Army Medical University, for providing abundant technical support. We also appreciate Dc. Kun Yang from the Army Medical University for editing and submission consultation.
Author contributions
Conceptualization: Junyu Liu, Qi Liu.
Data curation: Yaping Tang, Hui Fang, Chengwei Guo.
Formal analysis: Yaping Tang, Ruidi Xie.
Funding acquisition: Qi Liu.
Investigation: Yaping Tang.
Methodology: Yaping Tang, Yanmei Yan, Ruidi Xie, Qi Liu.
Project administration: Yaping Tang, Qi Liu.
Resources: Yanmei Yan, Qi Liu.
Software: Junyu Liu, Chengwei Guo.
Supervision: Qi Liu.
Visualization: Yaping Tang, Hui Fang, Chengwei Guo.
Writing – original draft: Yaping Tang.
Writing – review & editing: Qi Liu.
Footnotes
Abbreviations: 1,25VitD3 = 1,25-dihydroxyvitamin-D3, BCA = bicinchoninic acid, ELISA = enzyme-linked immunosorbent assay, FITC = fluorescein isothiocyanate, HbA1c = glycosylated hemoglobin, MAPK = mitogen-activated protein kinase, PBS = phosphate buffer saline, PD = periodontitis, PI = propidium iodide, PMSF = phenylmethylsulfonyl fluoride, p-p38 = phosphorylated p38, PVDF = polyvinylidene difluoride, qRT-PCR = real-time quantitative reverse transcription polymerase chain reaction, T2DM = type 2 diabetes mellitus.
This work was supported by a grant funded to Qi Liu from the National Natural Science Foundation of China (No. 81360168). The authors have no conflicts of interest to disclose.
References
- [1].Petersen PE, Ogawa H. The global burden of periodontal disease: towards integration with chronic disease prevention and control. Periodontology 2000 2012;60:15–39. [DOI] [PubMed] [Google Scholar]
- [2].He W, You M, Wan W, et al. Point-of-care periodontitis testing: biomarkers, current technologies, and perspectives. Trends Biotechnol 2018;36:1127–44. [DOI] [PubMed] [Google Scholar]
- [3].Potempa J, Mydel P, Koziel J. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat Rev Rheumatol 2017;13:606–20. [DOI] [PubMed] [Google Scholar]
- [4].Bosshardt DD. The periodontal pocket: pathogenesis, histopathology and consequences. Periodontology 20002018;76:43–50. [DOI] [PubMed] [Google Scholar]
- [5].Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers 2017;3:17038. [DOI] [PubMed] [Google Scholar]
- [6].Papapanou PN, Susin C. Periodontitis epidemiology: is periodontitis under-recognized, over-diagnosed, or both. Periodontology 2000 2017;75:45–51. [DOI] [PubMed] [Google Scholar]
- [7].Eke PI, Wei L, Borgnakke WS, et al. Periodontitis prevalence in adults>/= 65 years of age, in the USA. Periodontology 20002016;72:76–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Winning L, Patterson CC, Neville CE, et al. Periodontitis and incident type 2 diabetes: a prospective cohort study. J Clin Periodontol 2017;44:266–74. [DOI] [PubMed] [Google Scholar]
- [9].Obadan-Udoh E, Jordan S, Mudah O, et al. Gap analysis of older adults with type 2 diabetes receiving nonsurgical periodontal therapy. J Evid Based Dent Pract 2017;17:335–49. [DOI] [PubMed] [Google Scholar]
- [10].Swerdlow DI, Preiss D, Kuchenbaecker KB, et al. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet 2015;385:351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Uusitupa M. Remission of type 2 diabetes: mission not impossible. Lancet 2018;391:515–6. [DOI] [PubMed] [Google Scholar]
- [12].Hu Y, Zong G, Liu G, et al. Smoking cessation, weight change, type 2 diabetes, and mortality. New Engl J Med 2018;379:623–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chapple IL, Genco R. Working group 2 of joint EFPAAPw. Diabetes and periodontal diseases: consensus report of the joint EFP/AAP workshop on periodontitis and systemic diseases. J Clin Periodontol 2013;40suppl 14:S106–112. [DOI] [PubMed] [Google Scholar]
- [14].Nitta H, Katagiri S, Nagasawa T, et al. The number of microvascular complications is associated with an increased risk for severity of periodontitis in type 2 diabetes patients: Results of a multicenter hospital-based cross-sectional study. J Diabetes Investig 2017;8:677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chowdhury TA, Grant P. Drug therapies in type 2 diabetes: an era of personalised medicine. Clin Med 2016;16:441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Loe H, Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care 1993;16:329–34. [PubMed] [Google Scholar]
- [17].Jiang X, Fan X, Wu R, et al. The effect of care intervention for obese patients with type II diabetes. Medicine 2017;96:e7524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].El Kebir D, de Oliveira Lima Dos Santos E, Mansouri S, et al. Mild acidosis delays neutrophil apoptosis via multiple signaling pathways and acts in concert with inflammatory mediators. J Leukocyte Biol 2017;102:1389–400. [DOI] [PubMed] [Google Scholar]
- [19].Freire MO, Dalli J, Serhan CN, et al. Neutrophil resolvin E1 receptor expression and function in type 2 diabetes. J Immunol 2017;198:718–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang TF, Jen IA, Chou C, et al. Effects of periodontal therapy on metabolic control in patients with type 2 diabetes mellitus and periodontal disease: a meta-analysis. Medicine 2014;93:e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang J, Hossain M, Thanabalasuriar A, et al. Visualizing the function and fate of neutrophils in sterile injury and repair. Science 2017;358:111–6. [DOI] [PubMed] [Google Scholar]
- [22].Hegde V, Dworsky EM, Stavrakis AI, et al. Single-dose, preoperative vitamin-D supplementation decreases infection in a mouse model of periprosthetic joint infection. J Bone Jt Surg Am Vol 2017;99:1737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hoe E, Nathanielsz J, Toh ZQ, et al. Anti-inflammatory effects of vitamin D on human immune cells in the context of bacterial infection. Nutrients 2016;8: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mao X, Xing X, Xu R, et al. Folic acid and vitamins D and B12 correlate with homocysteine in Chinese patients with type-2 diabetes mellitus, hypertension, or cardiovascular disease. Medicine 2016;95:e2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ishii M, Yamaguchi Y, Isumi K, et al. Transgenic mice overexpressing vitamin D receptor (VDR) show anti-inflammatory effects in lung tissues. Inflammation 2017;40:2012–9. [DOI] [PubMed] [Google Scholar]
- [26].Nakashyan V, Tipton DA, Karydis A, et al. Effect of 1, 25 (OH)2 D3 and 20 (OH)D3 on interleukin-1beta-stimulated interleukin-6 and -8 production by human gingival fibroblasts. J Periodontal Res 2017;52:832–41. [DOI] [PubMed] [Google Scholar]
- [27].American Diabetes A 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes care 2018;41:S13–27. [DOI] [PubMed] [Google Scholar]
- [28].Soskolne WA, Klinger A. The relationship between periodontal diseases and diabetes: an overview. Ann Periodontol 2001;6:91–8. [DOI] [PubMed] [Google Scholar]
- [29].Rashidi Maybodi F, Haerian-Ardakani A, Vaziri F, et al. CPITN changes during pregnancy and maternal demographic factors ’impact on periodontal health. Iranian J Reprod Med 2015;13:107–12. [PMC free article] [PubMed] [Google Scholar]
- [30].Campbell IK, Leong D, Edwards KM, et al. Therapeutic targeting of the G-CSF receptor reduces neutrophil trafficking and joint inflammation in antibody-mediated inflammatory arthritis. J Immunol 2016;197:4392–402. [DOI] [PubMed] [Google Scholar]
- [31].Xu T, Weng Z, Pei C, et al. The relationship between neutrophil-to-lymphocyte ratio and diabetic peripheral neuropathy in Type 2 diabetes mellitus. Medicine 2017;96:e8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kaur PK, Narula SC, Rajput R, et al. Periodontal and glycemic effects of nonsurgical periodontal therapy in patients with type 2 diabetes stratified by baseline HbA1c. J Oral Sci 2015;57:201–11. [DOI] [PubMed] [Google Scholar]
- [33].Costa KL, Taboza ZA, Angelino GB, et al. Influence of periodontal disease on changes of glycated hemoglobin levels in patients with type 2 diabetes mellitus: a retrospective cohort study. J Periodontol 2017;88:17–25. [DOI] [PubMed] [Google Scholar]
- [34].Hayashi J, Hasegawa A, Hayashi K, et al. Effects of periodontal treatment on the medical status of patients with type 2 diabetes mellitus: a pilot study. BMC Oral Health 2017;17:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Herrera BS, Hasturk H, Kantarci A, et al. Impact of resolvin E1 on murine neutrophil phagocytosis in type 2 diabetes. Infect Immun 2015;83:792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Van der Velden U. What exactly distinguishes aggressive from chronic periodontitis: is it mainly a difference in the degree of bacterial invasiveness? Periodontology 2000 2017;75:24–44. [DOI] [PubMed] [Google Scholar]
- [37].Hienz SA, Paliwal S, Ivanovski S. Mechanisms of bone resorption in periodontitis. J Immunol Res 2015;2015:615486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hajishengallis E, Hajishengallis G. Neutrophil homeostasis and periodontal health in children and adults. J Dent Res 2014;93:231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nicu EA, Loos BG. Polymorphonuclear neutrophils in periodontitis and their possible modulation as a therapeutic approach. Periodontology 2000 2016;71:140–63. [DOI] [PubMed] [Google Scholar]
- [40].Manosudprasit A, Kantarci A, Hasturk H, et al. Spontaneous PMN apoptosis in type 2 diabetes and the impact of periodontitis. J Leukocyte Biol 2017;102:1431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Biosse Duplan M, Coyac BR, Bardet C, et al. Phosphate and vitamin D prevent periodontitis in X-linked hypophosphatemia. J Dent Res 2017;96:388–95. [DOI] [PubMed] [Google Scholar]
- [42].Zheng J, Chen S, Albiero ML, et al. Diabetes activates periodontal ligament fibroblasts via NF-kappaB in vivo. J Dent Res 2018;97:580–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Qu L, Liang X, Jiang B, et al. Risk factors affecting the prognosis of descending necrotizing mediastinitis from odontogenic infection. J Oral Maxillofac Surg 2018;76:1207–15. [DOI] [PubMed] [Google Scholar]
- [44].Balci Yuce H, Gokturk O, Aydemir Turkal H, et al. Assessment of local and systemic 25-hydroxy-vitamin D, RANKL, OPG, and TNF levels in patients with rheumatoid arthritis and periodontitis. J Oral Sci 2017;59:397–404. [DOI] [PubMed] [Google Scholar]
- [45].Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord 2017;18:153–65. [DOI] [PubMed] [Google Scholar]
- [46].Uwitonze AM, Murererehe J, Ineza MC, et al. Effects of vitamin D status on oral health. J Steroid Biochem Mol Biol 2018;175:190–4. [DOI] [PubMed] [Google Scholar]
- [47].Salvador-Bernaldez M, Mateus SB, Del Barco Barrantes I, et al. p38alpha regulates cytokine-induced IFNgamma secretion via the Mnk1/eIF4E pathway in Th1 cells. Immunol Cell Biol 2017;95:814–23. [DOI] [PubMed] [Google Scholar]
- [48].Park EJ, Park SW, Kim HJ, et al. Dehydrocostuslactone inhibits LPS-induced inflammation by p38MAPK-dependent induction of hemeoxygenase-1 in vitro and improves survival of mice in CLP-induced sepsis in vivo. Int Immunopharmacol 2014;22:332–40. [DOI] [PubMed] [Google Scholar]
- [49].Frasch SC, Nick JA, Fadok VA, et al. Henson PM. p38 mitogen-activated protein kinase-dependent and -independent intracellular signal transduction pathways leading to apoptosis in human neutrophils. J Biol Chem 1998;273:8389–97. [DOI] [PubMed] [Google Scholar]
- [50].Diomede F, Zingariello M, Cavalcanti M, et al. MyD88/ERK/NFkB pathways and pro-inflammatory cytokines release in periodontal ligament stem cells stimulated by Porphyromonas gingivalis. Eur J Histochem EJH 2017;61:2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Liu Y, Qingjuan S, Gao Z, et al. Circulating fibrocytes are involved in inflammation and leukocyte trafficking in neonates with necrotizing enterocolitis. Medicine 2017;96:e7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Batool H, Nadeem A, Kashif M, et al. Salivary levels of IL-6 and IL-17 could be an indicator of disease severity in patients with calculus associated chronic periodontitis. BioMed Res Int 2018;2018:8531961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mao CY, Wang YG, Zhang X, et al. Double-edged-sword effect of IL-1beta on the osteogenesis of periodontal ligament stem cells via crosstalk between the NF-kappaB, MAPK and BMP/Smad signaling pathways. Cell Death Dis 2016;7:e2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Malcolm J, Awang RA, Oliver-Bell J, et al. IL-33 exacerbates periodontal disease through induction of RANKL. J Dent Res 2015;94:968–75. [DOI] [PubMed] [Google Scholar]
- [55].Kim DS, Cha SB, Park MC, et al. Scopoletin stimulates melanogenesis via cAMP/PKA pathway and partially p38 activation. Biol Pharm Bull 2017;40:2068–74. [DOI] [PubMed] [Google Scholar]
- [56].Liu J, Zhu Y, Chen S, et al. Apocynin attenuates cobalt chloride-induced pheochromocytoma cell apoptosis by inhibiting P38-MAPK/Caspase-3 pathway. Cell Physiol Biochem 2018;48:208–14. [DOI] [PubMed] [Google Scholar]
- [57].Wang Q, Li H, Xie H, et al. 25-Hydroxyvitamin D3 attenuates experimental periodontitis through downregulation of TLR4 and JAK1/STAT3 signaling in diabetic mice. J Steroid Biochem Mol Biol 2013;135:43–50. [DOI] [PubMed] [Google Scholar]
- [58].Yang H, Long F, Zhang Y, et al. 1alpha,25-dihydroxyvitamin D3 induces neutrophil apoptosis through the p38 MAPK signaling pathway in chronic obstructive pulmonary disease patients. PLoS One 2015;10:e0120515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Li H, Wang Q, Xiao Y, et al. 25-Hydroxyvitamin D (3)-loaded PLA microspheres: in vitro characterization and application in diabetic periodontitis models. AAPS PharmSciTech 2013;14:880–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Poon CC, Li RW, Seto SW, et al. In vitro vitamin K (2) and 1alpha,25-dihydroxyvitamin D (3) combination enhances osteoblasts anabolism of diabetic mice. Eur J Pharmacol 2015;767:30–40. [DOI] [PubMed] [Google Scholar]
- [61].Bashutski JD, Eber RM, Kinney JS, et al. The impact of vitamin D status on periodontal surgery outcomes. J Dent Res 2011;90:1007–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hildebolt CF. Effect of vitamin D and calcium on periodontitis. J Periodontol 2005;76:1576–87. [DOI] [PubMed] [Google Scholar]
- [63].Alshouibi EN, Kaye EK, Cabral HJ, et al. Vitamin D and periodontal health in older men. J Dent Res 2013;92:689–93. [DOI] [PMC free article] [PubMed] [Google Scholar]


